Abstract
Aims
Although the linkages between aboveground photosynthates production and belowground respiration processes have been well studied, doubts remain as to the extent that photosynthate regulates soil respiration (Rsoil) and its generality throughout the growing season in a given ecosystem. This study aimed to test whether photosynthesis affected Rsoil at the diurnal scale and assess how the relation between them changed with changing vegetation composition.
Methods
We measured Rsoil and gross primary productivity (GPP using eddy covariance) in two consecutive growing seasons (2013–2014) in a desert ecosystem, western China. We compared Rsoil on sunny days with that on adjacent cloudy days in two periods with different vegetation compositions [herbs-shrub coexistence period (HSP) and purely shrubs dominated period (SDP)] to identify how photosynthesis affect Rsoil.
Results
GPP regulated diurnal variations of Rsoil conspicuously in spite of the strong correlation between soil temperature (Tsoil) and Rsoil, but such regulation was limited in HSP. 48.3% of the changes in daytime Rsoil between sunny and cloudy days was explained by changes in Tsoil together with changes of GPP in HSP. When spring annuals died, no differences in daily amplitude and average of Rsoil between sunny and cloudy days were found.
Conclusions
Our results suggested that effect of photosynthesis on Rsoil was not constant throughout the growing season. In the presence of herbaceous plants, Rsoil was directly related to photosynthesis. These findings highlighted the important role of photosynthesis played in Rsoil regulation and the importance of community composition in determining the extent that photosynthesis affects Rsoil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantifying ecosystem carbon budgets is essential for a sustainable future because CO2 plays an active role in Earth’s mass & energy budget. Soil respiration (Rsoil), representing a substantial source of CO2 emissions to the atmosphere (Raich and Potter 1995), can be a variable carbon flux, making its quantification important for improving our ability to predict ecosystem carbon dynamics (Barron-Gafford et al. 2014). Over the last decade, substantial progress has been made in modeling Rsoil by moving beyond the relationship between Rsoil and temperature (see Lloyd and Taylor 1994; Davidson et al. 2006a; Barron-Gafford et al. 2014; Cable et al. 2011; Liu et al. 2019) to develop frameworks and models incorporating with multiple biotic and abiotic effects, such as soil microbial biodiversity (Liu et al. 2018), and antecedent environmental conditions (Barron-Gafford et al. 2014). However, a notable amount of variation in Rsoil was still unexplained, and there remained significant challenges in mechanistically understanding carbon flux processes in soils. For example, doubts remain as to how aboveground–belowground linkages modulate carbon dynamics (Carbone and Trumbore 2007; Kayler et al. 2010), which has been reported to be largely responsible for the current limit of developing process-based models of Rsoil at short and medium temporal scales (Vargas et al. 2011; Han et al. 2014). Thus, understanding whether and how canopy photosynthesis regulates Rsoil has the potential to reveal the underlying determinants of Rsoil and improve our ability to quantify and predict ecosystem carbon balance in natural setting (Trumbore 2006; Barron-Gafford et al. 2014).
A tight linkage between Rsoil and aboveground photosynthesis has been widely reported for different ecosystems based on shading (Yan et al. 2011), clipping (Zhou et al. 2007), gridling (Hӧgberg et al. 2001), and/or isotopic labeling experiments (Hӧgberg et al. 2008; Bahn et al. 2009). In particular, the hysteretic relationship between Rsoil and temperature may partially result from abiotic forcing (Phillips et al. 2011), but may be also tied with the time delay in supply of recently assimilated products (Tang et al. 2005; Liu et al. 2006). Rsoil usually lags behind canopy photosynthesis hours even days (Tang et al. 2005; Kuzyakov and Gavrichkova 2010; Vargas et al. 2011; Yan et al. 2011; Han et al. 2014). The highly variable lag time between them has been suggested to be associated with plant functional type and the size of the plants (Kuzyakov and Gavrichkova 2010; Vargas et al. 2011), wherein large trees and shrubs tend to produce longer lag time than grasses or herbaceous plants.
Shifts in the distribution of vegetation is a widespread feature of global change, and uncovering the effect of such changes on ecosystem processes (e.g. ecosystem C flux) has been one of the core topics in modern ecology (Metcalfe et al. 2011). For example, shift from grass to woody plants, or vice versa, has been continually occurring in many regions of North and West America and other parts of the world (Van Auken 2000; Jackson et al. 2002; Carbone and Trumbore 2007), due to deforestation, desertification, and woody plant invasion. Ecosystem processes associated with such shifts, including carbon assimilation and plant-specific carbon use and/or allocation (Metcalfe et al. 2011), are modified as a result of changes in canopy structure and plant traits, such as leaf area index, root distribution (Jackson et al. 2000; Schenk and Jackson 2002) and maximum rooting depth (Breecker et al. 2012).
In term of Rsoil, the distribution and dominance of woody plants relative to grasses may not only influence the extent of photosynthetic influence on Rsoil but also the speed of the link between aboveground carbon fixation and belowground carbon release. Namely, the effect of photosynthesis on Rsoil varies with shifting vegetation composition, and this variation may also occur within a growing season owing to differences in plant life history and phenology (Huang and Li 2015). Unfortunately, previous studies associating Rsoil with aboveground carbon processes have mainly focused on a certain growth period (i.e., pulse labeling studies) or a particular target (e.g., grass or trees), and little is known about its continuity throughout the entire growth season in a mixed vegetation of woody/herb plants (Kuzyakov and Gavrichkova 2010).
In this study, we focused on C fluxes in the Gurbantonggut desert, the second largest desert in China. Its plant community comprises a typical “two-layer” structure (Liu et al. 2019) with considerable inter- or intra-annual variation in composition of herbs and shrubs (Fan et al. 2014; Huang and Li 2015), which provides a unique opportunate to test the effect of community composition on soil C fluxes. The main objectives of this study were to test the effect of canopy photosynthesis on Rsoil at a diurnal scale and to determine how such effect respond to shifting plant community composition. We accomplished this by automatic measurement of Rsoil and eddy covariance measurement of ecosystem photosynthesis with high resolution (e.g., hourly) in two consecutive growing seasons (2013 and 2014). We took advantage of natural variations in weather conditions and compared Rsoil on sunny days with that on adjacent cloudy days in two growing periods with distinct vegetation compositions (the dominance of herbs and shrubs from mid-Arip to late May, and purely shrubs dominance from early June to later October). We hypothesized that diurnal or day-to-day variation in Rsoil would be driven by a combination of temperature and canopy photosynthesis because canopy photosynthesis provides substrates for root and rhizosphere respiration (Bahn et al. 2008; Han et al. 2014). We also hypothesized that the control of canopy photosynthesis over diurnal Rsoil would weaken when plant community structure shifted from herbs and shrubs to only shrubs.
Material and methods
Study site
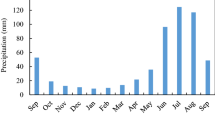
All fieldwork was conducted at the southern margin of the Gurbantonggut Desert, which is approximately 8 km away from the Fukang Station of Desert Ecology, Chinese Academy of Sciences (87°56′ E, 44°17′ N; 475 m a.s.l.). The plains in this area have a continental arid temperate climate with dry hot summers and cold winters. According to the meteorological data recorded in the Fukang Station of Desert Ecology over the last 30-years (1987–2016, data available at http://fkd.cern.ac.cn/), annual mean temperature is 6.6 °C and annual mean precipitation is ~160 mm. The soil is loamy sand (81.7% sand, 16.8% silt, and 1.5% clay) textured with high salinity and alkalinity (electrical conductivity >4dS m−1, pH > 8.2 for a soil solution at a soil:water ratio of 1:5), classified as Aridosols in the FAO/UNESCO soil classification system.
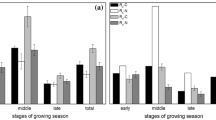
Plants in this area are mainly deep-rooted shrubs, such as Haloxylon ammodendron, and well-developed herbaceous plants, with maximal coverage of ca. 60%. More than 60% of the vegetation cover and up to 70–80% of herbaceous plant biomass are contributed by spring annuals from early spring to early summer (Fan et al. 2014). The dominant spring annuals mainly include Erodium oxyrrhynchum, Alyssum linifolium, and Nepeta micrantha, which account for more than 60% of herbaceous cover and 85% of biomass (Huang and Li 2015). Summer annuals and perennial herbs, including Ceratocarpus arenarius, Salsola foliosa, Descurainia Sophia, and Hyalea pulchella, usually coexist but only account for a very small portion of community biomass. Phenological observation revealed that spring annuals began to grow immediately after snow melt in late March, matured in early May, and usually died before late May (Fig. 1a). Growth of the dominant shrub H. ammodendron is usually slow in May, speeding up in early June until reaching its maximum biomass in late June or early July (Fig. 1b). Hence, the whole growing season could be divided into two periods: one in which herbs and shrubs coexisted (mid-April to late May; hereafter referred to as HSP), and one purely dominated by shrubs (early June to late October; SDP).
Dynamics of shrub leaf area index and herbaceous layer biomass
Shrub leaf area index (LAI) dynamics were determined by combining leaf (assimilating branch) relative growth rate with accumulated leaf biomass as described in Ma et al. (2014). Briefly, relative growth rate was derived from area changes in labeled assimilating branches, which were monitored by photographs at intervals of two weeks. Accumulated leaf biomass was measured using a harvesting method. At the end of the growing season, a 50 m × 3 m transect was randomly selected in the center of the footprint area of the eddy flux tower, and all assimilating branches in this transect were destructively sampled and dried (65 °C) to determine the accumulated leaf biomass. Based on the relationship between surface area and dry biomass, branch biomass was converted to branch area (per unit area). Coupled with variation in relative growth rate, seasonal LAI dynamics were determined.
Herbaceous plants were surveyed from mid-April to early October each year in 10 long-term monitoring quadrats (1 m × 1 m), which were random selected and fixed with iron wire in the footprint area of the flux tower at the beginning of the growing season in 2012. Plant coverage, abundance, and average height for each species were recorded synchronously. The frequency of plant survey was not fixed: at the beginning of the growing season, the biomass of spring ephemeral plants changed rapidly over time, and quadrats were surveyed every 12 days; whereas, when the spring ephemeral plants died, surveying frequency decreased to every 20 days. Finally, the dynamics of herbaceous plant biomass were determined using allometric equations as defined by Huang and Li (2015).
Root system investigation
The intact root system of H. ammodendron was excavated to investigate their vertical distribution. In total, five shrubs of approximately average size (average height, 2.15 m; average canopy diameter, 2.85 m) were randomly selected for excavation. A ring ditch with a diameter of 12 m was dug around each selected shrub. The soil surrounding the main root was removed manually at 0.1-m depth intervals to progressively expose the roots. In this way, the root could be traced layer by layer, and the diameter and length of lateral root segments could be measured section by section along the branches of the laterals with calipers and rulers. As the shape of the root segment resembled a cylinder, the root surface area was determined based on the recorded diameters and lengths of all root segments within each 0.1-m soil layer.
Root distribution of herbaceous plants was determined in late May when the biomass of herbaceous plants peaked (Fig. 1). Four quadrats, which were previously used to survey plants, were randomly selected to determine fine root biomass at intervals of 0.1 m.
Eddy covariance measurements
Ecosystem CO2 flux was monitored in 2013 and 2014 using an open-path eddy covariance (EC) system comprising a 3-D ultrasonic anemometer thermometer (STA-5055, KAIJO Corporation, Tokyo, Japan) and an open-path infrared gas (CO2/H2O) analyzer (LI-7500, LI-COR Inc., Lincoln, NE, USA). The former measured instantaneous fluctuations of the horizontal, vertical, and lateral wind speed and the virtual temperature. The latter measured instantaneous fluctuations in the concentration of CO2 and water vapor. These data were recorded with a data logger (CR23X, Campbell, Scientific Inc., UT, USA) at 30-min intervals. Additional meteorological parameters were measured simultaneously with an array of sensors, including photosynthetically active radiation (PAR) and net radiation (LI-190SB, Li-COR Inc., USA), air temperature and humidity (MP300, Campbell Scientific Ltd., Shepshed, UK), atmospheric pressure (CS106, Campbell Scientific Inc., USA), and precipitation (TE525 tipping bucket gauge, Texas Electronics, TX, USA). Soil temperature (Tsoil) was measured at six depths: 5, 10, 15, 20, 30, and 50 cm. Two soil heat-flux plates (HFP01SC, Hukseflux, the Netherlands) installed 5 cm below the soil surface monitored soil heat flux. All meteorological data were logged with another CR23X data logger. Soil water content (SWC) of the 0–10 cm soil layer was measured by a conventional balance-weighing and oven drying method at 10 a.m. with six replications.
Flux data processing
Raw EC data were processed in EdiRe (V.1.4.3.1186, www.geos.ed.ac.uk/abs/research/ micromet/EdiRe) to determine net ecosystem CO2 exchange (NEE). Data processing followed standard methods (Lee et al. 2004) and included despiking, coordinate rotation, auto-detection of the time delay between different sensors, spectral correction for sensor separation and sensor path length, and air density corrections. Additionally, 30-min flux data were rejected if the data were anomalous or measured under stable atmospheric conditions, which might have been derived from sensor maintenance, rain or snow, power failure, or insufficient mixing. Roughly 30–40% of the data were eliminated and gap-filling was required. Small gaps (i.e., less than 2 h) were filled by linear interpolation. Large gaps were filled separately for daytime and nighttime. For daytime, the relationship between photosynthetically active photon flux density and CO2 flux was used. Gap filling during the night was accomplished using the Lloyd-Taylor function between NEE (representing ecosystem respiration) and Tsoil. More details on flux data processing, quality control, and gap filling were described in Liu et al. (2012).
Based on the assumption that daytime ecosystem respiration (Reco,day) follows the same temperature response as during the night, 30-min Reco,day were determined using the same exponential functions (Lloyd-Taylor equation) developed for gap filling during the night:
where a and b are the fitting coefficients and Tsoil is soil temperature at 5 cm depth. Parameters for the temperature response of ecosystem respiration in different growing periods are listed in Fig. S1. Gross primary productivity (GPP) was determined by subtracting NEE from Reco.
Soil respiration measurements
Rsoil was monitored during the growing seasons (April 13–October 24, 2013; April 1–October 22, 2014), with an LI–8150 Automated Soil CO2 Flux System (LI-COR Inc., USA) equipped with six long-term monitoring chambers (LI-8100-104). Mosaic-distributed shrubs result in high levels of variation in soil physical and chemical characteristics, which are usually known as “fertilized islands” and “salty islands” (Li et al. 2007). To record such variations, which was also observed in Rsoil (Ma et al. 2012), measurements were taken under the H. ammodendron canopy (three monitoring chambers) and in the interplant space (the other three chambers) simultaneously. Shrubs of approximately average crown width and interplant sites where herbaceous plants were uniformly distributed were selected. To minimize disturbance, soil collars were installed at least 24 h prior to measurements and were left in place throughout the experiment. Measurements were taken every 30 min. Rsoil, at the ecosystem level, was estimated by area-weighted average with ratio of shrubs coverage (0.32) and interplant space (0.68) as weighting factors.
Leaf-level measurements of photosynthetic activity
The photosynthetic light-response curves of the dominant species (E. oxyrrhynchum and H. ammodendron) were measured using a LI-6400 portable photosynthesis system (LI-COR Inc., USA). In-chamber photosynthetic photon flux density (PPFDi) was controlled by a 20 × 30 mm2 leaf chamber with a red-blue light source, set as 0, 20, 50, 100, 150, 200, 300, 400, 600, 800, 1200, 1600, 1800, 2000 and 2200 μmol m−2 s−1. The gas flow rate was set as 400 μmol s−1 and chamber temperature was of 30 °C. A CO2-injecting device was attached to the system to control reference CO2 concentration at 400 μmol mol−1. For each species, leaves from three similar-sized individuals were selected and measurements were done in the morning (09:00–12:00) of the sunny days when the biomass of target species peaked (early May for E. oxyrrhynchum and late June for H. ammodendron). The photosynthetic light response curves were fitted with non-rectangle hyperbola models to obtain typical photosynthetic parameters: maximum net photosynthetic rate (Pmax), light saturation point (LSP) and light compensation point (LCP). More details about measurements and data processing are described in Xu and Li (2006).
Data analysis
To test the hypothesis that GPP contributes to diurnal or day-to-day Rsoil variations, we took advantage of natural weather transitions of sunny and cloudy days, and selected all paired days (20 in total) in these two growing seasons satisfying the following two criteria (Han et al. 2014): 1) the two days are adjacent, one was a sunny day and the other was cloudy, (2) no rain occurred during, and in five days before the two adjacent days. Under the conditions tested, it was reasonable to assume that changes in Rsoil on the two adjacent days were mainly determined by radiation through altering temperature and plant canopy photosynthesis, without any significant shifts in soil moisture, soil organic carbon, and plant condition (e.g., leaf area index and live biomass). We conducted a paired sample t-test to test the differences in the mean values of Rsoil and GPP between the sunny and adjacent cloudy days in the two growing periods (HSP and SDP), as well as the meteorological factors [PAR, relative humidity (RH), Tsoil, and SWC] between them. Linear and exponential regression analyses were used to evaluate the respective influences of GPP and Tsoil on daytime soil respiration on sunny and cloudy days respectively. Standardized major axis (SMA) regression analysis was performed to analyze the differences in slope of GPP–Tsoil between different growing periods. Additionally, multiple (stepwise) linear regression was applied to examine the effect of changes in GPP (ΔGPP) and changes in Tsoil (ΔTsoil) between sunny and cloudy days on the changes in daytime Rsoil (ΔRsoil). R2 change, a parameter indicating the contribution of a certain variable (ΔGPP or ΔTsoil) to the variations in Rsoil, was derived from the difference between model R2 of multiple linear regression with and without a certain variable. In all tests, a significance level of P = 0.05 was set.
As soil temperature variations are highly correlated with solar radiation, they would jointly affect GPP. Thus, it is important to determine the direct effect of GPP on Rsoil (Kuzyakov and Gavrichkova 2010). We accomplished this by separating temperature-dependent component (Rsoil_TD) from Rsoil using a similar approach as Liu et al. (2006), and then evaluated the direct effect of GPP on Rsoil based on a linear regression between GPP and temperature-independent component of Rsoil (Rsoil_TIN). Briefly, apart from the well-known role of Tsoil, many other temperature-independent factors such as photosynthesis, soil moisture, and substrate availability may also affect Rsoil. Namely, Rsoil comprises a temperature-dependent component and a temperature-independent component (Liu et al. 2006; Vargas and Allen 2008). We first established exponential functions between Rsoil and Tsoil in the two growing periods using data of the nighttime (23:00 and 06:00), during which temperature was assumed to be the only factor controlling Rsoil. Then, Rsoil_TD during daytime of sunny days was calculated by these functions based on Tsoil of the sunny days. Corresponding Rsoil_TIN was determined by the differences between directly measured Rsoil and Rsoil_TD.
SMA regression analyses were carried out with R 3.6.2 (R-package SMATR, http://www.r-project.org/). Other statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Root distribution
The vertical root distributions of herbaceous plants (expressed as root biomass per unit area) and H. ammodendron (expressed as surface area of feeder roots per individual plant) are presented in Fig. 2. The maximum rooting depth for herbaceous plants was found to be around 80 cm. The biomass of active absorbing roots decreased with depth, with more than 80% concentrated in the top 30 cm of soil (Fig.2a). In contrast, the main root of H. ammodendron extended to ~3 m, close to the 3.3-m groundwater table. More than 80% of absorbing roots were distributed below 40 cm and few feeder roots were found in the upper 0–30 cm (Fig. 2b).
Vertical distribution of roots for herbaceous plants (expressed as root biomass per unit area, a) and H. ammodendron (expressed as surface area of feeder roots per individual plant, b). Data are displayed as boxplots, where center is median, box top and bottom are 25th and 75th percentiles, hollow circles indicate 1th and 99th percentiles, solid squares indicate mean average and whiskers reflect standard deviation. Outliers are denoted with plus signs
Diurnal variation in Rsoil and GPP
Diurnal variation in Rsoil exhibited a single peak on both sunny and cloudy days with flux rates varying from 0.01 μmol∙m−2 s−1 to 1.74 μmol∙m−2 s−1 (Fig. 3). The amplitude of Rsoil differed considerably between sunny and cloudy days. From April to early June when shrubs coexisted with herbaceous plants, Rsoil was lower on cloudy days and fluctuated within a relatively narrow range compared with that on sunny days. By contrast, there were no apparent differences in Rsoil between sunny and cloudy days from mid-June to the end of the growing season, when shrubs dominated. GPP exhibited a bell-shaped curve on a sunny day, but no clear diurnal trend was observed on a cloudy day.
Diurnal variation in soil respiration (Rsoil) and gross primary productivity (GPP) on the 20 paired days in the growing seasons of 2013 and 2014. Grey shading indicates the cloudy days and the unshaded parts represented the sunny days. Rsoil was measured once every 30 min, and data were represented as mean ± standard error (n = 3). Data of GPP are represented as 30-min averages
To gain further insight into the diurnal and day-to-day variations in Rsoil under different radiation conditions and growing periods, data of GPP, Rsoil, and Tsoil were averaged from sunny and cloudy days during HSP (7 paired days in total) and SDP (12 paired days in total) (Fig. 4), respectively. Diurnal trends in Rsoil lagged behind GPP but preceded Tsoil on sunny days. In the mornings (07:00–09:30), Rsoil increased gradually with increasing GPP but Tsoil decreased. In the afternoons, Rsoil decreased following declining GPP (Fig. 4). Peak values of Rsoil were observed around 1 h (0–2.5 h) later than that of GPP (Table S1).
Weather conditions and carbon flux on sunny and adjacent cloudy days
On sunny days, PAR was significantly higher than that on corresponding cloudy days for both HSP and SDP (Fig. 5a). Accordingly, averaged Tsoil was significantly lowered by approximately 2.8 °C in HSP, and about 4.2 °C in SDP on cloudy days, compared to the corresponding sunny days. In contrast, there was no significant difference in SWC and RH between cloudy and sunny days in either period (Fig. 5b and d).
Comparisons of photosynthetically active radiation (PAR, a), relative humidity (RH, b), soil temperature at 5 cm depth (Tsoil, c), soil water content (SWC, d), gross primary productivity (GPP, e) and soil respiration (Rsoil, d) between sunny and adjacent cloudy days. Values are means of sunny and cloudy days in the herb-shrub coexistence period (HSP, n = 7) and shrub-dominated period (SDP, n = 12). Bars with the same letter do not significantly differ (P < 0.05). Data are displayed as boxplots, where center is median, box top and bottom are 25th and 75th percentiles, hollow circles indicate 1th and 99th percentiles, solid squares indicate mean average and whiskers reflect standard deviation. Outliers are denoted with plus signs
Cloudy conditions significantly lowered the leaf-level photosynthetic rates of dominant plants (Fig. S2) and thereby average values of GPP in both growing periods (P < 0.001; Fig. 5e and f). On cloudy days, GPP decreased by 46.09% in HSP (from 1.99 ± 0.23 μmol m−2 s−1 to 1.07 ± 0.16 μmol m−2 s−1) and ~50% in SDP (from 1.89 ± 0.23 μmol m−2 s−1 to 0.94 ± 0.16 μmol m−2 s−1), compared to the corresponding sunny days. Similarly, there was a significant decrease in Rsoil on cloudy days (0.336 ± 0.033 μmol m−2 s−1), compared to corresponding sunny days (0.54 ± 0.07 μmol m−2 s−1) in HSP (P < 0.001). However, during SDP, Rsoil was not significantly affected by cloudy conditions (all values were around 0.15 μmol m−2 s−1) (Fig. 5f).
Influence of GPP and Tsoil on daytime Rsoil variation
Daytime Rsoil on sunny days was positively correlated with GPP in both growing periods (Fig. 6; R2 = 0.518, P < 0.001 in HSP and R2 = 0.235, P < 0.001 in SDP) and the slope of Rsoil–GPP in HSP was significantly higher than that in SDP (P < 0.001). On cloudy days, daytime Rsoil exhibited the same relationship with GPP (Fig. 6; R2 = 0.443, P < 0.001 in HSP and R2 = 0.250, P < 0.001 in SDP) but no significant difference was observed in the slopes of the linear regression in these two periods (P = 0.431). Meanwhile, daytime Rsoil increased exponentially with Tsoil (R2 = 0.475, P < 0.001 on sunny days and R2 = 0.676, P < 0.001 on cloudy days in HSP; R2 = 0.485, P < 0.001 on sunny days and R2 = 0.425, P < 0.001 on cloudy days in SDP). In HSP, change in Rsoil between sunny and cloudy days was positively correlated with ΔGPP (R2 = 0.229, P < 0.001) and ΔTsoil (R2 = 0.308, P < 0.001; Fig. 7), respectively. However, ΔRsoil only increased with ΔTsoil in SDP. Multiple linear regression analysis also suggested that ΔTsoil (R2 change = 0.311, P < 0.001; R2 change indicating the contribution of a certain variable to explanation of the variations in Rsoil) and ΔGPP (R2 change = 0.177, P < 0.001) together explained 48.3% of the changes in daytime Rsoil between sunny and cloudy days in HSP, whereas only 19.9% of the changes in Rsoil was explained by change in Tsoil in SDP (Table 1).
To gain further insight into the direct effect of GPP, Rsoil_TD (temperature-dependent component of Rsoil) was removed based on the temperature response function (Fig. S3). Results showed that Rsoil_TIN (temperature-independent component of Rsoil) was an important component of Rsoil in HSP (Fig. 8), with daily average values from 0.058 μmol m−2 s−1 to 0.81 μmol m−2 s−1. More importantly, Rsoil_TIN was significantly correlated with GPP (R2 = 0.854, P < 0.001, Fig. 9).
Diurnal variation in directly observed soil respiration (Rsoil) and its temperature-dependent (Rsoil_TD) and temperature-independent components (Rsoil_TIN) on sunny days in the herb–shrub coexistence period. Rsoil_TIN was calculated as the difference between observed Rsoil and the fitted Rsoil_TD. Error bars of Rsoil_TD indicate 95% confidence intervals
Discussion
Differed effect of canopy photosynthesis on Rsoil in different growing periods
Our findings demonstrated that the influence of canopy photosynthesis on Rsoil differed for the two growing periods, indicating vegetation composition determined the magnitude of Rsoil and the contribution of GPP to diurnal Rsoil variations. Given the distinct traits (root distribution and plant height) of dominant plants for these two periods, such different effects of GPP were not surprising. Herbaceous plants survive on rain and snow melt water (Fan et al. 2014; Huang and Li 2015), and their roots are generally concentrated in upper 30 cm soil layer. Rsoil during HSP was thereby mainly driven by root respiration and corresponding rhizosphere respiration in surface soil layer, which would rapidly respond to changes in aboveground carbon assimilation due to shorter path lengths for photosynthates transport (Tang et al. 2005; Vargas and Allen 2008; Bahn et al. 2009; Han et al. 2014). In contrast, desert shrubs, mainly relying on deep soil water or groundwater for their survival (Dai et al. 2015), extend their roots into deep soil (Fig. 2b; Xu and Li 2006). Considerable Rsoil was derived from deeper soil layers (Breecker et al. 2012; Hirsch et al. 2002; Davidson et al. 2006b) and the time required for CO2 assimilation by shrubs, transport of photosynthates to roots, and subsequent diffusion of respired CO2 from soil would be significantly prolonged (Hӧgberg et al. 2008; Subke et al. 2009). For example, based on a radiocarbon (14C) pulse-chase labeling experiment, Warembourg and Paul (1973) found that labeled CO2 appeared 24 h after pulse labelling at 35 cm depth, while 100 h was needed at 60 cm. The deep rooting pattern of desert shrubs (Fig.2b, Xu et al. 2011) suggests that respired CO2 would need much longer time to diffuse out of the soil surface.
The nearly synchronous patterns of Rsoil and canopy photosynthesis in HSP suggested a tight link between recent photosynthates and Rsoil (Kuzyakov and Gavrichkova 2010; Han et al. 2014). More importantly, it also indicated that the speed for photosynthates translocating from the canopy to belowground might be faster than previously assumed. This finding was consistent with observations reported in many previous studies (Tang et al. 2005; Vargas and Allen 2008; Bahn et al. 2009; Yan et al. 2011; Han et al. 2014). Several potential mechanisms have been suggested to account for the sub-daily level links between plant photosynthesis and Rsoil. Firstly, the recent photoassimilates might be directly supplied to belowground and utilized in Rsoil processes with a sufficiently short time to influence diurnal Rsoil (Kuzyakov et al. 2001; Vargas and Allen 2008; Yan et al. 2011). Secondly, the indirect effect of photosynthesis on root respiration may also be rapid through ion uptake, phloem loading (or unloading), and root exudate production (Thompson and Holbrook 2004; Liu et al. 2006). Information at the physiological level could be rapidly transmitted to roots in the form of concentration or/and pressure waves, which might trigger immediate responses in the root tips (Thompson and Holbrook 2004; Davidson and Holbrook 2009).
With no significant influence of GPP on Rsoil at diel timescale in SDP, it is reasonable to expect time lags between photosynthesis and Rsoil at a timescale >1 day, which has been widely reported for woody vegetation (Hӧgberg et al. 2008; Moyano et al. 2008; Subke et al. 2009; Kayler et al. 2010; Kuzyakov and Gavrichkova 2010). Unfortunately, we were not be able to determine the exact lag time owing to the co-varied multiple factors for regulation of allocation, transport and utilization of photoassimilates, such as plant physiology, growth stages (Kaylor et al. 2010; Kuzyakov and Gavrichkova 2010; Savage et al. 2013) and variable respiration sources (e.g., the fast pool or storage pool; Carbone and Trumbore 2007), which has gone beyond the scope of the current study. Nevertheless, the distinct responses of Rsoil to decreasing canopy photosynthesis in different growing periods provided evidence that the response speed of Rsoil to photosynthesis is not constant throughout the growing season. More importantly, in the presence of herbaceous plants, Rsoil directly responded to canopy photosynthesis on a diurnal scale in this deep-rooted shrub-dominated desert plant community.
Diurnal variation in Rsoil
We found that Rsoil significantly reduced on cloudy days in HSP, which resulted from two mechanisms. For one thing, Rsoil decreased with decreasing Tsoil (Fig. 5). For another, decreased incoming radiation reduced canopy photosynthesis (Figs. 5 and 9) and the allocation of recent photosynthates to belowground, and finally lowered the rate of Rsoil (Wan and Luo 2003).
After removing the temperature-dependent component of Rsoil, changes in daytime Rsoil were ultimately attributed to the differences in GPP in HSP (Fig. 9). This finding was a valuable addition to the increasing evidence showing that photosynthesis played an important role in regulating diel Rsoil (Tang et al. 2005; Vargas and Allen 2008; Bahn et al. 2009; Davidson and Holbrook 2009; Kuzyakov and Gavrichkova 2010; Han et al. 2014). Canopy photosynthate regulated Rsoil mainly via its control over recent photosynthesis substrate supply. First, as discussed above, autotrophic Rsoil is a direct consequence of root respiration. Second, recent photosynthates via root exudates (sugars or other labile C components) support substantial microbial activity in the rhizosphere, which may significantly decrease without photosynthates supply (Butler et al. 2003; Hӧgberg et al. 2008; Kuzyakov 2010). Moreover, the amount and intensity of labile organic carbon input (by root exudation) potentially varies with plant productivity, and thereby strongly affects the ability of microorganisms to decompose soil organic carbon and the amount of CO2 emitted to the atmosphere. Hence, it is necessary to incorporate canopy photosynthesis or radiation (a proxy of photosynthesis) as one of the key drivers in Rsoil modeling (Vargas and Allen 2008; Bahn et al. 2009; Davidson and Holbrook 2009; Kuzyakov and Gavrichkova 2010; Han et al. 2014). Failure to consider these important but hidden regulatory roles of photosynthesis for Rsoil may lead to erroneous interpretations of belowground processes related to C turnover and of Rsoil data.
Characteristics and limitations of the current study
Our study has evaluated the influence of canopy photosynthesis on Rsoil by taking advantage of natural shifts in sunny and cloudy days. Compared with many other indirect methods (e.g., trenching, girdling, or clipping), this approach is based on undisturbed field measurements without any artificial treatments, avoiding the possible alterations of other important factors, such as biomass, LAI, soil organic matter, or moisture content (Fig. 5). Although isotope labeling method has the same advantage as the approach we used, it cannot avoid interference by substrate availability resulted from sudden changes in canopy photosynthesis (Mencuccini and Hӧlttӓ 2010) and is highly financial costs (Kuzyakov and Gavrichkova 2010). Despite the advantages, the approach used in this study also has some pitfalls. Since we are completely relying on natural weather conditions, the effect of canopy photosynthesis on Rsoil was only analyzed on 20 pairs of sunny and cloudy days, which did not strictly cover the whole growing season. Moreover, our study may not have been able to disclose the potential influence of phenological changes in plant photosynthesis, allocation of assimilation and other physiological parameters on seasonal Rsoil variations because of lack of phenological data. Hence, future studies should incorporate leaf - or individual -level physiological changes of dominant plants with a long-term, continueous datasets of CO2 flux (plant, soil and ecosystem) and combine different approaches, including isotopes, wavelet coherence analysis, and mechanism-based models.
Although this study was carried out in an arid environment, the inconsistent effects of photosynthesis on Rsoil observed in this study are likely to be pervasive in all other ecosystems experiencing considerable seasonal changes in plant composition and phenology. Given the projected changes in regional climate and vegetation distribution, our findings highlighted the importance of community composition in determining to what extent that photosynthesis are directly related to Rsoil.
References
Bahn M, Rodeghiero M, Anderson-Dunn M, Dore S, Gimeno C, Drosler M, Williams M, Ammann C, Berninger F, Flechard C, Jones S, Balzarolo M, Kumar S, Newesely C, Priwitzer T, Raschi A, Siegwolf R, Susiluoto S, Tenhunen J, Wohlfahrt G, Cernusca A (2008) Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems 11:1352–1367. https://doi.org/10.1007/s10021-008-9198-0
Bahn M, Schmitt M, Siegwolf R, Richter A, Bruggemann N (2009) Does photosynthesis affect grassland soil-respired CO2 and its carbon isotope composition on a diurnal timescale? New Phytol 182:451–460. https://doi.org/10.1111/j.1469-8137.2008.02755.x
Barron-Gafford GA, Cable JM, Bentley LP, Scott RL, Huxman TE, Jenerette GD, Ogle K (2014) Quantifying the timescales over which exogenous and endogenous conditions affect soil respiration. New Phytol 202:442–454. https://doi.org/10.1111/nph.12675
Breecker DO, McFadden LD, Sharp ZD, Martinez M, Litvak ME (2012) Deep autotrophic soil sespiration in shrubland and woodland ecosystems in Central New Mexico. Ecosystems 15:83–96. https://doi.org/10.1007/s10021-011-9495-x
Butler JL, Williams MA, Bottomley PJ, Myrold DD (2003) Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microb 69:6793–6800. https://doi.org/10.1128/Aem.69.11.6793-6800.2003
Cable JM, Ogle K, Lucas RW, Huxman TE, Loik ME, Smith SD, Tissue DT, Ewers BE, Pendall E, Welker JM, Charlet TN, Cleary M, Griffith A, Nowak RS, Rogers M, Steltzer H, Sullivan PF, van Gestel NC (2011) The temperature responses of soil respiration in deserts: a seven desert synthesis. Biogeochemistry 103:71–90. https://doi.org/10.1007/s10533-010-9448-z
Carbone MS, Trumbore SE (2007) Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns. New Phytol 176:124–135. https://doi.org/10.1111/j.1469-8137.2007.02153.x
Dai Y, Zheng XJ, Tang LS, Li Y (2015) Stable oxygen isotopes reveal distinct water use patterns of two Haloxylon species in the Gurbantonggut Desert. Plant Soil 389:73–87. https://doi.org/10.1007/s11104-014-2342-z
Davidson EA, Holbrook M (2009) Is temporal variation of soil respiration linked to the phenology of photosynthesis? Phenology of ecosystem processes. Springer, New York
Davidson EA, Janssens IA, Luo YQ (2006a) On the variability of respiration in terrestrial ecosystems: moving beyond Q(10). Glob Chang Biol 12:154–164. https://doi.org/10.1111/j.1365-2486.2005.01065.x
Davidson EA, Savage KE, Trumbore SE, Borken W (2006b) Vertical partitioning of CO2 production within a temperate forest soil. Glob Chang Biol 12:944–956. https://doi.org/10.1111/j.1365-2486.2005.01142.x
Fan LL, Tang LS, Wu LF, Ma J, Li Y (2014) The limited role of snow water in the growth and development of ephemeral plants in a cold desert. J Veg Sci 25:681–690. https://doi.org/10.1111/jvs.12121
Han GX, Luo YQ, Li DJ, Xia JY, Xing QH, Yu JB (2014) Ecosystem photosynthesis regulates soil respiration on a diurnal scale with a short-term time lag in a coastal wetland. Soil Biol Biochem 68:85–94. https://doi.org/10.1016/j.soilbio.2013.09.024
Hirsch AI, Trumbore SE, Goulden ML (2002) Direct measurement of the deep soil respiration accompanying seasonal thawing of a boreal forest soil. J Geophys Res-Atmos 108. https://doi.org/10.1029/2001jd000921
Hӧgberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792. https://doi.org/10.1038/35081058
Hӧgberg P, Hogberg MN, Gottlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, Linder S, Nasholm T (2008) High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol 177:220–228. https://doi.org/10.1111/j.1469-8137.2007.02238.x
Huang G, Li Y (2015) Phenological transition dictates the seasonal dynamics of ecosystem carbon exchange in a desert steppe. J Veg Sci 26:337–347. https://doi.org/10.1111/jvs.12236
Jackson RB, Schenk HJ, Jobbagy EG, Canadell J, Colello GD, Dickinson RE, Field CB, Friedlingstein P, Heimann M, Hibbard K, Kicklighter DW, Kleidon A, Neilson RP, Parton WJ, Sala OE, Sykes MT (2000) Belowground consequences of vegetation change and their treatment in models. Ecol Appl 10:470–483. https://doi.org/10.1890/1051-0761(2000)010[0470:Bcovca]2.0.Co;2
Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626. https://doi.org/10.1038/nature00910
Kaylor Z, Gessler A, Buchmann N (2010) What is the speed of link between aboveground and belowground processes? New Phytol 187:886–888. https://doi.org/10.1111/j.1469-8137.2010.03332.x
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Gavrichkova O (2010) Time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Glob Chang Biol 16:3386–3406. https://doi.org/10.1111/j.1365-2486.2010.02179.x
Kuzyakov Y, Ehrensberger H, Stahr K (2001) Carbon partitioning and below-ground translocation by Lolium perenne. Soil Biol Biochem 33:61–74. https://doi.org/10.1016/S0038-0717(00)00115-2
Lee X, Massman M, Law B (2004) Handbook of micrometeorology. A guide for surface flux measurement and analysis, Kluwer Academic, Boston
Li J, Zhao C, Zhu H, Li Y, Wang F (2007) Effect of plant species on shrub fertile island at an oasis-desert ecotone in the south Junggar Basin, China. J Arid Environ 71:350–361. https://doi.org/10.1016/j.jaridenv.2007.03.015
Liu Q, Edwards N, Post W, L, Ledford J, Lenhart S (2006) Temperature-independent diel variation in soil respiration observed from a temperate deciduous forest. Glob Chang Biol 12: 2136–2145. doi: https://doi.org/10.1111/j.1365-2486.2006.01245.x
Liu R, Li Y, Wang QX (2012) Variations in water and CO2 fluxes over a saline desert in western China. Hydrol Process 26:513–522. https://doi.org/10.1002/hyp.8147
Liu YR, Delgado-Baquerizo M, Wang JT, Hu HW, Yang ZM, He JZ (2018) New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol Biochem 118:35–41. https://doi.org/10.1016/j.soilbio.2017.12.003
Liu R, Li Y, Wang YG, Ma J, Cieraad E (2019) Variation of water use efficiency across seasons and years: different role of herbaceous plants in desert ecosystem. Sci Total Environ 647:827–835. https://doi.org/10.1016/j.scitotenv.2018.08.035
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8(3):315-323. https://doi.org/10.2307/2389824
Ma J, Zheng XJ, Li Y (2012) The response of CO2 flux to rain pulses at a saline desert. Hydrol Process 26:4029–4037. https://doi.org/10.1002/hyp.9204
Ma J, Liu R, Tang LS, Lan ZD, Li Y (2014) A downward CO2 flux seems to have nowhere to go. Biogeosciences 11:6251–6262. https://doi.org/10.5194/bg-11-6251-2014
Mencuccini M, Hӧlttӓ T (2010) The significance of phloem transport for the speed with which canopy photosynthesis and belowground respiration are linked. New Phytol 185:189–203. https://doi.org/10.1111/j.1469-8137.2009.03050.x
Metcalfe DB, Fisher RA, Wardle DA (2011) Plant communities as drivers of soil respiration: pathways, mechanisms, and significance for global change. Biogeosciences 8:2047–2061. https://doi.org/10.5194/bg-8-2047-2011
Moyano FE, Kutsch WL, Rebmann C (2008) Soil respiration fluxes in relation to photosynthetic activity in broad-leaf and needle-leaf forest stands. Agric For Meteorol 148:135–143. https://doi.org/10.1016/j.agrformet.2007.09.006
Phillips CL, Nickerson N, Risk D, Bond BJ (2011) Interpreting diel hysteresis between soil respiration and temperature. Glob Chang Biol 17:515–527. https://doi.org/10.1111/j.1365-2486.2010.02250.x
Raich JW, Potter CS (1995) Global patterns of carbon-dioxide emissions from soils. Global Biogeochem Cy 9:23–36. https://doi.org/10.1029/94gb02723
Savage K, Davidson EA, Tang J (2013) Diel patterns of autotrophic and heterotrophic respiration among phenological stages. Glob Chang Biol 19:1151–1159. https://doi.org/10.1111/gcb.12108
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494. https://doi.org/10.1046/j.1365-2745.2002.00682.x
Subke JA, Vallack HW, Magnusson T, Keel SG, Metcalfe DB, Hogberg P, Ineson P (2009) Short-term dynamics of abiotic and biotic soil 13CO2 effluxes after in situ 13CO2 pulse labelling of a boreal pine forest. New Phytol 183:349–357. https://doi.org/10.1111/j.1469-8137.2009.02883.x
Tang J, Baldocchi DD, Xu L (2005) Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob Chang Biol 11:1298–1304. https://doi.org/10.1111/j.1365-2486.2005.00978.x
Thompson MV, Holbrook NM (2004) Scaling phloem transport: information transmission. Plant Cell Environ 27:509–519. https://doi.org/10.1111/j.1365-3040.2003.01148.x
Trumbore S (2006) Carbon respired by terrestrial ecosystems - recent progress and challenges. Glob Chang Biol 12:141–153. https://doi.org/10.1111/j.1365-2486.2006.01067.x
Van Auken OW (2000) Shrub invasions of north American semiarid grasslands. Annu Rev Ecol Syst 31:197–215. https://doi.org/10.1146/annurev.ecolsys.31.1.197
Vargas R, Allen MF (2008) Environmental controls and the influence of vegetation type, fine roots and rhizomorphs on diel and seasonal variation in soil respiration. New Phytol 179:460–471. https://doi.org/10.1111/j.1469-8137.2008.02481.x
Vargas R, Baldocchi DD, Bahn M, Hanson PJ, Hosman KP, Kulmala L, Pumpanen J, Yang B (2011) On the multi-temporal correlation between photosynthesis and soil CO2 efflux: reconciling lags and observations. New Phytol 191:1006–1017. https://doi.org/10.1111/j.1469-8137.2011.03771.x
Wan SQ, Luo YQ (2003) Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochem Cy 17. https://doi.org/10.1029/2002gb001971
Warembourg FR, Paul EA (1973) Use of 14CO2 canopy techniques for measuring carbon transfer through plant-soil system. Plant Soil 38:331–345. https://doi.org/10.1007/Bf00779017
Xu H, Li Y (2006) Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant Soil 285:5–17. https://doi.org/10.1007/s11104-005-5108-9
Xu GQ, Li Y, Xu H (2011) Seasonal variation in plant hydraulic traits of two co-occurring desert shrubs, Tamarix ramosissima and Haloxylon ammodendron, with different rooting patterns. Ecol Res 26:1071–1080. https://doi.org/10.1007/s11284-011-0858-8
Yan LM, Chen SP, Huang JH, Lin GH (2011) Water regulated effects of photosynthetic substrate supply on soil respiration in a semiarid steppe. Glob Chang Biol 17:1990–2001. https://doi.org/10.1111/j.1365-2486.2010.02365.x
Zhou X, Wan SQ, Luo YQ (2007) Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob Chang Biol 13:761–775. https://doi.org/10.1111/j.1365-2486.2007.01333.x
Acknowledgments
The authors thank all staff of the Fukang Station of Desert Ecology for technique support and assistance in field work and three anonymous reviewers for many valuable suggestions. This study was funded by State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, CAS (G2016-02-02), National Science Foundation of China (41730638,41771121 and 41671114) and Xinjiang Province “Tianshan Youths” Project (2018Q081).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 0.98 mb)
Rights and permissions
About this article
Cite this article
Ma, J., Liu, R., Li, C. et al. Herbaceous layer determines the relationship between soil respiration and photosynthesis in a shrub-dominated desert plant community. Plant Soil 449, 193–207 (2020). https://doi.org/10.1007/s11104-020-04484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04484-6