Abstract
Aims
Phosphorus (P) removed in grains causes losses of P from fields each year. Reducing grain P may therefore improve the P efficiency of cropping systems. This study quantified impacts of reduced seed-P concentrations on rice seedling vigor and final yields and investigated whether this was influenced by soil P supply or genotype.
Methods
Seed batches with P concentrations ranging from 0.9 to 3.5 mg g−1 were produced by growing rice in field plots ranging from severely P-deficient to fully fertilized and used in glasshouse and field experiments to investigate effects on seedling vigor and final grain/straw yield.
Results
‘Genotype by seed-P concentration’ interactions were significant for seedling vigor but grain yield was generally not affected. This suggested some genotypes were sensitive to reduced seed-P concentration during the seedling stage while others with seed-P concentrations as low as 0.9 mg g−1 maintained rapid early vigor and high grain yield.
Conclusions
Results indicate it may be possible to reduce seed-P concentrations without having negative effects on seedling vigor or yield. The development of cultivars with reduced seed-P concentration, particularly if combined with rapid seedling root growth, could be a valid option to improve the sustainability of phosphate fertilizer use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The removal of phosphorus (P) from fields in the grain of crop plants at harvest is a major driver of the current global P cycle. Estimates suggest that the P removed in harvested products across the globe equates to around 85 % of the P applied each year as fertilizer (Lott et al. 2000). Only a small percentage of this removed P is recycled back onto crop land (Cordell et al. 2009), resulting in high requirement for P fertilizer application to cropping land (Rose and Wissuwa 2012).
At a global scale, cereal crops are responsible for the greatest removal of P from fields each year because of the large area cultivated (Lott et al. 2000) and because at maturity around 50–80 % of total plant P is located in seeds of staple crops such as wheat, maize and rice (Batten et al. 1986; Karlen et al. 1988; Rose et al. 2010). The removal of P from fields in the grains of major cereal crops therefore represents a major P flux in the global P cycle. In addition to driving demand for P fertilizer in high-inputs systems, the removal leads to negative soil P balances in low-input agriculture where P fertilizers are not accessible or are too costly (MacDonald et al. 2011). In Africa where little P is applied, such negative P balances contribute to declining soil fertility (Haefele et al. 2013).
Further, around 65–80 % of the total seed P is stored as phytic acid (PA), which cannot be digested by monogastric animals and humans. High P concentrations in human and animal wastes subsequently cause environmental problems such as eutrophication of water bodies (Raboy 2009). In light of this, attempts have been made to breed for reduced phytic acid content, particularly in feed grain. Numerous mutants with low phytic acid (lpa) in seeds have been identified across most major crop species, but with the exception of two mutants, seed total P concentrations remain unchanged (see review by Raboy et al. 2014). Thus, while the nutritional quality of the grain is enhanced, there is no reduction in the removal of P from fields in harvested grain.
In contrast, addressing seed total P directly by reducing the amount of P translocated to developing seeds during grain filling may be a feasible option for minimizing the removal of P from fields at harvest with ensuing environmental and economic benefits. It can be estimated that the 2010 global rice crop of 700 MT (FAO 2013) removed in excess of 1.4 MT P (using a conservative estimate for grain P concentrations of 2 mg g−1). The value of P removed in grain amounts to 7 billion US$ in fertilizer equivalents (at US$ 5 kg−1 P). If grain-P concentrations could be reduced by 20 %, and even if only a fraction of the ‘spared’ P is saved in terms of reduced fertilizer applications, it can be estimated that the potential economic benefit to rice growers is in the 100’s of millions of US$ annually.
A number of factors need to be considered with such an approach, including impacts on grain quality, human health and subsequent seedling growth. In a recent review, Rose et al. (2012) found limited published evidence that reductions in seed-P concentrations would impact grain quality or human health, but the impact on seedling vigor remained contentious. Several studies with lpa mutants that have perturbations to the phytic acid synthesis pathway have found reduced seed germination and seedling vigor in mutant plants compared to wild type plants (see Raboy 2009 and references therein). Further, a mutant line deficient in an acid purple phosphatase gene also showed reduced translocation of P to developing seeds, and subsequent issues with seed germination and seedling vigor were reported (Robinson et al. 2012). However, it is possible that the lower germination percentage and subsequent reductions in seedling vigor were due to impaired critical functions as a result of the mutations rather than insufficient P in the seeds per se (Rose et al. 2012). Support for this hypothesis comes from studies with the barley lpa1-1 mutant, which has a reduction in total grain P of 10–15 % as well as reduced phytic acid levels yet has no issues with seed germination and no significant loss of seedling vigor (Raboy et al. 2014).
A number of other studies have also reported reductions in seedling vigor when seeds low in P concentration have been cultivated in P-deficient soil (see review by White and Veneklaas 2012 and references therein). However, one of the major limitations with all work conducted to date on the impact of seed-P concentration on seedling vigor is that low-P seeds have typically been obtained from plants suffering from P deficiency stress and the lack of discussion on the potential implications this may have for the results. In the study of Zhu and Smith (2001), the low-P seeds differed by 50 % in mass to the high-P seeds, yet the authors concluded that seed-P concentration had a major impact on seed germination and seedling vigor. Two further papers also reported that the low-P seed from P-stressed parent plants was smaller than the high-P seed obtained from the P-replete parent plants (Burnett et al. 1997; Derrick and Ryan 1998). Of the remaining studies that have investigated the impact of cereal seed-P concentrations on seedling vigor, there is a notable lack of studies conducted on realistic agricultural soils. For example, Zhang et al. (1990) cultivated barley seedlings in sand culture supplemented with nutrient solution, while Bolland and Baker (1988) grew wheat plants in a newly-cleared virgin soil with a total P content of 58 mg kg−1. While it seems probable that high-P seed confers an advantage in natural ecosystems or on highly P-deficient soils, there is little published evidence to suggest that high-P seed levels are needed in soils that have a history of P fertilization. The first objective of the present study was therefore to investigate the impact of seed-P concentrations on seedling vigor and subsequent grain yields on agricultural soils. We focus specifically on seed-P concentration and not on total seed-P content, because the amount removed with harvested grain at the same grain yield level entirely depends on the concentration of P in the harvested biomass.
White and Veneklaas (2012) argue that greater seed-P reserves enable more rapid early root growth which then results in greater seedling vigor. This contrasts with observations in rice where genotype ‘Kasalath’, which has small seeds with a low P content of 34 μg seed−1, has greater initial root growth and subsequent seedling vigor than the larger seeded ‘Nipponbare’ with 44 μg seed−1 (Wissuwa and Ae 2001). Data further showed more rapid P translocation from seed to seedling in Kasalath, possibly indicating that genotypic differences in early P translocation rates are more important than total seed-P content in conferring early seedling vigor and subsequent tolerance to P deficiency (Wissuwa and Ae 2001). We hypothesized that the relationship between seed-P reserves and seedling biomass (root and shoot) may not be consistent across genotypes, and that genotypes with inherently high early root growth and shoot biomass production may be less susceptible to any impact of lower seed-P concentrations. The second objective was therefore to investigate whether any impact of seed-P concentrations on seedling vigor is genotype specific. We examined this relationship across a number of rice genotypes in both upland and transplanted lowland systems. In order to exclude seed effects not related to P, such as the reduced seed size typically seen in seeds produced under P-deficient conditions, batches with equal seed size but different P content were used in this study.
Materials and methods
A number of trials were conducted in greenhouse and field condition over multiple seasons in Asia (Japan) and West Africa (Benin and Burkina Faso) to investigate the impact of different seed-P concentrations in rice genotypes on seedling vigor, and biomass and grain yield in either up-land (direct seeded) or low-land (transplanted) systems.
Seed production and measurements of seed-P concentration
Several rice genotypes were grown under up-land conditions (from May to October of 2012 and 2013) in an Andosol soil under three levels of P availability in Tsukuba, Japan. Plants were cultivated on a severely P-deficient plot that had no history of P fertilization (>40 years); a P-deficient plot that has not received P fertilizer in the past 10 years; and a P-replete control plot fertilized with 50 kg ha−1 (as P2O5) annually. Previous experiments have typically shown a yield reduction of 70–90 % in the severely P-deficient plot and 30–50 % in the mildly P-deficient plot, relative to the fertilized control. Agronomic practices performed were common for the growing area. Fertilizers were applied (basal) in the equivalent amount to 50-0-50 or 50-50-50 kg ha−1 (N, P2O5, K2O, respectively) and an additional 25 kg N as top dressing. Seed lots produced for experiments in West Africa were harvested from variety screening trials in Gambia in 2012 and Nigeria and Burkina Faso in 2013. At each location, the genotypes were grown with and without P application (80-69-60 and 80-0-60 kg ha−1, as N, P2O5, K2O, respectively).
At maturity, whole plants were harvested in bundles and air-dried. Panicles were then threshed and seeds were manually cleaned by winnowing. Seeds harvested from each field were weighed, and typically seeds from P-deficient sites had lower hundred grain weights compared to those produced under P-fertilized conditions. In such cases shriveled or otherwise visibly smaller seeds were omitted and batches were weighed again until low-P seed batches were within 10 % of their high-P counterparts.
Seeds (approximately 250 mg) were digested in a mixture of 3:1:1 nitric:perchloric:sulfuric acid and the P concentration in the extract was determined using the colorimetric vanadomolybdate assay (Murphy and Riley 1962). A standard sample “Certified tea leaves II” (National Institute for Environmental Studies-Japan) with a P concentration of 0.472 ± 0.032 mg g−1 was used as a reference material. For African samples, seed-P concentrations were determined using a continuous-flow analysis system (Thomas et al. 1967) after wet digestion of the plant material in tubes at high temperature with sulfuric acid, salicylic acid, hydrogen peroxide, and selenium (Novozamsky et al. 1983).
Seedling and plant growth experiments
Using these seed batches differing in seed-P concentrations, several experiments were conducted to determine the impact of seed-P concentrations on seedling vigor and yield. The list of genotypes and their respective seed characteristics used in experiment 1, 2 and 3, and different locations at Japan and Africa are shown in Table 1.
Experiment 1
Effect of seed-P concentration on vigor of seedling grown in pots (trials were conducted at Japan and Africa sites)
In the first trial conducted in Japan in 2012, seeds from four rice genotypes (FKR43, GNF6, WAB and WPF2) containing different P concentration were pre-germinated for 1 day and sown in 80-L containers containing P-sufficient soil (fertilized soil). Water was applied regularly to simulate upland condition. Seedlings were gently removed and evaluated at 3 and 4 weeks after sowing (WAS). Only the data for 4 WAS are presented since they did not vary.
In the second trial in Benin in 2014, seeds batches from five genotypes (Mudgo, DJ123, Surjamkuhi, NERICA4 and Santhi Sufaid) were sown in 70 cm3 cups containing 3 P-deficient soils collected from different locations in Benin. Trays containing the cups were placed inside lower trays containing nutrient solutions with or without P. Cotton wads were positioned at the bottom of the cups to allow the nutrient solution to pass to the soil (Saito et al. 2015). The nutrient solution was composed of 2 mM KCl (minus-P treatment), 2 mM KH2PO4 (plus-P treatment), and 3 mM Ca(NO3)2, 2 mM KNO3, 0.75 mM MgSO4, 1 mM NH4NO3, 150 μM Fe sequestrene, 15 μM MnCl2, 1.5 μM ZnCl2, 2 μM CuCl2, 10 μM H3BO3 and 0.1 μM (NH4)6Mo7O24 (both treatments). The nutrient solution was replaced weekly. Fifteen days after sowing the seedlings were evaluated, and shoot and root dry matter obtained and reported as seedling biomass. For Mudgo, germination was poor, so only data from two soils was obtained.
Experiment 2
Effect of seed-P concentration on yield and dry matter of plants grown under up-land field condition (trials were conducted at Japan and Africa sites)
In Japan (2013), a set of five genotypes (BJ1, DJ123, N22, RTS14 and Surjamkuhi, Table 1) were sown directly in field plots with either P-deficient or P-replete soil (fertilized soil). Seed-P concentration data for these genotypes are given in Table 1. The field was either supplied or not with P fertilizer (75-0-50 and 75-50-50 kg ha-1 as N, P2O5, K2O, respectively). Plants were sown in 1 m-wide beds, with a planting density of 0.2 m × 0.1 m and grown to maturity. Thinning and weeding were performed accordingly, and plants were cultivated under up-land (aerobic) conditions with supplementary irrigation to avoid drought stress. Since the values from fertilized soil showed a two-fold increase respect to those of deficient-P level soil, the data were analyzed separately.
In Africa (2013), two field trials were installed at the Farako-Ba research station in Burkina Faso and in Bohicon, Benin, using seed batches of 3 genotypes (DJ123, Santhi Sufaid and Surkamkuhi) (Table 1). At each location, the field was either supplied or not with P fertilizer (80-0-60 and 80-69-60 kg ha-1 as N, P2O5, K2O, respectively). Seeds were sown at a hill density of 0.2 × 0.2 m with 3 seeds per hill in a plot size of 0.8 × 0.8 m. Plants were thinned to 1 plant per hill. At harvest, grain weight and straw dry weight was measured. In Benin, supplementary irrigation was provided whenever necessary. In Burkina Faso, irrigation was provided only at crop establishment, and terminal drought stress was observed.
Experiment 3
Effect of seed-P concentration on yield and dry matter of plant grown under low-land field condition (trials were conducted at Japan site)
In 2012, seeds of genotypes FKR43, GNF6, WAB and WPF2 from the same seed sources used in experiment 1 were sown into seedling cell trays filled with either common soil or commercial nursery growing media. At 30 days after sowing (DAS) seedlings were transplanted into a completely fertilized paddy field and cultivated up to maturity. Fertilization dose was 75-50-50 kg ha-1 (N, P2O5, K2O, respectively). Seedling measurements were not taken.
A similar trial was repeated in 2013 using a different set of genotypes: BJ1, DJ123, Hunan Early Dwarf 3, Kalubala Vee, N22, RTS14 and Surjamkuhi (Table 1). Seedlings were grown in cell trays filled with common soil, and their vigor was evaluated at transplanting time. At maturity whole plants were harvested and number of tillers, panicles and yield was evaluated
Statistical analysis
In Japan, the field experiments were set into a two- or three-way factorial arrangement on a randomized complete block (RCB) design. The analysis of variance (ANOVA) was performed using the Statistix software (version 9, Analytical Software). Pairwise comparison for traits determined in this study between seed batches differing in seed-P concentrations in the given genotype was carried out using Tukey’s range test. For the experiment in Africa, ANOVA was carried out using the SAS software. Seed-P concentration, genotype and P rate were included as fixed factors. Effects of seed-P concentrations within genotypes were tested using a set of contrasts.
Results
Seed production
Seed batches were harvested from the three field plots (severely P deficient, deficient, and replete) in Japan, and from the unfertilized vs. fertilized fields in Africa. Average seed weight was typically 15-20 % lower in batches produced in Japan under P deficiency compared to the control (data not shown). To obtain more comparable batches (within genotypes) shriveled or otherwise visibly smaller seeds were discarded prior to the analysis of P concentration. In general, the average seed weight remained lower in seed batches from P-deficient plots but these differences typically were around 5 % (Table 1). In Japan, the seed-P concentration ranged from 0.89 to 2.70 mg g−1 in seed produced in 2011 for 2012 experiments, and from 1.15 to 3.63 mg g−1 in seed produced in 2012 for 2013 experiments. Seeds produced for experiments in Africa ranged from 1.29 to 3.15 mg g−1 and seed weights did not differ between batches.
Seed weight ranged from below 20 mg seed−1 (Surjamkuhi, Santhi Sufaid and N22) to around 30 mg seed−1 (DJ123 and WAB) (Table 1). With differences in seed-P concentration and weight, large variation in total seed-P content was observed with extremes of 18.7 and 97.2 μg seed−1. For further ease of data presentation we grouped seed batches into three levels based on the range of seed-P concentrations reported by Dobermann and Fairhurst (2000): clearly below concentrations typically achieved in farmers’ fields (low, < 1.7 mg g−1); within the range one may observe in insufficiently fertilized farmers’ fields (medium, 1.7–2.3 mg g−1); or the more typical range seen in high-input agriculture (high, > 2.3 mg g−1).
Field and pot experiments
Experiment 1
Effect of seed-P concentration on seedling vigor in pot experiments (trials were conducted at Japan and Africa sites)
In a first pot trial conducted in Japan (2012) using P-fertilized soil, no significant impact of seed-P concentration on seedling biomass was detected in any of the four genotypes (Fig. 1). Notably, GNF6 seedlings produced from seeds with extremely low P concentrations (0.89 mg g−1) and P content (18.7 μg seed−1) showed no reduction in biomass compared to plants produced from seeds with 3-fold higher seed-P concentration. A significant (p < 0.001) effect of genotype on seedling vigor regardless of seed-P concentration was detected with higher general seedling vigor for genotype GNF6 than FKR43.
Seedling biomass at 28 days after sowing (DAS) as affected by their seed-P concentrations (Experiment 1-Japan). Batches of seeds from 4 genotypes with either low, medium, or high seed-P concentrations were grown in 80-L containers filled with fertilized upland soil. Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentrations within genotype; ns: non-significant difference (p < 0.05) FKR: FKR43, GNF: GNF6, WAB: WAB189-B, WPF: WPF2
In a second pot trial conducted in Africa (Benin-2014), seedlings were screened at two P rates (with or without P supply) in 3 P-deficient soils. Since the results were similar among these soils the mean of 3 soils is presented (Fig. 2). All genotypes responded to P application and the mean seedling biomass without P supply across all the genotypes and soils was only 42 % of that with P supply (Fig. 2). Seedling biomass differed 1.6- and 2.4-fold among genotypes without and with P supply, respectively, with Mudgo and DJ123 having higher seedling biomass than Surjamkuhi, NERICA4 and Santhi Sufaid. Besides the effects of genotype and P rate, there was a significant P rate x seed P x genotype interaction (p = 0.02; Fig. 2). In P-deficient soil seedling biomass was significantly reduced by using seeds with lower P concentrations for all genotypes except for Mudgo and NERICA4. In P-fertilized pots lower seed-P concentration reduced seedling biomass in two out of five genotypes (DJ123 and Surjamkuhi).
Seedling biomass of five rice genotypes as affected by their seed-P concentrations (Experiment 1-Africa, Benin). Seedlings were grown in 3 P-deficient soils either or not amended with P fertilizer (P-deficient or P-replete soil), and evaluated at 15 days after sowing (DAS). Data points are the mean values of 3 soils. Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentrations within genotype; ns: non-significant difference (p < 0.05) Mud: Mudgo, DJ: DJ123, Sur: Surjamkuhi, N4: NERICA4, SS: Santhi Sufaid
Experiment 2
Effect of seed-P concentration on yield and dry matter of plants grown under up-land field condition (trials were conducted at Japan and Africa sites)
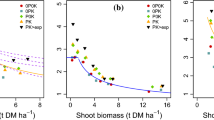
In Japan, seeds of five rice genotypes were sown directly to P-deficient or P-fertilized field plots and plants were grown to maturity to evaluate seed-P concentration effects on straw and grain yield. P deficiency was rather severe, reducing straw and grain yield by 75 and 50 %, respectively (Fig. 3). Seed-P concentration effects were not significant under P replete conditions for all genotypes, however, under severe P deficiency low seed-P concentrations led to reduced straw and grain yield of genotypes BJ1 and RTS14 (Fig. 3).
Effect of seed-P concentrations on straw (a) and panicle weight (b) of plants grown either in P-deficient or P-replete soil (Experiment 2-Japan). Batches of seeds from several genotypes with either low, medium, or high seed-P concentrations were grown in P-deficient or P-replete soil, under up-land condition (2013 data). Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentrations within genotype; ns: non-significant difference (p < 0.05) BJ: BJ1, DJ: DJ123, N22, RTS: RTS14, Sur: Surjamkuhi
Similar experiments in West Africa (Burkina Faso and Benin) did not show a significant response to P fertilization in both locations, and combined data (across P treatments) are therefore shown in Fig. 4. Grain and straw yields were lower in Burkina Faso than in Benin, due to difference in water condition (water stress was more severe in Burkina Faso). However, the difference in grain yield between two locations was genotype-dependent, being almost 50 % lower for DJ123 in Burkina Faso but hardly detectable in Santhi Sufaid. For all three genotypes, grain and straw yield remained unaffected by the seed-P concentration (Fig. 4).
Effect of seed-P concentrations on straw weight (a) and grain yield (b) of three rice genotypes grown in two field trials in Burkina Faso and Benin (Experiment 2-Africa). Trials were conducted both with and without P application but mean data are presented per site as no P response was observed. Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentrations within genotype; ns: non-significant difference (p < 0.05) DJ: DJ123, SS: Sunthi Sufaid, Sur: Surjamkuhi
Experiment 3
Effect of seed-P concentrations on seedling vigor and grain and straw yields under irrigated low-land field conditions (trials were conducted at Japan site)
Experiment 3 was conducted under low-land conditions using the same seed sources as respective pot and upland trials of experiments 1 and 2 in Japan (see Table 1). In the first trial (2012), seeds of the four genotypes were sown in seedling cell trays containing either common field soil or a commercial nursery substrate. Seedlings were then transplanted into a fully fertilized paddy field and cultivated up to maturity. Raising seedlings in the nursery soil significantly improved straw and panicle weights by 14 and 8 %, respectively (Fig. 5a-b) but seed-P concentrations had no effect. Even with extremely low seed-P concentrations of 0.89 mg g−1 genotype GNF produced high straw and panicle weights compared to other genotypes.
Effect of seed-P concentrations and nursery soils on straw (a) and panicle weight (b) (Experiment 3-Japan 2012). Batches of seeds from 4 genotypes with either low, medium, or high seed-P concentrations were grown in either commercial nursery or common soil. Seedling were transplanted to a fertilized paddy field and grown up to maturity. Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentrations within genotype; ns: non-significant difference (p < 0.05) FKR: FKR43, GNF: GNF6, WAB: WAB189-B, WPF: WPF2
In the second low-land trial (2013), a different set of genotypes was used compared to 2012 and genotype differences were highly significant at the seedling stage. Seedlings of the high seed-P concentration batches used for transplanting had about 15–50 % higher biomass compared to medium- or low-P seed batches, with the exception of genotype N22 (Fig. 6). However, at maturity seed-P concentration effects were no longer significant for straw weight (Fig. 7a) and only one out of seven genotypes (BJ1) had significantly lower panicle weight if medium or low P seed batches were used (Fig. 7b). Interestingly this negative effect was already observed at a P concentration of 2.32 mg g−1 in BJ1, which is probably within the normal range expected for seeds obtained from farmer’s field. A further reduction to 1.24 mg g−1 had no additional negative effect on panicle weight or seedling biomass (Figs. 6 and 7b, respectively).
Biomass of seedlings derived from seeds with either low, medium, or high seed-P concentrations grown in common field soil (Experiment 3-Japan 2013). Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentration within genotype; ns: non-significant difference (p < 0.05) BJ: BJ1, DJ: DJ123, HE: Hunan early dwarf No3, KV: Kalubala Vee, N22: N22, RTS: RTS14, Sur: Surjamkuhi
Straw (a) and panicle weight (b) of seven genotypes with either low, medium, or high seed-P concentrations grown in fertilized lowland soil (Experiment 3-Japan 2013). Error bars are standard errors of the mean. Letters refer to statistical difference of seed-P concentrations within genotype; ns: non-significant difference (p < 0.05) BJ: BJ1, DJ: DJ123, HE: Hunan early dwarf No3, KV: Kalubala Vee, N22: N22, RTS: RTS14, Sur: Surjamkuhi
Discussion
Assessing impacts of reduced seed-P concentration on seedling vigor and grain yield was driven by the view that i) rice and other grain crops tend to load more P into grains than actually needed for optimum germination and seedling establishment; ii) the removal of ‘excessive’ grain P drives the need for continuously high fertilizer inputs in high input systems, while reducing soil fertility due to continuous mining of P in low input systems; and iii) the high levels of P contained in grain are of no benefit to consumers but rather pose a large environmental threat by increasing P concentrations in human waste. While it has been postulated that grain-P concentrations of only 1 mg g−1 would be sufficient to support the physiological functions of a developing seedling (Raboy 2005), grain P concentrations are typically in excess of 2 mg g−1 and may reach 3 mg g−1 or higher under well-fertilized conditions (Dobermann and Fairhurst 2000; Rose et al. 2010). At present little is known as to whether a minimum seed-P concentration is needed to maintain seedling vigor and where such minimum concentration may lie.
Further, many previous studies on the impact of seed-P concentrations on seedling vigor investigated plants that were cultivated in artificial growing media with very low P supply (Zhang et al. 1990; Zhu and Smith 2001) or in newly cleared land with extremely low native soil P levels (Bolland and Baker 1988). Thus developing seedlings may have had little opportunity to overcome a P limitation by rapid uptake of P from their environment. We therefore investigated whether soil-P status played a role in the seedling vigor response of rice genotypes to reduced seed-P concentrations.
Effect of seed-P concentration and soil P status on rice seedling vigor and grain yield
Without P supply, three out of five genotypes showed reduced vigor in response to reduced seed-P concentration in the pot trial in Benin (Fig. 2) and six out of seven genotypes showed reduced seedling vigor in experiment 3, Japan (Fig. 6) in common agricultural soil that was not supplemented with P fertilizer. This is in general agreement with reductions in seedling vigor observed in previous studies when plants were cultivated in low-P media without P fertilizer addition (Bolland and Baker 1988; Zhang et al. 1990; Rose et al. 2012). While we previously observed no negative impact of lower seed-P concentration on seedling vigor when plants were cultivated in a P-fertilized agricultural soil in a direct-seeded pot trial (Rose et al. 2012), the addition of P fertilizer to soils did not guarantee optimum early growth of plants grown from low-P seeds in the present study. Seedling biomass was reduced by around 30 % in two genotypes (DJ123 and Surjamkuhi) in the P-fertilized soil in the pot trial in Benin (Fig. 2). However, even with these biomass reductions, seedlings of these genotypes still produced twice the biomass of their counterparts grown in the P-deficient soil (Fig. 2). Nadeem et al. (2012, 2013) also showed that the initiation and rate of P uptake by roots of maize seedlings was not impacted by seed-P concentration; rather, P uptake rates were driven by the concentration of P in the external growing media. Given that P availability in the external growing medium has a much greater impact on seedling vigor than seed-P concentration or content, we suggest that in low P soils, a potential reduction in seedling vigor due to reduced seed-P levels may be overcome by managing external P supply to the seedlings, such as supplementing the nursery soil with some form of P fertilizer in transplanted systems. Further, as seedling vigor and the effect of reduced seed-P concentration is genotype-specific, genotype selection for high general seedling vigor and ‘tolerance’ of reduced seed-P concentration should be a component of any cropping system pursuing reduced seed P.
That seedlings used for transplanting in experiment 3 were negatively affected by reduced seed-P concentration (Fig. 6) may be due to another aspect of growth conditions, namely the very small soil volume as each seedling was raised in small cells of dimensions of 2 × 2 × 4 cm (width x length x depth). It is possible that the small soil volume for root exploration and the lack of P fertilization limited P uptake from the growing medium sufficiently to give high-P seed an additional advantage.
Despite detecting a reduction in seedling vigor in roughly half the cases tested, no lasting impacts of seed-P concentrations on final grain yields were detected in any genotype in two experiments conducted in Japan and Africa (Figs. 4 and 5). Two further experiments detected negative effects in only one (Fig. 7) or two of the genotypes tested (Fig. 3). One may thus conclude that negative yield effects of reduced seed-P concentrations would not be expected in transplanted rice where competition from weeds are not problematic, but that potentially reduced seedling vigor could pose a problem in direct seeded rice unless sufficient genotypic variation for the ability to maintain unperturbed seedling vigor exists in the rice varieties used.
Effect of genotype and existence of a critical seed-P concentration
The results clearly showed that some rice genotypes can maintain normal seedling vigor with reduced seed-P concentration in both P-deficient and P-fertilized soils (Figs. 1 and 2). In the most extreme case genotype GNF6 showed no reduction in seedling vigor despite low-P seeds having a P concentration of only 0.89 mg g−1 (Fig. 1). The data indicate that a genotype component explaining tolerance to reduced seed-P concentrations exists but that growth conditions and soil P fertility do play a major role as well. As a result we were not able to detect a distinct threshold seed-P concentration that should be maintained in order to avoid seedling vigor loss. Should a threshold exist it would most likely be genotype-specific and possibly dependent on the P availability in the growth medium.
One additional factor that can be expected to have an influence on results is the impact of the level of P stress suffered by the parent plants on subsequent seed quality. To the best of our knowledge, all studies to date investigating the impact of seed P on seedling vigor have used low-P seeds obtained from plants that have suffered from some degree of P deficiency stress (see White and Veneklaas 2012 and references therein). That the growing environment during seed production has subsequent effects on seedling establishment and vigor of progeny has long been established (Derwyn et al. 1966; Walter and Jensen 1970), but received little mention in any the above-mentioned studies. It is highly likely that, even when similar-sized seed batches are selected, inherent differences in seed quality remain that are unrelated to P concentration but would confound the results of such studies. In particular, the P-deficiency stress suffered by parent plants may have a negative influence on the seed quality regardless of seed-P levels. Rose et al. (2012) obtained seeds of the same genotype that differed in seed-P concentrations from contrasting (but not P-deficient) growing environments, but it is still likely that the difference in growing environment of the parent plants influenced the results of the subsequent seedling vigor experiment. The only studies that we are aware of that have circumvented this issue were conducted by Bregitzer and Raboy (2006) using lpa mutant plants. Seeds differing in seed-P concentration (the lpa1-1 barley mutant contains 10–15 % less seed total P than wild type plants) were obtained from sib lines grown in the same environment, and no differences in seedling vigor were observed across a range of growing environments (Bregitzer and Raboy 2006). While seed batches of equal weight were selected for each experiment in the present study, it remains likely that some ‘maternal’ effect is present. One would ideally attempt to produce batches of seeds with contrasting seed-P concentrations in unstressed mother plants grown in the same environment as this would ultimately be the case in a hypothetical variety exhibiting reduced P loading. Unfortunately our understanding of the genetic and molecular processes involved in seed-P loading have not progressed to the point where a single gene could be targeted by mutation or knock-down to produce near identical rice lines only contrasting for seed-P concentrations. Once such lines are available and seeds produced in a favorable environment can be tested for linkages between reduced seed P and seedling vigor, it is likely that such linkages are found to be even weaker than in the present study.
In the absence of maternal effects we would hypothesize that genotypes with inherently high early vigor should be less susceptible to any impact of lower seed-P concentrations. Data in Fig. 1 support this hypothesis to some degree as genotype GNF6 had overall highest shoot biomass 4 weeks after sowing. On the other hand, genotype DJ123 did show reduced seedling vigor in the low-P seed treatment despite having high non-stress vigor (Figs. 2 and 6). However, seedling vigor is typically measured as shoot biomass or height, while a beneficial trait when seeds have a low P content may be root vigor. In a series of other experiments, we have observed that genotype DJ123 has inherent high shoot vigor (height and biomass) but has a relatively low root:shoot ratio, i.e., appears to have relatively low root vigor (M. Wissuwa, unpublished data). It is possible that the poor root vigor of DJ123 contributed to its poor performance in the low-P seed treatment. Early root and shoot vigor are now the subject of ongoing studies.
Conclusion
Our study demonstrates that seed sources with reduced seed-P concentration can be used in rice cultivation without affecting seedling vigor or grain yield. Any negative effect on seedling vigor appears to be genotype-specific and could therefore be avoided through selection and breeding of cultivars that tolerate low seed-P concentrations. Our study indicates such genotypes do exist. The potentially large benefits of reduced seed-P concentrations on the sustainability and profitability of phosphate fertilizer use suggest potentially large returns on investments in breeding efforts for reduced seed-P loading, particularly if coupled with selection for good seedling root vigor that would assure improved early P acquisition in seedlings.
References
Batten GD, Wardlaw IF, Aston MJ (1986) Growth and the distribution of phosphorus in wheat developed under various phosphorus and temperature regimes. Aust J Agric Res 37:459–469
Bolland MDA, Baker MJ (1988) High phosphorus concentrations in seed of wheat and annual medic are related to higher rates of dry matter production of seedlings and plants. Aust J Exp Agric 28:765–770
Bregitzer PP, Raboy V (2006) Effects of four independent low-phytate mutations on barley (Hordeum vulgare L.) agronomic performance. Crop Sci 46:1318–1322
Burnett VF, Newton PJ, Coventry DR (1997) Effect of seed source and seed phosphorus content on the growth and yield of wheat in north-eastern Victoria. Aust J Exp Agric 37:191–197
Cordell D, Drangert J, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305
Derrick JW, Ryan MH (1998) Influence of seed phosphorus content on seedling growth in wheat: implications for organic and conventional farm management in South East Australia. Biol Agric Hortic 16:223–237
Derwyn R, Whalley B, McKell CM, Green LR (1966) Effect of environmental conditions during the parent generation on seedling vigor of the subsequent seedlings of Oryzopsis miliacea (L.) Benth & Hook. Crop Sci 6:510–512
Dobermann A, Fairhurst TH (2000) Nutrient disorders and nutrient management. Potash and Phosphate Institute of Canada and International Rice Research Institute, Singapore, p 191
FAO (2013) Statistical yearbook 2013 – world food and agriculture. FAO, Rome, p 307
Haefele SM, Saito K, Ndiaye KM, Mussgnug F, Nelson A, Wopereis MSC (2013) Increasing rice productivity through improved nutrient use in Africa. In: Wopereis MCS., Johnson DE, Ahmadi N, Tollens E, Jalloh A (ed) Realizing Africa’s Rice Promise. CABI, pp 250–264
Karlen DL, Flannery RL, Sadler EJ (1988) Aerial accumulation and partitioning of nutrients by corn. Agron J 80:232–242
Lott JNA, Ockenden I, Raboy V, Batten GD (2000) Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci Res 10:11–33
MacDonald GK, Bennett EM, Potter PA, Ramankutty N (2011) Agronomic phosphorus imbalances across the world’s croplands. PNAS 108:3086–3091
Murphy J, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nadeem M, Mollier A, Morel C, Vives A, Prud’homme L, Pellerin S (2012) Seed phosphorus remobilization is not a major limiting step for phosphorus nutrition during early growth of maize. J Plant Nutr Soil Sci 175:805–809
Nadeem M, Mollier A, Morel C, Shahid M, Aslam M (2013) Maize seedling phosphorus nutrition: Allocation of remobilized seed phosphorus reserves and external phosphorus uptake to seedling roots and shoots during early growth stages. Plant Soil 371:327–338
Novozamsky J, Houba VJG, van Eck R, van Vark W (1983) A novel digestion technique for multi-element plant analysis. Commun Soil Sci Plant Anal 14:239–249
Raboy V (2005) Inositol phosphates in plants and the development of low phytate crops. Proceedings Bouyoucos Conference: Inositol phosphates in the soil-plant-animal system. Sun Valley, Idaho USA, August 2005
Raboy V (2009) Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci 177:281–296
Raboy V, Cichy K, Peterson K, Reichman S, Sompong U, Srinives P, Saneoka H (2014) Barley (Hordeum vulgare L.) Low phytic acid 1–1: an endosperm-specific, filial determinant of seed total phosphorus. J Hered 105:656–665
Robinson WD, Carson I, Ying S, Ellis K, Plaxton WC (2012) Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol 196:1024–1029
Rose TJ, Wissuwa M (2012) Rethinking internal phosphorus utilization efficiency: a new approach is needed to improve PUE in grain crops. Adv Agron 116:185–217
Rose TJ, Pariasca-Tanaka J, Rose MT, Fukuta Y, Wissuwa M (2010) Genotypic variation in grain phosphorus concentration, and opportunities to improve P-use efficiency in rice. Field Crop Res 119:154–160
Rose TJ, Pariasca-Tanaka J, Rose MT, Mori A, Wissuwa M (2012) Seeds of doubt: re-assessing the impact of grain P concentrations on seedling vigor. J Plant Nutr Soil Sci 175:799–804
Saito K, Vandamme E, Segda Z, Fofana M, Ahouanton K (2015) A screening protocol for vegetative-stage tolerance to phosphorus deficiency in upland rice. Crop Sci. doi:10.2135/cropsci2014.07.0521
Thomas RL, Sheard RW, Moyer JR (1967) Comparison of conventional and automated procedures for Nitrogen, Phosphorus, and Potassium analysis of plant material using a single digestion. Agron J 59:240–243
Walter LE, Jensen EH (1970) Effect of environment during seed production on seedling vigor of two alfalfa varieties. Crop Sci 10:635–638
White PJ, Veneklaas EJ (2012) Nature and nurture: the importance of seed phosphorus content. Plant Soil 357:1–8
Wissuwa M, Ae N (2001) Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed 120:43–48
Zhang M, Nyborg M, McGill WB (1990) Phosphorus concentration in barley (Hordeum vulgare L.) seed: influence on seedling growth and dry matter production. Plant Soil 122:79–83
Zhu YG, Smith SE (2001) Seed phosphorus (P) content affects growth, and P uptake of wheat plants and their association with arbuscular mycorrhizal (AM) fungi. Plant Soil 231:105–112
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim S. George.
Rights and permissions
About this article
Cite this article
Pariasca-Tanaka, J., Vandamme, E., Mori, A. et al. Does reducing seed-P concentrations affect seedling vigor and grain yield of rice?. Plant Soil 392, 253–266 (2015). https://doi.org/10.1007/s11104-015-2460-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2460-2