Abstract
Aims
To optimize assay conditions of two common methods for measuring potential free-living nitrogen-fixation (FLNF), acetylene reduction assay (ARA) and 15N2-incorporation (15N2), for use with soil/rhizosphere samples.
Methods
We tested the impact of different carbon (C) sources, oxygen concentrations (O2), and incubation times on FLNF rates of two low-fertility Michigan soils via ARA and 15N2.
Results
FLNF rates were greatest with addition of a C cocktail, at low O2, and with 7-day incubations for both methods. FLNF via ARA was 1700x greater with a C cocktail versus glucose only and via 15N2 was 17x greater with a C cocktail compared to other C sources and no-C controls. Specific O2 optimum varied by method and site. A 7-day incubation was needed for the ARA, but a 3-day incubation was suitable for 15N2. Lastly, we confirm previously identified issues with the ARA of acetylene-independent ethylene production/consumption resulting in potential FLNF measurement error of 1.3–52.3 μg N g−1 day−1.
Conclusions
We present an optimized method for measuring potential FLNF in soil/rhizosphere samples which will allow for consistent and comparable FLNF rate measurements. Researchers should account for C source, O2, and incubation time when assessing FLNF and use the ARA method with caution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free-living nitrogen fixation (FLNF), N-fixation by soil organisms not in symbiotic associations with plants, is prevalent across all ecosystems and contributes a measurable portion of N to terrestrial systems annually (Brouzes et al. 1969; Reed et al. 2011). Though FLNF can occur throughout the soil, limitations on carbon (C) availability likely constrain FLNF to C-rich regions such as the rhizosphere (Knowles 1965; Smercina et al. 2019). FLNF is of great interest in agricultural systems, especially low input cropping systems and bioenergy crop production (Roley et al. 2018), because of its potential to offset the need for external N inputs, potentially reducing or even eliminating waste N and the associated negative environmental impacts (e.g. eutrophication and N2O production; Vitousek et al. 1997).
While FLNF has been measured across natural and managed ecosystems (Reed et al. 2011; Roley et al. 2018), the methods employed have not been optimized to account for the wide diversity of diazotrophs (N-fixing organisms) and conditions present in soils and the rhizosphere. The most commonly employed method for measuring FLNF is the acetylene reduction assay (ARA; Hardy et al. 1968) because it is a relatively simple method with low analysis costs. This technique relies on the capacity of nitrogenase, the N-fixing enzyme, to reduce triple bonded molecules other than N2; thus, acetylene is reduced to ethylene and ethylene concentrations can then be easily measured using a standard gas chromatograph (GC) equipped with a flame ionization detector (Hardy et al. 1968). However, previous work suggests there are challenges to successfully applying the ARA to FLNF, particularly in the rhizosphere (Witty 1979; van Berkum and Bohlool 1980; Boddey 1987; Giller 1987; Smercina et al. 2019). Nitrogenase has a different affinity for acetylene than N2, often outcompeting N2 when in the same system (Brouzes and Knowles 1973), making measures of acetylene reduction only a proxy for N-fixation rates. To overcome this a conversion factor is employed, typically assumed to be three, to estimate N-fixation rates (Hardy et al. 1968). However, this conversion factor can actually range from less than 1 to over 30 depending on the form of nitrogenase (i.e. whether it is the typical nitrogenase with a molybdenum cofactor, or an alternative nitrogenase with a vanadium, or iron cofactor; Bellenger et al. 2014). In a soil, or more specifically the rhizosphere, the potential diversity of N-fixing bacteria, including diverse physiology and different forms of nitrogenase, can have a large impact on the difference between acetylene reduction rates and actual FLNF rates that no single conversion factor can capture (Smercina et al. 2019).
In addition, the measured product of the ARA, ethylene, can be both produced and consumed in soils independent of actual acetylene reduction (van Berkum and Bohlool 1980; Zechmeister-Boltenstern and Smith 1998). Ethylene is a plant hormone with wide-ranging effects and is naturally produced in soils by both plants and bacteria (Witty 1979; Nohrstedt 1983; Wang et al. 2002; Friesen et al. 2011). In fact, a study which added 14C-labeled acetylene to the headspace of soil core incubations found that only 43% of recovered ethylene carried the label, indicating that over half of the ethylene was produced endogenously by soil organisms (Witty 1979). Methanogenic bacteria have been shown to oxidize ethylene via the enzyme methane monooxygenase (de Bont 1976; Boddey 1987; Xin et al. 2017) and though few recent studies have been published on the topic, our calculations of Gibb’s free energy for the oxidation of ethylene to ethylene oxide reveal a favorable and spontaneous reaction at room temperature (ΔG° = −81.4 kJ mol−1). Finally, acetylene may inhibit ethylene oxidation and actually result in overestimation of FLNF as endogenously produced ethylene is not consumed (Witty 1979; van Berkum and Bohlool 1980). Although concerns regarding ethylene production and consumption during the ARA have been raised previously, experimental controls that account for non-acetylene reduction associated ethylene fluxes are often lacking or misrepresentative, which leads to inaccurate estimates of acetylene reduction (Boddey 1987). Despite these issues, the ARA still remains the standard and most accessible method for assessing FLNF, but its use may be weakening our understanding of this process.
In order to truly understand the potential contribution of FLNF to total available N in natural and managed systems, it is important to use a method that measures FLNF directly and has been optimized to account for the diversity of N-fixing microbes and the wide range of environmental conditions they face. The ARA is an indirect method for assessing N-fixation that was originally designed and optimized for assessing symbiotic N-fixation by rhizobia in association with legumes. ARA is suited for measurement of symbiotic N-fixation because N is fixed at high rates so that measurement duration can be short and all rhizobia utilize the molybdenum cofactor form of nitrogenase under microaerobic metabolism. In contrast, the 15N2 incorporation method directly assesses N-fixation by measuring the difference in 15N abundance of samples exposed to 15N2 and reference samples (Boddey 1987; Warembourg 1993; Weaver and Danso 1994). This method is more accurate and direct than the ARA (Myrold et al. 1999), though not without potential issues, such as contamination via 15N-nitrate, ammonium, and nitrous oxide of the 15N2 gas source (Dabundo et al. 2014). This contamination results in assimilation of 15N unrelated to FLNF and can occur at rates greater than or equal to 15N2 incorporation, thus leading to overestimates of FLNF (Dabundo et al. 2014). While 15N2 incorporation has predominately been used to determine the conversion factor needed for calculating FLNF rates from acetylene reduction rates (Myrold et al. 1999), it has not been optimized to assess FLNF of whole soil communities.
Optimization of either ARA or 15N2 incorporation methods to assess FLNF requires consideration of the diversity in organisms and growth strategies of bacteria performing FLNF. In particular, there is need for a method that measures FLNF potential in an informative way, based on conditions which may be expected in the study system (e.g. rhizosphere). Previous studies have recommended providing glucose as a C source to optimize N-fixing conditions (Brouzes et al. 1969; Brouzes and Knowles 1973; Gupta et al. 2014; Roley et al. 2018), however the form of C which is optimal for FLNF likely varies by the organisms present (Smercina et al. 2019). Therefore, it is important to find a C source that can support whole diazotroph communities rather than specific populations. Potentially favorable C sources may include sucrose, malic acid or citric acid, all of which are used to isolate diazotrophs from the environment (Baldani et al. 2014), as well as mannitol and calcium lactate which have been used in previous studies when assessing FLNF (O’Toole and Knowles 1973; Patriquin and Knowles 1975). Additionally, soil niche spaces are extremely heterogenous with regard to water content, gas exchange and nutrient availability. This likely influences the diazotroph community composition and therefore, the optimal conditions for FLNF.
In this study, we optimize conditions which support the greatest potential acetylene reduction via the ARA and potential FLNF via the 15N2 incorporation method. The goal of this work was to identify conditions under which FLNF can be routinely measured, relatively quickly and easily, with robust results that capture differences in the diazotroph community inked to edaphic factors. Specifically, we choose target conditions which mimic those that may be expected in the rhizosphere to provide “realistic” potential rates. We assess different C sources, known to support diazotroph growth, to determine which supports the most N-fixation. FLNF is also influenced by oxygen availability with nitrogenase being irreversibly inhibited by oxygen presence (Robson and Postgate 1980). Yet, many diazotrophs cannot grow under strictly anaerobic conditions and anaerobic incubations may not be appropriate (Silvester et al. 1982; Smercina et al. 2019). Therefore, it is also necessary to determine optimal oxygen concentrations (O2) for FLNF. Another important aspect of these assays is incubation time. Though no single incubation time is standard for assessing N-fixation rates, shorter incubations should be more favorable for limiting changes in microbial community structure (Weaver and Danso 1994; Goldfarb et al. 2011; Oliverio et al. 2017). In this work, we investigate different incubation lengths to determine the shortest incubation time that yields detectable FLNF rates. Lastly, we test for issues with the ARA including background ethylene production and consumption to determine its suitability for soil and rhizosphere samples. Overall, we present an optimized method of assessing potential FLNF rates which attempts to account for and incorporate the wide diversity of diazotrophs and their growth requirements. This is extremely important if we want to systematically study FLNF across systems in order to better understand factors controlling diazotrophs and rates of FLNF.
Materials and methods
Sample collection
Soils were collected from two Michigan field sites, Lux Arbor (LUX; 42.476365, −85.451887) and Lake City (LC; 44.296098, −85.199612), with different soil texture, climate, and land use history (Table 1). These field sites are maintained as part of the Great Lakes Bioenergy Research Center’s marginal land experiment (https://www.glbrc.org/). Each field site has four replicate split plots of switchgrass (Panicum virgatum L.; cv. Cave-in-Rock) monoculture which have been maintained since 2013. Switchgrass is known to associate with diazotrophs (Bahulikar et al. 2014) and FLNF has been measured in association with the switchgrass rhizosphere (Roley et al. 2018; Smercina et al. unpublished data). Split plots are divided into fertilized (+ 56 kg urea-N ha−1 yr−1) and unfertilized (no added N) halves. We sampled both fertilized and unfertilized soils to allow us to optimize assay conditions for high and low N across different soil and climate conditions.
Because the ARA is relatively more accessible due to lower costs with regard to materials and analysis equipment, we conducted our initial optimization efforts using this method (described below). For ARA tests, soils were collected using a shovel to a depth of 10 cm from edges of switchgrass plots in July of 2016. Soils were also collected from plot edges in March of 2017 for comparison of ethylene production and consumption on fresh versus stored soils (see below). Soils were kept cool until returning to the lab where they were stored at 4 °C until analysis. Soils were sieved (4 mm mesh) and homogenized prior to analysis.
15N2 incorporation method optimization was conducted on intact soil cores collected using a 1.5 cm diameter turf corer to a depth of 5 cm (Fig. S1). Cores were collected in March of 2017 (incubation time test only, see below) and 2018 (C source and oxygen concentration tests, see below). These intact soil cores were sized to fit within 20 ml gas vials (Wheaton, DWK Life Sciences, Millville, NJ, USA), so the samples could be immediately transferred to their incubation vials at the time of collection in order to minimize soil disturbance between collection and analysis (Fig. S1). Ten replicate cores were collected from three randomly chosen points within each split plot for a total of 240 cores per field site. We also collected four reference cores, one per replicate block, per site. Cores were collected, immediately transferred to vials and placed in a cooler until returning to the lab. Cores were stored at 4 °C until analysis and all assays were started within 72 h of sample collection. Extra soil was also collected for measurement of soil moisture and water holding capacity (WHC; described below).
Soil moisture and water holding capacity
Soils were sieved (4 mm mesh) prior to soil moisture and WHC analysis. Soil moisture was determined on all soils (2016, 2017, and 2018) using 5 g of soil dried at 60 °C for at least 24 h. WHC was determined using 10 g of field moist soil. Soil was placed inside a funnel and saturated. Soils were covered with plastic wrap to prevent evaporation; three small holes were poked in the top of the plastic wrap to maintain atmospheric pressure within the funnel and flask. Soils were allowed to drain for 24 h, then weighed to determine 100% WHC.
Acetylene reduction
Optimization of the ARA was conducted in January 2017 on soils collected in July of 2016 (Hardy et al. 1968; Myrold et al. 1999). Soils were stored at 4 °C from time of collection to time of analysis, for a total of 5 months. While this storage time is likely to reduce microbial activity and therefore may reduce ARA rates, methodological comparisons between samples which have experienced similar conditions should not be hindered and are still valid for this methods study. Ten grams of sieved soils, collected in July 2016, were weighed into 4 oz. (127 ml) mason jars (Ball® Corp., Broomfield, CO, USA). Jars were loosely capped and pre-incubated at 25 °C for one week prior to analysis to revive the microbial community after long cool-storage. During the pre-incubation, 100 μg glucose C g−1 dry soil were added each day, a rate of C input matching that expected in the rhizosphere (Baudoin et al. 2003), to support the microbial community. At the start of the analysis, 4 mg C ml−1 glucose solution was added to the soils in a ratio of 1 ml solution to 1 g dry soil, matching the rate used by Gupta et al. (2014) in a similar study of FLNF in grasses. This rate of C addition results in the equivalent of ~570 μg of C per day over 7 days which is approximately 5x the amount which may be available in situ. Though greater than what might be expected in situ, these C additions ensure C limitation does not occur. After C addition, jars were then tightly sealed and 10% of the headspace was replaced with acetylene generated in the lab from additions of calcium carbide to nanopure water. Addition of the acetylene gas marked incubation start time. ARA optimization test variables included incubation time, oxygen concentration, C source, and checks of background ethylene production and consumption (Table 2).
To test incubation time, jars were sampled 1, 4, 6, 18, 24, and 168 (7 days) hours after incubation start for LUX soils and 6, 18, 24, and 168 h after incubation start for LC soils. At each time point, 10 ml of gas were withdrawn from each jar and injected into pre-evacuated 10 ml gas vials (Thomas Scientific, Swedsboro, NJ, USA). We used two jars per sample to ensure no more than three gas samples were taken from one jar. Thus, a total of 80 jars (5 replicates * 4 oxygen concentrations * 2 site * 2 jars) were used to measure ARA rates at each of the six time points representing a total of 40 samples. It is important to note that 30 ml of gas were removed at each sampling, without replacement. This represents a removal of ~24% of the total headspace. While this headspace removal does not affect the comparison of relative rates, as all samples were treated the same, measures of actual ARA may be negatively impacted. When headspace is removed and not replaced, resulting back pressure can cause an influx of air that dilutes ethylene and acetylene concentrations within the jars. When measuring actual ARA rates, researchers should be sure to replace the volume of sampled headspace with He or other appropriate gas to maintain pressure and account for the resulting dilution. Gas samples were analyzed for ethylene concentration on a Trace™ 1310 gas chromatograph equipped with a flame ionization detector (Thermo Fisher, Waltham, MA, USA). Acetylene reduction rates were calculated as the difference in ethylene gas concentration between each time point and the first sampling point divided by the incubation time (in days) and grams of dry soil (μg C2H4 g −1 dry soil day−1).
The effect of oxygen concentration on acetylene reduction was tested on replicate jars at 0, 5, 10, and 20% oxygen. These oxygen concentrations were chosen with consideration to soil and rhizosphere conditions, microbial growth optima, and N-fixation optima. Soil and rhizosphere oxygen concentrations are highly variable, making it difficult to mimic soil/rhizosphere conditions with just one oxygen concentration. For example, some rhizospheres are oxygen-rich (Pedersen et al. 1998; Blossfeld et al. 2011), while others are oxygen-depleted (Tschiersch et al. 2012; Minett et al. 2013). It is also difficult to optimize oxygen concentrations for microbial growth and N-fixation because nitrogenase is deactivated by oxygen, but many diazotrophs are aerobic organisms requiring at least some oxygen to grow (Bottomley and Myrold 2014). Therefore, the chosen oxygen concentrations span a range of potential oxygen optima from anaerobic to ambient. To create these concentrations, jars were evacuated via vacuum manifold and the headspace replaced with Ultra High Purity Helium (UHP-He). Then, 5.6, 11.2, and 22.4 ml of headspace gas were removed and replaced with identical amounts of pure O2 to create 5, 10, and 20% oxygen concentrations, respectively. No headspace gas was replaced with O2 in the 0% oxygen treatment. Finally, we replaced 10% of the jar headspace with acetylene. Gas samples were collected from jars at 1, 4, 6, 18, 24, and 168 h after acetylene addition and analyzed as described above. Each oxygen concentration was replicated five times and acetylene reduction rates were calculated as described above.
We also tested the impact of C source on acetylene reduction rates. A second set of LUX soil jars receiving the oxygen concentration treatments described above were duplicated. These soils received a C cocktail containing glucose, sucrose, and malic acid represented in equal proportions based on C content. These C sources were chosen based on recommendations for isolating diazotrophs from soils (Baldani et al. 2014) and all represent compound groups which are found in the rhizosphere (i.e. carbohydrates and organic acids; Baudoin et al. 2003). Though many studies only use glucose as a C source (Gupta et al. 2014), diazotrophs are a diverse community and are isolated using a wide variety of C sources. We attempted to simulate the diversity of C sources available in the rhizosphere using a mixture of C compounds. The 4 mg C ml−1 solution was added as described above and jars were incubated for 18 h. Gas samples were collected and analyzed as described above.
Lastly, we examined the potential for background ethylene production and consumption in our soils from LUX. Ten grams of soil collected in July 2016 (stored) or March 2017 (fresh) were weighed into ten replicate jars. Jars containing soil from July 2016 were pre-incubated as described above, while jars containing soil from March 2017 were pre-incubated at room temperature for 24 h prior to incubation start. After pre-incubation, a glucose solution was added as described above and jars were sealed. To test background production, five replicate jars were incubated without addition of acetylene gas. To test background consumption, 10% of the headspace in five replicate jars was replaced with an ethylene standard, but no acetylene was added. Gas samples were taken 6, 18, and 24 h and analyzed as described above. Results from these samples are reported as μg C2H4 g−1 dry soil.
15N2 Incorporation method
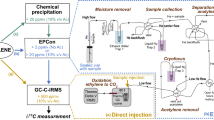
FLNF rates were measured based on net changes in 15N of soil incubated with 15N2 gas (Gupta et al. 2014). Cores were pre-incubated at room temperature for 24 h prior to 15N analysis to ensure a fully active microbial community at the time of 15N2 addition. Following pre-incubation, C source (or water) was added to each core using a syringe and 21-gauge needle to bring the cores to 60% WHC. The syringe and needle allowed the solution to be more evenly distributed along the intact core. Vials were then capped with aluminum rings and septa and evacuated. Immediately following evacuation experimental vials received 1 mL of 98 atom% acid-washed 15N2 gas (Sigma-Aldrich, Inc., St. Louis, MO, USA) and reference vials received UHP-N2. This results in a pN2 of ~1.87 atm, more than sufficient to saturate N-fixing sites. Knowles (1980) recommends achieving 0.8 to 1.0 atm pN2 and a minimum of 0.4 atm. 15N2 was acid washed with 5% sulfuric acid prior to addition to vials to ensure no contamination with 15N-NH3 and other gases. Following N2 addition, oxygen was added to the vials to achieve the appropriate concentration (see below). Vial atmospheres were balanced with UHP-He. Samples were incubated at room temperature for one, three, or seven days. After incubation, vials were uncapped and samples were placed in a 60 °C drying oven for 48 h. After drying, samples were ground into a fine powder on a roller mill, weighed into tin capsules, and then analyzed following standard procedures at Washington State University’s Stable Isotope Core Laboratory (Pullman, WA). Briefly, tinned samples were combusted to N2 with an ECS 4010 elemental analyzer (Costech Analytical, Valencia, CA) and analyzed on a Delta PlusXP continuous flow isotope ratio mass spectrometer (Thermofinnigan, Bremen) equipped with a 3 m GC column. FLNF rates were calculated in μg N fixed g−1 dry soil day−1 as:
where AEi represents atom percent access of sample against an unenriched reference sample, TNi represents total nitrogen content in sample, AEatm represents atom percent excess in the vial atmosphere (98 atom% in our case), and t is incubation time in days (Warembourg 1993; Roley et al. 2018).
15N2 Incorporation carbon source testing
We tested the impact of three C sources including sucrose, sucrose plus malic acid and a C cocktail, and a no-C (water) control on FLNF rates on intact cores collected in March of 2018 (Table 2). For these C source tests, we expanded on the ARA work by including both a no-C control and a 2-C source mixture in order to more thoroughly understand the role of C source in stimulating FLNF. In place of a glucose-only treatment, we chose to use sucrose as it is metabolized to glucose and is a favored C source for diazotrophs (Baldani et al. 2014). Our C cocktail contains glucose, sucrose, malic acid, and citric acid adjusted to a pH of 7 using potassium hydroxide. Each source in the C cocktail provides an equivalent amount of C (1 mg C g−1 dry soil). C sources were added at concentration of 4 mg C g−1 dry soil (Gupta et al. 2014). All solutions were added in a volume that brought soil moisture content to 60% water holding capacity.
15N2 Incorporation C solution pH
Checks of C solution pH revealed significant decreases in pH following addition of organic acids (malate and citrate). Solution pH when unbuffered was ~3.0 (data not shown). We wanted to test the impact on FLNF of adding buffered versus unbuffered C cocktail to soils. Thirty-six extra intact cores were sampled from fertilized halves of split-plots (9 cores per split-plot) at LUX. Samples were divided into three treatments (n = 12 per treatment): buffered C cocktail, unbuffered C cocktail, or water control. The pH of the buffered C cocktail was adjusted as described above, while unbuffered C cocktail pH was not altered. C cocktail or water was added to cores as described above and FLNF rates were assessed following the 15N2 incorporation method described above.
15N2 Incorporation oxygen concentration testing
We tested four oxygen concentrations including, anaerobic (0%), 5%, 10% and 20% on intact cores collected in March of 2018 (Table 2). UHP-O2 was added to evacuated vials following addition of N2 to bring atmospheric oxygen concentrations to one of the four concentrations above. Oxygen concentration tests were conducted on soils provided a C cocktail as the C source and were incubated for three days.
15N2 Incorporation incubation time testing
To determine how long samples should be exposed to 15N2, we tested incubation times of one, three, and seven days on intact cores collected in March of 2017 (Table 2). Longer incubations can result in large microbial community shifts which can bias results (Weaver and Danso 1994). Further, longer incubations increase the probability of fixed 15N being denitrified and thus lost from the soil. Alternatively, incubations that are too short may result in too little 15N being incorporated into the soil and thus not obtaining measurable differences between enriched and reference soils. Incubation test cores were provided C cocktail as the C source and received 10% oxygen.
Data analysis
Results from ARA testing were analyzed by one-way ANOVA followed by Tukey’s post hoc with test variable (i.e. incubation time, oxygen concentration, C source) as a fixed effect using the R stats package (R core team 2018). Where applicable, test variables were analyzed individually by site. Differences between test variable groups were considered significant at α ≤ 0.05. Ethylene production and consumption results were analyzed by one-way ANOVA followed by Tukey’s post hoc.
Results of 15N optimization tests were analyzed by two-way ANOVA with Tukey’s post hoc. Analysis was carried out by site with test variables (i.e. C source, oxygen concentration or incubation time) as a fixed effect and fertilizer treatment as a random effect nested within field block using the nlme R package (Pinheiro et al. 2018). Site was a significant factor (p < 0.05), therefore all analyses of test variable were carried out separately for each site. Fertilizer treatment was not significant, therefore we pooled results from all samples within a site such that each test variable group is represented by n = 24. Significant differences between test variable groups were considered significant at α ≤ 0.05. C cocktail buffering data was analyzed by one-way ANOVA with Tukey’s post hoc with buffering treatment as a fixed effect.
Due to differences in collection date and sample handling, no statistical analyses were performed comparing ARA and 15N2 results. We also did not perform statistical analyses comparing 15N2 results for intact cores collected in March 2017 to March 2018.
Results
Acetylene reduction
Optimization of the ARA included testing of incubation time, oxygen concentration, C source, and ethylene background controls. Acetylene reduction rates were measured at 1, 4, 6, 18, and 24 h and 7 days after incubation start (Fig. 1). Acetylene reduction rates did not differ significantly at LUX for the first 24 h. After 7 days, acetylene reduction rates were significantly greater than all other time points for LUX (P < 0.0001). There was a spike in rates after 6 h of incubation at LC. Acetylene reduction rates after 6 h and 7 days of incubation were not significantly different and were both greater than all other measured time points. Acetylene reduction rates were also tested under oxygen concentrations of 0, 5, 10, and 20%. Testing of O2 was done at all incubation times, but there was no relationship between oxygen concentration and acetylene reduction rates before 7 days of incubation. However, using a 7-day incubation, we found that acetylene reduction rates decreased steadily with increasing oxygen concentration with 0% having the greatest reduction rates and 20% have the lowest (Fig. 2). 10% oxygen concentration was not significantly different from 5% or 20%. C source tests were conducted at all tested oxygen concentrations over an 18 h incubation. Because there was no effect of oxygen concentration, we report averages across all oxygen concentrations. Acetylene reduction rates with glucose addition were very low, averaging ~0.5 μg C2H4 g−1 dry soil day−1 (Fig. 3). On average, rates were over 1700 times higher when C cocktail was added versus glucose alone (P = 0.00122). Lastly, we examined soil samples for background production or consumption of ethylene. Soils incubated without addition of acetylene still produced measurable concentrations of ethylene, and this was not significantly affected by incubation time (Fig. 4a). Incubations of soils that included additions of ethylene gas, but not acetylene demonstrated both production and consumption of ethylene, with significant differences in ethylene concentration over time (Fig. 4b). These changes in ethylene concentrations through time would equate to acetylene reduction rates ranging from 3.8 to 157.0 μg C2H4 g−1 soil day−1 or using 3 as a conversion factor, N-fixation rates of 1.3 to 52.3 μg N g−1 soil day−1.
– Acetylene reduction in μg ethylene (C2H4) g−1 dry day−1 rates at different incubation times (hours) for (a) LUX and (b) LC. Bars shows average acetylene reduction (n = 20) with standard error bars. Lowercase letters indicate significant differences between incubation times at p ≤ 0.05. Inset figure provides a zoom in on 1- and 4-h incubation time at LUX. Samples were incubated at 0, 5, 10, and 20% oxygen concentrations with glucose as a carbon source
– Acetylene reduction in μg ethylene (C2H4) g−1 dry soil day−1 rates at different oxygen concentrations (%) for LUX at 7-day incubation time point. Bars represent average acetylene reduction (n = 5) with standard error bars. Lowercase letters indicate significant differences at p ≤ 0.05. Samples were incubated for 7 days with glucose as a carbon source
- Acetylene reduction in μg ethylene (C2H4) g−1 dry soil day−1 rates with addition of different C sources. Bars represent average acetylene reduction (n = 20) with standard error bars. Lowercase letters indicate significant differences at p ≤ 0.05. Inset figure provides zoom in on glucose addition treatment. Samples were incubated for 18 h at 0, 5, 10, and 20% oxygen
– Background ethylene (a) production and (b) consumption of fresh and stored soil samples incubated with addition of ethylene standard. Bars represent average ethylene concentration in μg ethylene g−1 dry soil (n = 5) with standard error bars. Horizontal line represents initial ethylene concentration (206.8 μg ethylene g−1 dry soil; B only). Lowercase letters indicate significant differences at p ≤ 0.05
15N2 Incorporation carbon source
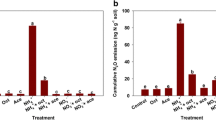
We tested the effect of four different C sources in the following combinations: sucrose, sucrose plus malate, and the C cocktail (glucose, sucrose, malic acid, and citric acid), on rates of FLNF over a 3-day incubation at 10% O2 relative to a no-C control. Additions of C cocktail stimulated the greatest FLNF at both field sites.
FLNF rates were significantly greater under additions of the C cocktail (P < 0.0001), than other C sources or no-C controls additions at LUX (Fig. 5a). C cocktail additions were 13.3x and 9.7x greater than sucrose (P < 0.0001) or sucrose plus malate (p < 0.0001) additions respectively. FLNF rates under the C cocktail treatment were 48.7x greater than the no-C treatment (P < 0.0001). There were no significant differences in FLNF rates between no-C controls and sucrose or sucrose plus malate treatments at LUX.
– FLNF rates measured via 15N2 incorporation method under additions of different C sources at (a) LUX and (b) LC. Bars represent average N-fixation in μg N g−1 dry soil day−1 (n = 24) with standard error bars. Lowercase letters indicate significant differences at p ≤ 0.05. Samples were incubated at 10% oxygen for three days
In LC soils, additions of C cocktail resulted in 7.96x greater FLNF than no-C controls (P = 0.00348) and 4.8x greater FLNF than sucrose plus malate (P = 0.01026; Fig. 5b). FLNF rates with C cocktail additions were 1.3x greater than for sucrose additions, but this increase was not significant. FLNF rates were not significantly different between sucrose, sucrose plus malate, and no-C treatments.
We also tested the importance of buffering the pH of the C cocktail before adding it to the soils. Checks of C cocktail solution pH revealed that the presence of malic acid decreased pH to acidic levels near 2–3 (data not shown). To test whether this pH change impacted FLNF rates, we measured the impact of buffered (pH ≈ 7.0) versus unbuffered (pH ≈ 3.0) C cocktail relative to no-C controls on FLNF for a subset of samples from LUX (n = 12 per treatment). Buffering the C cocktail resulted in 2.2x greater FLNF rates than unbuffered C cocktail (P = 0.04373) and 5.8x greater FLNF rates than no-C controls (P = 0.00185; Fig. 6). FLNF rates from unbuffered C cocktail additions were not significantly different from the no-C treatment (P = 0.4339).
15N2 Incorporation oxygen concentration
We tested the effect of four oxygen concentrations on FLNF rates ranging from anaerobic (0% oxygen) to ambient (20%) during a 3-day incubation with C cocktail additions. Optimal oxygen concentration varied between sites, with 10% oxygen being most favorable at LUX and 5% oxygen being most favorable at LC (Fig. 7).
– FLNF rates measured via 15N2 incorporation method under additions of oxygen concentrations (%) at (a) LUX and (b) LC. Bars represent average N-fixation in μg N g−1 dry soil day−1 (n = 24) with standard error bars. Lowercase letters indicate significant differences at p ≤ 0.05. Samples were incubated with carbon cocktail as the carbon source for three days
FLNF rates at LUX were greatest under 10% oxygen (P < 0.0001) followed by 20% oxygen (Fig. 7a). 10% oxygen concentrations resulted in 5.8x greater FLNF compared to 0% O2 (P < 0.0001) and 5.6x greater FLNF compared to 5% O2 (P < 0.0001). FLNF rates under 0% and 5% oxygen did not differ significantly from 20% oxygen.
5% oxygen resulted in significantly greater FLNF rates than all other test oxygen concentrations at LC (Fig. 7b). FLNF rates were 3x and 9.5x greater under 5% oxygen than 0% (P < 0.0001) and 20% (P < 0.0001) oxygen, respectively. 5% oxygen resulted in 39.3x greater FLNF rates than those measured under 10% oxygen (P < 0.0001) at LC. There were no significant differences in FLNF rates between 0%, 10%, or 20% oxygen concentrations.
15N2 Incorporation incubation time
We examined FLNF rates at three different incubation times (1, 3, and 7 days). FLNF rates were greatest after 7 days of incubation at both LUX (P < 0.0001; Fig. 8a) and LC (P < 0.0001; Fig. 8b). At LUX, a 7-day incubation resulted in 164x greater FLNF rates than a 1-day (P < 0.0001) incubation and 3.8x greater FLNF rates than a 3-day (P < 0.0001) incubation. Though a 3-day incubation resulted in 43.8x greater FLNF rates than a 1-day incubation at LUX, this difference was not significant (P = 0.194). At LC, a 7-day incubation resulted in 201.2x greater FLNF rates than a 1-day (P < 0.0001) incubation and 24.1x greater FLNF rates than a 3-day (P < 0.0001) incubation.
– FLNF rates measured via 15N2 incorporation method at different incubation times (days) at (a) LUX and (b) LC. Bars represent average N-fixation in μg N g−1 dry soil day−1 (n = 24) with standard error bars. Lowercase letters indicate significant differences at p ≤ 0.05. Samples were incubated with carbon cocktail as the carbon source and at 10% oxygen
Discussion
In this study, we optimized conditions for measurement of potential FLNF via 15N2 incorporation in bulk and rhizosphere soils. We also identified conditions which are optimal for measurement of potential acetylene reduction via the ARA. The 15N2 incorporation method is free of the issues associated with the ARA and is the most direct method of assessing N-fixation rates (Warembourg 1993). Our work illustrates several issues with the ARA; we found significant amounts of endogenous ethylene production and evidence of ethylene consumption in our soils, thus confirming past work which suggested these processes are at work in soil systems (Witty 1979; Nohrstedt 1983; Boddey 1987; Zechmeister-Boltenstern and Smith 1998). Ethylene production and consumption can complicate results of the ARA and the addition of ethylene positive and negative controls is often not adequate to account for these background processes (Witty 1979; van Berkum and Bohlool 1980; Nohrstedt 1983; Sloger and van Berkum 1988). Though we recommend avoiding use of the ARA, it may be preferred because it has lower cost and is relatively high-throughput with automated GC systems. The ARA may still be useful for identifying important drivers of FLNF at high temporal and spatial resolution if the proper controls are used and absolute quantification of FLNF or N-budgeting are not the primary research goals. ARA may also be appropriate for short-term measurements in simplified systems, but should be validated with the 15N2 incorporation method. If the ARA is used to assess potential rates of FLNF, researchers can counter some of the inherent issues with the ARA by using high replication, including both positive and negative control samples to account for background ethylene production and consumption, and using the optimized conditions we suggest for measuring the maximum acetylene reduction potential.
Optimization of the ARA and 15N2 incorporation included testing of incubation time. Because the ARA is highly sensitive, shorter incubation times are often recommended (Brouzes et al. 1971), however required incubation times may vary by study system (Myrold et al. 1999). Therefore, we chose to assess acetylene reduction across a variety of incubation times to determine which allowed us to measure potential FLNF most precisely. At LUX, there were no significant differences in incubation time until the 7-day sampling, while at LC 6-h and 7-day incubations resulted in the greatest acetylene reduction rates and 18-h and 24-h incubations had significantly lower rates. These results indicated that incubation time was an important determinant of potential FLNF rates and that optimal incubation time was likely site-dependent. Based on our study system, we found that longer incubation times (e.g. 7 days rather than the suggested range of 1 to 24 h) were needed to achieve consistently detectable results when using the ARA. However, it is important to note that use of long incubation times, particularly when using the ARA, can result in large overestimates of FLNF (Silvester et al. 1982). This is thought to be the result of de-repression by which inhibition of FLNF by acetylene causes bacterial N-deprivation which then stimulates increased FLNF activity or nitrogenase synthesis (Silvester et al. 1982; Rai et al. 1992). This lag in ARA activity may also result from a lag in diazotroph population growth as these organisms adapt to the incubation conditions (van Berkum and Bohlool 1980). Given this issue, we strongly encourage researchers to optimize incubation time for their own systems, reducing times whenever feasible.
FLNF rates measured via 15N2 incorporation were also greatest after 7 days of incubation. We were surprised to find that differences in FLNF rates between 7 days and 3 days were so great, with FLNF rates being approximately 4 and 24 times greater at 7 days than at 3 days for LUX and LC, respectively. This may indicate that after 7 days of incubation, diazotroph communities have shifted to a dominance of organisms well-suited to assay conditions. Others have shown that communities can change drastically over the course of these incubations (Weaver and Danso 1994; Goldfarb et al. 2011; Oliverio et al. 2017). Therefore, 7-day incubations, though demonstrating high FLNF rates, may not accurately predict the potential for the endemic diazotroph community to fix N.
At the other extreme, a 1-day incubation resulted in negligible incorporation of 15N into the soil with values in the pg N g−1 dry soil day−1 range. Though a 1-day incubation is likely to have the least changed microbial community, it does not appear to be long enough to ensure that 15N incorporation is great enough to be well above the background levels of a relatively large soil total N pool and well above measurement detection and sensitivity limits of the elemental analyzers and mass spectrometers routinely used for these analyses. Therefore, in our study system, a 3-day incubation is optimal, allowing for adequate incorporation of 15N label into the soil while minimizing changes to the microbial community. In systems with potentially high FLNF rates, such as tropical ecosystems (Reed et al. 2011), shorter incubation times may be feasible. However, in systems which are likely to have lower FLNF rates, such as tundra and temperate forests (Reed et al. 2011), incubation times of up to 7 days may be required. In these cases, researchers should acknowledge the potential for (or ideally, measure) microbial community shifts. In summary, as with the ARA, we again recommend optimization of incubation times for each unique study system.
We optimized oxygen concentration for the ARA and 15N2 incorporation by testing four concentrations ranging from anaerobic to ambient including 0, 5, 10, and 20% oxygen. Acetylene reduction rates were greatest under anaerobic conditions and decreased as oxygen concentration increased. This finding is generally supported by previous studies showing anaerobic conditions to be more favorable than ambient O2 concentrations for acetylene reduction of sieved soils (Chang and Knowles 1965; Brouzes et al. 1969; Brouzes et al. 1971). However, we were surprised that microaerobic conditions were not more favorable to ARA. Previous work has indicated that microaerobic conditions are optimal for supporting the diversity of oxygen needs within the diazotroph community (Brouzes et al. 1971; Silvester et al. 1982). These microaerobic conditions likely balance the need to protect nitrogenase from oxygen damage with aerobic growth. (Boyd et al. 2015; Smercina et al. 2019). There are several potential explanations for our results. First, acetylene can inhibit oxygen use in aerobic soils (Knowles et al. 1973), though acetylene can also reduce growth of some anaerobic diazotrophs and would likely also suppress N-fixation in our anaerobic incubations (Knowles et al. 1973). Second, it is also important to note that the ARA was performed on homogenized and sieved soils, therefore soil microsites were likely disturbed. Soil structure is a key factor influencing soil oxygen concentration (Tiedje et al. 1984) and microsites may be of particular importance in maintaining optimal oxygen concentrations for FLNF (Smercina et al. 2019). Anaerobic conditions may be more favorable for FLNF in disturbed soils because the microsites favorable to strict anaerobes and microaerophilic organisms may be exposed. Lastly, the ARA was performed on saturated soils which due to reduced oxygen diffusion tend to be oxygen depleted (Tiedje et al. 1984) and therefore more favorable to anaerobic organisms.
These results highlighted both the importance of maintaining soil structure and choosing an appropriate soil moisture content when assessing FLNF. Therefore, we chose to use intact cores and adjust to 60% WHC, an optimal moisture content for microbial activity (Linn and Doran 1984), for 15N2 incorporation optimization work. Following Weaver and Danso’s (1994) ARA in grass systems, we advocate for the removal of soil homogenization from N-fixation assay protocols, using instead intact soil cores, in order to maintain the rich variety of niche space and activity of a diverse array of N-fixing organisms. However, if researchers conduct work on homogenized soils or with the ARA, we recommend low oxygen concentration or anaerobic conditions be used. Researchers should also be advised that ARA conducted under low oxygen availability has been associated with FLNF de-repression, particularly for longer incubation times (Silvester et al. 1982; Rai et al. 1992).
With intact soil cores, we again tested the same four oxygen concentrations using the 15N2 incorporation method. We found that low, but not anaerobic oxygen concentrations are most favorable for potential FLNF when assessed on intact cores held at 60% WHC. Specific oxygen optima varied by site likely due to a variety of factors including differences in soil texture and soil moisture content (Tiedje et al. 1984). At LUX, potential FLNF was greatest around 10% oxygen while at LC 5% oxygen promoted the greatest potential FLNF. This may have been strongly influenced by soil texture and resulting integrity of intact cores from each site. While cores from LUX were likely to maintain their structure, cores from LC, where soils are sandier, frequently lost some structure once the C cocktail was added. As discussed above, loss of structure can expose microsites resulting in more exposure of anaerobic and microaerophilic diazotrophs to oxygen. Differences in oxygen optima may also be the result of differences in diazotroph community composition, though we did not assess community composition in this study. Researchers should consider evaluating how oxygen concentration and water content impact FLNF in their study system before choosing the values of these parameters used in their assays.
Finally, we tested how C source impacts potential FLNF rates. We found that supplying a C source with more C forms supports greater potential FLNF than C sources with fewer compounds regardless of the method used to measure FLNF. ARA results indicated that addition of C cocktail increased potential acetylene reduction rates compared to glucose only. Using the 15N2 incorporation method, we found C cocktail to increase potential FLNF by 8 to 48 times above no-C controls depending on site. It is not surprising that a C cocktail supports greater FLNF than single sources of C as diazotrophs are diverse and often isolated with C sources other than glucose (Baldani et al. 2014). Surprisingly, additions of a single C source (sucrose) did result in similar rates of FLNF to the C cocktail at LC. However, FLNF did not differ significantly between sucrose and no-C treatments at either site. In contrast, Roley et al. (2018) found additions of glucose solution to increase FLNF by nearly 5 times compared to no glucose additions. While this suggests that at some sampling times and sites one C source may sufficiently stimulate the microbial community, we recommended use of the C cocktail for both the ARA and 15N2 incorporation methods as it is likely to stimulate FLNF across a wide range of conditions and study sites. We also note that it is important to adjust the pH of the C cocktail solution as we observed a decrease in FLNF rates when unbuffered, acidic C cocktail was used versus buffered, neutral C cocktail. Unbuffered C cocktail may place selective pressure on the diazotroph community as both diversity and structure of diazotroph communities have been shown to be influenced by soil pH (Fan et al. 2018).
Lastly, our work adds to the growing body of evidence that FLNF occurs in many systems and can contribute largely to plant N demands (Bormann et al. 1993; Reed et al. 2011; Ladha et al. 2016; Roley et al. 2018). Up to of 48% of N demand for grasses such as maize, rice, and wheat were found to come from sources other than soil and fertilizer N (Ladha et al. 2016), and others have shown N-fixation can contribute significantly (> 50% in some cases) to maize N demands (Chalk 2016; Kuan et al. 2016). In switchgrass systems, like those studied in this work, Roley et al. (2018) found N deficits at upwards of 35–58 kg N ha−1 yr−1 and extrapolated FLNF rates indicated this process could meet 80 to 100% of the deficit. By extrapolating our average FLNF rates from μg N fixed g−1 dry soil day−1 to kg N ha−1 yr −1, we found FLNF at LUX has the potential to contribute 0.25 to 11.0 kg N ha−1 yr −1. These rates are up to 2x greater than the estimated contribution of N from symbiotic N-fixation in temperate grasslands (Reed et al. 2011) and meet approximately 19–31% of the N deficit identified by Roley et al. (2018). Extrapolation of FLNF rates at LC results in much lower annual N contributions from FLNF with potential contributions of only 0.04 to 0.35 kg N ha−1 yr −1. These large differences in potential N contribution from FLNF across sites only serve to highlight the need for better estimation of FLNF and better understanding of controls on this important N source. Although these extrapolated rates are based on optimized conditions for potential FLNF rates, they are still useful for estimating potential contribution of FLNF to the overall N budget, as is done with other potential N-cycle process rates (e.g. N-mineralization, nitrification, denitrification), with the understanding that budget numbers generated using these potential rates are likely overestimates.
Conclusions
As demonstrated in past work, we confirmed that issues with the ARA, such as background ethylene production and consumption, limit its applicability to measuring FLNF in soils. While we recommend avoiding use of the ARA, researchers could overcome some of its issues by including high sample replication and controls for background ethylene production and consumption. If ARA is chosen, we recommend longer incubation times, anaerobic conditions, and the addition of a C cocktail. In addition, we would caution against using the ARA results to estimate N budgets as it is not a direct measure of N-fixation or N accumulation, but of potential nitrogenase enzyme activity.
We strongly recommend that FLNF potential be measured using the 15N2 incorporation method and be conducted on intact soil cores. To ensure optimal conditions for FLNF across a wide variety of diazotrophs, we recommend use of a C cocktail containing a mixture of glucose, sucrose, malic acid, and citric acid, and a 3-day incubation to allow for adequate incorporation of the 15N label over the shortest incubation time. Researchers should consider testing incubation time in their own system as the time needed to achieve adequate 15N incorporation will vary by activity of diazotroph communities at each site. In some cases, incubation times may be reduced, thereby reducing changes to the microbial community during the incubation. We also recommend incubating samples at low oxygen concentrations (between 5 and 10%) as this promoted the greatest potential FLNF compared to ambient or anaerobic conditions in our study. However, because optimal oxygen concentration is variable across sites with different soil texture, we recommend that researchers optimize oxygen concentration for each new site tested.
The ARA has been used for decades and without proper positive and negative controls has likely hindered our ability to investigate FLNF across systems. While the ARA could be successfully employed in some cases – i.e. initial testing to reduce cost of optimization work, ultimately a more direct measurement method such as 15N2 incorporation and consideration of the optimal conditions for a given study system are needed to move our understanding of this important process forward.
References
Bahulikar RA, Torres-Jerez I, Worley E, Craven K, Udvardi MK (2014) Diversity of nitrogen-fixing bacteria associated with switchgrass in the native tallgrass prairie of northern Oklahoma. Appl Environ Microbiol 80(18):5636–5643
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431
Baudoin E, Benizri E, Guckert A (2003) Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol Biochem 35(9):1183–1192
Bellenger JP, Xu Y, Zhang X, Morel FMM, Kraepiel AML (2014) Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N 2-fixing bacteria in soils. Soil Biol Biochem 69:413–420
Blossfeld S, Gansert D, Thiele B, Kuhn AJ, Lösch R (2011) The dynamics of oxygen concentration, pH value, and organic acids in the rhizosphere of Juncus spp. Soil Biol Biochem 43(6):1186–1197
Boddey RM (1987) Methods for quantification of nitrogen fixation associated with gramineae. Crit Rev Plant Sci 6(3):209–266
Bormann BT, Bormann FH, Bowden WB, Piece RS, Hamburg SP, Wang D et al (1993) Rapid N^ 2 fixation in pines, Alder, and locust: evidence from the sandbox ecosystems study. Ecology 74(2):583–598
Bottomley PJ, Myrold DD (2014) Biological N inputs. Soil microbiology, ecology and biochemistry 3:365–388
Boyd ES, Costas AMG, Hamilton TL, Mus F, Peters JW (2015) Evolution of molybdenum nitrogenase during the transition from anaerobic to aerobic metabolism. J Bacteriol 197(9):1690–1699
Brouzes R, Knowles R (1973) Kinetics of nitrogen fixation in a glucose-amended, anaerobically incubated soil. Soil Biol Biochem 5:223–229
Brouzes R, Lasik J, Knowles R (1969) The effect of organic amendment, water content, and oxygen on the incorporation of 15N2 by some agricultural and forest soils. Can J Microbiol 15(8):899–905
Brouzes R, Mayfield CI, Knowles R (1971) Effect of oxygen partial pressive on nitrogen fixation and acetylene reduction in a sandy loam soil amended with glucose. Plant. Soil. (Special):481–494
Chalk PM (2016) The strategic role of 15 N in quantifying the contribution of endophytic N 2 fixation to the N nutrition of non-legumes. Symbiosis 69(2):63–80
Chang PC, Knowles R (1965) Non-symbiotic nitrogen fixation in some Quebec soils. Can J Microbiol 11(1):29–38
De Bont JAM (1976) Oxidation of ethylene by soil bacteria. Antonie Van Leeuwenhoek 42(1–2):59–71
Dabundo, R., Lehmann, M. F., Treibergs, L., Tobias, C. R., Altabet, M. A., Moisander, P. H., & Granger, J. (2014). The contamination of commercial 15N2 gas stocks with 15N–labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PloS one, 9(10), e110335.
Fan K, Weisenhorn P, Gilbert JA, Shi Y, Bai Y, Chu H (2018) Soil pH correlates with the co-occurrence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil Biol Biochem 121:185–192
Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, Martinez-Romero E (2011) Microbially mediated plant functional traits. Annu Rev Ecol Evol Syst 42:23–46
Giller KE (1987) Use and abuse of the acetylene reduction assay for measurement of “associative” nitrogen fixation. Soil Biol Biochem 19(6):783–784
Goldfarb KC, Karaoz U, Hanson CA, Santee CA, Bradford MA, Treseder KK et al (2011) Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front Microbiol 2:94
Gupta VVSR, Kroker SJ, Hicks M, Davoren CW, Descheemaeker K, Llewellyn R (2014) Nitrogen cycling in summer active perennial grass systems in South Australia: non-symbiotic nitrogen fixation. Crop and Pasture Science 65:1044–1056
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene–ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207
Kasmerchak, C.S., & Schaetzl, R. (2018). Soils of the GLBRC Marginal Land Expeirment (MLE) Sites. Zenodo. https://doi.org/10.5281/zenodo.2578238
Knowles R (1965) The significance of nonsymbiotic nitrogen fixation. Soil Sci Soc Am Proc 29(2):223
Knowles R (1980) Nitrogen fixation in natural plant communities and soils. Nitrogen fixation in natural plant communities and soils:557–582
Knowles R, Brouzes R, O'toole P (1973). Kinetics of nitrogen fixation and acetylene reduction, and effects of oxygen and of acetylene on these processes, in a soil system. Bulletins from the Ecological Research Committee, (17), 255–262
Kuan KB, Othman R, Rahim KA, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS One 11(3):e0152478
Ladha JK, Tirol-Padre A, Reddy CK, Cassman KG, Verma S, Powlson DS et al (2016) Global nitrogen budgets in cereals: a 50-year assessment for maize, rice, and wheat production systems. Sci Rep 6:19355
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils 1. Soil Sci Soc Am J 48(6):1267–1272
Minett DA, Cook PL, Kessler AJ, Cavagnaro TR (2013) Root effects on the spatial and temporal dynamics of oxygen in sand-based laboratory-scale constructed biofilters. Ecol Eng 58:414–422
Myrold DD, Reuss R, Klug MJ (1999) Dinitrogen fixation. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 241–257
Nohrstedt HÖ (1983) Natural formation of ethylene in forest soils and methods to correct results given by the acetylene-reduction assay. Soil Biol Biochem 15(3):281–286
O’Toole P, Knowles R (1973) Oxygen inhibition of acetylene reduction (nitrogen fixation) in soil effect of glucose and oxygen concentrations. Soil Biol Biochem 5:783–788
Oliverio AM, Bradford MA, Fierer N (2017) Identifying the microbial taxa that consistently respond to soil warming across time and space. Glob Chang Biol 23(5):2117–2129
Patriquin DG, Knowles R (1975) Effect of oxygen, mannitol and ammonium concentrations on nitrogenase (C2H2) activity in a marine skeletal carbonate sand. Mar Biol 32:49–62
Pedersen O, Borum J, Duarte CM, Fortes MD (1998) Oxygen dynamics in the rhizosphere of Cymodocea rotundata. Mar Ecol Prog Ser 169:283–288
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–137, URL: https://CRAN.R-project.org/package=nlme
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Rai AN, Borthakur M, Bergman B (1992) Nitrogenase derepresssion, its regulation and metabolic changes associated with diazotrophy in the non-heterocystous cyanobacterium Plectonema boryanum PCC 73110. Microbiology 138(3):481–491
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512
Robson RL, Postgate JR (1980) Oxygen and hydrogen in biological nitrogen fixation. Annual Reviews in Microbiology 34:183–207
Roley SS, Duncan DS, Liang D, Garoutte A, Jackson RD, Tiedje JM, Robertson GP (2018) Associative nitrogen fixation (ANF) in switchgrass (Panicum virgatum) across a nitrogen input gradient. PLoS One 13(6):e0197320
Silvester WB, Sollins P, Verhoeven T, Cline SP (1982) Nitrogen fixation and acetylene reduction in decaying conifer boles: effects of incubation time, aeration, and moisture content. Can J For Res 12(3):646–652
Sloger C, van Berkum P (1988) Endogenous ethylene production is a potential problem in the measurement of nitrogenase activity associated with excised corn and sorghum roots. Plant Physiol 88(1):115–118
Smercina DN, Evans SE, Friesen ML, Tiemann LK (2019) To fix or not to fix: controls on free-living nitrogen fixation in the rhizosphere. Appl Environ Microbiol 85(6):e02546–e02518
Tiedje JM, Sexstone AJ, Parkin TB, Revsbech NP (1984) Anaerobic processes in soil. Plant Soil 76(1–3):197–212
Tschiersch H, Liebsch G, Borisjuk L, Stangelmayer A, Rolletschek H (2012) An imaging method for oxygen distribution, respiration and photosynthesis at a microscopic level of resolution. New Phytol 196(3):926–936
Van Berkum P, Bohlool BB (1980) Evaluation of nitrogen fixation by bacteria inassociation with roots of tropical grasses. Microbiol Rev 44:491–517
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7(3):737–750
Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:s131–s151
Warembourg FR (1993) Nitrogen fixation in soil and plant systems. Nitrogen isotope techniques:127–156
Weaver RW, Danso SK (1994) Dinitrogen fixation. Methods of Soil Analysis: Part 2—Microbiological and Biochemical Properties, (methodsofsoilan2), 1019–1045
Witty JF (1979) Acetylene reduction assay can overestimate nitrogen-fixation in soil. Soil biology and biochemistry
Xin JY, Xu N, Ji SF, Wang Y, Xia CG (2017) Epoxidation of Ethylene by Whole Cell Suspension of Methylosinus trichosporium IMV 3011. Journal of Chemistry, 2017
Zechmeister-Boltenstern S, Smith KA (1998) Ethylene production and decomposition in soils. Biol Fertil Soils 26(4):354–361
Acknowledgments
We would like to thank all members of the Tiemann, Evans, and Friesen labs, including Steve Gougherty, Kristen Przano, Ethan Rocklin, and Expery Omollo, who helped to collect and process samples. We also thank Sarah Roley, three anonymous reviewers and K. Giller for thoughtful review and comments that greatly improved this manuscript. This work was conducted under the MMPRNT project, funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research award DE-SC0014108. This work was also funded in part by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research (DOE BER Office of Science DE-SC0018409 and DE-FC02-07ER64494).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Additional information
Responsible Editor: Euan K. James.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 136 kb)
Rights and permissions
About this article
Cite this article
Smercina, D.N., Evans, S.E., Friesen, M.L. et al. Optimization of the 15N2 incorporation and acetylene reduction methods for free-living nitrogen fixation. Plant Soil 445, 595–611 (2019). https://doi.org/10.1007/s11104-019-04307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04307-3