Abstract
Eucalyptus clones have wide plasticity and worldwide dispersion, growing satisfactorily in different edaphoclimatic conditions. Furthermore, clones with higher phosphorus (P) uptake efficiency can grow in soils with lower P availability, due to their fast growth, biomass, and wood production. These traits can promote reduced use of phosphate fertilizers in nurseries and cultivation areas, thus lowering input costs and the likelihood of soil contamination. However, Eucalyptus spp. clones with higher P uptake efficiency are still relatively unknown. Thus, the aim of this study was to investigate whether morphological, physiological, and biochemical characteristics of Eucalyptus plants influence nutritional P efficiency and influence plant growth. Three concentrations of P were used, 10% P (very low level P), 30% P (low level P) and 100% (standard level P), of the standard concentration of P in Hoagland and Arnon solution and three Eucalyptus spp. clones (Eucalyptus dunnii, Eucalyptus saligna, and Eucalyptus urograndis). Morphological variables associated with shoot and root systems, photosynthetic variables, chlorophyll a fluorescence, photosynthetic pigments, antioxidant enzyme, and acid phosphatase activity were evaluated. Phosphorus concentration of 10% did not promote the production of plants with desirable morphological traits. The highest acid phosphatase values were found in the shoots of E. urograndis and E. saligna grown in the concentration of 10% P. Seedlings of E. urograndis were the most efficient in absorbing P. Furthermore, the concentration of 30% P was the one recommended for plant production to reach economic and ecological benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Eucalyptus is one of the most relevant and studied genera on the planet, making it prominent in the forestry industry (Canal et al. 2023). It contains about 700 species originating from Australia, Papua New Guinea, East Timor, Indonesia, and the Philippines, in addition to several hybrids developed to exploit different plant characteristics (Brune 2023). Fast growth, adaptability to a wide range of tropical and subtropical regions, combined with the versatile wood properties for energy, lamination, sawmilling, pulp, and paper, ensure its central position in modern forestry (Dai et al. 2023; Estopa et al. 2023).
Eucalyptus dunnii Maiden, Eucalyptus saligna Smith, and the hybrid Eucalyptus urograndis (E. urophylla ST Blake x E. grandis Hill ex Maiden) are grown in forests around the world. E. dunnii and E. saligna are economically important because they are fast-growing, have high-quality wood and are tolerant to cold and frost (Kulmann et al. 2021; Bird et al. 2022). E. urograndis has excellent productivity rates, in addition to good wood characteristics for several industrial purposes and adaptability to different forest sites (Dahali et al. 2022).
Eucalyptus cultivation is usually carried out in areas with highly weathered and leached soils, which have low nutrient availability for plants (Sousa et al. 2022). However, forest improvement programs allow us to obtain genotypes that have better productivity, even in environments with low nutrient availability (Alvarez et al. 2020). Thus, it is necessary to consider the costs of resources, especially mineral nutrients, to which an alternative would be the use of more nutritionally efficient genetic materials (Kulmann et al. 2021).
Among the essential elements, phosphorus (P) is one of the nutrients that most limits plant growth in the initial development phase due to its participation in energy-rich compounds, such as adenosine triphosphate (ATP) (Awad-Allah et al. 2023). In addition, P plays an effective role in plant vitality, essential in reproduction, cell division, and plant metabolism (respiration, photosynthesis ,and synthesis of organic substances) (Hyder et al. 2023).
On the other hand, P deficiency in the induction and root formation phases significantly reduces root length, resulting in reduced biomass and consequently reduced seedling quality (Lopez et al. 2023). Plants grown in environments with low P concentrations may present higher concentrations of reactive oxygen species (ROS), which damage lipids, enzymes, and photosynthetic pigments (Kulmann et al. 2023). When this happens, plants may develop response mechanisms, such as the production of guaiacol peroxidase (GPX), superoxide dismutase (SOD), and acid phosphatase (APase) to reestablish homeostasis within these plants (Meng et al. 2021). GPX, SOD, and APase are then activated under conditions of nutritional deficiency to restore the plant internal balance and consequently allow vital plant growth processes to continue, even under stress conditions. Thus, special attention must be given to the use of P, due to its limited availability in the soil, which may result in plant physiological and biochemical changes.
Low P availability limits plant growth in numerous soils around the world, particularly in developing countries where access to P-based fertilizers is limited (Ibrahim et al. 2022). P-based fertilizers are mainly derived from phosphate rocks, whose reserves are a finite and non-renewable resource. Forecasts indicate that phosphate rock reserves will run out within the next 50 years (Antunes et al. 2022). As a result, there is a concern for a more sustainable use of P in agriculture and for this reason it is necessary to improve the efficiency with which P fertilizers are used in different agricultural systems (Lopez et al. 2023).
Improving the efficiency of P-fertilizer use for crop growth requires increased P uptake by plants (P uptake efficiency), and improved P use in processes that lead to faster growth and greater allocation of biomass to harvestable parts (internal P use efficiency) (Nadeem et al. 2022). As a response to nutritional limitation, plants may invest a greater proportion of their biomass in the root system to exploit a greater volume of soil, increase the physiological absorptive capacity of the root (through more effective absorption systems), or increase nutrient use efficiency (Trentin et al. 2022). On the other hand, internal use efficiency must specifically consider the amount of biomass that can be generated in a given period of time by the amount of nutrients present in plant tissues (Nadeem et al. 2022). Thus, it is essential to select plants that can adapt to soils with low P availability, as they could reduce dependence on fertilizer use.
Eucalyptus clones such as E. dunnii, E. saligna, and E. urograndis can adapt to environments with different P concentrations, especially because they have different morphological, physiological, and even biochemical characteristics that provide them with higher or lower P uptake efficiency (Bulgarelli et al. 2019). For this reason, it is important to assess morphological, physiological, and biochemical mechanisms in such clones grown in environments with different P concentrations. This way, we will be able to define the genotypes which best adapt to environments with higher or lower P concentrations and understand the reason for this adaptation (Alvarez et al. 2020). Furthermore, the results of this study can help to rationalize the use of phosphate fertilizers in plant nurseries (Honorato et al. 2020), or even in field plantations, which would reduce production costs and the potential for contamination of soil and surface waters adjacent to cultivation areas (Haque 2021).
Varying responses observed for Eucalyptus spp. fertilized with P are explained by genotypic characteristics expressed by morphological, physiological, and metabolic aspects which involve processes associated with the mineral nutrition of each species (Kulmann et al. 2021). Eucalyptus dunnii, Eucalyptus saligna, and Eucalyptus urograndis are typically grown in the same areas. However, they often differ from one another in terms of growth rates. Therefore, our hypothesis was that Eucalyptus clones have different growth rates due to morphological, physiological, and biochemical characteristics or differences which increase nutritional efficiency (especially of P), in addition to increasing P uptake or use efficiency. Thus, this study aimed to examine whether morphological, physiological, and biochemical properties of Eucalyptus species influence nutritional P efficiency and influence plant growth.
Material and Methods
Plant Material
The Eucalyptus spp. (E. dunnii, E. saligna, and E. urograndis) used in the study were provided by the CMPC (Celulose Riograndense) company. The seedlings were produced by vegetative propagation in a clonal mini-garden system according to the methodology adopted by the company itself. Thus, the minicuttings were collected and submitted to the rooting in a greenhouse shortly after collection. Minicuttings were 12 cm long and had three upper buds, leaves were cut in the center of the blade (preserving 50% of the photosynthetic area) to reduce transpiration. Seedlings were grown in 55 cm3 plastic tubes made of non-toxic polypropylene. The substrate used for seedling production was Carolina Soil®, composed of Sphagnum sp. and vermiculite with the addition of 30% carbonized rice husk. Base fertilization was carried with Osmocote® controlled-release fertilizer (CRF) which consisted of 15% N; 9% P2O5; 12% KCl; 1% Mg; 2.3% S; 0.05% Cu; 0.06% Mn; 0.45% Fe; and 0.2% Mo. When seedlings were approximately 25 cm in height, 90 days after rooting, they were sent to the greenhouse at the Federal University of Santa Maria (UFSM) for acclimatization, until the time of the experiment. During this period, the seedlings were irrigated daily until the experiment was set up.
Study Site and Experimental Design
The study was carried out in a greenhouse at the Federal University of Santa Maria (UFSM), city of Santa Maria, state of Rio Grande do Sul (RS), Southern Brazil. The average temperature inside the greenhouse was 25 °C and air humidity was 60%.
The experiment was a completely randomized design, with three repetitions. Three concentrations of P were used, 10% P (very low level P), 30% P (low level P) and 100% (standard level P), of the standard concentration of P in Hoagland and Arnon solution and three Eucalyptus spp. clones (Eucalyptus dunnii, Eucalyptus saligna, and Eucalyptus urograndis). Phosphorus concentrations of 100% (control), 30%, and 10% corresponded to 31, 9.3, and 3.1 mg P L−1, respectively. The P concentrations present in the nutrient solution were defined using preliminary tests, previous experiments carried out in our laboratory, and reviewing scientific literature.
The seedlings were carefully removed from the tubes 98 days after rooting and roots were thoroughly washed to remove substrate. Each seedling was placed in a 6L pot filled with Hoagland and Arnon complete nutrient solution (1950). One Styrofoam sheet with three central holes was added to the surface of each pot to allow plants to pass through. The Styrofoam sheet provided support to plants and reduced solution evaporation in each pot.
Seedlings were left to acclimate for 15 days in 100% Hoagland and Arnon nutrient solution (1950). Solution aeration in each pot was done using PVC microtubes connected to an air compressor. Microtubes were inserted into the solution through the Styrofoam sheet. The nutrient solution, in its original form, consisted of the following concentrations (in mg L−1): NO3− = 196; NH4 = 14; P = 31; K = 234; Ca = 160; Mg = 48.6; S = 70; Fe-EDTA = 5; Cu = 0.02; Zn = 0.15; Mn = 0.5; B = 0.5; and Mo = 0.01.
Treatments were applied after the acclimation period was over. Eucalyptus clones were subjected to different conditions of P availability for 18 days, totaling 33 days in the hydroponic system. The nutrient solution in each pot was replaced twice a week and pH adjusted daily to 5.5 ± 0.2, using 1.0 mol L−1 HCl or 1.0 mol L−1 NaOH.
Morphological Variables
Shoot height and root length were measured with ruler at the start and at the end of the experiment. A digital caliper was used to measure collar diameter. The increase in shoot (SI), root (RI), number of leaves (NLI), and collar diameter (CDI) were calculated based on the measurements. Leaf area was measured through WinRhizo 2013 software, which uses the methodology of Tennant (1975). Samples were digitized in a professional scanner (EPSON Expression 11,000) and TIFF-format images were analyzed.
Shoots and roots were washed in running water and then in distilled water. Plant parts were dried in a forced air circulation oven at 65 °C until constant weight was reached. The results were used to calculate shoot dry weight (SDW), root dry weight (RDW), and total dry weight (TDW = SDW + RDW).
Root morphological characterization was done through digitized images in WinRhizo Pro 2013 software coupled to EPSON Expression 11,000 scanner equipped with additional light (TPU) at 600 DPI resolution. Total root length (cm plant−1), root surface area (cm2 plant−1), root volume (cm3 plant−1), and average root diameter (mm) were determined.
Photosynthetic Variables
The third fully expanded leaf was used to evaluate photosynthetic variables in an infrared gas analyzer (IRGA) (Li-COR® 6400 XT) at 1500 μmol m−2 s−1 photosynthetic radiation and CO2 concentration of 400 μmol mol−1. Measurements were taken in the morning between 8:00 am and 10:00 am, before plants were collected for growth analysis. The following variables were determined at that time: net photosynthetic rate (A), transpiration rate (E), stomatal conductance (Gs), intercellular CO2 concentration (Ci), instantaneous carboxylation efficiency (A/Ci: ratio between photosynthetic rate and intercellular CO2 concentration), and water use efficiency (WUE: ratio between photosynthetic and transpiration rates).
Chlorophyll a Fluorescence
Chlorophyll a fluorescence was examined in three plants per treatment. Analyses were performed on fully expanded leaves collected from the middle third in the morning between 8:00 and 11:30 am with a portable light-modulated fluorometer (Junior-Pam Chlorophyll Fluorometer WalzMess-und-Regeltechnik, Germany). Leaves were preadapted to the dark for 30 min before determining initial fluorescence (Fo). Subsequently, samples were subjected to a saturating light pulse (10.000 µmol m−2 s−1) for 0.6 s to determine maximum fluorescence (Fm) and electron transport rate (ETRm). Maximum quantum yield of PSII (Fv/Fm) was obtained from the ratio between variable fluorescence (Fv = Fm–Fo) and maximum fluorescence.
Tissue P Analysis
Shoot and root dry matter was ground in a Wiley mill, passed through a 2-mm mesh sieve, then digested with nitric-perchloric acid (EMBRAPA 1999). Phosphorus (P) content in the extract was determined based on the method by Murphy and Riley (1962) in a UV–visible spectrophotometer (1105, Bel Photonics) at 880 nm. Subsequently, the following P efficiency indices were determined (Siddiqi and Glass 1981):
-
a)
P absorption efficiency (PAE) = (µg of P accumulated in plants) / (mg of root dry weight)−1;
-
b)
P use efficiency in roots (Root PUE) = (mg of root dry weight)2 / (µg of P accumulated in roots)−1;
-
c)
P use efficiency in the shoots (Shoot PUE) = (mg of shoot dry weight 2 / (µg of P accumulated in the shoot)−1;
-
d)
P use efficiency in plants (Total PUE) = (mg of total dry weight)2 / (µg of P accumulated in plants)−1.
Biochemical Variables
Seedlings were collected and separated into parts. Leaves and roots were washed in distilled water, then immediately placed in aluminum foil envelopes and frozen in liquid N to avoid sample degradation. These samples were kept in an ultrafreezer at − 80 °C until analysis, when they were macerated in liquid N, homogenized in a specific buffer, and assessed.
Chlorophyll a, total chlorophyll, and carotenoid concentrations in leaves were extracted based on the method by Hiscox and Israelstam (1979) and estimated through Lichtenthaler (1987). Superoxide dismutase (SOD) activity was determined using spectrophotometric method described by Giannopolitis and Ries (1977), while guaiacol peroxidase (GPX) activity was determined based on Zeraik et al. (2008).
Acid phosphatase (APase) activity was determined based on Tabaldi et al. (2007). The reaction was triggered by adding a substrate (PPi at final concentration of 3.0 mM) and stopped by adding 200 μL of TCA 10% at a final concentration of 5%. Inorganic phosphate (Pi) was quantified at 630 nm, using malachite green as a colorimetric reagent and KH2PO4 as a standard for the calibration curve.
Statistical Analysis
Error distribution normality was investigated through Shapiro–Wilk test and error variance homogeneity through the Bartlett test (Storck and Lopes 2011). Both tests were done to all experimental variables. Whenever assumptions were met, analysis of variance and Tukey test were applied to all treatments at 5% probability of error, using Sisvar statistical software (Ferreira 2019).
Furthermore, data were subjected to multivariate principal component analysis (PCA) in R software (R Core Team 2021) using the Facto Extra package (Kassambara and Mundt 2017) to investigate correlation effects between response variables and treatment distribution. Principal component analysis was performed according to a set of principal components (PC1 and PC2 were used in this case), which consisted of a set of standardized linear and orthogonal combinations which explained the original data variability altogether.
Results
Morphological Variables
There was significant interaction (p ≤ 0.05) between the factors (Eucalyptus clones and P concentrations) for the morphological variables, and this finding showed that the joint action of these factors influenced the expression of these characteristics.
The highest increases in shoot (SI) (3.35 cm) and root (RI) (13.45 cm) were observed in E. saligna at the concentration of 30% P, which was statistically different from 100% P (Fig. 1a and Fig. 1b). Thus, there was an increase of 54.25% in SI and 51.64% in RI of plants subjected to 30% P compared to control plants of E. saligna (100% P in nutrient solution) (Fig. 1a and Fig. 1b). The highest RI for E. urograndis was also found at the concentration of 30% P, though the opposite was observed for SI. Phosphorus concentrations did not affect SI, RI, and number of leaves (NLI) in E. dunnii (Fig. 1c), but the lowest increases in collar diameter (CDI) for E. urograndis, E. saligna, and E.dunnii were found at the lowest P concentration (10% P) (Fig. 1d).
Mean values recorded for the increase in shoot (SI) (a), root (RI) (b), number of leaves (NLI) (c), and collar diameter (CDI) (d), of Eucalyptus clones grown in three phosphorous concentrations. Different letters between treatments within the same clones represent statistical difference in the Tukey test at 5% probability of error. Bars represent mean ± standard deviation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
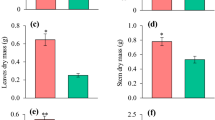
Shoot dry weight (SDW), root dry weight (RDW), and total dry weight (TDW) in E. saligna was not statistically different between 30% P and 100% P (control) (Fig. 2). However, the highest RDW and TDW for E. urograndis were observed in both P deficiency concentrations (10% P and 30% P) (Fig. 2b and Fig. 2c). Root dry weight in E. dunnii did not differ among P concentrations (Fig. 2c). The highest leaf area values (159, 875 cm2) were found in E. urograndis at 30% P. This result represented an increase of 27.38% in comparison to plants at 100% P (Fig. 2d). The highest leaf area values for E. dunnii were in plants subjected to 10% P, though P concentrations did not influence leaf area in E. saligna (Fig. 2d).
Mean values recorded for shoot dry weight (SDW) (a), root dry weight (RDW) (b), total dry weight (TDW) (c), leaf area, and (d) of Eucalyptus clones grown in three phosphorus concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
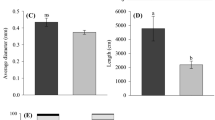
The highest root length and root surface area values were found in E. urograndis at 30% P. These variables presented an increase of 69.8% and 41.09%, respectively, in comparison to those of the control (Fig. 3a and Fig. 3b). However, the opposite was found for E. saligna, which had the lowest average root length at 30% P and was statistically different from the control.
The highest average root length and root diameter in Eucalyptus dunnii were found at 10% P (Fig. 3a and Fig. 3c). The highest root diameter and volume values were found in E. urograndis at 30% P (Fig. 3c and Fig. 3d). Root diameter of E. urograndis increased by 24.85% and root volume increased by 57.39% in comparison to plants at 30% P.
Physiological Variables
The interaction between the factors (Eucalyptus clones and P concentrations) had a significant effect (p ≤ 0.05) on the physiological variables.
The highest values of net photosynthetic rate (A) and transpiration rate (E) for E. dunnii were found at the lowest P concentration (Fig. 4a and b). Eucalyptus urograndis presented its highest net photosynthetic rate (21.937 μmol CO2 m−2 s−1) and lowest transpiration rate (5.735 mmol H2O m−2 s−1) at the concentration of 30% P, which led to an increase of 16.57% in net photosynthetic rate and a reduction of 14.52% in transpiration rate compared to the control (100% P) (Fig. 4).
Mean values recorded for net photosynthetic rate (A) (a), transpiration rate (E) (b), stomatal conductance (Gs) (c), intercellular CO2 concentration (Ci) (d), water use efficiency (WUE) (e), and instantaneous carboxylation efficiency (by Rubisco) (A/Ci) (f) of Eucalyptus clones grown in three phosphorus concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
The highest values for stomatal conductance (Gs) and intercellular CO2 concentration (Ci) were found in E. urograndis subjected to the lowest P concentration (10% P), which led to differences of 28.33% and 1.76%, respectively, compared to those recorded for plants grown in 30% P (Fig. 4c and d). The highest values of Gs and Ci for E. saligna were found at 30% P (Fig. 4).
The highest values for water use efficiency (WUE) and instantaneous carboxylation efficiency (by Rubisco) (A/Ci) were observed for E. dunnii at 10% P (Fig. 4e and f). Eucalyptus urograndis grown in this same solution had the lowest values of WUE and A/Ci.
The highest initial fluorescence (Fo) values were found for E. dunnii grown in the lowest P concentration (10% P) (Fig. 5a). Maximum fluorescence (Fm) values were not statistically different between E. urograndis grown in 10% P and 100% P (Fig. 5b).
Mean values recorded for initial fluorescence (Fo) (a), maximum fluorescence (Fm) (b), electron transport rate (ETRm) (c), and maximum quantum yield of PSII (Fv/Fm) (d) of Eucalyptus clones grown in three phosphorus concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
The lowest electron transport rate (ETRm) values were found for E. dunnii grown in 10% P (Fig. 5c). The highest ETRm values for E. saligna were found at 30% P. ETRm values for E. urograndis did not change after P application. The lowest maximum quantum yield of PSII (Fv/Fm) values for E. saligna were observed at lower P concentrations (10% and 30% P). Eucalyptus urograndis and E. dunnii showed the lowest Fv/Fm values at 30% P (Fig. 5d).
Biochemical Variables and Tissue P Analysis
The interaction between the factors (Eucalyptus clones and P concentrations) showed a significant effect (p ≤ 0.05) on the biochemical variables. The highest values for total chlorophyll (3.588 mg g−1 FW) and carotenoids (0.449 mg g−1 FW) for E. saligna were found at the lowest P concentration (10% P) (Fig. 6). Chlorophyll a values for E. saligna and E. dunnii were not statistically different among P concentrations. Similarly, total Chl and carotenoid values for E. urograndis were not statistically different among P concentrations (Fig. 6).
Superoxide dismutase (SOD) activity in shoots and roots of E. saligna was not affected by P concentrations (Fig. 7a). The highest SOD activity in shoots and roots of E. urograndis was found at 30% P. The highest guaiacol peroxidase (GPX) activity was observed in shoots of E. saligna and E. urograndis at 30% P (Fig. 7c). On the other hand, the lowest GPX activity in shoots of E. dunnii was found at 30% P. Root GPX activity in E. saligna and E. urograndis was not impacted by P concentrations (Fig. 7d).
Mean values recorded for superoxide dismutase activity in shoots (Shoot SOD) (a) and roots (Root SOD) (b), guaiacol peroxidase activity in shoots (Shoot GPX) (c) and roots (Root GPX) (d) and acid phosphatase activity in shoots (Shoot APase) (e) and roots (Root APase) (f) of Eucalyptus clones grown in three phosphorus concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
The highest acid phosphatase (APase) activity in shoots of E. urograndis and E. saligna was found at 10% P (Fig. 7e and f). The same was observed in roots of E. urograndis at 10% P (Fig. 7f). Root APase activity for E. saligna was not affected by P application (Fig. 7f). The lowest APase activity was observed in shoots of E. dunnii at 10% P. However, the lowest APase activity in roots of E. dunnii was found at 30% P (Fig. 7f).
The highest P concentrations in shoots and roots of E. saligna and E. urograndis were found at 30% P, while the lowest at 10% P (Fig. 8). The highest P concentration in shoots was found for E. dunnii grown in 10% P (Fig. 8a). Eucalyptus saligna grown in 30% P had the highest average P accumulation in shoots and in the whole plant, though it did not differ from the control (Fig. 8c and e). Eucalyptus urograndis grown in 30% P also showed the highest average P accumulation in roots but was not statistically different from plants grown in 10% P (Fig. 8d and e). On the other hand, E. dunnii did not show significant difference in P accumulation in shoots, roots and plants among P concentrations (Fig. 8). Similarly, P accumulation in shoots of E. urograndis and in roots of E. saligna did not show significant differences (Fig. 8c and d).
Mean values recorded for P concentrations in shoots (a) and roots (b), P accumulation in shoots (c) and roots (d), and total P accumulation (e) of Eucalyptus clones grown in three phosphorus concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
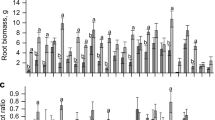
The highest P use efficiency in shoots and plants was found in E. saligna at the lowest P concentration (10%) (Fig. 9c and d). The highest P use efficiency in shoots of E. dunnii was found at 100% P (Fig. 9c), but was not significantly different from plants at 30% P. However, E. urograndis and E. dunnii did not show any difference in P uptake and use efficiency among P concentrations (Fig. 9a and d). None of the clones showed statistically significant differences in P use efficiency in roots among P concentrations (Fig. 9b).
Mean values recorded for P absorption efficiency (PAE) (a), P use efficiency in roots (Root PUE) (b), P use efficiency in shoots (Shoot PUE) (c), Total P use efficiency (d), of Eucalyptus clones grown in three P concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Principal Component Analysis (PCA)
Principal component analysis (PCA) was performed by only extracting the first two components from each eucalyptus clone (Fig. 10). PCA of E. saligna explained 48.6% of data variability in principal component 1 (PC1) and 24.2% in principal component 2 (PC2) (Fig. 10a). PCA allowed us to observe the behavior of the effect of P concentration on response variables. Phosphorus concentration of 10%, which is on the left in the spatial distribution, showed positive correlation to the increase in number of leaves, root dry weight, P use efficiency in shoots, roots and total P use efficiency, acid phosphatase activity in shoots, and initial fluorescence. Phosphorus concentration of 30% was positioned to the right in the spatial distribution and showed positive correlation to shoot increase and root increase, P concentration in shoots and roots, P absorption efficiency, P accumulation in shoots, roots and total P accumulation, superoxide dismutase activity in shoots and roots, guaiacol peroxidase activity in shoots, maximum fluorescence, electron transport rate, stomatal conductance, intercellular CO2 concentration, net photosynthetic rate, water use efficiency, chlorophyll a, and carotenoids. Finally, P concentration of 100% was influenced by root surface area, root volume, root diameter, root length, GPX in roots, APase in roots, instantaneous carboxylation efficiency, and transpiration rate.
Relationship between principal component 1 (PC1) and principal component 2 (PC2) of the parameters of nutritional (total P in shoot (P Shoot) and roots (P Roots); P use efficiency in shoot (PUE Shoot), roots (PUE Roots) and total (PUE Total); P absorption efficiency (PAE); P accumulated in shoot (PA Shoot), roots (PA Roots) and total (PA Total)), morphological (shoot increase (SI), number of leaves (NLI), diameter collar (CDI), roots (RI); shoot dry matter (SDM), roots (RDM), and total (TDM); surface area roots (SA Roots), volume (V Roots), diameter (D Roots), and length (L Roots)), enzymatic (SOD in shoot (SOD Shoot) and roots (SOD Roots); GPX in shoot (GPX Shoot) and roots (GPX Roots); phosphate acid in shoot (Phosphatase Shoot) and roots (Phosphatase Roots)) and physiological initial fluorescence (Fo), maximum fluorescence (Fm), electron transport rate (ETRm), maximum quantum yield of PSII (Fv/Fm), net photosynthetic rate (E), stomatal conductance (Gs), intercellular CO2 concentration (Ci), transpiration rate (A), water use efficiency (WUE), instantaneous carboxylation efficiency (A/Ci), chlorophyll a (Chl a), total (Chl total), carotenoids (Carot) of Eucalyptus saligna (a), Eucalyptus dunnii (b), and Eucalyptus urograndis (c)
PCA of E. dunnii explained 41.4% of data variability in PC1 and 36.5% in PC2 (Fig. 10b). PCA provided three groups of variables based on P concentration. Phosphorus concentration of 10% was influenced by P content in shoots, leaf area, shoot increase, root surface area, root volume, root diameter, root length, APase in roots, initial fluorescence, maximum fluorescence, chlorophyll a, and total chlorophyll. Phosphorus concentration of 30% showed positive correlation to collar diameter increase and root increase, GPX in roots, P use efficiency in roots and total P use efficiency, P accumulation in roots, and intercellular CO2 concentration. Lastly, P concentration of 100% was influenced by P concentration in roots, P use efficiency in shoots, P absorption efficiency, P accumulation in shoots and total P accumulation, shoot dry weight, total dry weight, SOD in shoots, GPX in shoots, APase in shoots, and electron transport rate.
PCA of E. urograndis explained 44.8% of data variability in PC1 and 35.5% in PC2 (Fig. 10c). PCA provided three groups of response variables according to P concentrations. The concentration of 10% P was influenced by shoot increase, shoot dry weight, root dry weight, total dry weight, P use efficiency in shoots, roots and total P use efficiency, leaf area, APase in shoots, APase in roots, stomatal conductance, and intercellular CO2 concentration. The concentration of 30% P had a positive correlation to P concentration in shoots, P concentration in roots, P accumulation in shoots, roots and total P accumulation, increase in number of leaves and root, root surface area, root volume, root diameter, root length, SOD in shoots, SOD in roots, GPX in shoots, net photosynthetic rate, water use efficiency, and chlorophyll a. Finally, the concentration of 100% P was influenced by the increase in collar diameter, electron transport rate, maximum fluorescence, maximum quantum yield of PSII, transpiration rate, GPX in roots, instantaneous carboxylation efficiency, total chlorophyll, and carotenoids.
Discussion
The highest values of increase in root length and diameter for E. dunnii were found in plants grown in the concentration of 10% P (Fig. 3a and c). This is due to the transport of signaling molecules from shoots to roots, in addition to important photoreceptors, such as phytochrome A (phyA) activation, which transfers auxin from the stem system to the roots, stimulating root production (Kulmann et al. 2021; Siqueira et al. 2023). This positively influences plant H2PO4− uptake and induces Pi transport proteins and the regulation of Pi uptake kinetics (Nadeem et al. 2022). Furthermore, with low tissue P content, plants can exude organic acids capable of chelating Al3+, Fe3+ and Ca2+, decreasing the likelihood of P complexation and precipitation (Zhou et al. 2021; Wan et al. 2023). Plants can also release OH− to the solution, which is an important strategy to maintain higher pH levels that promote increased P availability (Shen et al. 2018).
However, the highest values of root length, surface area, and volume, as well as root increase in E. urograndis (Fig. 3a, b, and d) were found in plants grown in 30% P (Fig. 1b). Similar behavior was observed for the root increase in E. saligna. This response was caused by the increased P content available in the solution which enables part of the nutrient to move closer to the outer surface of roots, enhancing the absorption of chemical P species and increasing content in roots (Kulmann et al. 2023). This happens because greater root–soil contact leads to a larger absorption area with larger root systems which play an important role in the uptake of relatively immobile nutrients such as P (Berghetti et al. 2021).
The highest net photosynthetic rates in E. urograndis and E. saligna grown in 30% P (Fig. 4a) may have contributed to increase shoot biomass production (Fig. 2a). This led to higher P accumulation (Fig. 8a and e) and maximized CO2 availability per leaf area, increasing CO2 fixation in leaves (Loudari et al. 2022). Thus, the greater efficiency in nutrient translocation to the shoots enabled nutrient supply to the photosynthetically active sites in plants and increased photosynthetic rates (Kulmann et al. 2023).
Eucalyptus urograndis grown in 30% P showed lower transpiration rate than that of the control plants (Fig. 4b). This may have happened because plants with more CO2 in leaf intercellular spaces often close their stomata, decreasing stomatal conductance and increasing water use efficiency (WUE) (Aguilar et al. 2023). However, E. dunnii subjected to 10% P had the highest values of net photosynthetic rate, WUE and A/Ci (Fig. 4a, e, and f).
The highest leaf area values of E. urograndis were found in plants grown in 30% P. This response may have occurred because the concentration of 30% P stimulated energy metabolism, cell division, and elongation, resulting in increased leaf area (Fig. 2d). This can also lead to greater leaf area per unit of leaf dry weight, increasing the capacity to capture and assimilate CO2 per plant and the ability to produce new leaves (Marques et al. 2020). On the other hand, E. saligna did not show increased leaf area with decreasing P content (Fig. 2d). This likely happened because part of the P absorbed by roots could have been immediately incorporated into Rubisco to catalyze C assimilation. Therefore, leaves did not need to adjust morphology to enable greater P acquisition, only increase photosynthetic pigment concentration (Fig. 9a and Fig. 6).
The highest concentrations of chlorophyll a in E. urograndis (Fig. 6a) and total chlorophyll (Fig. 6b) in E. saligna subjected to 30% P may have promoted an increase in biomass production. This occurred because increased amounts of pigments enable plants to absorb more light radiation and convert it into carbohydrates, resulting in increased biomass production (Roca et al. 2018). Thus, a reduction in P concentration (30% P) did not cause significant damage to the photosystem of E. urograndis and E. saligna seedlings, as there was no degradation or reduced production of chloroplast pigments, contributing to a higher net assimilation rate.
Similar results for pigment content were observed for E. dunnii in 10% P (Fig. 6). Because of this, there may have been a greater transfer of resonance energy from antenna complexes to reaction centers, where it was used for photochemical reactions responsible for biomass production (Berghetti et al. 2021). Despite the reduction in P availability (10% P), E. dunnii seedlings were able to maintain maximum quantum yield of PSII (Fv/Fm) values similar to those of plants grown in the control (Fig. 5d), indicating the resistance in the primary reactions of photosynthesis, even under stress conditions (Gonçalves et al. 2019).
There was a greater decrease in maximum fluorescence (Fm) than in initial fluorescence (Fo) for E. urograndis grown in 30% P (Fig. 5b and Fig. 5a). This resulted in a lower Fv/Fm ratio which reduced the efficiency of radiant energy captured by photosystem II (PSII) (Singh and Reddy 2015). However, electron transport rate (ETRm) values for E. urograndis were not statistically different between plants grown in solutions with low P concentrations and those in the control (Fig. 5c), which suggests the stress caused by low P availability did not inhibit electron transport in PSII (Gonçalves et al. 2019). Thus, the decrease in Fv/Fm ratio suggests excess energy dissipation by non-photochemical extinction or non-radioactive mechanisms in the PSII reaction center (Singh and Reddy 2015; Sperdouli et al. 2021).
The photosynthetic capacity of P-deficient plants may result in greater accumulation of photon energy under high irradiance. Excess photons cause an increase in reactive oxygen species (ROS) production as a by-product of photosynthesis (Kuinchtner et al. 2023). However, there is often an increase in antioxidant enzyme activity to eliminate ROS produced in plants subjected to stress (Mattos et al. 2023).
The main antioxidant enzymes responsible for eliminating ROS to maintain homeostasis in plant cells are superoxide dismutase (SOD) and guaiacol peroxidase (GPX) (Kulmann et al. 2023). Thus, the increased ROS rate in E. urograndis seedlings grown in 30% P was likely followed by high SOD and GPX activity in shoots and roots (Fig. 7). Thus, stress-tolerant genotypes may at least partly depend on an increased antioxidant defense system (Chen et al. 2020). The lack of an increase in GPX, SOD and acid phosphatase (APase) activity in E. saligna at 30% P may be associated with metabolic adjustments induced by P deficiency, such as the use of PPi-dependent enzymes in the glycolysis pathway and the replacement of membrane phospholipids by sulfolipids (Hoyos and Fonseca 2019). Reduced phosphorus addition (10% P) in E. dunnii decreased the activity of SOD and GPX (Fig. 7). This may have occurred due to increased photosynthetic pigment synthesis and changes in chlorophyll a fluorescence, with less photochemical energy dissipation (Ferreira et al. 2018).
Increased APase activity in shoots of E. urograndis and E. saligna at 10% P and in roots of E. urograndis at 10% P may have been a result of the low P concentration in plant tissue (Fig. 7e, f, Fig. 8a and b). The induction of intracellular APase activity occurs to maintain the internal homeostasis of inorganic phosphate (Pi) and supply it to organic P compounds in the cytoplasm and vacuoles (Han et al. 2022). There was no significant effect of APase activity on E. urograndis grown in the control and 30% P concentrations. This result may indicate greater P remobilization at these concentrations, confirmed by P content in tissues and higher P accumulation in roots and plants (Fig. 8). However, APase activity in E. dunnii decreased in shoots of plants subjected to 10% P (Fig. 8e and f). This may have occurred because, at a molecular level, P starvation-induced genes that encode enzymes help to prioritize internal P use and maximize P acquisition by inducing high-affinity Pi transporters in roots (Vasconcelos et al. 2022).
There was no significant difference in P use efficiency in roots of the Eucalyptus clones assessed in this study (Fig. 9b). This possibly occurred because P use efficiency in roots subjected to P deficiency conditions increased as a result of changes in enzyme activity in the glycolysis pathway, which start operating in alternative carbohydrate catabolism pathways during P deficiency, contributing to more efficient P use through recycling, reduced consumption, and the use of other nutrient fractions (Iqbal et al. 2023).
There was no significant difference in P absorption efficiency and total P use efficiency in E. urograndis and E. dunnii among P concentrations (Fig. 9a and d). This happened because P reduction in the nutrient solution promoted a more efficient functioning of the roots in the nutrient uptake process. Phosphorus absorption efficiency may also be related to the positive effect of P supply on nutrient accumulation in shoots, as indicated by the positive correlations between these two variables (Khan et al. 2023). This was somewhat expected, as P demand in shoots acts as the main regulating factor in the amounts of absorbed nutrients. One of the responses that also condition greater adaptation to low P availability and contribute to increase P absorption efficiency is the secretion of acid phosphatase by the root system, as it promotes the breakdown of phosphate esters and releases inorganic P into the solution, making it available for uptake by the roots (Martins et al. 2021).
PCA of the Eucalyptus clones helped to explain the effect of P concentration on response variables. Eucalyptus urograndis grown in the concentration of 30% P was more efficient in the use of resources, resulting in increased root surface area, volume, and length. As a result, greater amounts of P were absorbed by plants under these conditions, which was confirmed by the positive relationship with P content in shoot tissues (Roca et al. 2018).
Phosphorus concentrations of 30% and 100% in E. dunnii showed a positive correlation to increased collar diameter, root length or dry weight, shoot dry weight, and total dry weight. On the other hand, 100% P in E. urograndis may have provided adequate amounts of P in the solution, as these concentrations showed a negative correlation to shoot increase, shoot dry weight, root dry weight, and leaf area. This happened because P concentration in the solution may have been enough to meet plant demand, promoting an increase in leaf area production per unit of leaf dry mass. As a result, this increased plant ability to capture and assimilate CO2 and contributed to the maintenance of plant energy (Kayoumu et al. 2023).
PCA of E. saligna showed concentrations of 30% P and 100% P promoted an increase in shoot biomass production, which provided greater CO2 fixation in leaf tissue (Loudari et al. 2022). This was confirmed by the positive relationship between the concentrations of 30% P and 100% P with electron transport rate, stomatal conductance, intercellular CO2 concentration, net photosynthetic rate, water use efficiency, instantaneous carboxylation efficiency, transpiration rate, chlorophyll a, and carotenoids.
Thus, Eucalyptus clones showed differences in growth due to variations in morphological, physiological, and biochemical characteristics that influenced the nutritional efficiency of P, which confirmed our initial hypothesis. Thus, genetic differences may play a significant role in determining morphological, physiological, and biochemical characteristics which lead to different levels of nutrient absorption efficiency in Eucalyptus clones. Based on our results, E. urograndis was the genotype that adapted to environments with low P concentrations the best, as this adaptation process is associated with increased root growth, physiological efficiency (e.g., photosynthetic rate), antioxidant capacity, and P absorption efficiency. This response is essential to rationalize the use of phosphate fertilizers, thus reducing production costs and the potential for soil and water contamination.
Conclusion
Eucalyptus urograndis showed the highest P absorption efficiency as it did not have limitations in biomass production and root growth at a low P concentration (30% P). This finding revealed that E. urograndis requires low P availability and can grow in environments with low P concentrations. Although E. saligna did not show decreased shoot dry weight and total dry weight and increased P absorption efficiency at 30% P, there was decreased root growth and root dry matter production. Eucalyptus dunni had the lowest electron transport rate and P use efficiency in shoots at 10% P. However, it was necessary to use the standard P concentration in nutrient solution to promote plant growth for E. dunnii.
Morpho-physiological and biochemical characteristics are directly linked to the nutritional efficiency of P. Thus, this can be used to select Eucalyptus clones for environments with low P availability, as well as to help to develop strategies focused on phosphate fertilization under different edaphic conditions.
Data Availability
Not applicable for that section.
References
Aguilar MVM, Kuinchtner CC, Wertonge GS et al (2023) Tolerance and sensitivity of Inga marginata and Allophylus edulis to copper excess. Trees-Struct Funct 10:1–17. https://doi.org/10.1007/s00468-022-02383-0
Alvarez JA, Cortizo SC, Gyenge JE (2020) Yield stability and phenotypic plasticity of Populus spp. clones growing in environmental gradients: I-yield stability under field conditions. For Ecol Manage 463:01–08. https://doi.org/10.1016/j.foreco.2020.117995
Antunes E, Vuppaladadiyam AK, Kumar R et al (2022) A circular economy approach for phosphorus removal using algae biochar. Clean Circ Bioecon 1:01–09. https://doi.org/10.1016/j.clcb.2022.100005
Awad-Allah EFA, Elsokkary HE, Lindberg SM (2023) Phosphorus protects against cadmium-induced phytotoxicity by stimulating plasma membrane H+-ATPase activity. J Plant Nutr 46:3918–3932. https://doi.org/10.1080/01904167.2023.2220709
Berghetti ÁLP, Araujo MM, Tabaldi LA et al (2021) Effects of nitrogen fertilization on the growth and on photochemical efficiency in plants of Handroanthus heptaphyllus. J Plant Nutr 44:2464–2475. https://doi.org/10.1080/01904167.2021.1899216
Bird MG, Hardner CM, Dieters M et al (2022) Global genotype by environment trends in growth traits for Eucalyptus dunnii. New for 53:101–123. https://doi.org/10.1007/s11056-021-09846-1
Brune A (2023) Eucalypt alternatives to Acacia mangium in wet tropics. J Trop for Sci 35:01–15
Bulgarelli RG, Silva FMO, Bichara S, Andrade SAL, Mazzafera P (2019) Eucalypts and low phosphorus availability: between responsiveness and efficiency. Plant Soil 445:349–368. https://doi.org/10.1007/s11104-019-04316-2
Canal AS, Alonso L, Picos J et al (2023) Automatic tree detection and attribute characterization using portable terrestrial lidar. Trees 37:963–979. https://doi.org/10.1007/s00468-023-02399-0
Chen Z, Khan A, Shia X, Hao X, Tan DKY, Luo H (2020) Water-nutrient management enhances root morpho-physiological functioning, phosphorus absorption, transportation and utilization of cotton in arid region. Ind Crops Prod 143:02–09. https://doi.org/10.1016/j.indcrop.2019.111975
Dahali R, Lee SH, Tahir PM et al (2022) Influence of Chrysoporthe deuterocubensis canker disease on the machining properties of Eucalyptus urograndis. Forests 13:01–14. https://doi.org/10.3390/f13091366
Dai X, Lin Y, Zhou T et al (2023) Natural annual transcriptome dynamics of Eucalyptus reveal seasonal adaptation of tropical/sub-tropical trees Get access Arrow. Tree Physiol 43:658–674. https://doi.org/10.3389/fpls.2022.1067498
Empresa Brasileira De Pesquisa Agropecuária (EMBRAPA). Centro Nacional de Pesquisa Informática Agropecuária. Manual de nálises químicas de solos, plantas e fertilizantes. Brasília: Embrapa/CNPTIA, 1999. 370p.
Estopa RA, Paludeto JGZ, Müller BSF et al (2023) Genomic prediction of growth and wood quality traits in Eucalyptus benthamii using different genomic models and variable SNP genotyping density. New for 54:343–362. https://doi.org/10.1007/s11056-022-09924-y
Ferreira DF (2019) SISVAR: A computer analysis system to fixed effects split plot type designs. Revista Brasileira De Biometria 37:529–535
Ferreira PAA, Ceretta CA, Tiecher T et al (2018) Rhizophagus clarus and Phosphorus in Crotalaria juncea: Growth, Glomalin Content and Acid Phosphatase Activity in a Copper-Contaminated Soil. Rev Bras Ciênc Solo 42:01–16. https://doi.org/10.1590/18069657rbcs20170245
Giannopolitis CN, Ries SK (1977) Purification and quantitative relationship with water-soluble protein in seedlings. J Plant Physiol 48:315–318. https://doi.org/10.1104/pp.59.2.315
Gonçalves KS, Paz VPS, Silva FL, Hongyu K, Almeida WF (2019) Potassium phosphite and water deficit: physiological response of Eucalyptus using multivariate analysis. J Agric Sci 11:565–574. https://doi.org/10.5539/jas.v9n11p99
Han Y, White PJ, Cheng L (2022) Mechanisms for improving phosphorus utilization efficiency in plants. Ann Bot 129:247–258. https://doi.org/10.1093/aob/mcab145
Haque SE (2021) How effective are existing phosphorus management strategies in mitigating surface water quality problems in the U.S.? Sustainability 13:01–13. https://doi.org/10.3390/su13126565
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1132–1334. https://doi.org/10.1139/b79-163
Hoagland DR, Arnon DI. 1950. The water culture method for growing plants without soil. Berkeley, CA: Agric. Exp. Stn., Univ. Of California. (Circ. 347).
Honorato AC, Oliveira JRG, Passos AM, Yano-Melo AM (2020) Mycorrhizal inoculation on the production of seedlings of native Caatinga species. Floresta e Ambiente 27:01–09. https://doi.org/10.1590/2179-8087.124017
Hoyos GR, Fonseca LM (2019) Physiological and antioxidant responses of cape gooseberry (Physalis peruviana L.) seedlings to phosphorus deficiency. Agronomia Colombiana 37:03–11. https://doi.org/10.15446/agron.colomb.v37n1.65610
Hyder S, Rizvi ZF, Santos-Villalobos S et al (2023) Applications of plant growth-promoting rhizobacteria for increasing crop production and resilience. J Plant Nutr 46:2551–2258. https://doi.org/10.1080/01904167.2022.2160742
Ibrahim M, Iqbal M, Tang YT et al (2022) Phosphorus mobilization in plant-soil environments and inspired strategies for managing phosphorus: a review. Agronomy 12:01–17. https://doi.org/10.3390/agronomy12102539
Iqbal A, Qiang D, Xiangru W et al (2023) Phosphorus and carbohydrate metabolism contributes to low phosphorus tolerance in cotton. BMC Plant Biol 23:01–20. https://doi.org/10.1186/s12870-023-04100-6
Kassambara A, Mundt F. 2017. Factoextra: extract and visualize the results of multivariate data analyses. R package version, 1(4).
Kayoumu M, Iqbal A, Muhammad N et al (2023) Phosphorus availability affects the photosynthesis and antioxidant system of contrasting low-p tolerant cotton genotypes. Antioxidants 12:01–23
Khan F, Siddique AB, Shabala S et al (2023) Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 12:01–29. https://doi.org/10.3390/plants12152861
Kuinchtner CC, Aguilar MVM, Senhor DF et al (2023) Morpho-physiological and biochemical mechanisms of copper tolerance in Handroanthus heptaphyllus. Ciência e Agrotecnologia 47:01–13. https://doi.org/10.1590/1413-7054202347011322
Kulmann MSS, Paula BV, Sete PB et al (2021) Morphological and kinetic parameters of the absorption of nitrogen forms for selection of Eucalyptus clones. J for Res 32:1599–1611. https://doi.org/10.1007/s11676-020-01195-7
Kulmann MSS, Aguilar MVM, Tassinari A et al (2023) Effects of increasing soil phosphorus and association with ectomycorrhizal fungi (Pisolithus microcarpus) on morphological, nutritional, biochemical, and physiological parameters of Pinus taeda L. For Ecol Manage 544:01–14. https://doi.org/10.1016/j.foreco.2023.121207
Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Packer L, Douce R (eds). Methods in Enzimology, 148: 350–381. doi: https://doi.org/10.1016/0076-6879(87)48036-1
Lopez G, Ahmadi SH, Amelung W et al (2023) Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Front Plant Sci 13:01–18. https://doi.org/10.3389/fpls.2022.1067498
Loudari A, Mayane A, Zeroual Y et al (2022) Photosynthetic performance and nutrient uptake under salt stress: Differential responses of wheat plants to contrasting phosphorus forms and rates. Front Plant Sci 13:01–16. https://doi.org/10.3389/fpls.2022.1038672
Marques ACR, Oliveira LB, Brunetto G, Tavares MS, Quadros FLF, Nicoloso FT (2020) Interaction between growth strategies and phosphorus use efficiency in grasses from South America natural grasslands. Revista Ceres 67:062–069. https://doi.org/10.1590/0034-737X202067010009
Martins NP, Fuchslueger L, Fleischer K et al (2021) Fine roots stimulate nutrient release during early stages of leaf litter decomposition in a Central Amazon rainforest. Plant Soil 469:287–303. https://doi.org/10.1007/s11104-021-05148-9
Mattos JPO, Aguilar MVM, Alves JS et al (2023) Selenium does not ease growth inhibition caused by aluminum in seedlings. J Plant Nutr 10:1–18. https://doi.org/10.1080/01904167.2023.2206427
Meng X, Chen W, Wang Y et al (2021) Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 16:01–20. https://doi.org/10.1371/journal.pone.0246944
Murphy J, Riley JPA (1962) Modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nadeem M, Wu J, Ghaffari H et al (2022) Understanding the adaptive mechanisms of plants to enhance phosphorus use efficiency on podzolic soils in boreal agroecosystems. Front Plant Sci 13:01–23. https://doi.org/10.3389/fpls.2022.804058
Qi S, Wen Z, Dong Y, Li H, Miao Y, Shen J (2018) The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB PLANTS 10:01–11. https://doi.org/10.1093/aobpla/ply054
R core team. 2021. R: A language and environment for statistical computing. Available at https://www.r-project.org/.
Roca LF, Romero J, Bohórquez JM, Alcántara E, Fernández-Escobar R, Trapero A (2018) Nitrogen status affects growth, chlorophyll content and infection by Fusicladium oleagineum in olive. Crop Prot 109:80–85. https://doi.org/10.1016/j.cropro.2017.08.016
Siddiqi MY, Glass ADM (1981) Utilization index: a modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J Plant Nutr 4:289–302. https://doi.org/10.1080/01904168109362919
Singh SK, Reddy VR (2015) Response of carbon assimilation and chlorophyll fluorescence to soybean leaf phosphorus across CO2: Alternative electron sink, nutrient efficiency and critical concentration. J Photochem Photobiol, B 151:276–284. https://doi.org/10.1016/j.jphotobiol.2015.08.021
Siqueira JA, Zsögön A, Fernie AR et al (2023) Does day length matter for nutrient responsiveness? Trends Plant Sci 28:1113–1123. https://doi.org/10.1016/j.tplants.2023.04.012
Sousa RN, da Silva BA, da Costa VV et al (2022) Limestone and phosphogypsum are key drivers of eucalypt production in the highly weathered soils of Brazil. Plant Soil 10:01–21. https://doi.org/10.1007/s11104-022-05801-x
Sperdouli I, Andreadis S, Moustaka J et al (2021) Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 26:01–13
Storck L, Lopes S (2011) Experimentação vegetal, 3rd edn. Editora da UFSM, Santa Maria, RS
Tabaldi LA, Ruppenthal R, Cargnelutti D, Morsch VM, Pereira LB, Schetinger MRC (2007) Effects of metal elements on acid phosphatase activity in cucumber (Cucumis sativus L.) seedlings. Environ Exp Bot 59:43–48. https://doi.org/10.1016/j.envexpbot.2005.10.009
Tennant DA (1975) A test of a modified line intersect method of estimating root length. J Ecol 63:995–1001. https://doi.org/10.2307/2258617
Trentin E, Ferreira PAA, Ricachenevsky FK et al (2022) The tolerance of grapevine rootstocks to copper excess and to the use of calcium and phosphorus to mitigate its phytotoxicity. Environ Sci Pollut Res 29:82844–82854. https://doi.org/10.1007/s11356-022-21515-0
Vasconcelos MJV, Figueiredo JEF, Oliveira MF et al (2022) Expression analysis of phosphate induced genes in contrasting maize genotypes for phosphorus use efficiency. Braz J Biol 82:01–13. https://doi.org/10.1590/1519-6984.261797
Wan X, Ai S, Liao H (2023) Deciphering interactions between phosphorus status and toxic metal exposure in plants and rhizospheres to improve crops reared on acid soil. Cells 2023(12):01–17. https://doi.org/10.3390/cells12030441
Zeraik AE, Souza FS, Fatibello-Filho O (2008) Desenvolvimento de um spot test para o monitoramento da atividade da peroxidase em um procedimento de purificação [Spot test development to monitor peroxidase activity in purification procedure]. Quim Nova 31:731–734. https://doi.org/10.1590/S0100-40422008000400003
Zhou M, Chen W, Zhao M, Li Y, Li M, Hu X (2021) Genome-wide characterization and evolutionary analyses of Purple Acid Phosphatase (PAP) gene family with their expression profiles in response to low phosphorus stresses in Moso Bamboo (Phyllostachys edulis). Forests 12:01–19. https://doi.org/10.3390/f12030326
Acknowledgements
We are grateful to the Federal University of Santa Maria - UFSM and CMPC team for their support in the execution of this research.
Funding
This study was funded by Coordination for the Improvement of Higher Education Personnel (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
MVMA: Conceptualization, Writing—Revision, Project administration, carrying out the experiments, biochemical and physiological analysis, and data analysis. CCK: Research, supervision, programming, carrying out experiments, and biochemical and physiological analysis. DFS: Software, formal analysis, supervision and performance of experiments, and biochemical and physiological analysis. TPB: Visualization, validation, project administration, conducting experiments, and biochemical and physiological analysis. CdSL: Validation, formal analysis, and performance of the experiment. MSdSK: Methodology, Software, Formal Analysis, Supervision, and Writing—Revision. MMA: Supervision, Visualization, Writing—Revision, and Co-supervision of the project. ÁLPB: Writing—Review, Visualization, and Validation. GB: Supervision, Fundraising, Project Co-supervision, Writing, and Review. LAT: Project Orientation, Conceptualization, Fundraising, Biochemical and Physiological Analysis, and Writing—Revision.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable for that section.
Consent to Participate
Not applicable for that section.
Consent for Publication
Not applicable for that section.
Additional information
Handling Editor: Mikihisa Umehara .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda Aguilar, M.V., Kuinchtner, C.C., Senhor, D.F. et al. Selecting Eucalyptus spp. Clones to Enable Higher Phosphorus Uptake Efficiency. J Plant Growth Regul 43, 854–870 (2024). https://doi.org/10.1007/s00344-023-11145-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11145-2