Abstract
Aim
We aimed to explore the general response patterns of plant biomass allocation to grazing disturbance and to test two important hypotheses, optimal partitioning and isometric allocation, for explaining potential mechanisms by which grazing controls biomass distribution in an alpine grassland on the Tibetan Plateau.
Methods
We identified 57 relevant papers about grazing on the Tibetan Plateau, from which 366 data sets suitable for the meta-analysis were extracted. Effect sizes were assessed by computing natural log-converted response ratios of response variables. Percentage change relative to control was used for each estimate of grazing effects.
Results
The aboveground biomass, soil water content (SWC), soil organic carbon, soil total nitrogen, and soil total phosphorus significantly decreased with increased grazing intensities, while plant species richness (SR), soil bulk density (SBD) and the ratio of root to shoot exhibited the opposite tendency. Belowground biomass (BGB) showed no significant differences under light and high grazing intensities while apparently increased under moderate grazing intensity (MG) that verifies the biomass transfer hypothesis. BGB was positively related to SBD and SR but was negatively associated with SWC.
Conclusions
The biomass transfer in MG supports the optimal partitioning hypothesis that plants partition biomass among various organs to maximize growth rate responding to environmental stress. The findings suggest that the primary mechanisms leading to the enhancement of BGB in MG are compensatory growth of individual plants, a dwarfing tendency within the plant community, a significant increase in species richness, and changes in soil microbial communities resulting from grazing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Tibetan Plateau, more than 85% covered by alpine grassland, holds approximately 44% of the total grassland in China (Piao et al. 2012), which is equal to 6–8% of the global grassland area, and accounts for 9–16% of the total world grassland carbon stocks (Ni 2002). Moreover, the alpine grassland ecosystem is of great importance for ecological security in China (Li et al. 2014). This ecosystem is highly sensitive to climate change and anthropogenic activities, due to the extremely harsh environment and unique alpine climate (Chapin et al. 2008; Sun and Wang 2016). Nevertheless, grazing is the major human activity in alpine grassland on the Tibetan Plateau and plays a crucial role in supporting the livelihood of Tibetan herdsmen (Dong et al. 2010; Feng et al. 2010).
Grazing is considered to have extensive and profound effects on alpine grassland ecosystem succession by causing remarkable changes in the composition and structure of plant communities (Sun et al. 2018; Sun et al. 2014). Livestock numbers have recently expanded, intensifying the grazing pressure, as a result of rapid economic development and population increases since the late 1970s (Yan et al. 2013). A report showed that the overgrazing rates in Tibet, Qinghai, Sichuan, and Gansu provinces were 38%, 25%, 37% and 36%, respectively (Zhang et al. 2014). Overgrazing has led to serious degradation and made the fragile alpine grassland ecosystem more sensitive to climate changes (Lu et al. 2015; Sun et al. 2014).
Vegetation biomass acts as the major source of soil carbon (C) input, regulating terrestrial ecosystem C cycling and storage (Li et al. 2014; Ma et al. 2008; Sun et al. 2013). Aboveground and belowground components of plants are closely related, and their interactions greatly influence ecosystem processes and functions (Bardgett et al. 2005; Fan et al. 2015; Wardle et al. 2004). As an important parameter of plant physiological ecology (Mokany et al. 2006), biomass allocation represents photosynthate allocation between aboveground and belowground biomass (BGB), which could reflect a plant’s adaptation to habitat and disturbance after a long-term life history (Shipley and Meziane 2002). Information about vegetation biomass and allocation changes in response to grazing is of great value not only for the development of better grazing management practices and protection of grasslands (Dong et al. 2015) but also for the assessment of terrestrial ecosystem C budgets (Dong et al. 2010).
Some scholars have determined that grazers should be managed differently in different regions to mitigate greenhouse gas emissions (Mcsherry and Ritchie 2013). Additionally, numerous studies have explored the impacts of grazing on vegetation biomass and allocation. For example, grazing has shown positive (Mcnaughton 1979, 1983; Milchunas and Lauenroth 1993), negative (Chen et al. 2006; Yan et al. 2013), or no (Mcnaughton et al. 1989) effects on aboveground biomass (AGB), as well as increased (Mcnaughton 1979, 1983; Milchunas and Lauenroth 1993) or decreased (Biondini et al. 1998; Yan et al. 2013) BGB in different investigations. Nevertheless, researchers generally accept the grazing optimization theory, which suggests that vegetation biomass can exhibit a unimodal pattern with plant compensatory growth increasing as a consequence of grazing intensity, reaching a peak value at a moderate rate of herbivory (Dyer et al. 1986; Hilbert et al. 1981; Yan et al. 2013).
However, the regulating mechanisms by which plants respond to grazing disturbance are still much debated (Sun et al. 2014; Yan et al. 2013). Two important hypotheses of biomass allocation, optimal partitioning and isometric allocation, have been proposed (Mccarthy and Enquist 2007; Müller et al. 2000; Sun and Wang 2016). The optimal partitioning hypothesis states that plants partition biomass among various organs to maximize growth rate in response to environmental stress (Bloom et al. 1985; Chapin et al. 1987; Sun et al. 2014). For instance, the optimal partitioning hypothesis proposes that plants experiencing arid and barren soil conditions should reduce the proportion of AGB and allocate more photosynthetic products to belowground parts to maximize water and nutrient uptake, whereas under ample water supply plants should allocate more to the aboveground portion to maximize plant growth to better access light resources (Dukes et al. 2005; Ma et al. 2010). Conversely, the isometric allocation hypothesis proposes that there is an isometric net primary productivity ratio of roots to shoots based on individual plant size, regardless of plant species or community types (Cheng and Niklas 2007; Enquist and Niklas 2002).
In fact, both hypotheses have some limitations and are debated. Although the optimal partitioning hypothesis emphasizes the impact of environmental factors on plants’ photosynthate allocation, it does not account for individual plant size (Mccarthy and Enquist 2007). As for the latter, the isometric allocation hypothesis considers vegetation biomass allocation as size-independent but cannot explain how environmental factors influence photosynthate allocation (Genard et al. 2008). Sun et al. (2017) proposed a “self-restraint combined with environment self-adaptation” hypothesis, where biomass allocation would follow the optimal partitioning hypothesis under environmental stress while complying with the isometric allocation hypothesis when environmental resources are sufficient. Biomass allocation is often, but not always, mediated by environmental factors; for instance, previous studies have suggested that biomass allocation patterns are system-specific and change along with the composition of plant species once the system is subject to anthropogenic disturbance (Der Maarel et al. 1989).
To date, no conclusion has been drawn on whether a uniform theory is applicable for describing the variation in biomass allocation in response to different grazing intensities in the cold and sensitive alpine grassland ecosystem. We hypothesized that there would be a biomass transfer from aboveground to belowground under a certain intensity of grazing stress, based on the optimal partitioning hypothesis. Thus, a meta-analysis was performed to explore the general response patterns of vegetation biomass and allocation to different grazing intensities on the Tibetan Plateau. We aimed to test the two abovementioned opposing hypotheses and answer the following questions: (i) does the hypothesis-suggested biomass transfer occur in response to grazing disturbance? (ii) what are potential mechanisms by which grazing disturbance drives variation in biomass allocation? The answers would provide a valuable ecological understanding of the effects of different grazing strategies and are meaningful for appropriate management of livestock and grassland on the Tibetan Plateau.
Materials and methods
Data collection
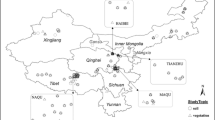
In this paper, ISI Web of Science and China National Knowledge Infrastructure (1990–2019) were used to collect research literature on grassland grazing experiments on the Tibetan Plateau. The Tibetan Plateau is located in southwest China, starting from Kunlun mountain range in the north, ending in Qilian mountain range in the northeast, Hengduan mountain range in the east, and Himalayan mountain range in the southwest. The topographic areas mainly include the northern Tibet plateau, the Qaidam basin, the Brahmaputra valley (the southern Tibet valley) and a series of mountain ranges (Wang et al., 2006). The following key words were used to select the studies: grazing, Tibet Plateau, grassland biomass, grassland productivity, and soil environment. Studies were included based on the following criteria: (1) the experimental data must have been collected from field experiments of grazing on the Tibetan Plateau; (2) the experiments had to have included grazing and control treatments, and the grazing patterns were obviously indicated in the description of the grazing treatment (i.e., grazing intensity, grazing model and grazing with soil depth); (3) the biomass and soil environment were studied during the peak growing season; and (4) each preliminary study gave the mean, standard deviation (SD) and/or standard error (SE), and confidence intervals for grazing and control conditions. In addition, we assembled background information related to the data from each article and conducted detailed analysis and comparisons. For instance, the grazing intensities and soil depths were collected from journal articles. We acquired the initial data directly from tables of published papers or used the GetData Graph Digitizer (ver.2.25, <www.getadata-graph-digitizer.com/>) to extract data from digitized charts. Therefore, the sample observation on line 598 conforms to the sampling standards; these were derived from 57 published articles (Supplementary). The locations of sample sites of the data sets were shown in Fig. 1. To confirm that grazing patterns expressed the impacts of grazing on grassland ecosystems, we grouped the data according to grazing intensity and soil depth (i.e., 0–10 cm depth, 10–20 cm depth and > 20 cm soil depth). The grazing intensity was divided into three degrees including light grazing (LG), moderate grazing (MG), and heavy grazing (HG) considering several indicators such as utilization of forage grass, number of sheep and yak, and distance from the source of water, etc. based on previous studies (Biondini et al., 1998, Gao et al. 2008). The detail information was shown in Table S2 in the Supplementary.
Data analysis (meta-analysis)
Based on the techniques reported in a previous study (Hedges et al. 1999), the meta-analysis was performed using the MetaWin 2.1 software package to calculate the response ratios (RR) of the response variables (Rosenburg et al., 2000). The response of each variable to the change in grazing intensity was evaluated by calculating the RR of each group as follows:
where \( {\overline{X}}_t \) and \( {\overline{X}}_c \) represent the treatment and control groups, respectively. The variance (v) of the RR was calculated by:
where nt and nc symbolize the sample sizes, and St and Sc are the SDs of the variable under consideration in the treatment and control groups, respectively. The inverse of the variance (\( \mathrm{w}=\frac{1}{v} \)) was deemed to be the weight (W) of each RR (Eldridge et al. 2011).
The mean response ratio (RR++) was computed from the individual RR of each paired comparison between control and treatments, RRij (i = 1,2,3…,m; j = 1,2,3…,k), with the weight of each RR. In our study, m refers to the number of groups, and k is the number of comparisons. The RR++ was computed with Eq. 3:
Our study calculated the entire RR++ and the 95% bootstrap confidence intervals (CIs) for an entire dataset. The result of grazing control treatment is considered statistically significant if the 95% bootstrap CIs does not overlap zero, while the difference between categorical variables (i.e., between light or heavy effects of grazing) is considered significant if the bootstrap CIs do not overlap with each other (Lu et al., 2016).
Principal Component Analysis (PCA) was carried out with the soil physicochemical property data to explore the explanatory powers of variance in soil properties between grasslands with different grazing intensities, and the packages of “FactoMineR”, “factoextra” and “corrplot” in software R (CoreTeam, 2016) were used for PCA. Pearson correlation analyses were performed to examine the relationships between the RR of the BGB and the RR of species richness (SR). The two analyses were carried out with R version 3.3.2 (CoreTeam, 2016) and SigmaPlot 14.0 software (Systat Software, Inc., Chicago, IL, USA), respectively.
Results
Response patterns of plant biomass to different grazing intensities
Our results showed that, with increasing grazing intensity, the mean value of AGB exhibited a decreasing trend from 226 to 99 g m−2 (Fig. 2a). However, BGB (0–30 cm) and total biomass (TB) fluctuated within a certain range with grazing intensity increasing, and there were no significant trends (Fig. 2b and c). Interestingly, the mean value of the ratio of root to shoot (R/S) responded well to grazing intensity, increasing from 6.5–9.4 (Fig. 2d).
a Aboveground biomass (AGB), b belowground biomass (BGB) in 0–30 cm, c total biomass (TB) and d the ratio of root to shoot (R/S) of different treatments. CK, LG, MG and HG represent control group, light grazing, moderate grazing and heavy grazing, respectively. Small cube of each box represented the mean values
Across all the studies, grazing had a significant negative effect on AGB. However, the intensity of this negative effect varied with grazing patterns (Fig. 3a); as the intensity of grazing increased, the negative response of AGB to grazing increased. Moreover, we found that MG had a significant positive effect on BGB, with an increase of 5.38% (Fig. 3b). The effects of grazing duration on AGB and BGB were similar to those of grazing intensity. Conversely, LG and HG had no significant effects on BGB. The smallest observed effects on TB were observed in response to LG and MG, but TB declined significantly under HG (Fig. 3c).
Response ratios (RR) of biomass in response to different grazing intensities. Grazing intensity was divided into three levels: light, moderate, and heavy intensity, respectively. LG, light grazing; MG, moderate grazing; HG, heavy grazing; AGB, above-ground biomass; BGB, below-ground biomass; TB, total biomass; R/S, the ratio of root to shoot. Error bars are the 95% bootstrapped confidence intervals. The numbers outside the parentheses the number of observation samples
Grazing patterns not only affected grassland biomass but also affected the allocation of above- and below-ground materials (Fig. 3d). The RR of R/S increased with intensified grazing. The results showed that HG led to a larger proportion of belowground material allocation. In addition, when the data were classified according to the soil depth, there were significant differences among the subgroups. The response ratio of BGB increased with soil depth only under MG (Fig. 4). However, the opposite result was found for LG and HG. Regardless of the depth of the soil, LG and HG had no significant effect on BGB.
Response patterns of soil properties to different grazing intensities
The PCA results indicated that two components explained 58.1% of the total variance. Specifically, the first principal component (PC1) was highly relevant to soil bulk density (SBD), soil water content (SWC) and BGB; PC2 was highly relevant to soil organic carbon (SOC), total nitrogen (STN) and total phosphorus (STP). It was obvious that the orientations of SBD and BGB exhibited the smallest angle, which indicated that SBD promoted the increase of BGB under MG. Conversely, BGB had the largest angle, with SWC, which illustrated that SWC had negative effects on BGB (Fig. 5).
Figure 6 showed that the responses of soil characteristics to grazing increased with intensified grazing. There was positive responses of SBD and SR to grazing, while grazing had negative impacts on SWC, SOC, STN and STP (Fig. 6). Additionally, when the data were subdivided based on soil depth, the responses of SOC to grazing differed significantly between different soil layers (Fig. 6d). For example, LG significantly increased SOC in the 0–10 cm depth but reduced that in the >20 cm soil depth.
Response ratios (RR) of soil properties for three soil depth intervals (0–10, 10–20 and > 20 cm) and response ratios (RR) of species richness(SR) in response to different grazing intensities. GI, grazing intensity; LG, light grazing; MG, moderate grazing;HG, heavy grazing; SWC, soil water content; SBD, soil bulk density; SR, species richness, SOC, soil organic carbon; STN, soil total nitrogen; STP, soil total phosphorus
The differences in RR for SOC and STN under different grazing intensities is most marked in the top 10 cm of soil and gradually decreases for the 10–20 and 20–30 cm depths. Regardless of soil depth, SWC generally decreased under grazing conditions (Fig. 6a). There were decreasing tendencies in the RR of most indicators with increased soil depth except for SBD (Fig. 6b, d and e). Correlation analysis showed that BGB was positively correlated with SR (R2 = 0.58, P = 0.01) (Fig. 7).
Discussion
The biomass transfer hypothesis was verified under moderate grazing practice
In the present paper, grazing shows no significant effects on TB in the MG condition, where the reduction of AGB was offset by increased BGB (Fig. 3a–c). This result provides evidence for the biomass transfer hypothesis under MG, which, however, is not well supported under LG and HG conditions. BGB under LG and HG changes insignificantly while remarkably increasing under MG and 1–2 years duration (Fig. 3b), supporting the grazing optimization hypothesis in that BGB exhibits a unimodal curve with increasing grazing intensity and peaked under MG due to compensatory growth (Dyer et al. 1986; Hilbert et al. 1981).
The regional-scale response patterns are similar to the results of Milchunas & Lauenroth (1993) at the global scale, but conflict with those of Yan et al. (2013) who found that grazing showed a substantial negative effect on TB. The outcome of grazing greatly depends on grassland productivity (Osem et al. 2010), with effects of grazing switching from a decrease in plant biomass in regions with lower productivity to an increase in biomass in more productive areas (Yan et al. 2013). The alpine grassland has a short growing season and relatively low productivity due to the cold climate on the Tibetan Plateau (Sun et al. 2019), contributing to the insignificant effects on TB under LG and MG. However, TB dramatically decreases when the HG condition prevails (Fig. 3c), suggesting that alpine grassland ecosystems can tolerate some extent of grazing disturbance but become unstable once the disturbance surpasses particular thresholds (Villnas et al. 2013).
Contrary to our expectation based on a surface soil fertilization effect caused by faeces which is beneficial for allocating greater BGB to surface soil, we found that the increases in BGB in subsoil were higher than those in topsoil under MG (Fig. 4), which is consistent with other observations (Yan et al. 2013). The effects of grazing on BGB allocation patterns at different soil depths might be related to the original soil nutritional conditions, plant species composition, and grazing duration (Yan et al. 2013).
The habitat determines biomass dynamics
We identify several mechanisms responsible for biomass transfer in response to grazing that have been proposed in the past. In general, our results provide no evidence for the size-independent isometric allocation hypothesis, regardless of grazing intensity. Instead, the enhancement of BGB under MD (Fig. 3b) supports the optimal partitioning hypothesis that plants allocate photosynthate among various organs to maximize growth rate under unfavourable growth conditions (Bloom et al. 1985; Chapin et al. 1987). The grazing-induced decreases in vegetation coverage greatly promote light availability at ground level, causing a competitive release for light resources (Buis et al. 2009).
In contrast, there were significant decreases in SWC, SOC, STN, and STP, while SBD increased under MG (Fig. 6), indicating a worse soil environment resulting from grazing disturbance (Gao et al. 2008). Soil properties play vital roles in plant growth, for example, SWC can regulate nutrient availability which affects soil nutrient supply ability and drives plant succession in the alpine steppe (Hou et al. 2018). STN and SOC are closely related to the physiological activities of plants such as photosynthesis and respiration (Bennett and Klironomos 2018). SBD is greatly associated with the soil porosity which further affects plant root respiration providing energy for protein synthesis within the roots (Sun et al. 2018). Based on the optimal partitioning hypothesis, plants reduce the proportion of AGB and allocate more photosynthetic products to BGB so they better access water and nutrients in deeper soils to adapt to the relatively arid and barren soil environment (Dong et al. 2010; Dukes et al. 2005; Ma et al. 2010).
The regrowth capacity and allocation patterns of individual plants following grazing are considered to represent the adaptive evolution of phenotypic plasticity for the variable environments experienced (Juenger and Bergelson 2000; Stowe and Marquis, 2000). At the individual level, plants need to absorb more nutrients to complete their life histories with a maximum life span by compensatory growth in response to grazing damage (Rautio et al. 2005; Roa-Fuentes et al. 2012). Hence, plants tend to overcompensate more frequently under unfavourable growth conditions (Hawkes and Sullivan 2001). The enhancement of root growth under poor resource conditions was conducive to the promotion of plants’ ability to compete for water and nutrients (Sun et al. 2014). Soil physical and chemical properties may mediate the responses of BGB to grazing disturbance (Gao et al. 2008; Wu et al. 2011), but the effects of soil physical properties were much stronger than those of nutrient resource deficiency in the present study (Fig. 5), showing that SBD promoted the increase in BGB while SWC was negatively correlated with BGB under MG.
At the community level, plant height is an important trait in determining response patterns to grazing (Diaz and Cabido 2010). As a strategy for avoiding ingestion by herbivores, a plant community would exhibit a dwarfing tendency to resist herbivores when grazing occurs, demonstrated by taller plants that allocate a large portion of photosynthate to AGB typically declining in response to grazing, which inversely favours shorter plant species that allocate more to BGB (Diaz et al. 2007; Evju et al. 2009), perhaps leading to a significant increase in BGB under MG intensity (Fig. 3b).
The significant increase in SR under MG (Fig. 6c), in agreement with previous studies that demonstrate that grazing positively affects SR through weakening the dominance of the principal functional group (Denyer et al. 2010; Wang et al. 2018), is also responsible for the enhancement of BGB by grazing, suggested by the dramatic positive correlation between BGB and SR shown in Fig. 7. Grazing alters plant species composition, which is tightly linked with biomass allocation patterns (Gao et al. 2011; Stahlheber and D’Antonio 2013; Wu et al. 2009). In the presence of grazing, forbs that are unpalatable and resistant to trampling replace native graminoids with high palatability (Liu et al. 2011; Wan et al. 2011), likely due to competitive release from dominance by graminoids (Borer et al. 2014; Knapp et al. 2012). The exotic and remaining native forbs are more deeply rooted than graminoids with crowd-short rhizomes (Wang et al. 2004), perhaps leading to the higher BGB in subsoil than topsoil under MG (Fig. 4).
Additionally, grazing-induced changes in plant communities affect soil microbial communities and related processes by modifying the environment of decomposers (Klumpp et al. 2009). Different plant species support different components and abundances of soil microorganisms and microbe-feeding fauna because of variation in both quantity and quality of litter returned to soil (Groffman et al. 1996). In return, decomposer communities exert great control over decomposition rates, further affecting nutrient availability for plant growth as a feedback loop (Wang et al. 2014). Moreover, soil animal activity around the plant rhizosphere, which benefits nutrient bioavailability and aggregate formation, is intimately associated with plant root growth (Rashid et al. 2016). Thus, we infer that MG might promote root-associated microbes and fauna that lead to an increase in BGB under MG (Wardle 2010). On the other hand, increased photosynthate allocation to roots would enhance the resistance of plants impaired by herbivores to arbuscular mycorrhizal infection of roots (Strauss and Agrawal 1999).
Limitations of the current study
In the current study, we mainly focused on the general effects of grazing intensity on plant biomass allocation. However, responses of grasslands to grazing are affected not only by grazing intensity but also by other factors (e.g., air temperature, precipitation, etc.). Previous studies also indicated that the effects of grazing might shift from negative to positive with increasing temperature or precipitation providing heat and moisture conditions for plant growth (Yan et al. 2013). In addition, soil type might also be a strong determinant of plant response patterns to grazing, as it influences plant community structure and the original nutritional conditions of soil (Stahlheber and D’Antonio 2013). Additionally, the responses of plants may vary with land use history (Foster et al. 2003), grazing duration (Yan et al. 2013), and grazing patterns such as rotational grazing or continuous grazing (Kirkman 2002). Unfortunately, most of these data are not available in studies we collected, which limits the quantitative assessments of effects on response patterns of plants to grazing. Further studies should allocate more effort to the elucidation of regional-scale response patterns of plants to grazing considering the abovementioned key factors, which is of great significance for formulating appropriate region-specific management of grasslands on the Tibetan Plateau.
Conclusions
The biomass transfer in alpine grasslands in response to grazing in the present study supports the optimal partitioning hypothesis. Compensatory growth of individual plants, a dwarfing tendency of the plant community, a significant increase in species richness, and changes in soil microbial communities caused by grazing are the primary mechanisms leading to the enhancement of BGB in MG. Our results provide insight into the general response patterns of plant biomass to different grazing intensities, which is of great theoretical value for alpine grassland sustainable management and carbon budget assessment on the Tibetan Plateau.
References
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20:634–641
Bennett JA, Klironomos JN (2018) Climate, but not trait, effects on plant–soil feedback depend on mycorrhizal type in temperate forests. Ecosphere 9
Biondini ME, Patton BD, Nyren PE (1998) Grazing intensity and ecosystem processes in a northern mixed-grass prairie, USA. Ecol Appl 8:469–479
Bloom AJ, Chapin SF, Mooney HA (1985) Resource limitation in plants-an economic analogy. Annu Rev Ecol Evol Syst 16:363–392
Borer ET, Seabloom EW, Gruner DS, Stanley WH, Helmut H, Lind EM, Adler PB, Juan A, Michael TA, Bakker JD (2014) Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–520
Buis GM, Blair JM, Burkepile DE, Burns CE, Chamberlain AJ, Chapman PL, Collins SL, Fynn RWS, Govender N, Kirkman KP (2009) Controls of aboveground net primary production in Mesic savanna grasslands: an inter-hemispheric comparison. Ecosystems 12:982–995
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental FactorsPhysiological ecology provides tools for studying how interacting environmental resources control plant growth. BioScience 37:49–57
Chapin FS, Randerson JT, Mcguire AD, Foley JA, Field CB (2008) Changing feedbacks in the climate–biosphere system. Front Ecol Environ 6:313–320
Chen Y, Lee P, Lee G, Mariko S, Oikawa T (2006) Simulating root responses to grazing of a Mongolian grassland ecosystem. Plant Ecol 183:265–275
Cheng D, Niklas KJ (2007) Above- and below-ground biomass relationships across 1534 forested communities. Ann Bot 99:95–102
CoreTeam R (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Denyer JL, Hartley SE, John EA (2010) Both bottom-up and top-down processes contribute to plant diversity maintenance in an edaphically heterogeneous ecosystem. J Ecol 98:498–508
Der Maarel EV, Titlyanova A, Der Maarel EV, Titlyanova A (1989) Above-ground and below-ground biomass relations in steppes under different grazing conditions. Oikos 56:364–370
Diaz S, Cabido MN-MI (2010) Can grazing response of herbaceous plants be predicted from simple vegetative traits? J Appl Ecol 38:497–508
Diaz S, Lavorel S, Mcintyre S, Falczuk V, Casanoves F, Milchunas D, Skarpe C, Rusch G, Sternberg M, Noy-Meir I (2007) Plant trait responses to grazing - a global synthesis. Glob Chang Biol 13:313–341
Dong SK, Wen L, Zhu L, Li X (2010) Implication of coupled natural and human systems in sustainable rangeland ecosystem management in HKH region. Front Earth Sci Chin 4:42–50
Dong Q, Zhao X, Wu G, Chang X (2015) Optimization yak grazing stocking rate in an alpine grassland of Qinghai-Tibetan plateau, China. Environ Earth Sci 73:2497–2503
Dukes JS, Chiariello NR, Cleland EE, Moore LA, Shaw MR, Thayer SS, Tobeck T, Mooney HA, Field CB (2005) Responses of grassland production to single and multiple global environmental changes. PLoS Biol 3
Dyer MI, Deangelis DL, Post WM (1986) A model of herbivore feedback on plant productivity. Bellman Prize Math Biosci 79:171–184
Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol Lett 14:709–722
Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning in seed plants. Science 295:1517–1520
Evju M, Austrheim G, Halvorsen R, Mysterud A (2009) Grazing responses in herbs in relation to herbivore selectivity and plant traits in an alpine ecosystem. Oecologia 161:77–85
Fan HB, Wu JP, Liu WF, Yuan YH, Hu L, Cai QK (2015) Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 392:127–138
Feng R, Long R, Shang Z, Ma Y, Dong S, Wang Y (2010) Establishment of Elymus natans improves soil quality of a heavily degraded alpine meadow in Qinghai-Tibetan plateau, China. Plant Soil 327:403–411
Foster DR, Swanson FJ, Aber JD, Burke IC, Brokaw N, Tilman D, Knapp AK (2003) The importance of land-use legacies to ecology and conservation. BioScience 53:77–88
Gao YH, Luo P, Wu N, Chen H, Wang GX (2008) Impacts of grazing intensity on nitrogen pools and nitrogen cycle in an alpine meadow on the eastern Tibetan plateau. Appl Ecol Environ Res 6:69–79
Gao YH, Schumann M, Zeng XY, Chen H (2011) Changes of plant communities and soil properties due to degradation of alpine wetlands on the Qinghai-Tibetan plateau. J Environ Prot Ecol 12:788–798
Genard M, Dauzat J, Franck N, Lescourret F, Moitrier N, Vaast P, Vercambre G (2008) Carbon allocation in fruit trees: from theory to modelling. Trees Struct Funct 22:269–282
Groffman PM, Eagan P, Sullivan WM, Lemunyon JL (1996) Grass species and soil type effects on microbial biomass and activity. Plant Soil 183:61–67
Hawkes CV, Sullivan JJ (2001) The impact of herbivory on plants in different resource conditions: a meta-analysis. Ecology 82:2045–2058
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hilbert DW, Swift DM, Detling JK, Dyer MI (1981) Relative growth rates and the grazing optimization hypothesis. Oecologia 51:14–18
Hou G, Sun J, Wang JN (2018) Dynamics and controls of carbon use efficiency across China's grasslands. Pol J Environ Stud 27:1541–1550
Juenger T, Bergelson J (2000) the evolution of compensation to herbivory in scarlet gilia, Ipomopsis aggregata: herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution 54:764–777
Kirkman KP (2002) The influence of various types and frequencies of rest on the production and condition of sourveld grazed by sheep or cattle. 2. Vigour. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa 19: 93–105
Klumpp K, Fontaine S, Attard E, Roux XL, Gleixner G, Soussana J (2009) Grazing triggers soil carbon loss by altering plant roots and their control on soil microbial community. J Ecol 97:876–885
Knapp AK, Hoover DL, Blair JM, Buis G, Burkepile DE, Chamberlain A, Collins SL, Fynn RWS, Kirkman KP, Smith MD (2012) A test of two mechanisms proposed to optimize grassland aboveground primary productivity in response to grazing. J Plant Ecol 5:357–365
Li YY, Dong SK, Wen L, Wang X, Wu Y (2014) Soil carbon and nitrogen pools and their relationship to plant and soil dynamics of degraded and artificially restored grasslands of the Qinghai–Tibetan plateau. Geoderma 213:178–184
Liu YS, Pan QM, Liu HD, Bai YF, Simmons M, Dittert K, Han XG (2011) Plant responses following grazing removal at different stocking rates in an Inner Mongolia grassland ecosystem. Plant Soil 340:199–213
Lu X, Yan Y, Sun J, Zhang X, Chen Y, Wang X, Cheng G (2015) Short-term grazing exclusion has no impact on soil properties and nutrients of degraded alpine grassland in Tibet, China. Solid Earth 6:1195–1205
Lu X, Wang L, McCabe MF (2016) Elevated CO2 as a driver of global dryland greening. Sci Rep 6:20716
Ma WH, Yang YH, He JS, Zeng H, Fang JY (2008) Above- and belowground biomass in relation to envi-ronmental factors in temperate grasslands, Inner Mongolia. Sci Chin-Life Sci 51:263–270
Ma WL, Shi PL, Li WH, He YT, Zhang XZ, Shen ZX, Chai SY (2010) Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai-Tibetan plateau. SciChin-Life Sci 53:1142–1151
Mccarthy MC, Enquist BJ (2007) Consistency between an Allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21:713–720
Mcnaughton SJ (1979) Grazing as an optimization process: grass-ungulate relationships in the Serengeti. Am Nat 113:691–703
Mcnaughton SJ (1983) Compensatory plant growth as a response to herbivory. Oikos 40:329
Mcnaughton SJ, Oesterheld M, Frank DA, Williams KJ (1989) Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 341:142–144
Mcsherry ME, Ritchie ME (2013) Effects of grazing on grassland soil carbon: a global review. Glob Chang Biol 19:1347–1357
Milchunas DG, Lauenroth WK (1993) Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol Monogr 63:327–366
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root : shoot ratios in terrestrial biomes. Glob Chang Biol 12:84–96
Müller I, Schmid B, Weiner J (2000) The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect Plant Ecol Evol Syst 3:115–127
Ni J (2002) Carbon storage in grasslands of China. J Arid Environ 50:205–218
Osem Y, Perevolotsky A, Kigel J (2010) Grazing effect on diversity of annual plant communities in a semi-arid rangeland: interactions with small-scale spatial and temporal variation in primary productivity. J Ecol 90:936–946
Piao SL, Tan K, Nan H, Ciais P, Fang JY, Tao W, Vuichard N, Zhu B (2012) Impacts of climate and co2 changes on the vegetation growth and carbon balance of Qinghai–tibetan grasslands over the past five decades. Glob Planet Chang 98:73–78
Rashid M, Mujawar LH, Shahzad T, Almeelbi T, Ismail IMI, Oves M (2016) Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res 183:26–41
Rautio P, Huhta A, Piippo S, Tuomi J, Juenger T, Saari M, Aspi J (2005) Overcompensation and adaptive plasticity of apical dominance in Erysimum strictum (Brassicaceae) in response to simulated browsing and resource availability. Oikos 111:179–191
Roa-Fuentes LL, Campo J, Parra-Tabla V (2012) Plant biomass allocation across a precipitation gradient: an approach to seasonally dry tropical Forest at Yucatán, Mexico. Ecosystems 15:1234–1244
Rosenburg MS, Adams DC, Gurevitch J (2000) Metawin. Statistical software for meta-analysis, version 2
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16:326–331
Stahlheber KA, D’Antonio CM (2013) Using livestock to manage plant composition: a meta-analysis of grazing in California Mediterranean grasslands. Biol Conserv 157:300–308
Stowe KA, Marquis RJ, imms HEL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31: 565–595
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Sun J, Wang HM (2016) Soil nitrogen and carbon determine the trade-off of the above- and below-ground biomass across alpine grasslands, Tibetan plateau. Ecol Indic 60:1070–1076
Sun J, Cheng G, Li W (2013) Meta-analysis of relationships between environmental factors and aboveground biomass in the alpine grassland on the Tibetan plateau. Biogeosciences 10:1707–1715
Sun J, Wang X, Cheng G, Wu J, Hong J, Niu S (2014) Effects of grazing regimes on plant traits and soil nutrients in an alpine steppe, northern Tibetan plateau. PLoS One 9:e108821
Sun J, Niu SL, Wang JN (2017) Divergent biomass partitioning to aboveground and belowground across forests in China. J Plant Ecol:1–9. https://doi.org/10.1093/jpe/rtx021
Sun J, Ma BB, Lu XY (2018) Grazing enhances soil nutrient effects: trade-offs between above and below-ground biomass in Alpine grasslands of the Tibetan plateau. Land Degrad Dev 29:770
Sun J, Zhang ZC, Dong SK (2019) Adaptive management of alpine grassland ecosystems over Tibetan plateau. Pratacult Sci 36:1–6. https://doi.org/10.11829/j.issn.1001-0629.2019-0224
Villnas A, Norkko J, Hietanen S, Josefson AB, Lukkari K, Norkko LA (2013) The role of recurrent disturbances for ecosystem multifunctionality. Ecology 94:2275–2287. https://doi.org/10.2307/23597376
Wan HW, Bai YF, Schönbach P, Gierus M, Taube F (2011) Effects of grazing management system on plant community structure and functioning in a semiarid steppe: scaling from species to community. Plant Soil 340:215–226
Wang CT, Wang QJ, Long RJ, Jing ZC, Shi HL (2004) Changes in plant species diversity and productivity along an elevation gradient in an alpine meadow. Acta Phytoecol Sin 28:240–245
Wang G, Wang Y, Kubota J (2006) Land-cover changes and its impacts on ecological variables in the headwaters area of the Yangtze River, China. Environ Monit Assess 120:361–385
Wang XX, Dong SK, Gao QZ, Zhou HK, Liu SL, Su XK, Li YY (2014) Effects of short-term and long-term warming on soil nutrients, microbial biomass and enzyme activities in an alpine meadow on the Qinghai-Tibet plateau of China. Soil Biol Biochem 76:140–142
Wang YX, Sun YI, Wang ZF, Chang SH, Hou FJ (2018) Grazing management options for restoration of alpine grasslands on the Qinghai-Tibet plateau. Ecosphere 9
Wardle DA (2010) Communities and ecosystems. Linking the aboveground and belowground components. Aust Ecol 29:358–359
Wardle DA, Bardgett RD, Klironomos JN, Setala H, Der Putten WHV, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Wu GL, Du GZ, Liu ZH, Thirgood S (2009) Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan plateau. Plant Soil 319:115–126
Wu GL, Ren GH, Wang D, Shi ZH, Warrington DN (2011) Above- and below-ground response to soil water change in an alpine wetland ecosystem on the Qinghai-Tibetan plateau, China. J Hydrol 476:120–127
Yan L, Zhou GS, Zhang F (2013) Effects of different grazing intensities on grassland production in China: a meta-analysis. PLoS One 8:e81466
Zhang Y, Zhang X, Wang X, Liu N, Kan H (2014) Establishing the carrying capacity of the grasslands of China: a review. Rangel J 36:1–9
Acknowledgements
This research was joint funded by the National Key Research Project of China (Grant No.2019QZKK0405-05), the Science and Technology Service Network Initiative (KFJ- STS-ZDTP-036), the State Key Research Development Program of China (Grant No. 2016YFC0501803 and 2016YFC0501802), and the National Natural Science Foundation of China (No. 41871040 and 41501057).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Zhanhuan Shang.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Zhan, T., Liu, M. et al. Verification of the biomass transfer hypothesis under moderate grazing across the Tibetan plateau: a meta-analysis. Plant Soil 458, 139–150 (2021). https://doi.org/10.1007/s11104-019-04380-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04380-8