Abstract
In situ carbon flux studies are typically rare over the Himalaya but are important to understand carbon (C) balance. We investigated night-time canopy respiration (Rnc) and soil respiration (Rs) of a deciduous coniferous forest in response to environmental factors. A comprehensive investigation has been carried out on C balance indicators by employing systematic and concurrent measurements over an annual growth cycle of pine (Nov 2010–Dec 2011). The study site consists of uniformly distributed young deciduous Pinus roxburghii plantation having understory as Lantana camara (an invasive shrub). Results underlined that both Rnc and Rs fluxes were highest in the post-monsoon season. Evaporative fraction (EF) and temperature explained maximum variability of fluxes during warm-moist monsoon. Our key finding depicts an inverse significant correlation between day-time canopy photosynthesis (Ac) and Rnc across the seasons (r = 0.83–0.99). This can be explained by the mechanistic physiological phase of optimal anabolism (Ac) with favorable environmental conditions and minimum level of catabolism (Rnc). The respiration-photosynthesis ratio (Rnc/Ac) typically ranged from 0.25 ± 0.11 (peak growing season) to 0.71 ± 0.16 (winter season) with mean of 0.26 ± 0.10. The ratio Rs/Ac was highest during the winter season (2.69 ± 0.43), while minimum during peak growing season (0.64 ± 0.29). The Rnc/Ac ratio and night-time temperature (AT) also revealed that the ratio could increase when AT crossed 24 °C. These responses indicate that under climate warming, it may have a significant influence on net plant C uptake. Presence of understory shrub minimizes the Rnc/Ac ratio, and indicative of a more positive C-balance. Nevertheless, the observations could certainly lend useful insight into C-balance and ecological function in the region. Further, it may be useful in parameterizing and validating C-cycle models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mechanisms controlling the terrestrial carbon (C) cycle components and their responses to the changing environment across the Himalayan ecosystems are usually unknown. Over the last few decades, ecosystems of the Himalaya have experienced three times more warming than the global average (Bhutiyani et al. 2007; Xu et al. 2009; Shrestha et al. 2012). Increased diurnal temperature and change in precipitation patterns in response to climate warming have altered the regional vegetation in Himalaya (Yadav et al. 2004; Basistha et al. 2009). In view of such environmental change and their impact on C-cycle components, it demands field-based measurements data for understanding the underlying C-cycle processes and controlling environmental factors. In this viewpoint, Indian Space Research Organization (ISRO) undertook an initiative to characterize energy–water–carbon exchange, and their control on mid-altitudinal western Himalayan forests by establishing an INSAT (Indian National Satellite) linked micrometeorological tower of 13 m height at the Forest Research Institute campus, Dehradun, India (Bhattacharya et al. 2013).
The deciduous conifer forest, Pinus roxburghii (sarg.) is the dominant vegetation in the lower western Himalayan region (elevation of 500–2000 m) that covers ~ 18,650 km2 area, and constitutes ~ 6% of the total forested area of India (Forest Survey of India 2011). Given its climatic and ecological importance, understanding on energy-water-carbon exchange fluxes becomes critical to ascertain various environmental controlling factors. In our earlier studies, radiation-energy balance and inter-linkages of C (canopy photosynthesis, Ac), and water fluxes were investigated across the seasons. Results underlined that the degree of coupling between water and C exchange was stronger during post-monsoon and spring seasons (Singh et al. 2014a, b). In this study, we aimed to investigate seasonal dynamics of the canopy (plant) and soil respiration fluxes based on in situ field measurements data and to characterize the dynamics of these fluxes in relation to physical environmental factors, biophysical, and ecophysiological variables.
One of the critical C-balance components is plant respiration, which is a pivotal constituent of forest C-balance. Change in this component would alter atmospheric CO2 concentration, and thereby, it may have a significant effect on global C-cycle strength. Photosynthetically fixed C is allocated to a variety of plant usages, and 50–80% of C is returned immediately to the atmosphere through canopy respiration (Hagihara and Hozumi 1991; Turnbull et al. 2002; Atkin et al. 2007; DeLucia et al. 2007). It is well known that the day-time and night-time canopy respiration (Rnc) are not equal in magnitude, and the Rnc alone constitutes a major fraction of canopy respiration, as day-time respiration is strongly inhibited by light (Sun et al. 2015; Wehr et al. 2016). Measurement of Rnc becomes essential to understand net ecosystem C-balance as the respiration rates obtained during night-time is more stable because the impact of sugar synthesis and transport is minimum (Will and Ceulemans 1997). It is also likely that the temperature-induced changes in Rnc and soil respiration (Rs) could have a large impact on C-exchange and storage (Cox et al. 2000; Piao et al. 2010; Anderegg et al. 2015). Further, there is seasonal constancy in respiration-photosynthesis (Rnc/Ac) ratio until unless perturbed by some external factors (Gifford 2003; Atkin et al. 2007; DeLucia et al. 2007; Van Oijen et al. 2010). It would be interesting to investigate environmental controls on respiration fluxes in the Himalaya, where seasonal transition phases are prominent and changes in annual phenology are drastic.
Rs is the flux of microbially and plant root-respired CO2 from the soil surface to the atmosphere, and it represents a major flux of C into the atmosphere (Schlesinger and Andrews 2000; Bond-Lamberty and Thomson 2010). The variability of Rs is affected by a multitude of factors; however, soil temperature and moisture are the prime determinants (Baldocchi et al. 2001; Curtin et al. 2000). Changes in precipitation-temperature and consequent changes in vegetation phenology-productivity may also influence the variability of Rs. It is thus crucial to understand how annual dryness-wetness cycle, temperature-moisture conditions influence Rs in the subtropical coniferous forests.
Several studies have analyzed the patterns of leaf respiration to reduce C-exchange uncertainty in different biomes over the globe (Wright et al. 2004; Reich et al. 2006; Atkin et al. 2008, 2015). Nonetheless, Himalayan ecosystems remain elusive with respect to C-balance, and even respiration-photosynthesis ratio for basic model parameterizations. Thus, in situ field data are needed to understand the likely effects of future climate change on pine forests. Further, we have limited knowledge, as of how Rnc and Rs respond to environmental factors, such as, moisture/wetness conditions (dewfall, night-time vapor pressure deficit, evaporative fraction) and temperature conditions (night-time air/soil temperature). To characterize these environmental controls on Rnc and Rs in a young homogenously distributed Pinus roxburghii forest ecosystem having understory, we have used 14 months’ of micrometeorological, ecophysiological, and biophysical data comprising an annual growth cycle from November 2010 to December 2011.
Given the complex topography, canopy structure of the pines with understory, we utilized manually intensive chamber-based methodology (Speckman et al. 2015), however, at a longer time-interval (continuous 24 h cycles at 10-day interval). Hereafter, dekads should be read as 10 days in a month, for instance, Nov1d can be read as data over 1st to 10th of November. The uncertainty of eddy-covariance (EC) technique during night poses an additional challenge to understand seasonal variation of Rnc and Rs (Berkelhammer et al. 2013; Speckman et al. 2015), but the chamber-based measurements are useful when individual ecosystem components and environmental factors or disturbances need to be discerned (Speckman et al. 2015). To best of our knowledge, this is the initial comprehensive study with chamber-based field measurements over the conifers in the western Himalaya to characterize environmental controls on seasonal variations of plant and soil respiration fluxes.
Materials and methods
Site description and climate
The experimental site was located within reserve forest at the Forest Research Institute (FRI), Dehradun, India (30°20′04″N and 78°00′02″E) with a mean elevation of 640 m (Fig. S1). The site has a micrometeorological tower (13 m) with multi-level, slow-response sensors, designed to measure sub-hourly radiative-convective flux, state parameters and sub-surface thermal profiles. The biophysical (LAI: leaf area index, PAR: photosynthetically active radiation, phenology) and ecophysiological properties (photosynthesis, leaf respiration, and soil respiration) of young (~ 8.5 years old), homogenously distributed chir pine (Pinus roxburghii Sarg.) plantation patch (~ 5 hectares) were measured at 10-day interval through systematic manual sampling. The patch has an understory cover of Lantana camara L. (Verbenaceae), which was cleared during 2010 (October–November). To study the effects of understory cover on ecosystems carbon, water, and energy fluxes, it was not cleared in the year 2011. To have systematic biophysical and ecophysiological measurements, the site was homogenously divided into nine sampling quadrats (10 m × 10 m) in different directions. The micrometeorological tower was placed centrally.
Climate of study site
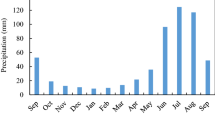
Study site lies in a heavy rainfall zone in the western Himalayan region (70-year average: 2020 ± 423 mm). Typically, cold winter season comprises December to February, while hot summer season comprises March to May. The summer monsoon season months are from June to September, while the post-monsoon season months are October and November (Basistha et al. 2009). The seasonal pattern of temperature (AT) and precipitation (P) accorded concurrently with peaks of both P and AT (mean 25.5 °C) during July–September (Fig. S2). Such coincident variation (moist–warm condition) is the environmental characteristic of the region.
Vapour pressure deficit (VPD: 1.1 ± 0.85 kPa) remain high (3.7 kPa) only during dry summer (April–May) and minimum (0.37 kPa) during the summer monsoon. Soil water content (w/w; mean of 3 depth: 0.1, 0.2 and 0.45 m, manually collected only once in a month, because of failure of automatic field sensor) during the study period varied between 11% (May) and 24% (August). Sunshine hours vary from 4.4 to 9.3 h day−1 with a minimum during July–August and maximum during May. Mean monthly open pan evaporation varies from 1.2 to 7.2 mm with lowest during winter and highest during hot summer. Seasonal VPD and soil moisture dynamics were found inversely but significantly related (R2 = 0.69, p < 0.001). Evaporative fraction (EF: the ratio between the evapotranspiration (ET) and the available energy at the land surface), an indicator of annual dryness–wetness cycle (range 0–1), remained above 0.38 throughout the study period with a mean of 0.5 ± 0.16. It varied between 0.38 (winter dekads) and 0.9 (summer monsoon dekads). Further, stronger ET and AT relationship than ET–VPD relation, defined this subtropical pine ecosystem as energy-limited rather than water–limited. The energy and water limitation phase during an annual growth cycle occurs in continuum with a shorter water limited period (confined only to the summer months: April–June) (Fig. S3).
Ecophysiological measurements and data processing
Night-time canopy respiration (R nc)
Rnc rates of both over and understory were measured from the active tissues (foliage) except the woody tissues (stem) of young pine (understory growth has a little woody tissue). Rates of respiration were manually but intensively measured at 10–day interval from 10th November 2010 to 31st December 2011 for 12 h (18:30 h–05:30 h) using the LI-6400 XT portable photosynthesis system (LI-COR, Lincoln, NE, model LI-6400 XT, Standard leaf chamber area: 2 cm × 6 cm). During measurements, block temperature and VPD was maintained under prevailing ambient conditions. The airflow rate and reference CO2 was set at 400 and 380 µmol mol−1, respectively.
The hourly sampling of a species (overstory: Pine and understory: Lantana) consisted of 360 to 450 readings made from a portable photosynthesis system (LI-Cor, Lincoln, NE, model LI-6400 XT). Hourly readings of the species represent three plants sampled in three different quadrats. Each plant was divided into three-vertical layers (top, middle, and bottom). Three to five needles/leaves per vertical layer of a plant around each canopy position were measured from the photosynthesis system. About 40–50 readings were made within 1.5–2.0 min on each layer. Thus, each plant in a quadrat was sampled for about 6 min consisted of 120–150 readings.
Leaf-level respiration data from photosynthesis system were up-scaled to canopy level on the basis of LAI (Law et al. 2001; Cavaleri et al. 2008) as well as on total canopy leaf area basis (“Biophysical measurements”) (Chambers et al. 2004).
Soil respiration (R s)
Rs rates were measured for 24 h at 10-day interval using a soil chamber (LI-6400-09) combined with a LI-6400 IRGA (Li-COR, Lincoln, NE, USA), randomly in different quadrats for maximum possible spatial coverage. This data were also supplemented intermittently by applying another closed, static chamber based (25 cm × 25 cm) on infrared gas analyzer (IRGA) CO2 sensor (GasAlertMicroIR5, BW Tech., Honeywell Inc., CA, USA). Within a quadrat, systematic sampling was done to cover both root and non–root zone (based on the distance from trees). Chambers were slipped into collars inserted into the soil 24 h prior to the measurements.
Biophysical measurements
Phenology
At weekly intervals, digital field photographs taken from all quadrats were analyzed to determine the transition dates of growth stages of Pine. The green stage of Pine corresponds to May–September and characterized by green, matured and elongated needles. During this period, the crown was mostly covered by green needles so that total photosynthetic area (computed by mechanical counting of all needles in the crown) was equated with green vegetation fraction (GVF) of 1.0 (as 100%). Traces of brown needles began to appear in October, and thus GVF of October was 0.92. As winter approached, browning of leaves increased rapidly because of a physiologically dormant stage and accordingly, the GVF was calculated. By the end of March, the GVF was calculated ~ 0.17. The emergence of new needle buds commenced in March, while the growing season referred the period from April to October.
The understory Lantana camara was cleared in the last week of October 2010. New leaves were emerged from the tuberous underground root in the onset of spring. By March end, Lantana attained a height of 0.3–0.4 m. Lantana canopy was completely developed before the onset of summer monsoon, and attained a height of 1.0 m. It attained maximum height (~ 1.5 m) during the post-monsoon. In Nov–Dec, 2011, when Lantana was not cleared, leaf browning was observed after November.
Leaf area index (LAI)
The LAI was measured at the 10-day interval in overstory pine and in understory Lantana. We used plant canopy analyzer (PCA) (LAI-2000; Li-Cor, Inc., Lincoln, NE, USA) for measuring the LAI. The detailed sampling plan and the correction factor for the clumping of needles in the pine canopy were described in Singh et al. (2014b). Overall, we followed the procedure as per LAI-2000 PCA instruction manual.
Net canopy photosynthesis (A c): measurements, upscaling and errors
Measurements
The samplings of photosynthesis and night respiration were carried out at 10-day interval during the growth cycle (i.e., Nov 1d 2010 to Dec 3d 2011) continuously for 24-h. On each day of measurement, daytime (06:00–18:00 h LMT) net photosynthesis and respiration in the next night (18:30–05:30 h LMT) were measured hourly. Hourly sampling of a species (overstory: Pine and understory: Lantana) consisted of 360–450 readings made from a portable photosynthesis system (LI-Cor, Lincoln, NE, model LI-6400 XT). Hourly readings of the species represent three plants sampled in three different quadrats. Each plant was divided into three-vertical layers (top, middle and bottom). Three to five needles/leaves per vertical layer of a plant around each canopy position were measured from photosynthesis system. About 40–50 readings were made within 1.5–2.0 min on each layer. Thus, each plant in a quadrat was sampled for about 6 min consisted of 120–150 readings. The readings from each layer were stored separately in the photosynthesis system for data processing and upscaling.
Upscaling
Leaf level photosynthesis (Lnar) data acquired with LI-6400 XT portable photosynthesis system was upscaled to canopy level according to Campbell and Norman (1998) by assuming single light assimilation response relation for all the leaves in the canopy. Canopy was divided into sunlit and shaded class based on canopy sub-division into three layers (top–mid–bottom). Based on observations on LAI and light penetration, almost always leaves/needles at lowest canopy positions were considered as a shaded class. Photosynthesis contributions from each class (sunlit and shaded) were computed separately. Assimilation rate was summed up according to the fraction of leaf area in each class:
Sunlit leaf area index (LAIS) in the vegetation canopy (m2 leaf m−2ground) can be expressed as:
where K = cos(θ), θ = leaf angle (mean tilt angle) and \(I_{{\text{A}}}^{'}=1 - \exp ~\left( { - k \times {\text{LAI}}} \right)\), k for over and understory vegetation were computed from LAI and PAR measurements above and below the canopy of understory and overstory as per Lagergren et al. (2005).
The shaded LAI was calculated as:
The day-time net canopy assimilation (Ac) calculated as gross primary production \(\left( {{\text{GP}}{{\text{P}}_{{\text{day}}}}} \right)\) minus day-time plant respiration \({\text{(R}}{{\text{d}}_{{\text{day}}}}{\text{)}}\). Mathematically, it can be written as:
Seasonal variability of Ac has been also provided in Text S1 (supplementary) and may be referred to Singh et al. (2014b).
Using Ac, we have computed two indicators, namely, Rnc/Ac (an indicator of canopy carbon balance) and Rs/Ac (an indicator of soil carbon balance). These indicators were described in “Seasonal variation of Ac and respiration-photosynthesis ratios”. The total ecosystem respiration (Re) was computed as sum of Rnc and Rs. Further, the ecosystem respiration-photosynthesis ratio (Re/Ac) was computed that indicative of net canopy C balance.
We have also provided associated errors of canopy photosynthesis in supplementary (text S2) and Table S1.
Micrometeorological observations
Sensors on the micrometeorological tower provide automated measurements of basic variables and radiation components. ET was computed following Bowen ratio energy balance (BREB) method. It consists of various multi-level probes comprising both above-ground (net radiometer, rain gauge, anemometers, AT-RH sensors) and below-ground sensors (soil heat flux plates, thermometers) and details are discussed in Singh et al. (2014a). In our previous study, we analyzed half-hourly averaged micrometeorological data, where we computed ET and EF based on BREB method (Singh et al. 2014b).
Notably, this study has analyzed new environmental variables in the canopy environment, such as, night-time air/soil temperature, night-time VPD, night-time latent heat flux (hereafter, called as “dewfall”). Night-time latent heat (W m−2) flux was computed as the sum of daytime ET subtracted from the 24 h sum divided by night-time hours.
Statistical analysis and uncertainty estimate
To examine the relationship between C-cycle components and environmental-biophysical factors, Pearson’s correlation analysis was performed. Additionally, one to one interaction between significantly correlated C components and environmental-biophysical predictors were assessed using linear regression analysis. We used multiple regression and ANOVA to identify the best environmental-biophysical factors as predictor variables. Significant statistical differences were set with p < 0.001 unless otherwise stated. All statistical analyses were performed using SPSS (ver. 14) and MATLAB R2012 (The MathWorks, Inc., Natick, MA, USA) software. Further, we explored the possibility of temporal coupling between canopy photosynthesis (Ac) and soil respiration (Rs) using wavelet coherence analysis in wavelet analysis software (Torrence and Compo 1998; Grinsted et al. 2004).
Results
Seasonal variations of R nc associated with environmental variables
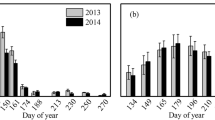
The annual behaviour of Rnc across the seasons, such as winter 2010 (no understory), pre- to post-monsoon (comprising growing period: April–October), and Nov–Dec, 2011 (with understory) has been shown in Fig. 1. The corresponding standard errors (SE) were also shown. The results showed that annual behaviour of Rnc was unimodal (maximum in post-monsoon). Rnc was maximum (4.44 µmol CO2 m−2 s−1) in the mid–August, while it was minimum (0.76 µmol CO2 m−2 s−1) in March (no understory). Combined mean Rnc of both over and understory during growing season was 3.47 ± 0.48 µmol CO2 m−2 s−1, while with understory (Nov–Dec, 2011), it was two times higher (3.41 ± 0.21 µmol CO2 m−2 s−1) than without it during winter 2010 (1.55 ± 0.29 µmol CO2 m−2 s−1).
Variations in night-time canopy respiration (Rnc ± SE) in relation to night-time air temperature (AT) at 10-day (dekads) interval over the study period (November 2010 to December 2011). Solid circles indicate growing period (April–October), dotted triangle denotes winter 2010 (no understory), but solid triangle denotes November–December, 2011 (with understory). It may be noted that only November month data points have been aggregated with winter season as the canopy browning was observed in November
Monthly variations of Rnc and night-time AT were shown in Table 1, wherein, Rnc rates were separated as overstory (Pine) and understory (Lantana). Results showed that Rnc rates were consistently higher from the understory. Mean condition (March–October) indicates that the Rnc rates from understory were higher by a factor of 1.70. But, the uncertainty associated with understory Rnc was also higher (SD ± 1.8). Month-wise corresponding standard deviation (SD) and coefficient of variation (CV) were also tabulated along with both Rnc and night-time AT. Maximum rates of Rnc were observed during the month of July and August (summer monsoon season). Therefore, these results indicate that peak values were associated with wetness conditions (soil moisture 20–24%) rather than the night-time AT.
During the winter season, Rnc and AT (night-time) were negatively correlated, which might be due to senescence induced higher respiratory fluxes that increased with the decline in temperature (Fig. 2a). By contrast, during hot summer season (March–May) and peak growing season (June–October), Rnc and AT (night-time) were positively correlated (r = 0.89 and 0.64) that suggestive of an increase in Rnc under increasing night-time AT. Albeit, the relationships were statistically significant with moderate r value (0.64) especially during peak growing season, it suggests that night-time AT alone may not control the rates of Rnc (explained only ~ 40% of the variability). So, in this energy-limited environment, other wetness factors, such as dewfall and EF had a major role to play.
Relationship between Rnc with night-time AT (a) and night-time VPD (b). Solid circles indicate across peak growing season with understory (June–October), dotted triangle denotes winter 2010 (no understory), solid triangle denotes November–December, 2011 (with understory), and square denotes hot summer season (March–May). In case of night-time VPD (b), September–October data were shown separately with open circles. Relationship between Rnc with night-time latent heat fluxes (dewfall) and evaporative fraction (EF) were shown in subplot (c) and (d), respectively
Night-time latent heat flux [expressed as latent heat (W m−2) or dewfall] is an important component of the daily water cycle, but the least studied phenomenon in the Himalayan ecosystem. Typically, dewfall and rainfall (or soil moisture) were inversely correlated. It was observed that dewfall and night-time VPD were positively correlated (r = 0.83, p < 0.001) during the growing period (April–October). We observed that the night-time VPD (kPa) and Rnc were negatively correlated across all seasons except the hot summer season (Fig. 2b). Their relationships were quite identical along with Rnc and dewfall (Fig. 2c). Moreover, Rnc and dewfall were negatively correlated irrespective of winter or growing seasons (Fig. 2c). Their relationships were stronger during peak growing season (r = 0.92, p < 0.001), while moderate during winter 2010 (r = 0.69, p < 0.01). With understory during Nov–Dec, 2011, their relationship was weaker. By contrast, in hot summer season (March–May), we observed a positive correlation (r = 0.73, p < 0.001), and it suggests that with increasing dewfall, the Rnc rate increases. Rnc and EF were strongly and positively correlated (Fig. 2d) during growing and hot summer seasons (r = 0.85–0.90, p < 0.001). However, during the winter season 2010 (no understory), there was no correlation as most of the data points were clustered, when EF ranged from 0.4 to 0.5. In the case of Nov–Dec, 2011, it becomes negatively correlated (r = 0.94). Inverse Rnc–EF relation during winter might be due to the senescence-induced higher respiratory fluxes with an overall decrease in temperature and EF. Notably, under increased wetness conditions, subtropical pine forests exhibited an increase in Rnc rates.
Multiple linear regression (ANOVA) analysis between Rnc and combinations of night-time AT, dewfall, VPD (night-time), and EF environmental variables could explain 83% of the Rnc variability (p < 0.001) across the peak growing season. Nevertheless, we infer that about 17% of this unexplained variation of Rnc may be related to other environmental variables including species-specific physiological traits. Hence, these relationships have significance in predicting Rnc in relation to any alteration in the environmental parameters.
Seasonal variations of R s associated with environmental variables
The annual behaviour of Rs across the seasons over the study period has been shown in Fig. 3. Results showed that the annual cycle of Rs was bimodal (maximum in post-monsoon and pre-monsoon). The mean Rs across the 14 months period was 7.97 ± 2.4 µmol CO2 m−2 s−1 (CV: 30%). A smaller peak (7–8 µmol CO2 m−2 s−1) was observed in March–April as the ecosystem entered growing season. The highest peak (12–15 µmol CO2 m−2 s−1) was noted in post-monsoon season, concurrent with the peak in plant respiration (Rnc) (Fig. 1). Rs declined with the advancement of summer season (April–May), may be due to decreasing soil moisture. It again gradually increased with the arrival of summer monsoon before declining to a lowest in winter (November–February) (Fig. 3). During winter 2010 and Nov–Dec, 2011, Rs was 6.5 ± 0.70 and 7.25 ± 0.59 µmol CO2 m−2 s−1, respectively. The CVs in these periods were 10.7% and 8.2%, respectively, reflecting the feeble role of photosynthates from understory and overstory on Rs during winter physiological dormancy.
Variations in night-time soil respiration (Rs ± SE) in relation to night-time soil temperature (ST) and soil moisture (SM) at 10-day interval over the study period (November 2010–December 2011). Solid circles indicate growing period (April–October), dotted open triangle denotes winter 2010 (no understory), but solid triangle denotes November–December, 2011 (with understory)
The relationships between Rs and environmental variables were shown in Fig. 4. The results showed that irrespective of the seasons, both soil and air temperatures have a substantial control on Rs rates, wherein r values exceeded 0.81 (p < 0.001) (Fig. 4a). In winter season 2010 and in Nov–Dec, 2011, we observed a positive correlation between Rs and temperatures with r-value of 0.71 in 2010 (no understory), while 0.95 in Nov–Dec, 2011 (with understory). In case, if we plot all seasons together (excluding hot summer water-limited period), the relationship between Rs and temperatures (soil and air) becomes stronger, wherein night-time ST (or AT) predominantly explained 85% of the Rs flux variation (Fig. 4b). During hot summer season water-limitation period (April–May), Rs rates declined with increasing temperature. Thereby, we infer that temperature has no control over Rs rates. As mentioned earlier, during this period, soil moisture mostly controlled Rs variability.
Variations of Rs in relation to night-time soil temperature (ST) and day-time air temperature (AT) at 10-day interval over the period from November 2010 to December 2011 (a, b). Variations of Rs in relation to soil moisture (SM) and EF were plotted in subplots (c), (d). Open circles indicate growing season (June–July), while solid circles indicate growing season (August–October). In all plots, hot summer season covering the period March–May was excluded
In this moisture abundant environment, Rs was moderately correlated with soil moisture (r = 0.56, p < 0.05) across all seasons except the hot summer (Fig. 4c). It is worth to repeat here that because of the failure of the automatic field moisture sensor, we collected monthly soil moisture samples. Excluding hot summer time water-limitation period, EF alone explained 85% of the flux variability (Fig. 4d). During growing season, over the period of June–July and August–October, r–values were observed as 0.98, and 0.90, respectively, between Rs and EF. In winter season 2010 (no understory), there was no correlation, while in Nov–Dec, 2011, it showed a strong correlation under the presence of understory (r = 0.98). We also performed multiple regression analysis between Rs and environmental controlling factors (night-time soil temperature and EF), and the results underlined that these two factors could explain about 89% (p < 0.001) of the Rs variability. These results reasonably indicate that the seasonal transitions phases are critically important for the Rs flux.
Seasonal variation of A c and respiration-photosynthesis ratios
Overstory pine Ac varied between 1.99 and 10.5 µmol CO2 m−2 s−1, while for understory (Lantana camara), it was always higher that varied from 3.7 to 17.3 µmol CO2 m−2 s−1. Ac was lowest during the winter season because of physiological dormancy and combined effects of low temperature, LAI, net radiation and PAR. During growing season (April–October) and with an account of understory growth, mean Ac observed as 15.2 ± 5.7 µmol CO2 m−2 s−1 with a peak of 25–26 µmol CO2 m−2 s−1. Peak was observed in the post-monsoon (September–October) on account of optimal conditions of environmental variables, such as clear sky, moderate VPD (0.6–0.8 kPa), and high soil moisture availability (20–24%) including understory growth (peak in LAI cycle). In winter 2010 (no understory), mean Ac was 2.39 ± 0.10 µmol CO2 m−2 s−1. Compared to winter season (2010), Ac was observed as 11.33 ± 4.5 µmol CO2 m−2 s−1 in Nov–Dec, 2011, which was five-times higher owing to the presence of understory.
The Rnc/Ac (an indicator of canopy carbon balance) and Rs/Ac (an indicator of soil carbon balance) were shown for month-wise and season-wise in the Table 2. These results showed that Rnc/Ac varied from 0.14 (± 0.01) to 0.87 (± 0.02) across seasonal phenophases (minimum during post-monsoon and maximum in winter’10). During the growing season, it varied from 0.14 (± 0.01) to 0.35 (± 0.05). These results suggest that the higher fraction of Rnc/Ac observed during winter’10 season (no understory), while the lower fraction of Rnc/Ac observed during growing season. The higher fraction of Rnc/Ac during winter season was because of lower rates of Ac. During Nov–Dec, 2011, the respiration-photosynthesis ratio was largely lower (0.29 ± 0.19) than the winter’10 season (0.71 ± 0.16), and this may be explained by the presence of understory. In addition, the respiration-photosynthesis ratio was found much higher for overstory than for understory (Lantana camara). Thus, it will be interesting to note the substantial effect of understory vegetation on respiration-photosynthesis ratio (Table 2).
During winter and hot summer seasons, the pattern of Rs/Ac (an indicator of soil carbon balance) was similar as compared to the Rnc/Ac, wherein we observed higher soil respiration losses (Table 2). The Rs/Ac ratio was observed as 2.69 (± 0.43), 1.45 (± 1.14), and 0.74 (± 0.30) during winter’10, hot summer’11, and Nov–Dec, 2011, respectively. As discussed earlier, the higher fraction of Rs/Ac during winter season was attributed to lower rates of Ac owing to physiological dormancy. During winter’10 and hot summer periods, it can be noted that the Rs rates exceed the Ac that leads to negative C-balance. Nevertheless, the Rs/Ac ratio stabled at 0.64 (± 0.29) during peak growing season, which is suggestive of gain in C-balance.
Seasonal relationship between A c and R nc rates
The relation between Rnc and Ac is shown in Fig. 5 and these results depict an inverse and significant relationship across the seasons. The Rnc rates were subtle during winter season 2010 (no understory), while significantly higher during Nov–Dec, 2011 (with understory). In Nov–Dec, 2011, Rnc and Ac showed a negative significant correlation (r = 0.83), which indicates senescence induced higher respiratory CO2 release. In particular, the understory has a higher influence on Rnc than overstory. During the growing season, we observed that Rnc and Ac were inversely related (r = 0.83). It suggests that during a phase of optimal anabolism (e.g., Ac), catabolism process (e.g. Rnc) reduces to a minimum. As a result, the process could help for constructive canopy C-balance. However, during the hot summer season (March–May), when the canopy of both overstory and understory started developing, an increase in Ac as well as in Rnc was observed (r = 0.93).
Discussion
The contribution of night-time plant and soil respiration is potentially immense and worth considering at fine scale to understand the carbon balance dynamics and to improve the global climate-carbon models (Gifford 2003; Atkin et al. 2015; Wehr et al. 2016). There exist a multi-temporal correlations and occasional phase reversal between photosynthesis and soil respiration (Vargas et al. 2011) as the day-time plant respiration is much less than the night-time due to light-inhibition (Sun et al. 2015; Wehr et al. 2016). During night-time, Rnc rate is also stable because of the minimum impact of sugar synthesis and transport (Will and Ceulemans 1997). Hence, we examined respiratory fluxes in understudied but dominant vegetation in the mid–altitudinal Himalayan range (500–2000 m) (Forest Survey of India 2011) i.e. the chir pine ecosystem (Pinus roxburghii). Measurements were made on night-time canopy respiration (Rnc) and soil respiration (Rs) in a homogenously distributed young chir pine plantation (with and without understory).
Our key findings underlined that at daily time-scale over the active growing season, night-time canopy respiration (Rnc) and day-time canopy photosynthesis (Ac) are inversely correlated. It suggests that during the phase of optimal anabolism, catabolism reduces to a minimum and environmental conditions which induce a decline in Ac, may concurrently increase Rnc and vice-versa. Soil respiration and Ac relations were only sufficiently prominent at seasonal time-scale as evidenced by their de-coupling during hot summer-time water stress. Evaporative fraction-temperature explained maximum variability in fluxes over this energy-limited ecosystem.
The environmental control on the Rnc is interesting in this subtropical energy-limited environment with a short distinct phase of hot summer time water-limitation. We found that in growing season, environmental wetness (dew, evaporative fraction) was the strongest statistical driver of Rnc. To understand the underlying physiological mechanism, we presented abridgedly the biochemistry of plants (Buchanan et al. 2002). Nocturnal decoupling of leaf water potential with the root water is a common phenomenon in non-water limiting environment (Simonin et al. 2009). This is because of the lowered VPD and absorption of moisture by leaf surface including stomata (Stone 1957; Breshears et al. 2008). Thus, stomata are hypothesized to remain open during the night, however, at a minimal constant level to get the moisture absorbed. Accordingly, if environmental moisture is sufficient, then leaf water potential may reach near saturation. In this water, saturated extracellular condition Rnc may increase due to the release of additional CO2 (in addition to normal TCA/krebs cycle) in the course of formation of pyruvate from malate. Excess extracellular moisture condition and increased HCO3− lead to an alternative route of phosphoenolpyruvate oxidation (instead of the normal route of pyruvate formation from phosphoenolpyruvate) (Buchanan et al. 2002). This causes an additional amount of night-time CO2 efflux in the period of excess moisture availability, and formed the biochemical basis of the observed relation between moisture content and Rnc (Fig. 2). This alternative route of phosphoenolpyruvate oxidation becomes the normal way of respiration to replenish the reducing equivalents in the plant mitochondria (Buchanan et al. 2002). It could be concluded that under increased temperature and wetness conditions, subtropical pine forests exhibited an increase in Rnc rates. It may be suggestive of an increase in Rnc under rising night-time temperature over the Himalaya. This finding is in line with previous observation over evergreen broadleaf tropical forest (Anderegg et al. 2015). So, night-time warming may differentially affect Rnc more than photosynthesis (Ac), thereby, reducing C uptake (Peng et al. 2013; Xia et al. 2014).
In the annual cycle, Rnc and Rs peaked during post-monsoon concurrent with the peak in productivity. However, on a daily time-scale, our findings indicated a significant inverse relationship between day-time net canopy assimilation and night-time canopy respiration (Fig. 5) except during spring-summer time over and understory canopy development and during winter season (2010) in absence of understory. This inverse relationship could be explained based on mechanistic physiological behavior of optimal anabolism (Ac) and the minimum level of catabolism (Rnc) during favorable environmental conditions. Biochemically, Buchanan et al. (2002) had explained that during the phase of optimal anabolism, catabolism (Rnc) usually reduces to a minimum. Our findings are also consistent with the temperature manipulation experiment by Turnbull et al. (2002). Sun et al. (2015) found an inverse relation in a conifer species (Pinus koraiensis) in north-eastern China. Nevertheless, the relation between the processes of photosynthesis and respiration is a subject of debate, usually linked mechanistically through the impacts that each process has on leaf carbohydrate status (Azcon-Bieto 1992; Goldschmidt and Huber 1992).
Remarkably, hot summer time water stress had an effect on photosynthesis and respiration relationship as it was significantly inverse when both over and understory canopy was still developing. During spring and hot summer seasons, when the canopy of both overstory and understory was developing, we observed an increase in Ac as well as in Rnc. This positive relation may be explained by heterotrophy and anaplerotic uptake of CO2 (dark CO2 fixation). Heterotrophy is a period in the life cycle of plants when autotrophic inputs are suspended and carbon substrate requirement is met through the remobilization of stored carbohydrate reserves. This state even occurs on a diurnal time-scale and on seasonal time-scale during the spring time bud/leaf burst in plants (Bathellier et al. 2007). During periods of active growth (canopy development), an additional inorganic carbon supply is essential in the tricarboxylic acid cycle (TCA or Krebs cycle) to generate carbon intermediates required for amino acid synthesis. Additional carbon can be sourced either through the scavenging of intracellular respired CO2 or through the uptake of CO2 from surrounding atmosphere (or soil air-pores in the case of plant roots). Further, it may be noted that the developing leaves have a higher rate of catabolism (Rnc) than mature leaves (Buchanan et al. 2002), therefore, a positive correlation was obtained when photosynthesis and respiration both were increasing with canopy growth initialization. Besides, higher LAI and Ac in understory than overstory may have contributed towards significant positive correlation during the recovery phase in these months. During winter 2010, Rnc and Ac showed no clear relation because overstory pine was undergoing physiological dormancy and understory was absent.
Our study found that the presence of understory vegetation tended to reduce respiration-photosynthesis ratio. The understory Lantana camara (an invasive shrub species) contributed significantly to the system C balance, as evident by its higher rate of Ac as well as Rnc (Table 1; Fig. 2). During growing season, Rnc consumed ~ 23% of the net canopy assimilation that increased to 30% during Nov–Dec, 2011 when understory was present but senescing, while, in the same time-period in winter 2010, in the absence of understory, Rnc represented ~ 70% of the Ac. Thus, given that pine (overstory) phenology behaved similarly in both the years, the contribution of understory component in C balance in a forest ecosystem is evident.
The ratio Rnc/Ac can be used to evaluate canopy C balance in response to variations of environmental factor. Photosynthesis–respiration relation is largely driven by environmental factors than inter–specific genetic variation (Lewis et al. 2011). Canopy respiration usually consumes about half of the fixed carbon, and the remainder supports plant growth (Amthor and Baldocchi 2001). The mean value of 0.28 during the growing season is suggestive of lower respiratory carbon losses from this coniferous ecosystem. The mean varied from 0.12 to 0.52 across the growing season. These results were consistent with the previous studies that reported in the range of 0.1 to 0.3 for different species including herbs, shrubs and trees in the subtropical ecosystems in southeast China (Chu et al. 2011). Ryan et al. (1994) reported that the Rnc/Ac ratio varies from 0.32 to 0.64 in pine trees, but from a Himalayan region (pine) we observed it at about 0.44. In line to this, prior studies have evaluated Rnc/Ac ratio along with temperature increase and underlined that the ratio increased in response to temperature as well as drought (Zaragoza-Castells et al. 2008; Catoni and Gratani 2014; Reich et al. 2016). Accordingly, our findings were also consistent with those previous studies. Further, based on night-time temperature manipulation experiment, Turnbull et al. (2002) discussed that Rnc rate could increase substantially with higher temperature and thereby, the increment in respiration-photosynthesis ratio. These responses indicate that an increase in night-time temperatures under climate warming may have a significant influence on net plant C uptake.
Additionally, we have analyzed Rnc/Ac ratio with respect to night-time AT (Fig. 6) and these results indicated that with increasing night-time AT, the respiration-photosynthesis (Rnc/Ac) ratio increased especially during growing season and with understory even during Nov–Dec, 2011. Their relationships were significantly positive, which is indicative of the gain of canopy C balance with increasing temperatures. Our results were consistent with previous studies that have reported about the temperature sensitivity on respiration-photosynthesis ratio (Rnc/Ac), which discussed the possible adverse impacts on canopy C balance, owing to temperature change (Atkin et al. 2006; Chi et al. 2013).
Soil respiration (Rs) was bimodal in the annual cycle. First, a smaller peak was observed when the ecosystem left winter, entered summer and a secondary (highest) peak was observed in the post-monsoon at the peak of the growing season. The peak in Rs matched with the concurrent optimal mix of the summer monsoon environmental conditions (warm-moist) and soil metabolic activities. We observed a tight link between plant productivity and soil respiration, which was most in the post-monsoon (Yuan et al. 2011). Interestingly, we noted a complete reversal in Rs and Ac relation (Fig. 1b, Fig. S4), when winter-time temperature-limited conditions changed to water-limitation period during hot summer seasons. This behaviour was probably due to the reduction in photosynthate availability (as over and understory canopy was still developing) as well as moisture stress-induced reduction in soil metabolism. Anaplerotic uptake of CO2 could be another reason (Dark CO2 fixation: when additional inorganic carbon is essential for amino acid synthesis during plant development, it is either sourced through the scavenging of respired CO2 or through the uptake of CO2 from surrounding atmosphere or through the soil air-pores in the case of plant roots). It is reasonable to conclude that during hot summer season, Rs decouples from photosynthetic carbon input and it reduces due to the combined effect of photosynthate availability and water deficit, while over rest of the growing season, R2 was greater than 0.65 (0.8 in post-monsoon, p < 0.001). Understory also played a role in determining the strength of Rs–Ac relation, even during winters. As it was weakest (R2 = 0.12, p = 0.22) when no understory was present (winter 2010) and stronger (R2 = 0.94, p < 0.001) during Nov–Dec, 2011 with understory (Fig. 1b, Fig. S4). Rs fluxes especially during the summer monsoon season were higher because of the presence of large populations of earthworms, termites, and associated fungi at the site (Bradford et al. 2014) in comparison to other studies from the region (Rout and Gupta 1989). Based on EC technique, Verlinden et al. (2013) suggested that Rs could consume about 46% of Ac of poplar (Populus) trees. Over European forests, Janssens et al. (2001) reported that the mean annual Rs could consume about 55% of Ac. In comparisons to the above estimates, our findings indicate that Rs could consume ~ 67% (range 38–108%) of the Ac in pine trees during the growing season with understory. Multiple regression analysis of Rs and abiotic factors (soil/air temperature, soil moisture, and EF) indicated that the temperatures and EF have a larger control on Rs flux during peak growing season (Wangdi et al. 2017), while soil moisture has a limited control. But, soil moisture has a larger role during hot summer water-limited period. Thus, seasonal transition phases of Rs fluxes in this region were critically associated with soil/air temperature, soil moisture, and EF.
It is also pertinent to mention here the possible source of uncertainty in the measurements and methods. Diffusion-leak may become a significant source of error in the chamber-based methodology. We carefully considered it every time while operating the photosynthesis system and ensured it by ‘valve matching’, as it was routinely performed every 30 min to maintain prevailing ambient conditions and to correct any analyzer offsets. Hourly Rnc measurements of over and understory comprised of 360–450 measurements made from a portable photosynthesis system (LI–6400 XT). These probably tended to reduce random measurement error (coefficient of variation). Lack of spatial representativeness is the most important source of systematic error in soil respiration (Rs) measurements. To have sufficient spatial representativeness of both root- and non-root-zone with a single measurement system, these zones were hourly and alternatively sampled in different random quadrats. We were also carried out, another static chamber (25 cm × 25 cm) IRGA-based parallel and periodic soil respiration measurement. Despite all these precautions in systematic chamber-based measurements, the systematic error of ~ 15 to 20% associated with chamber-based methodology (Luo and Zhou 2010) has not been excluded in this study. A systematic source of uncertainty in this study was that we were not able to measure the respiration from woody tissue such as branches and stems. The shrub understory has little woody tissues; however, the woody tissue respiration from chir pines (overstory) must be significant. Several authors (Law et al. 2001; Wang et al. 2004; Acosta et al. 2007), indicated that it can vary between 1 and 15% depending on the age. We, therefore, assumed it as 5% of total ecosystem respiration (Rnc + Rs) (because of young age: 8.5 year).
Conclusion
We addressed two major carbon balance indicators, namely, night-time canopy respiration (Rnc) and soil respiration (Rs) of the deciduous coniferous (chir pine) forest in response to environmental factors. This unique and comprehensive study has employed a combination of systematic ecophysiological measurements and gradients of micrometeorological data within and above the canopy system. It was possible to characterize respiratory carbon fluxes at temporal scales of hours, days and one complete annual phenological cycle from a relatively understudied region of the world. Observations could certainly lend useful insight into carbon balance and ecological functions in the region. Our key findings suggest that at daily time-scale over the active growing seasons, night-time canopy respiration and day-time canopy photosynthesis are inversely correlated. Soil respiration and productivity (Ac) relations are dynamic and maximum coupling was observed during post-monsoon, whereas complete reversal (de-coupling) during hot summer-time water stress. Evaporative fraction-temperature explained maximum variability in fluxes over this energy-limited ecosystem. Furthermore, we found that the Rnc/Ac ratio has increased with an increase in night-time temperature that indicative of temperature sensitivity on respiration-photosynthesis ratio. These responses indicated that increase in temperature under climate warming may have a significant influence on net plant C uptake. Alterations in environmental variables at seasonal time-scale to inter-season transition periods may have a significant effect on the C-balance in the region.
Presence of understory (Lantana) vegetation largely influenced both Rnc and Ac, whereas, it can minimize the respiration-photosynthesis ratio. Thus, the reduced ratio helps in accumulation of C in pine forest across subtropical Himalaya region. Removal of understory is a prevalent forest management practice in the Himalaya, and we propose that this may not be a viable tool for a forest carbon balance point of view. On the contrary, Lantana also commonly identified as the worst weed worldwide, which could pose serious problems in the plantation forestry. It can obstruct the growth of prime vegetation and may become dominant species causing a threat to the native flora.
Time-series of data are needed to assess the degree of inter-annual variability in response to environmental conditions. Improved understanding of short and long-term behaviour of C-exchange and regulations in relation to environmental conditions will provide a basis for improving models and exploring the effects of climate change on C-cycling. Nonetheless, these findings may be useful in understanding components of C balance indicators and further parameterizing and validating C-cycle models.
Author contribution statement
NS conceived and designed the experiments; NS and BRP analyzed the results and wrote the article.
Abbreviations
- R nc :
-
Night-time canopy respiration
- R s :
-
Soil respiration
- LE:
-
Latent heat
- SH:
-
Sensible heat
- ET:
-
Evapotranspiration
- EF:
-
Evaporative fraction
- AT:
-
Air temperature
- ST:
-
Soil temperature
- SM:
-
Soil moisture
- VPD:
-
Vapor pressure deficit
- RH:
-
Relative humidity
- C:
-
Carbon
- CO2 :
-
Carbon dioxide
- A c :
-
Day-time canopy photosynthesis
- GPP:
-
Gross primary production
- Rdday :
-
Day-time plant respiration
- R e :
-
Ecosystem respiration
- LAI:
-
Leaf area index
- 10-days interval:
-
Dekads
- EC:
-
Eddy-covariance
References
Acosta M, Pavelka M, Pokorny R et al (2007) Seasonal variation in CO2 efflux of stems and branches of Norway spruce trees. Ann Bot 101:469–477. https://doi.org/10.1093/aob/mcm304
Amthor JS, Baldocchi DD (2001) Terrestrial higher plant respiration and net primary productivity. In: Roy J et al (eds) Terrestrial global productivity. Academic, New York, pp 33–59
Anderegg WRL, Ballantyne AP, Smith WK et al (2015) Tropical nighttime warming as a dominant driver of variability in the terrestrial carbon sink. Proc Natl Acad Sci 112:15591–15596. https://doi.org/10.1073/pnas.1521479112
Atkin OK, Scheurwater I, Pons TL (2006) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol 12:500–515. https://doi.org/10.1111/j.1365-2486.2006.01114.x
Atkin OK, Scheurwater I, Pons TL (2007) Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. N Phytol 174:367–380. https://doi.org/10.1111/j.1469-8137.2007.02011.x
Atkin OK, Atkinson LJ, Fisher RA et al (2008) Using temperature-dependent changes in leaf scaling relationships to quantitatively account for thermal acclimation of respiration in a coupled global climatevegetation model. Glob Change Biol 14:2709–2726. https://doi.org/10.1111/j.1365-2486.2008.01664.x
Atkin OK, Bloomfield KJ, Reich PB et al (2015) Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. N Phytol 206:614–636. https://doi.org/10.1111/nph.13253
Azcon-Bieto J (1992) Relationships between photosynthesis and respiration in the dark in plants. In: Medrano H (ed) Trends in photosynthesis research. Intercept Ltd, Andover, Hampshire, pp 241–253
Baldocchi D, Falge E, Gu L, et al (2001) FLUXNET: a new tool to study the temporal and spatial variability of ecosystem–scale carbon dioxide, water vapor, and energy flux densities. Bull Am Meteorol Soc 82:2415–2434. https://doi.org/10.1175/1520-0477(2001)082%3C2415:FANTTS%3E2.3.CO;2
Basistha A, Arya DS, Goel NK (2009) Analysis of historical changes in rainfall in the Indian Himalayas. Int J Climatol 29:555–572. https://doi.org/10.1002/joc.1706
Bathellier C, Badeck F-W, Couzi P et al (2007) Divergence in δ13C of dark respired CO2 and bulk organic matter occurs during the transition between heterotrophy and autotrophy in Phaseolus vulgaris plants. N Phytol 177:406–4018. https://doi.org/10.1111/j.1469-8137.2007.02246.x
Berkelhammer M, Hu J, Bailey A et al (2013) The nocturnal water cycle in an open-canopy forest: nocturnal forest water. J Geophys Res Atmos 118:10,225–10,242. https://doi.org/10.1002/jgrd.50701
Bhattacharya BK, Singh N, Bera N et al (2013) Canopy-scale dynamics of radiation and energy balance over short vegetative systems. Scientific Report SAC/EPSA/ABHG/IGBP/EME-VS/SR/02/2013
Bhutiyani MR, Kale VS, Pawar NJ (2007) Long-term trends in maximum, minimum and mean annual air temperatures across the Northwestern Himalaya during the twentieth century. Clim Change 85:159–177. https://doi.org/10.1007/s10584-006-9196-1
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582. https://doi.org/10.1038/nature08930
Bradford MA, Warren IIRJ, Baldrian P et al (2014) Climate fails to predict wood decomposition at regional scales. Nat Clim Change 4:625–630. https://doi.org/10.1038/nclimate2251
Breshears DD, McDowell NG, Goddard KL et al (2008) Foliar absorption of intercepted rainfall improves woody plant water status most during drought. Ecology 89:41–47. https://doi.org/10.1890/07-0437.1
Buchanan B, Gruissem W, Jones RL (eds) (2002) Biochemistry & molecular biology of plants. Wiley, New York, p 682 (ISBN: 978-0-943088-39-6)
Campbell GS, Norman JM (1998) Introduction to environmental biophysics. Springer Science + Business Media Inc., New York, p 71
Catoni R, Gratani L (2014) Variations in leaf respiration and photosynthesis ratio in response to air temperature and water availability among Mediterranean evergreen species. J Arid Environ 102:82–88. https://doi.org/10.1016/j.jaridenv.2013.11.013
Cavaleri MA, Oberbauer SF, Ryan MG (2008) Foliar and ecosystem respiration in an old-growth tropical rain forest. Plant Cell Environ 31:473–483. https://doi.org/10.1111/j.1365-3040.2008.01775.x
Chambers JQ, Tribuzy ES, Toledo LC et al (2004) Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecol Appl 14:72–88. https://doi.org/10.1890/01-6012
Chi Y, Xu M, Shen R et al (2013) Acclimation of foliar respiration and photosynthesis in response to experimental warming in a temperate steppe in Northern China. PLoS One 8:e56482. https://doi.org/10.1371/journal.pone.0056482
Chu Z, Lu Y, Chang J et al (2011) Leaf respiration/photosynthesis relationship and variation: an investigation of 39 woody and herbaceous species in east subtropical China. Trees 25:301–310. https://doi.org/10.1007/s00468-010-0506-x
Cox PM, Betts RA, Jones CD et al (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187. https://doi.org/10.1038/35041539
Curtin D, Wang H, Selles F et al (2000) Tillage effects on carbon fluxes in continuous wheat and fallow–wheat rotations. Soil Sci Soc Am J 64:2080. https://doi.org/10.2136/sssaj2000.6462080x
De Lucia EH, Drake JE, Thomas RB, Gonzalez-Meler M (2007) Forest carbon use efficiency: is respiration a constant fraction of gross primary production? Glob Change Biol 13:1157–1167. https://doi.org/10.1111/j.1365-2486.2007.01365.x
Forest Survey of India (2011) Indian State of Forest Report 2011, Ministry of Environment and Forests, Government of India, Dehra Dun, India
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171. https://doi.org/10.1071/FP02083
Goldschmidt E, Huber S (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99:1443–1448
Grinsted A, Moore JC, Jevrejeva S (2004) Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys 11:561–566. https://doi.org/10.5194/npg-11-561-2004
Hagihara A, Hozumi K (1991) Respiration. In: Ragavendra AS (ed) Physiology of trees. Wiley, New York, pp 87–100
Janssens IA, Lankreijer H, Matteucci G et al (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol 7:269–278. https://doi.org/10.1046/j.1365-2486.2001.00412.x
Lagergren F, Eklundh L, Grelle A et al (2005) Net primary production and light use efficiency in a mixed coniferous forest in Sweden. Plant Cell Environ 28:412–423. https://doi.org/10.1111/j.1365-3040.2004.01280.x
Law B, Kelliher F, Baldocchi D et al (2001) Spatial and temporal variation in respiration in a young ponderosa pine forest during a summer drought. Agric For Meteorol 110:27–43. https://doi.org/10.1016/S0168-1923(01)00279-9
Lewis JD, Phillips NG, Logan BA et al (2011) Leaf photosynthesis, respiration and stomatal conductance in six Eucalyptus species native to mesic and xeric environments growing in a common garden. Tree Physiol 31:997–1006. https://doi.org/10.1093/treephys/tpr087
Luo Y, Zhou X (eds) (2010) Soil respiration and the environment. Academic, Elsevier
Peng S, Piao S, Ciais P et al (2013) Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 501:88–92. https://doi.org/10.1038/nature12434
Piao S, Luyssaert S, Ciais P et al (2010) Forest annual carbon cost: a global-scale analysis of autotrophic respiration. Ecology 91:652–661. https://doi.org/10.1890/08-2176.1
Reich PB, Tjoelker MG, Machado J-L, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439:457–461. https://doi.org/10.1038/nature04282
Reich PB, Sendall KM, Stefanski A et al (2016) Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531:633–636. https://doi.org/10.1038/nature17142
Rout S, Gupta S (1989) Soil respiration in relation to abiotic factors, forest floor litter, root biomass and litter quality in forest ecosystems of Siwaliks in northern India. Acta Oecol 10:229–244
Ryan MG, Linder S, Vose JM, Hubbard RM (1994) Dark respiration of pines. Ecol Bull (Copenhagen) 43:50–63
Schlesinger W, Andrews J (2000) Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20
Shrestha UB, Gautam S, Bawa KS (2012) Widespread climate change in the himalayas and associated changes in local ecosystems. PLoS One 7:e36741. https://doi.org/10.1371/journal.pone.0036741
Simonin KA, Santiago LS, Dawson TE (2009) Fog interception by Sequoia sempervirens (D. Don) crowns decouples physiology from soil water deficit. Plant Cell Environ 32:882–892. https://doi.org/10.1111/j.1365-3040.2009.01967.x
Singh N, Bhattacharya BK, Nanda MK et al (2014a) Radiation and energy balance dynamics over young chir pine (Pinus roxburghii) system in Doon of western Himalayas. J Earth Syst Sci 123:1451–1465. https://doi.org/10.1007/s12040-014-0480-6
Singh N, Patel NR, Bhattacharya BK et al (2014b) Analyzing the dynamics and inter-linkages of carbon and water fluxes in subtropical pine (Pinus roxburghii) ecosystem. Agric For Meteorol 197:206–218. https://doi.org/10.1016/j.agrformet.2014.07.004
Speckman HN, Frank JM, Bradford JB et al (2015) Forest ecosystem respiration estimated from eddy covariance and chamber measurements under high turbulence and substantial tree mortality from bark beetles. Glob Change Biol 21:708–721. https://doi.org/10.1111/gcb.12731
Stone EC (1957) Dew as an ecological factor: II. The effect of artificial dew on the survival of Pinus ponderosa and associated species. Ecology 38:414–422
Sun J, Guan D, Wu J et al (2015) Day and night respiration of three tree species in a temperate forest of northeastern China. iForest Biogeosci For 8:25–32. https://doi.org/10.3832/ifor0982-007
Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bull Am Meteorol Soc 79:61–78
Turnbull MH, Murthy R, Griffin KL (2002) The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ 25:1729–1737. https://doi.org/10.1046/j.1365-3040.2002.00947.x
Van Oijen M, Schapendonk A, Höglind M (2010) On the relative magnitudes of photosynthesis, respiration, growth and carbon storage in vegetation. Ann Bot 105:793–797. https://doi.org/10.1093/aob/mcq039
Vargas R, Baldocchi DD, Bahn M et al (2011) On the multi-temporal correlation between photosynthesis and soil CO2 efflux: reconciling lags and observations. N Phytol 191:1006–1017. https://doi.org/10.1111/j.1469-8137.2011.03771.x
Verlinden MS, Broeckx LS, Zona D et al (2013) Net ecosystem production and carbon balance of an SRC poplar plantation during its first rotation. Biomass Bioenergy 56:412–422. https://doi.org/10.1016/j.biombioe.2013.05.033
Wang K-Y, Kellomaki S, Zha TS, Peltola H (2004) Component carbon fluxes and their contribution to ecosystem carbon exchange in a pine forest: an assessment based on eddy covariance measurements and an integrated model. Tree Physiol 24:19–34. https://doi.org/10.1093/treephys/24.1.19
Wangdi N, Mayer M, Nirola MP et al (2017) Soil CO2 efflux from two mountain forests in the eastern Himalayas, Bhutan: components and controls. Biogeosciences 14:99–110. https://doi.org/10.5194/bg-14-99-2017
Wehr R, Munger JW, McManus JB et al (2016) Seasonality of temperate forest photosynthesis and daytime respiration. Nature 534:680–683. https://doi.org/10.1038/nature17966
Will RE, Ceulemans R (1997) Effects of elevated CO2 concentration on photosynthesis, respiration and carbohydrate status of coppice Populus hybrids. Physiol Plant 100:933–939. https://doi.org/10.1111/j.1399-3054.1997.tb00020.x
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Xia J, Chen J, Piao S et al (2014) Terrestrial carbon cycle affected by non-uniform climate warming. Nat Geosci 7:173–180. https://doi.org/10.1038/ngeo2093
Xu J, Grumbine RE, Shrestha A et al (2009) The melting Himalayas: cascading effects of climate change on water, biodiversity, and livelihoods. Conserv Biol 23:520–530. https://doi.org/10.1111/j.1523-1739.2009.01237.x
Yadav RR, Park W-K, Singh J, Dubey B (2004) Do the western Himalayas defy global warming? Western Himalayas defy global warming. Geophys Res Lett 31:L17201. https://doi.org/10.1029/2004GL020201
Yuan W, Luo Y, Li X et al (2011) Redefinition and global estimation of basal ecosystem respiration rate: basal ecosystem respiration rate. Glob Biogeochem Cycles. https://doi.org/10.1029/2011GB004150
Zaragoza-Castells J, Sánchez-Gómez D, Hartley IP et al (2008) Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of acclimation in both high-light and shaded habitats. Funct Ecol 22:172–184. https://doi.org/10.1111/j.1365-2435.2007.01355.x
Acknowledgements
This work has been carried out under the project titled ‘Energy and Mass Exchange in Vegetative Systems (EMEVS)’ in ISRO-Geosphere-Biosphere Programme. The authors are grateful to the Directors of Forest Research Institute (FRI), Dehradun and Space Applications Centre (SAC), Ahmedabad, India. The Wadia Institute of Himalayan Geology is thankfully acknowledged for all the logistical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Communicated by Grams.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, N., Parida, B.R. Environmental factors associated with seasonal variations of night-time plant canopy and soil respiration fluxes in deciduous conifer forest, Western Himalaya, India. Trees 33, 599–613 (2019). https://doi.org/10.1007/s00468-018-1804-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-018-1804-y