Abstract
Background and aims
Intraspecific aggregation of plant individuals can promote species coexistence by delaying competitive exclusions. However, such impacts may differ among species with contrasting spatial architecture and rely on the spatial distribution of resources.
Methods
We grew a phalanx clonal plant Carex neurocarpa (with aggregated ramets) and a guerilla one Bolboschoenus planiculmis (with diffused ramets) in monocultures or in 1:1 mixtures with an even or a clustered distribution pattern of the two species in homogeneous or heterogeneous soils.
Results
After 16 months, shoot biomass and ramet number were greater in mixtures than in monocultures in C. neurocarpa, but smaller in B. planiculmis. However, the growth of neither C. neurocarpa nor B. planiculmis differed between even and clustered mixtures. Soil nutrient heterogeneity did not significantly affect the growth of either species, but increased relative yield of B. planiculmis and decreased that of C. neurocarpa.
Conclusions
The relative importance of intra- vs. interspecific competition depends on the spatial architecture of plants, and soil nutrient heterogeneity slows down competitive exclusion by decreasing differences in competitive ability between plants. However, our results do not support the idea that intraspecific aggregation of individuals alters competitive interactions between species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraspecific aggregation of plant individuals is a common phenomenon in plant communities (Greig-Smith 1979; Herben and Hara 2003; Lara-Romero et al. 2016). It can result from limited seed or clonal dispersal (Bolker et al. 2003; Seidler and Plotkin 2006), competitive interactions (Bolker and Pacala 1999; Xue et al. 2013), environmental heterogeneity (Lara-Romero et al. 2016; Seabloom et al. 2005) and positive plant-soil feedbacks (Hartnett and Wilson 1999; O’Connor et al. 2002). Spatial aggregation of conspecific plant individuals has profound impacts on ecological patterns and processes. For instance, it may change light interception and water use (Mokany et al. 2008), soil nutrient accumulation (Derner and Briske 2001) and litter decomposition (Yu et al. 2011). Hence, spatial aggregation of conspecific individuals can alter the relative importance of intra- vs. interspecific competition, affecting species coexistence (Bolker et al. 2003; Houseman 2014; Lenssen et al. 2005; Stoll and Prati 2001; Tilman and Pacala 1993; Wassmuth et al. 2009). So far, studies testing the impact of spatial intraspecific aggregation of plant individuals have been mostly conducted in homogeneous environments (e.g. Lenssen et al. 2005; Monzeglio and Stoll 2008; Stoll and Prati 2001), without considering the inherent nature of environmental heterogeneity.
Natural environments are ubiquitously heterogeneous and essential resources for plant growth are commonly patchily distributed (Stuefer et al. 1996). A clonal plant may place more ramets in patches of higher resources by shortening inter-ramet distance (foraging strategy of a single clonal fragment, e.g. Dong et al. 2015; Peng et al. 2013; Slade and Hutchings 1987). A clonal plant may also increase the size of ramets in the higher resource patches by producing more leaves and roots, but show no change of inter-ramet distance (consolidate strategy of a singel clonal fragment, e.g. Alpert and Mooney 1996; Birch and Hutchings 1994; de Kroon and Schieving 1990; Lovett-Doust 1987). When several independent clonal fragments grow together, they may all sense and thus put more new ramets and/or increase ramet size in higher resource patches (foraging or consolidate strategies of several independent clonal fragments, e.g. Day et al. 2003; Fransen et al. 2001; Liu et al. 2017; Wang et al. 2016). Such responses, in turn, increase intraspecific aggregation (Lara-Romero et al. 2016; Maestre et al. 2003; Maestre and Cortina 2002; Seabloom et al. 2005), and may further alter competitive interactions between intra- and interspecific individuals (Lara-Romero et al. 2016; Maestre and Reynolds 2007; Monzeglio and Stoll 2008; Skaer Thomason and Rice 2017; Wijesinghe and Hutchings 1999). We therefore hypothesized that environmental heterogeneity will enhance the impact of intraspecific aggregation on competitive interactions between plant species. While many studies have tested the impacts of either environmental heterogeneity or spatial aggregation of intraspecific individuals on plant growth and competitive interactions, few have considered these two impacts simultaneously.
Plant species vary greatly in their spatial architectures and two contrasting spatial architectures have been identified for clonal plants, i.e., phalanx and guerilla (Lovett-Doust 1981). Clonal plants with a phalanx architecture produce no or short spacers connecting adjacent asexual individuals (ramets), so that ramets of the same genetic individual (genet) are spatially highly aggregated (Humphrey and Pyke 1998; Navas and Garnier 1990; Ye et al. 2006). By contrast, clonal plants with a guerilla architecture produce long spacers so that ramets of the same genet are widely spaced (Humphrey and Pyke 1998; Navas and Garnier 1990; Ye et al. 2006). Phalanx plants are expected to show advantages in acquiring local resources and thus may have competitive advantages in more crowded (with a higher spatial aggregation of individuals), homogeneous environments (Humphrey and Pyke 1998; Navas and Garnier 1990; Lopp and Sammul 2017; Saiz et al. 2016; Ye et al. 2006). By contrast, guerilla plants may have an advantage in exploiting open or high resource patches in heterogeneous environments through foraging (i.e. selective placement of ramets in high resource patches), but may benefit less in closed, homogeneous environments (Humphrey and Pyke 1998; Navas and Garnier 1990; Saiz et al. 2016; Sammul 2011; Ye et al. 2006). We are not aware of any studies that have tested simultaneously effects of environmental heterogeneity and spatial aggregation on the growth and competitive interactions of plants with contrasting spatial architectures. We hypothesized that impacts of environmental heterogeneity and intraspecific aggregation are different in guerilla and phalanx plants so that they alter competitive interactions between phalanx and guerilla plants.
To test our hypothesis, we grew a phalanx plant Carex neurocarpa and a guerilla plant Bolboschoenus planiculmis in monocultures and mixtures. In mixtures, the plants were grown either with an even or a clustered distribution pattern of the two species, and in either homogeneous soils or in heterogeneous soils consisting of high and low nutrient patches. Specifically, we addressed the following questions. (1) Does intraspecific aggregation of individuals affect the growth and competitive interactions of the two plants? (2) Does soil nutrient heterogeneity affect the growth and competitive interactions of the two plants with contrasting spatial architecture? (3) Is there an interactive effect of soil nutrient heterogeneity and intraspecific aggregation on the growth and competitive interactions of the two plants?
Materials and methods
Plant species
Both the phalanx clonal plant Carex neurocarpa Maxim. and the guerilla clonal plant Bolboschoenus planiculmis (F. Schmidt) T. V. Egorova (synonym: Scirpus planiculmis F. Schmidt) are perennial sedges of the Cyperaceae family (Chen et al. 1999). Carex neurocarpa is a tussock-forming clonal plant and produces very short rhizomes (inter-ramet distance <1 cm) and ramets of the same clone are closely spaced (Chen et al. 1999). In contrast, B. planiculmis forms long rhizomes (inter-ramet distance is up to 17 cm) so that ramets of the same clone are widely spaced (Chen et al. 1999). Rhizomes of B. planiculmis can branch intensively (Xue et al. 2013). Ramet height of C. neurocarpa is 0.2 to 1.0 m and that of B. planiculmis is 0.6 to 1.0 m. These two species are widely distributed and often coexist in wetlands in China (Chen et al. 1999).
Sampling and cultivation
On 15 June 2012, we collected more than 1800 ramets of C. neurocarpa and 1800 ramets of B. planiculmis from 20 natural communities along the north bank of Miyun reservoir in Beijing (40.533° N, 117.016° E). We then cut each ramet at 10 cm above shoot base and planted it in a small pot (10 cm in diameter) in an experimental garden (40.547° N, 117.010° E) a few kilometers away from the sampling places. After one month of cultivation, most of the ramets survived and produced new leaves. We then selected 864 similar-sized ramets of both C. neurocarpa and B. planiculmis and used them in the experiment described below. Initial biomass of the ramets was 0.132 ± 0.019 g (mean ± SE, n = 21) for C. neurocarpa and 0.119 ± 0.014 g (mean ± SE, n = 31) for B. planiculmis.
Experimental design
We pressed 48 wooden frames (50 cm wide × 50 cm long × 30 cm deep) into the soil to a depth of 25 cm in the experimental garden. The distance between adjacent frames was at least 0.5 m. The soil inside the wooden frames was removed and replaced with the experimental soil described below. Each frame was thereafter referred to as a plot.
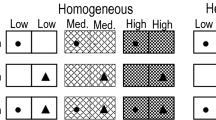
The experiment consisted of two levels of soil heterogeneity (homogeneous vs. heterogeneous) crossed with four levels of planting types (monoculture of C. neurocarpa, monoculture of B. planiculmis, an even mixture of C. neurocarpa and B. planiculmis, and a clustered mixture of C. neurocarpa and B. planiculmis; Fig. 1). There were eight treatments in total and six replicates (plots) in each treatment.
Schematic representation of the experimental design. The experiment consisted of four homogeneous and four heterogeneous treatments with ramets grown in monocultures or mixtures, with the two species planted evenly or in clusters. In monocultures, 36 ramets of Carex neurocarpa (phalanx) or Bolboschoenus planiculmis (guerilla) were planted at the cross-points of the patches within each frame, and in mixtures, 18 ramets of both species were intraspecifically segregated or aggregated within each frame. In the heterogeneous treatments, open and shaded patches represent patches with low and high nutrients, respectively. Black and open dots mark the positions where the ramets of C. neurocarpa and B. planiculmis were initially planted
In the heterogeneous treatments, each plot was divided into 49 equal patches (7.1 cm × 7.1 cm each) using a metal grid. Patches were filled with either an 1:1 (v:v) mixture of potting compost (total N: 13.39 g kg−1; total P: 6.34 g kg−1; total K: 24.45 g kg−1) and sand (total N: 0.23 g kg−1; total P: 1.01 g kg−1; total K: 22.34 g kg−1) (hereafter refer to as high nutrient soil) or an 1:9 (v:v) mixture of the compost and sand (hereafter refer to as low nutrient soil; Fig. 1). High and low nutrient soil patches were filled alternately. In total, 25 patches were filled with high nutrient soil (high nutrient patches) and 24 patches with the low nutrient soil (low nutrient patches) in each plot in the heterogeneous treatments. Thus, the high and low nutrient soils differed greatly in total N and total P. In the homogeneous treatments, the plot was also divided into 49 equal patches (7.1 cm × 7.1 cm), and in each patch, we filled a 25:24 (v:v) mixture of the high and low nutrient soils (Fig. 1). In this way, the total amount of nutrients per plot was the same in the homogeneous and the heterogeneous treatments. After filling the plots, we removed the metal grid so that roots could grow across patches. Before filling the plots with the soil mixtures, we placed, at the bottom of each plot, a piece of non-woven fiber (50 cm × 50 cm) which is widely used as rooting cloth to block roots from growing outside the plot but allow vertical movement of water.
We then planted ramets of C. neurocarpa and B. planiculmis at the cross-points of the patches within each plot (Fig. 1). In monocultures we planted 36 ramets of C. neurocarpa or B. planiculmis within a plot, and in mixtures we planted 18 ramets of both C. neurocarpa and B. planiculmis (Fig. 1). In even mixtures, ramets of the two species were planted in alternate positions (Fig. 1). In clustered mixtures, the 36 planting positions in a plot were divided into four clusters with nine planting positions each, and nine ramets of each species were planted within a cluster (Fig. 1). Thus, in even and cluster mixtures the 18 ramets of both C. neurocarpa and B. planiculmis were conspecifically segregated and aggregated, respectively (Fig. 1).
The experiment was maintained for 16 months (from 17 July 2012 to 4 November 2013). During the experiment, the mean precipitation from June to September was 329 mm in 2012 and 407 mm in 2013. Water was added to the plots when drought occurred in summer.
Harvest and measurement
Aboveground parts of each species were harvested at the end of experiment on 4 November 2013. We counted ramets of each species and harvested their aboveground shoots by cutting all plant material at ground level in each plot. For the guerilla plant (B. planiculmis) we counted ramets and harvested aboveground shoots in each type of soil patches (high vs. low nutrient patches) separately in the heterogeneous treatments. In the homogeneous treatments we also counted ramets of B. planiculmis and harvested the aboveground shoots in the same way as in the heterogeneous treatment, i.e. separately in patches that were located at the same places as the high and low nutrient soil patches in the heterogeneous treatment. As the ramets of the phalanx plant (C. neurocarpa) did not grow off the locations where it was planted, we harvested aboveground shoot biomass for this species in each plot but not separately for the two types of soil patches within each plot. Dry mass of all plant parts was determined after oven-dried at 70 °C for at least 48 h.
Data analysis
At the plot level, we first calculated shoot mass and ramet number per initial ramet of C. neurocarpa and B. planiculmis separately in each plot. Since the growth of the two species in the mixtures was not independent, we performed separate two-way ANOVAs to test the effects of soil nutrient heterogeneity (homogeneous vs. heterogeneous) and planting type (monoculture vs. even mixture vs. clustered mixture) on the growth measures of each of the two species at the plot level. Following ANOVA, planned contrasts were conducted to further separate the effect of planting type into the effect of competition type [intra- vs. interspecific competition, i.e. monoculture vs. (even mixture plus clustered mixture)] and the effect of intraspecific aggregation of plant individuals (even mixture vs. clustered mixture).
To directly examine the competitive interaction between the two species, we calculated relative yield of each species by dividing its shoot mass per initial ramet in each mixture (even or clustered mixture) by mean shoot mass per initial ramet in monocultures across the six replicates. We used two-way ANOVA to test the effects of soil nutrient heterogeneity and intraspecific aggregation of plant individuals (even mixture vs. clustered mixture) on relative yield of each of the two species separately.
At the patch level, we first calculated shoot mass and ramet number per initial ramet per patch of the guerilla clonal plant B. planiculmis in both types of soil patches within the plots. We performed three-way ANOVA with repeated measures to test the effects of soil nutrient heterogeneity, planting type and patch type (high vs. low nutrient patches) within the plots on the growth of B. planiculmis. Following ANOVA, planned contrasts were conducted to further separate the effect of planting type into the effect of competition type and that of intraspecific aggregation. In this analysis, patch type was treated as a repeated variable as data in the high and low nutrient soil patches in the same plot were not independent.
Before analysis, data of shoot mass and number of ramets at the plot level and data of shoot mass of B. planiculmis at the patch level were transformed to square root to improve normality and homoscedasticity. All analyses were performed with R (version 3.3.2; http://www.r-project.org) in RStudio (version 1.0.44; http://rstudio.org).
Results
Shoot mass and ramet number were greater in mixtures than in monocultures in C. neurocarpa (Table 1a; Fig. 2a-b), but smaller in B. planiculmis (Table 1b; Fig. 2c-d). However, intraspecific aggregation significantly affected the growth of neither C. neurocarpa nor B. planiculmis (Table 1; Fig. 2). Soil nutrient heterogeneity or its interactions with planting type did not significantly affect shoot mass or ramet number (Table 1; Fig. 2).
Relative yield was significantly greater in homogeneous than in heterogeneous soils in C. neurocarpa (Table 2a; Fig. 3a), but tended to be significantly smaller in B. planiculmis (Table 2b; Fig. 3b). Intraspecific aggregation of individuals or its interactions with soil nutrient heterogeneity did not significantly affect relative yield (Table 2).
There were significant effects of soil nutrient heterogeneity × patch type on the growth measures of B. planiculmis (Table 3). Shoot mass and ramet number of B. planiculmis were greater in the high than in the low nutrient patches in the heterogeneous soil treatments, but did not differ between the mirrored high and low nutrient patches in the homogeneous soil treatment (Table 3; Fig. 4).
Shoot biomass (a) and number of ramets (b) per initial ramet per patch of Bolboschoenus planiculmis (guerilla) in the high and low nutrient patches on homogeneous and heterogeneous soils. Mean values (± 1 SE) are given. The high and low nutrient soil patches in the homogeneous soil treatment represent the mirrored high and low nutrient patches at identical locations as those in the heterogeneous soil treatment
Discussion
Spatial architecture of plants can to some extent determine the uptake and the use of essential resources (Ikegami et al. 2009; Lopp and Sammul 2017; Nacry et al. 2013; Sammul 2011; Xie et al. 2014; Ye et al. 2006) and thus may affect competitive interactions between plant species (Humphrey and Pyke 1998; Liao et al. 2014; Lopp and Sammul 2017; Sammul 2011; Schmid and Harper 1985). Clonal plants can differ greatly in horizontal spatial architecture based on the distribution pattern of ramets of the same clone (Lovett-Doust 1981; Ye et al. 2006). Phalanx clonal plants show an aggregated distribution of ramets and are supposed to show competitive advantages when directly competing with other species (such as guerilla clonal plants with diffused distribution of ramets; Humphrey and Pyke 1998; Lopp and Sammul 2017; Navas and Garnier 1990; Saiz et al. 2016). By contrast, guerilla clonal plants show advantages to explore open areas by means of foraging to increase resource uptake in heterogeneous environments (Cahill and McNickle 2011; Dong et al. 2015; Lopp and Sammul 2017; Rajaniemi and Reynolds 2004; Sammul 2011; Xue et al. 2013). Thus, the relative importance of intra- vs. interspecific competition is expected to differ between phalanx and guerilla clonal plants (Humphrey and Pyke 1998; Navas and Garnier 1990). We indeed found that the phalanx clonal plant C. neurocarpa and the guerilla clonal plant B. planiculmis showed contrasting responses to intra- vs. interspecific competition, i.e. the growth of the C. neurocarpa was greater in mixtures than in monocultures, but that of B. planiculmis was the opposite. Our results thus provide support for the view that the spatial architecture of plants can affect the relative importance of intra- vs. interspecific competition and thus the competitive interactions between plant species (Humphrey and Pyke 1998; Navas and Garnier 1990; Saiz et al. 2016).
Individuals of many plant species are distributed in aggregation, and such intraspecific aggregation of individuals is expected to alter the competitive ability of plants (Hart and Marshall 2009; Lenssen et al. 2005; Monzeglio and Stoll 2005; Skaer Thomason and Rice 2017; Stoll and Prati 2001). However, our results did not show any evidence that spatial aggregation of conspecific individuals affected the growth and competition ability of the two clonal plants, even though several previous studies showed that intraspecific aggregation benefited weaker competitors (Hart and Marshall 2009; Lamošová et al. 2010; Stoll and Prati 2001; Wassmuth et al. 2009). Intraspecific aggregation of plant individuals can influence plant growth because it can alter the relative importance of intra- vs. interspecific competition and slow down the competitive exclusion process. However, the phalanx clonal plant C. neurocarpa produced much more biomass than the guerilla clonal plant B. planiculmis at harvest. The overwhelming dominance of the phalanx clonal plant may have covered the potential positive effect of intraspecific aggregation on the competitive performance of the guerilla clonal plant. Consequently, we did not detect any impact of intraspecific aggregation. Therefore, the weaker competitor may not benefit from spatial aggregation of conspecific individuals due to the overwhelming suppression by the stronger competitors.
As expected, soil nutrient heterogeneity had little impact on the growth of the phalanx clonal plant C. neurocarpa. Unexpectedly, however, soil nutrient heterogeneity did not affect the growth of the guerilla plant B. planiculmis at the plot level. Guerilla clonal plants are thought to be able to benefit from soil nutrient heterogeneity because they can selectively place more roots/ramets in high nutrient patches (Birch and Hutchings 1994; Dong et al. 2015; Zhou et al. 2012), and exchange resources between interconnected ramets in patches of different resource levels through clonal integration (Alpert 1991; Song et al. 2013; Wang et al. 2017). The absence of soil heterogeneity effects on the growth of the guerrilla clonal plant at the plot level could be due to the mismatch between patch size and inter-ramet distance. However, we did find increased shoot mass and ramet number of B. planiculmis in the high nutrient patches at the patch level (i.e. showing foraging responses; Birch and Hutchings 1994; Maestre and Reynolds, 2007; Rajaniemi and Reynolds 2004; Wijesinghe et al. 2001; Zhou et al. 2012), indicating that the guerilla clonal plant could respond to the heterogeneity treatment in our study. One possibility is that the benefits gained from foraging responses and resource integration may be offset by the presence of the conspecific and heterospecific competitors (Benot et al. 2013; Xue et al. 2013). At the end of the experiment, spaces were mostly occupied by the phalanx clonal plant, and hence only small patches of resource may remain. Thus, the effectiveness of exploiting resources for the guerilla clonal species may have decreased (Hutchings et al. 2003; Wijesinghe and Hutchings 1999; Xue et al. 2013). Despite that, we found that soil nutrient heterogenity increased the relative competitive ability of the guerilla clonal plant B. planiculmis and decreased that of the phalanx clonal plant C. neurocarpa. This result indicates that soil nutrient heterogeneity may delay the competitive exclusion process though equalizing the competitive ability of the competing species.
Environmental heterogeneity in resource supply may have different effects on the growth of plants when their individuals are arranged in different spatial patterns (i.e. intraspecific aggregation or not). This is because intraspecific aggregation of plant individuals may alter their intra- and interspecific competitions in communities and thus affect their responses to environmental heterogeneity (Damgaard 2010; Lara-Romero et al. 2016; Monzeglio and Stoll 2008). Unexpectedly, however, we did not find an interactive effect of soil nutrient heterogeneity and spatial aggregation of conspecific individuals on the performance of the phalanx or the guerilla clonal plant. Our results suggest that the responses of clonal plants to soil nutrient heterogeneity may not depend on the spatial patterns of the individuals.
We conclude that the relative importance of intra- vs. interspecific competition depends on the spatial architecture of plants, and soil nutrient heterogeneity can slow down the competitive exclusion through decreasing the relative difference in competitive ability between plants. However, our results do not support the idea that intraspecific aggregation of plant individuals can alter competitive interactions between species.
References
Alpert P (1991) Nitrogen sharing among ramets increases clonal growth in Fragaria chiloensis. Ecology 72:69–80
Alpert P, Mooney HA (1996) Resource heterogeneity generated by shrubs and topography on coastal sand dunes. Plant Ecol 122:83–93
Benot ML, Bittebiere AK, Ernoult A, Clement B, Mony C (2013) Fine-scale spatial patterns in grassland communities depend on species clonal dispersal ability and interactions with neighbours. J Ecol 101:626–636
Birch CPD, Hutchings MJ (1994) Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. J Ecol 82:653–664
Bolker BM, Pacala SW (1999) Spatial moment equations for plant competition: understanding spatial strategies and the advantages of short dispersal. Am Nat 153:575–602
Bolker BM, Pacala SW, Neuhauser C (2003) Spatial dynamics in model plant communities: what do we really know? Am Nat 162:135–148
Cahill JF, McNickle GG (2011) The behavioral ecology of nutrient foraging by plants. Annu Rev Ecol Evol Syt 42:289–311
Chen J-R, Z-Y W, Raven PH (1999) Flora of China. Science Press, Beijing
Damgaard C (2010) Intraspecific aggregation does not increase species richness in dune grasslands. J Ecol 98:1141–1146
Day KJ, John EA, Hutchings MJ (2003) The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Funct Ecol 17:454–463
de Kroon H, Schieving F (1990) Resource partitioning in relation to clonal growth strategy. In: van Groenendael J, de Kroon H (eds) Clonal growth in plants: regulation and function. SPB academic publishing, The Hague, pp 113–130
Derner J, Briske D (2001) Below-ground carbon and nitrogen accumulation in perennial grasses: a comparison of caespitose and rhizomatous growth forms. Plant Soil 237:117–127
Dong B-C, Wang J-Z, Liu R-H, Zhang M-X, Luo F-L, Yu F-H (2015) Soil heterogeneity affects ramet placement of Hydrocotyle vulgaris. J Plant Ecol 8:91–100
Fransen B, de Kroon H, Berendse F (2001) Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 82:2534–2546
Greig-Smith P (1979) Pattern in vegetation. J Ecol 67:755–779
Hart SP, Marshall DJ (2009) Spatial arrangement affects population dynamics and competition independent of community composition. Ecology 90:1485–1491
Hartnett DC, Wilson GW (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80:1187–1195
Herben T, Hara T (2003) Spatial pattern formation in plant communities. In: Sekimura T, Noji S, Ueno N, Maini PK (eds) Morphogenesis and pattern formation in biological systems-experiments and models. Springer Verlag, Berlin
Houseman GR (2014) Aggregated seed arrival alters plant diversity in grassland communities. J Plant Ecol 7:51–58
Humphrey LD, Pyke D (1998) Demographic and growth responses of a guerrilla and a phalanx perennial grass in competitive mixtures. J Ecol 86:854–865
Hutchings MJ, John EA, Wijesinghe DK (2003) Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology 84:2322–2334
Ikegami M, Whigham D, Werger MA (2009) Ramet phenology and clonal architectures of the clonal sedge Schoenoplectus americanus (Pers.) Volk. Ex Schinz and R. Keller. Plant Ecol 200:287–301
Lamošová T, Doležal J, Lanta V, Lepš J (2010) Spatial pattern affects diversity-productivity relationships in experimental meadow communities. Acta Oecol 36:325–332
Lara-Romero C, Cruz M, Escribano-Ávila G, García-Fernández A, Iriondo JM (2016) What causes conspecific plant aggregation? Disentangling the role of dispersal, habitat heterogeneity and plant-plant interactions. Oikos 125:1304–1313
Lenssen JPM, Hershock C, Speek T, During HJ, de Kroon H (2005) Experimental ramet aggregation in the clonal plant Agrostis stolonifera reduces its competitive ability. Ecology 86:1358–1365
Liao J, Tao M, Jiang M (2014) Spatial arrangements affect suppression of invasive Alternanthera philoxeroides by native Hemarthria compressa. Acta Oecol 59:46–51
Liu L, Dong B-C, Aplert P, Yu F-H (2017) Effects of soil substrate heterogeneity and moisture on interspecific competition between Alternanthera philoxeroides and four native species. J Plant Ecol 10:528–537
Lopp J, Sammul M (2017) The impact of timing of resource availability on clonal propagation of species with different growth forms. Folia Geobot 203:1–12
Lovett-Doust L (1981) Population dynamics and local specialization in a clonal perennial (Ranunculus repens): I. The dynamics of ramets in contrasting habitats. J Ecol 69:743–755
Lovett-Doust L (1987) Population dynamics and local specialization in a clonal perennial (Ranunculus repens). III. Responses to light and nutrient supply. J Ecol 75:555–568
Maestre FT, Cortina J (2002) Spatial patterns of surface properties and vegetation in a Mediterranean semi-arid steppe. Plant Soil 241:279–291
Maestre FT, Reynolds JF (2007) Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology 88:501–511
Maestre FT, Cortina J, Bautista S, Bellot J, Vallejo R (2003) Small-scale environmental heterogeneity and spatiotemporal dynamics of seedling establishment in a semiarid degraded ecosystem. Ecosystems 6:630–643
Mokany K, Ash J, Roxburgh S (2008) Effects of spatial aggregation on competition, complementarity and resource use. Austral Ecol 33:261–270
Monzeglio U, Stoll P (2005) Spatial patterns and species performances in experimental plant communities. Oecologia 145:619–628
Monzeglio U, Stoll P (2008) Effects of spatial pattern and relatedness in an experimental plant community. Evol Ecol 22:723–741
Nacry P, Bouguyon E, Gojon A (2013) Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370:1–29
Navas ML, Garnier E (1990) Demography and growth forms of the clonal perennial Rubia peregrina in Mediterranean vineyard and unmanaged habitats. J Ecol 78:691–712
O’Connor PJ, Smith SE, Smith FA (2002) Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol 154:209–218
Peng Y-K, Luo F-L, Li H-L, Yu F-H (2013) Growth responses of a rhizomatous herb Bolboschoenus planiculmis to scale and contrast of soil nutrient heterogeneity. Chinese J Plant Ecol 37:335–343
Rajaniemi TK, Reynolds HL (2004) Root foraging for patchy resources in eight herbaceous plant species. Oecologia 141:519–525
Saiz H, Bittebiere AK, Benot ML, Jung V, Mony C (2016) Understanding clonal plant competition for space over time: a fine-scale spatial approach based on experimental communities. J Veg Sci 27:759–770
Sammul M (2011) Length of the spacer rather than its plasticity relates to species distribution in various natural habitats. Folia Geobot 46:137–115
Schmid B, Harper JL (1985) Clonal growth in grassland perennials I. Density and pattern-dependent competition between plants with different growth forms. J Ecol 73:793–808
Seabloom EW, Bjørnstad ON, Bolker BM, Reichman OJ (2005) Spatial signature of environmental heterogeneity, dispersal, and competition in successional grasslands. Ecol Monogr 75:199–214
Seidler TG, Plotkin JB (2006) Seed dispersal and spatial pattern in tropical trees. PLoS Biol 4:e344
Skaer Thomason MJ, Rice KJ (2017) Spatial pattern and scale influence invader demographic response to simulated precipitation change in an annual grassland community. PLoS One 12:e0169328
Slade AJ, Hutchings MJ (1987) Clonal integration and plasticity in foraging behaviour in Glechoma hederacea. J Ecol 75:1023–1036
Song Y-B, Yu F-H, Keser LH, Dawson W, Fischer M, Dong M, van Kleunen M (2013) United we stand, divided we fall: a meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 171:317–327
Stoll P, Prati D (2001) Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 82:319–327
Stuefer JF, de Kroon H, During HJ (1996) Exploitation of environmental heterogeneity by spatial division of labour in a clonal plant. Funct Ecol 10:328–334
Tilman D, Pacala S (1993) The maintenance of species richness in plant communities. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities. University of Chicago Press, Chicago
Wang Y-J, Müller-Schärer H, van Kleunen M, Cai A-M, Zhang P, Yan R, Dong B-C, Yu F-H (2017) Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New Phytol 216:1072–1078
Wang Y-J, Shi X-P, Meng X-F, Wu X-J, Luo F-L, Yu F-H (2016) Effects of spatial patch arrangement and scale of covarying resources on growth and intraspecific competition of a clonal plant. Front Plant Sci 7:753.
Wassmuth BE, Stoll P, Tscharntke T, Thies C (2009) Spatial aggregation facilitates coexistence and diversity of wild plant species in field margins. Perspect Plant Ecol 11:127–135
Wijesinghe DK, Hutchings MJ (1999) The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale. J Ecol 87:860–872
Wijesinghe DK, John EA, Beurskens S, Hutchings MJ (2001) Root system size and precision in nutrient foraging: responses to spatial pattern of nutrient supply in six herbaceous species. J Ecol 89:972–983
Xie X-F, Song Y-B, Zhang Y-L, Pan X, Dong M (2014) Phylogenetic meta-analysis of the functional traits of clonal plants foraging in changing environments. PLoS One 9:e107114
Xue W, Huang L, Dong B-C, Zhang M-X, Yu F-H (2013) Patchy distributions of competitors affect the growth of a clonal plant when the competitor density is high. PLoS One 8:e78221
Ye X-H, Yu F-H, Dong M (2006) A trade-off between guerrilla and phalanx growth forms in Leymus secalinus under different nutrient supplies. Ann Bot 98:187–191
Yu F-H, Schütz M, Page-Dumroese DS, Krüsi BO, Schneller J, Wildi O, Risch AC (2011) Carex sempervirens tussocks induce spatial heterogeneity in litter decomposition, but not in soil properties, in a subalpine grassland in the central alps. Flora 206:373–379.
Zhou J, Dong B-C, Alpert P, Li H-L, Zhang M-X, Lei G-C, Yu F-H (2012) Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides. Ann Bot 109:813–818
Acknowledgements
We thank Rui Zhu, Bi-Cheng Dong, Pu Wang, Yong-Yang Wang, Jia-Yuan Li, Yong-Hong Gao and Xing-Xing Jiang for assistance with the experiment, and Jasper van Ruijven and two anonymous reviewers for valuable comments on the earlier version of the manuscript. This work was supported by NSFC (31570413 and 31761123001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeff R. Powell.
Rights and permissions
About this article
Cite this article
Xue, W., Huang, L., Yu, FH. et al. Intraspecific aggregation and soil heterogeneity: competitive interactions of two clonal plants with contrasting spatial architecture. Plant Soil 425, 231–240 (2018). https://doi.org/10.1007/s11104-018-3578-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3578-9