Abstract
Aims
Plant growth is often limited by low soil phosphorus (P) availability, soil nitrogen (N) availability may affect plants’ responses to P supply. We studied the growth and physiological responses of alfalfa to soil P supply at different N levels.
Methods
Alfalfa (Medicago sativa L.) plants were grown in an alkaline soil supplied with different levels of P (0, 5, and 20 mg kg−1) as monopotassium phosphate, and N (50 and 100 mg kg−1) as ammonium nitrate.
Results
Plant biomass and P concentrations always showed positive responses to P addition but not to N addition, nodulation was inhibited by lower P supply and higher N supply. Roots released more phosphatase and carboxylates, mainly tartrate, into the rhizosheath at lower soil P supply and higher N supply. Roots always acidified the rhizosheath, but rhizosheath pH did not vary considerably among treatments.
Conclusions
This study demonstrates the release of tartrate as a major carboxylate as affected by soil P supply and N supply, and highlights the importance of investigating plant adaptive strategies for P acquisition from soil with different N availability for proper application of P and N fertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a key element required as a component of nucleic acids, phospholipids, and sugar phosphates; it plays important roles in plant growth and metabolism (Lambers et al. 2008; Raghothama 1999). Due to the sorption of phosphate (Pi) to iron (Fe) and aluminum (Al) oxides and hydroxides in acid soils and calcium (Ca) in alkaline soils, Pi in soil is highly immobile, making P the least accessible macronutrient for plants (Hinsinger 2001). Low P availability is often a major constraint for crop production worldwide, especially in many low-input agricultural systems (Richardson et al. 2011; Simpson et al. 2015; Vance et al. 2003). To overcome soil P deficiency and increase agricultural productivity for a rising global population, large amounts of P fertilizers are applied to agricultural soils every year. However, P fertilizers are used very inefficiently, with only 15–30% of applied fertilizer P worldwide being taken up by crops in the year of its application (Syers et al. 2008). High P-fertilizer application relative to crop P use has led to P surpluses, resulted in P-saturated soils and caused serious environmental problems such as eutrophication of aquatic ecosystems, but P deficiency still limits agricultural productivity in many regions (Carpenter 2005; MacDonald et al. 2011; Mekonnen and Hoekstra 2018). Most P fertilizers are derived from rock phosphate reserves, which are a finite, nonrenewable resource, and there are concerns that we are gradually running out of global rock phosphate resources (Fixen and Johnston 2012; Johnston et al. 2014). To supply sufficient P to meet agricultural demands without wasting P resources and degrading the environment is a great challenge, and improving crop P-use efficiency is considered a promising solution to this challenge (Cordell and White 2015; MacDonald et al. 2011; Pang et al. 2018).
In response to P deficiency, plants exhibit a series of morphological, physiological, biochemical, and molecular adjustments to enhance their capacity to acquire P (Lambers et al. 2006; Plaxton and Tran 2011; Raghothama and Karthikeyan 2005). Root-mediated changes of pH in the rhizosphere can affect P availability. Protons released into the rhizosphere by legumes, especially those fixing atmospheric nitrogen (N), can result in rhizosphere acidification and consequently increase P availability in alkaline soils (Hinsinger et al. 2003). Increased release of carboxylates from roots into the rhizosphere is a key physiological P-mining strategy to mobilize sparingly-soluble soil P, because carboxylates compete with both inorganic P (Pi) and organic P (Po) for binding sites in soil (Lambers et al. 2008; Pang et al. 2018). Increased release of phosphatases, which hydrolyze soil Po to release Pi, is another physiological strategy to mobilize P (Nuruzzaman et al. 2006; Playsted et al. 2006).
In addition to P, N is often a growth-limiting nutrient in many regions, and large amounts of N fertilizers are applied worldwide to nourish the growing world population (Zhang et al. 2015). Many forage legumes are capable of symbiotic N-fixation, which can greatly reduce or eliminate the need for N fertilizer. Both the formation and N-fixation activity of nodules are suppressed by high levels of soil N, suggesting that the application of N fertilizer in the fields of forage legumes should be managed with caution (Murray et al. 2017; Regus et al. 2017). Soil P composition and availability can be altered and plant growth may be affected by N input. Adding N to soil in some ecosystems, including forests and agro-ecosystems, depletes soil P pools, especially soil labile P fractions, suggesting that N addition may lead to greater P limitation (Chen et al. 2018; Pasley et al. 2019; Zhao and Zeng 2019). When N is sufficient, plant growth and demand for P is not responsive to N addition (Perring et al. 2008). N addition can induce a greater demand for P, thus leading to the induction of P-acquisition mechanisms such as increased root exudation of protons, phosphatase enzymes, and carboxylates such as citrate and malate (Maistry et al. 2015; Phoenix et al. 2003). Legume nodules have a high requirement for P, soil P availability affects nodule development. Nodulation, as well as N acquisition and metabolism, is inhibited at low P supply (Prodhan et al. 2019; Valentine et al. 2017). For the fynbos legume Podalyria calyptrate, nodulation is inhibited by higher N supply, and such inhibition is greater at low P supply than at high P supply (Maistry et al. 2015).
Alfalfa (Medicago sativa L.) is an important perennial legume forage crop grown widely. Like many grain crops, the productivity of alfalfa is often limited by P, and there are a number of studies on the P-acquiring strategy of alfalfa (Fan et al. 2015; He et al. 2017; Pang et al. 2015; Suriyagoda et al. 2010). However, the growth and physiological responses of alfalfa to P deficiency at different soil N levels are less well understood. The objective of this study was to investigate the effects of soil P supply, N supply, and their interaction on alfalfa growth and nodulation, leaf and stem P concentrations, aboveground P-utilization efficiency (PUE), and root physiological traits such as release of protons, carboxylates, and phosphatases. We tested three hypotheses: (i) plant biomass and nodule number would be greater at higher P supply, but plant biomass would not be significantly affected by N supply, nodulation would be inhibited by lower P supply and higher N supply; (ii) leaf and stem P concentrations would increase with increasing soil P supply, but not vary considerably with N supply; (iii) release of protons, carboxylates, and phosphatase would be more at lower soil P supply and higher N supply.
Materials and methods
Plant cultivation and harvest
The substrate used in the study was a mixture of an aeolian sandy soil and the weathering product of Pisha sandstone. The Pisha sandstone is a type of loose rock that is present as an interbedded sandstone composed of thick layers of sandstone, sandy shale and shale formed in the Permian, Triassic, Jurassic, and Cretaceous. It has been found that addition of the weathering product of Pisha sandstone to the aeolian sandy soil can improve the water- and nutrient-retention capacity of the aeolian sandy soil (Sun and Han 2018). A large area of sandy soil in the Mu Us Sandland on the Loess Plateau in China has been amended with the weathering product of Pisha sandstone, and plants such as alfalfa are grown on such amended soil for both agricultural and revegetation purposes. Physicochemical properties of the aeolian sandy soil and the weathering product of Pisha sandstone are listed in Table 1.
Air-dried aeolian sandy soil and weathering product of Pisha sandstone were passed through a 2-mm sieve separately, then mixed at a weight ratio of 3:1. About 4 kg of the mixture (hereafter referred to as soil) was added to a PVC tube of 15-cm diameter and 20-cm height lined with a plastic bag, and a total of 24 pots were filled for the experiment. The soil in all pots was supplied with potassium (K) at 150 mg kg−1 as a K2SO4 solution. Two levels of N were supplied, the soil in 12 pots was supplied with N at 50 mg kg−1 (hereafter referred to as 50 N), while that in the other 12 pots was supplied with N at 100 mg kg−1 (hereafter referred to as 100 N), with all N supplied as a NH4NO3 solution. For each N treatment, there were three P levels, i.e. 0, 5, and 20 mg kg−1 (hereafter referred to as 0P, 5P, and 20P), with each level of P supplied to eight pots as a KH2PO4 solution. The soil in all pots was kept at 14.5% gravimetric soil water content for 15 days by weighing and supplying deionized (DI) water every three days; then the soil was taken together with the plastic bag out of the pot to let the soil air dry. The air-dried soil in each pot was passed through a 2-mm sieve again and then used to fill a PVC tube of 11-cm diameter and 40-cm height. A 5-mm thick cotton wick (100 cm long) was inserted into the pot beforehand to cover the bottom of the pot and extend along the wall of the pot vertically to the bottom of a plastic cup fixed outside the pot, of which the top was level with the top of the cup. The water management method of the experiment is shown in Fig. S1.
Plants were grown from late April to late September in 2018 in a glasshouse in the Institute of Soil and Water Conservation (34°16′33″N, 108°04′13″E), Yangling, Shaanxi, China. The experiment was arranged as a two-factorial (soil P supply and N supply) completely randomized design with four replicates. Seeds of alfalfa (Medicago sativa L. cv Golden Empress) were sterilized in 30% (v/v) hydrogen peroxide solution for 5 min, then rinsed with DI water three times, and placed on moistened filter papers in Petri dishes overnight (He et al. 2017). Twenty pre-germinated seeds were sown directly in each pot of the 100 N treatment in late April, 2018, without the application of rhizobia inoculants. At the same time, another 20 pre-germinated seeds, which were soaked in rhizobia inoculants, were sown in each pot of the 50 N treatment; then a few drops of the same rhizobia inoculant were further supplied to each pot using a plastic pipette; the rhizobia inoculant was supplied again to each pot at 15 days after sowing (DAS). The rhizobia inoculant (Sinorhizobium meliloti, accession number ACCC17501) was obtained from the Agricultural Culture Collection of China. The soil in all pots was watered to 14.5% gravimetric soil water content after sowing by weighing the pots and supplying DI water to the cup every two or three days, depending on the rate of water loss. Seedlings in each pot were thinned to 10 plants at 40 DAS.
Plants were harvested twice during the experiment. The first harvest was 100 DAS, when shoots of plants in all pots were cut at 0.5 cm above the soil surface and separated into leaves and stems, which were oven-dried at 75 °C for 72 h and weighed; roots were not harvested to allow resprouting. The second harvest was 55 days after the first harvest. Shoots were cut at the base, then separated into leaves and stems and treated in the same way as in the first harvest. The dry mass of all leaves and stems in both harvests of each pot was summed to obtain the shoot dry mass (SDM). For plants in each pot, about 1.0 g fresh fine roots and the attached rhizosheath soil, i.e. soil that was still attached to the roots after gently shaking, was extracted in a known volume of 0.2 mM CaCl2 solution to collect the rhizosheath carboxylates (He et al. 2017). The soaked roots were collected and washed thoroughly with tap water, then oven-dried at 60 °C to obtain the dry mass. A subsample of the extract was filtered through a 0.22-μm syringe filter into a 1-mL HPLC vial, then acidified by a drop of concentrated phosphoric acid and kept at −20 °C. The pH of the remaining unfiltered extract was measured using a pH meter. About 1.0 g fine roots in each pot was collected and washed with DI water, then quickly frozen in liquid nitrogen and stored at −80 °C for further assay of root acid phosphomonoesterase (PME) activity. Roots not extracted were washed first with tap water, then with deionized water to remove soil particles, and finally oven-dried at 60 °C for 72 h and weighed. Bulk soil in each pot was thoroughly mixed, and a small portion of the bulk soil was collected to air-dry and pass through a 2-mm sieve for measurement of pH in a 1:5 soil:water (w/w) suspension using a pH meter (Little 1992).
Analysis of rhizosheath carboxylates
Analysis of rhizosheath carboxylates was performed using a Waters 1525 HPLC equipped with a Waters 2489 detector and Waters Symmetry C18 reverse phase column (Waters, Milford MA, USA). The mobile phase was 20 mM KH2PO4, adjusted to pH 2.5 with concentrated H3PO4, with a flow rate of 0.6 mL min−1, and 100% methanol with a flow rate of 0.01 mL min−1. The working standards included oxalic, malic, citric, acetic, malonic, succinic, and tartaric acids to identify carboxylates at 210 nm (Cawthray 2003). The injection volume of each sample was 10 μL, and the run time for each sample was 16 min. Acetate and succinate were only detected in a few samples; therefore, they were not included in the calculation. The amounts of rhizosheath carboxylates were expressed in mol per unit root dry mass.
Assay of root acid phosphomonoesterase activity
Root PME activity was measured using para-nitrophenyl phosphate (pNPP) as substrate, according to the method described by Png et al. (2017), with minor modifications. About 0.5 g of the frozen roots were ground in 8 mL of sodium acetate buffer (pH 5.8) in an ice bath, and transferred to a centrifuge tube to centrifuge for 15 min at 4 °C, 12000 rpm. 0.2 mL of the supernatant was transferred to centrifuge tube; then 2 mL pNPP substrate was added and incubated at 30 °C for 30 min. A second identical supernatant for each sample was prepared as a control, in which 2 mL of the buffer was added instead of the pNPP substrate. The reaction was terminated by adding 4 mL 0.5 M NaOH. The concentration of paranitrophenol (pNP) in the final solution was determined by measuring the absorbance at 405 nm using a 3900H UV-VIS spectrophotometer (Hitachi Limited, Tokyo, Japan). Root PME activity was calculated and expressed on a root fresh mass basis.
Determination of leaf and stem P concentrations and calculation of aboveground PUE
Dried leaf and stem samples of each harvest were analyzed for P concentration separately. Each sample was finely ground using a stainless steel coffee grinder, then about 0.1 g subsample of each ground sample was digested in hot concentrated H2SO4:HClO4 (4:1). P concentration in the digested solution was determined using a 3900H UV-VIS spectrophotometer (Hitachi Limited, Tokyo, Japan) by the malachite green method (Motomizu et al. 1983). Shoot P content per pot was calculated according to the following formula:
Shoot P content = LDMH1 × leaf [P]H1 + SDMH1 × stem [P]H1 + LDMH2 × leaf [P]H2 + SDMH2 × stem [P]H2.
where LDM is the leaf dry mass, SDM is the stem dry mass, leaf [P] is the leaf P concentration, stem [P] is the stem P concentration, H1 and H2 represents the first and the second harvest, respectively. Aboveground P-utilization efficiency is defined as plant biomass production per unit of P uptake (Richardson et al. 2011). In this study, we calculated the aboveground PUE as shoot dry mass (the sum of leaf dry mass and stem dry mass in both harvests) per pot divided by shoot P content per pot as calculated according to the above formula.
Statistics
Means and standard errors of means (SEM) of shoot dry mass, root dry mass, nodule number, leaf and stem P concentrations, aboveground PUE, pH of the bulk soil and rhizosheath extract, amounts of rhizosheath carboxylates, and root PME activity of each treatment were obtained using the One-sample t-test in the IBM SPSS Statistics 22.0 software package (IBM, Montauk, New York, USA). The effects of soil P supply, N supply, and their interaction on the above-mentioned parameters were examined by performing a two-way (P × N) ANOVA, using the general linear model in the IBM SPSS Statistics 22.0 software package. When significant effect of soil P supply was found for a certain parameter mentioned above, the data for each soil N supply level were analyzed separately by performing a one-way ANOVA to compare the means between treatments with different P supply, and least significant difference (LSD) test for post hoc means comparison was used when significant (P ≤ 0.05) differences was found.
Results
Biomass and nodule development.
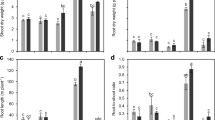
Soil P supply had a significant effect on shoot dry mass (SDM), root dry mass (RDM), and nodule number (NN) per pot (all P < 0.001) (Fig. 1, Table S1), but soil N supply only had a significant effect on NN (P < 0.001). For none of the SDM, RDM, and NN, there was a significant interaction between P and N. Adding P to the soil significantly enhanced the biomass accumulation of alfalfa, but increasing N supply did not promote plant growth, indicating that P, rather than N, was the growth-limiting factor for alfalfa in the studied soil. Mean NN at 5P and 20P was 3.88 times (P = 0.001) and 4.69 times (P < 0.001) greater than that at 0P, but there was no significant difference between NN at 5P and 20P (P = 0.399). Mean NN at 100 N was 35% less than that at 50 N. A higher P supply, and lower N supply together with rhizobia inoculation, favored the development of nodules.
Shoot dry mass (a), root dry mass (b), and nodule number (c) of alfalfa grown in the soil with different phosphorus (P) and nitrogen (N) supply. Data are presented as means + SEM (n = 4). 0P, 5P, and 20P represents that P was supplied at 0, 5, and 20 mg kg−1 as KH2PO4, respectively; 50 N shows that N was supplied at 50 mg kg−1 as NH4NO3 and plants were inoculated with rhizobia, while 100 N shows that N was supplied at 100 mg kg−1 as NH4NO3 and plants were not inoculated with rhizobia. Different letters above the bars indicate significant differences between treatments with different soil P supply for each N level separately, according to one-way ANOVA. The same for other figures
Stem and leaf P concentrations, and aboveground PUE
The effects of soil P supply, N supply, and their interaction on plant P concentration varied between different organs of the plant. Both stem [P] and leaf [P] increased significantly (both P < 0.001) (Fig. 2, Table S1) with increasing soil P supply, but only stem [P] was considerably affected by soil N supply (P = 0.001) and by the interaction between P and N (P < 0.001). At 0P, stem [P] was 12% lower at 100 N than at 50 N, while at 5P and 20P, it was 24% and 25% higher at 100 N than at 50 N, respectively.
Aboveground PUE was 0.37–0.85 g mg−1, and declined markedly with increasing soil P supply (P < 0.001) (Fig. 3, Table S1). Neither soil N supply, nor the interaction between P and N, significantly affected aboveground PUE.
pH of the bulk soil and rhizosheath extract
Soil P supply, N supply, and their interaction all considerably affected the pH of the bulk soil (all P < 0.001), but none of them caused a significant change in the pH of the rhizosheath extract (Fig. 4, Table S1). pH of the rhizosheath extract was 6.95–7.29, much lower than that of the bulk soil, which was 8.66–8. 91, suggesting that the rhizosheath was considerably acidified.
Rhizosheath carboxylate amounts and root PME activity
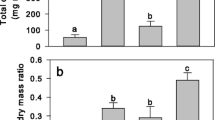
The amount of rhizosheath oxalate, malate, citrate, malonate, and tartrate at 50 N was 0.077–0.085, 0.103–0.147, 0.192–0.268, 0.219–0.263, and 0.180–1.6 mmol g−1 RDM, respectively, and that at 100 N was 0.059–0.131, 0.165–0.507, 0.194–0.264, 0.221–0.369, and 0.592–19.7 mmol g−1 RDM, respectively (Fig. 5). None of the soil P supply, N supply, and their interaction considerably affected the amount of oxalate, citrate, and malonate, but all of them significantly affected the amounts of malate and tartrate (all P ≤ 0.001) (Fig. 5, Table S1). At 50 N, the amount of malate was 17% higher at 5P than at 0P, but 19% lower at 20P than at 0P; at 100 N, it was 67% and 63% lower at 5P and 20P than at 0P, respectively. At 0P, 5P, and 20P, the amount of malate was 302%, 12%, and 83% higher at 100 N than at 50 N, respectively. At 50 N, the amount of tartrate was 89% and 89% lower at 5P and 20P than at 0P, respectively; at 100 N, it was 94% and 97% lower at 5P and 20P than at 0P, respectively. At 0P, 5P, and 20P, the amount of tartrate was 11.3, 6.0, and 2.3 times higher at 100 N than at 50 N, respectively. Tartrate was a major carboxylate released under the current experimental condition. At 0P at 50 N, and at all P levels at 100 N, tartrate was the most abundant carboxylate in the rhizosheath, suggesting that exudation of tartrate by roots was stimulated by low soil P and high soil N.
The amounts of rhizosheath carboxylates, including oxalate, malate, citrate, and malonate (a), and tartrate (b) relative to root dry mass (RDM) of alfalfa grown in the soil with different phosphorus (P) and nitrogen (N) supply. Data are presented as means + SEM (n = 4, except for some treatments in which certain carboxylate was not detectable in some sample(s)). Different letters above the bars of malate in panel (a) indicate significant differences in the amount of malate between treatments with different soil P supply for each N level separately, according to one-way ANOVA
Root PME activity was significantly affected by both soil P supply (P < 0.001) and N supply (P = 0.034), but not by the interaction between P and N (Fig. 6, Table S1). Mean root PME activity declined with increasing soil P supply, it was 47% and 59% lower at 5P and 20P than at 0P, respectively, while it was 26% higher at 100 N than at 50 N.
Discussion
The results of the present study supported most, but not all our hypotheses. The hypothesis that (i) plant biomass and nodule number would be greater at higher P supply, but plant biomass would not be significantly affected by N supply, nodulation would be inhibited by lower P supply and higher N supply was fully supported. Most plants, including alfalfa, tend to show a positive growth response to increasing P supply when soil P is deficient (Elser et al. 2007; He et al. 2017; Pang et al. 2010). It is likely that N supply was sufficient for alfalfa, even at 50 mg kg−1, so plant growth was not further enhanced when N supply was increased to 100 mg kg−1. There are other studies demonstrating that plant growth do not respond positively to increasing N supply when N is already sufficient (Perring et al. 2008). The different growth responses to increasing P supply and N supply suggest that P is a more growth-limiting factor for alfalfa in this study. High levels of soil N (Murray et al. 2017; Regus et al. 2017), and low levels of soil P (Prodhan et al. 2019; Valentine et al. 2017) often suppress nodulation of legumes. The higher nodule number at 50 N was likely partly due to the lower soil N level and partly to the inoculation of plants with rhizobia (Valentine et al. 2017). It has been reported that the inhibition of nodulation by higher N supply is greater at low P supply than at high P supply (Maistry et al. 2015). In the present study, there was no significant interaction between soil P supply and N supply on the nodulation of alfalfa plants. However, a different result in such interaction may be obtained if plants were inoculated with rhizobia in the same way at different N supply levels.
Our hypothesis (ii) was that leaf and stem P concentrations would increase with increasing soil P supply, but not vary considerably with N supply. This hypothesis was not fully supported. It has been reported that increased N supply and N:P supply ratio can reduce plant tissue P concentration, thus inducing a greater demand for P and capacity for plant uptake of P (Fageria 2001; Maistry et al. 2015). However, our results showed that both leaf and stem P concentrations always increased with increasing P supply, but leaf P concentration was not markedly affected by N supply, while stem P concentration was considerably affected by N supply and by the interaction between P and N, suggesting an organ-specific response of P demand to changes in the N:P supply ratio in soil.
The hypothesis (iii) that release of protons, carboxylates, and phosphatase would be more at lower soil P supply and higher N supply was partly supported. In the present study, plants in all treatments acidified the rhizosheath soil, although we did not quantify and compare the amounts of protons released by roots in different treatments. pH of the rhizosheath extract in the present study was between 7.0 and 7.3, a range within which P is readily available to plants (Lambers et al. 2008). In the study of He et al. (2017), pH of the rhizosheath extract ranged between 6.8 and 7.2, and there was no significant difference in rhizosheath pH among soil P levels when alfalfa was grown in an aeolian sandy soil and a loessial soil supplied with 0–160 mg kg−1 P as calcium superphosphate. The lack of differences in rhizosheath pH across soil P levels and N supply suggests that plants can sense the rhizosheath pH and alter it to a range suitable for P uptake. The release of phosphatases, as shown by root PME activity, was greater at lower soil P supply and higher N supply. It has been observed that addition of N to soils deficient in P increases the phosphatase activity of roots and soil, due to N-induced higher demand for P by plants (Maistry et al. 2015; Phoenix et al. 2003; Treseder and Vitousek 2001). A higher phosphatase activity suggests that plants tend to increase the acquisition of P from organic P fractions in soils with low availability of P (Lambers et al. 2006; Png et al. 2017).
The hypothesis that release of carboxylates would be greater at lower soil P supply and higher N supply was confirmed, mainly because tartrate, the major carboxylate in most cases in the present study, was released in a considerably higher amount at lower P supply and higher N supply. Release of a large amount of tartrate was also observed in the rhizosheath of alfalfa grown in other alkaline soils supplied with different levels of P and N (Figs. S2–S5), and these results, together with those in the present study, suggest that significantly more tartrate will be released from roots of plants grown in P-deficient soils with a high N supply as NH4NO3. Unlike malate and citrate, which are often reported in plant responses to P deficiency and thought to play an important role in mobilizing both inorganic and organic P in soil (Lambers et al. 2008; Pang et al. 2018), tartrate is seldom reported in studies on plant-soil interaction under P deficiency, although it has been identified in the exudates of roots and rhizosphere bacteria, and in aqueous extracts of forest litter (He et al. 1999; Strobel 2001). This is the first report on the exudation of tartrate as a major carboxylate by roots, especially under P deficiency at high N supply. Furthermore, tartrate is an important component of the secretion of rhizosphere phosphate-solubilizing bacteria (Shahid et al. 2015), of which the population may be increased and the activity may be stimulated under P deficiency, thus more organic anions may be secreted to increase P availability in soil (Ibarra-Galeana et al. 2017; Liu et al. 2014). The amounts of oxalate, malate, citrate, and malonate in the present study were also very large compared with those in other studies on alfalfa (Fan et al. 2015; Pang et al. 2015; Suriyagoda et al. 2010), likely due to the high pH of the soil we used, and the repeated removal of the aboveground parts. Release of large amounts of carboxylates may be a considerable carbon (C) cost for plants over the growth cycle. In the present study, the exudation of a greater amount of tartrate could cause the plants to accumulate less biomass and grow more slowly, as the magnitude of the tartrate exudation relative to total C fixed very likely increased markedly, and less C fixed was available for growth, although we were not able to quantify the amount of total C fixed and the amount of C exudated as carboxylates based on our data.
There are reports that tartrate has the potential to reduce P adsorption and increase soil P availability, but its capacity to mobilize P is weaker than that of citrate, but greater than that of oxalate (Dye 1995; He et al. 1999; Wang et al. 2008). However, the effects of organic anions on P sorption depend on the mineral composition of soil particles. For example, citrate had a more pronounced effect on P sorption by Fe-rich sites, but tartrate and citrate had a similar effect on P sorption by Al-rich sites (Earl et al. 1979). According to Dye (1995), the types of P-sorption site that is attacked in the presence of tartrate and citrate are different, as citrate dissolves sites at which P is held, while tartrate occupies the high affinity sites to reduce P sorption. Therefore, a greater amount of rhizosphere tartrate would lead to more efficient use of added P by plants, in contrast, a greater amount of rhizosphere citrate would help plants to utilize more fixed P. The mixture of different organic anions is more effective in reducing P sorption than an individual organic anion alone under the same concentrations of organic ligands, likely because more high-affinity sites are occupied by different anions than by an individual anion alone (He et al. 1999). However, the amounts of organic anions in the rhizosphere should reach a certain threshold for effective reduction of P sorption by soil particles (Dye 1995; Earl et al. 1979).
In conclusion, plant growth and P concentrations always showed positive responses to P addition, but not to N addition, likely due to N was sufficient. Roots always acidified the rhizosheath, rhizosheath pH was altered to a range within which P is readily available to plants. There were interactions between soil P supply and N supply on plant physiology, N addition can induce a greater demand for P, thus leading to the induction of P-acquisition mechanisms. Roots released more phosphatase and carboxylates, mainly tartrate, the most abundant carboxylate in most cases, into the rhizosheath at lower soil P supply and higher N supply. Our work suggests that N addition may lead to greater P limitation, and highlights the importance of investigating plant adaptive strategies for P acquisition from soil with different N availability for proper application of P and N fertilizers.
References
Carpenter SR (2005) Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc Natl Acad Sci U S A 102:10002–10005
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240
Chen H, Chen M, Li D, Mao Q, Zhang W, Mo J (2018) Responses of soil phosphorus availability to nitrogen addition in a legume and a non-legume plantation. Geoderma 322:12–18
Cordell D, White S (2015) Tracking phosphorus security: indicators of phosphorus vulnerability in the global food system. Food Secur 7:337–350
Dye C (1995) Effect of citrate and tartrate on phosphate adsorption by amorphous ferric hydroxide. Fertilizer Research 40:129–134
Earl KD, Syers JK, McLaughlin JR (1979) Origin of the effects of citrate, tartrate, and acetate on phosphate sorption by soils and synthetic gels. Soil Sci Soc Am J 43:674–678
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fageria VD (2001) Nutrient interactions in crop plants. J Plant Nutr 24:1269–1290
Fan JW, Du YL, Turner NC, Wang BR, Fang Y, Xi Y, Guo XR, Li FM (2015) Changes in root morphology and physiology to limited phosphorus and moisture in a locally-selected cultivar and an introduced cultivar of Medicago sativa L. growing in alkaline soil. Plant Soil 392:215–226
Fixen PE, Johnston AM (2012) World fertilizer nutrient reserves: a view to the future. J Sci Food Agric 92:1001–1005
He H, Peng Q, Wang X, Fan C, Pang J, Lambers H, Zhang X (2017) Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 416:565–584
He JZ, De Cristofaro A, Violante A (1999) Comparison of adsorption of phosphate, tartrate, and oxalate on hydroxy aluminum montmorillonite complexes. Clay Clay Miner 47:226–233
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hinsinger P, Plassard C, Tang CX, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Ibarra-Galeana JA, Castro-Martínez C, Fierro-Coronado RA, Armenta-Bojórquez AD, Maldonado-Mendoza IE (2017) Characterization of phosphate-solubilizing bacteria exhibiting the potential for growth promotion and phosphorus nutrition improvement in maize (Zea mays L.) in calcareous soils of Sinaloa, Mexico. Ann Microbiol 67:801–811
Johnston AE, Poulton PR, Fixen PE, Curtin D (2014) Phosphorus: its efficient use in agriculture. Adv Agron 123:177–228
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Lambers H, Thijs LP, Chapin FS (2008) Plant physiological ecology. Springer, New York, USA
Little IP (1992) The relationship between soil pH measurements in calcium chloride and water suspensions. Aust J Soil Res 30:587–592
Liu FP, Liu HQ, Zhou HL, Dong ZG, Bai XH, Bai P, Qiao JJ (2014) Isolation and characterization of phosphate-solubilizing bacteria from betel nut (Areca catechu) and their effects on plant growth and phosphorus mobilization in tropical soils. Biol Fertil Soils 50:927–937
MacDonald GK, Bennett EM, Potter PA, Ramankutty N (2011) Agronomic phosphorus imbalances across the world's croplands. Proc Natl Acad Sci U S A 108:3086–3091
Maistry PM, Muasyaa AM, Valentineb AJ, Chimphangoa SBM (2015) Balanced allocation of organic acids and biomass for phosphorus and nitrogen demand in the fynbos legume Podalyria calyptrata. J Plant Physiol 174:16–25
Mekonnen MM, Hoekstra AY (2018) Global anthropogenic phosphorus loads to freshwater and associated grey water footprints and water pollution levels: a high-resolution global study. Water Resour Res 54:345–358
Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst 108:361–367
Murray JD, Liu CW, Chen Y, Miller AJ (2017) Nitrogen sensing in legumes. J Exp Bot 68:1919–1926
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120
Pang J, Ryan MH, Lamberst H, Siddique KHM (2018) Phosphorus acquisition and utilisation in crop legumes under global change. Curr Opin Plant Biol 45:248–254
Pang J, Tibbett M, Denton MD, Lambers H, Siddique KHM, Bolland MDA, Revell CK, Ryan MH (2010) Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant Soil 328:133–143
Pang JY, Yang JY, Lambers H, Tibbett M, Siddique KHM, Ryan MH (2015) Physiological and morphological adaptations of herbaceous perennial legumes allow differential access to sources of varyingly soluble phosphate. Physiol Plant 154:511–525
Pasley HR, Cairns JE, Camberato JJ, Vyn TJ (2019) Nitrogen fertilizer rate increases plant uptake and soil availability of essential nutrients in continuous maize production in Kenya and Zimbabwe. Nutr Cycl Agroecosyst 115:373–389
Perring MP, Hedin LO, Levin SA, McGroddy M, de Mazancourt C (2008) Increased plant growth from nitrogen addition should conserve phosphorus in terrestrial ecosystems. P Natl Acad Sci USA 105:1971–1976
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2003) Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol 161:279–289
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiol 156:1006–1015
Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170:491–500
Png GK, Turner BL, Albornoz FE, Hayes PE, Lambers H, Laliberté E (2017) Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J Ecol 105:1246–1255
Prodhan MA, Finnegan PM, Lambers H (2019) How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci 24:69–82
Raghothama KG (1999) Phosphate acquisition. Ann Rev Plant Physiol Mol Bio 50:665–693
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49
Regus JU, Wendlandt CE, Bantay RM, Gano-Cohen KA, Gleason NJ, Hollowell AC, O'Neill MR, Shahin KK, Sachs JL (2017) Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 414:159–170
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156
Shahid M, Hameed S, Tariq M, Zafar M, Ali A, Ahmad N (2015) Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann Microbiol 65:1525–1536
Simpson RJ, Stefanski A, Marshall DJ, Moore AD, Richardson AE (2015) Management of soil phosphorus fertility determines the phosphorus budget of a temperate grazing system and is the key to improving phosphorus efficiency. Agric Ecosyst Environ 212:263–277
Strobel BW (2001) Influence of vegetation on low-molecular-weight carboxylic acids in soil solution - a review. Geoderma 99:169–198
Sun Z, Han J (2018) Effect of soft rock amendment on soil hydraulic parameters and crop performance in mu us Sandy land, China. Field Crop Res 222:85–93
Suriyagoda LDB, Ryan MH, Renton M, Lambers H (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Ann Bot 105:755–767
Syers J, Johnston A, Curtin D (2008) Efficiency of soil and fertilizer phosphorus use. Reconciling changing concepts of soil phosphorus behaviour with agronomic information. Fertilizer and plant nutrition bulletin no 18 Rome, Italy: FAO
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N, P in Hawaiian rain forests. Ecology 82:946–954
Valentine AJ, Kleinert A, Benedito VA (2017) Adaptive strategies for nitrogen metabolism in phosphate deficient legume nodules. Plant Sci 256:46–52
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Wang Y, He Y, Zhang H, Schroder J, Li C, Zhou D (2008) Phosphate mobilization by citric, tartaric, and oxalic acids in a clay loam Ultisol. Soil Sci Soc Am J 72:1263–1268
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528:51–59
Zhao Q, Zeng DH (2019) Nitrogen addition effects on tree growth and soil properties mediated by soil phosphorus availability and tree species identity. Forest Ecol Manag 449:117478
Acknowledgements
This work was supported by The National Key Research and Development Plan of China (2017YFC0504504), The Natural Science Basic Research Program of Shaanxi Province (2019JM-411), The National Natural Science Foundation of China (41301570), The Light of West China Program of Chinese Academy of Sciences, and Fundamental Research Funds for Central Universities in China. We thank Xiyan Chen, College of Life Sciences, Northwest A&F University, for helping the analysis of rhizosheath carboxylates using HPLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Martin Weih.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 637 kb)
Rights and permissions
About this article
Cite this article
He, H., Wu, M., Guo, L. et al. Release of tartrate as a major carboxylate by alfalfa (Medicago sativa L.) under phosphorus deficiency and the effect of soil nitrogen supply. Plant Soil 449, 169–178 (2020). https://doi.org/10.1007/s11104-020-04481-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04481-9