Abstract
Background and aims
Roots of the lowest branch orders have the highest mortality rate, and may contribute predominately to plant carbon (C) and nutrient transfer into the soil. Yet patterns and controlling factors of the decomposition of these roots are poorly understood.
Methods
We conducted a two-year field litterbag study on different root orders and leaf litter in four temperate and four subtropical tree species.
Results
Five species showed slower decay rates in lower- (order 1–2) than higher-order (order 3–5) roots, and all species showed slower decay rates in lower-order roots than leaf litter. These patterns were strongly related to higher acid-insoluble fraction in lower- than higher-order roots, and in roots than in leaf litter, but were unrelated to initial N concentration. Litter N was predominantly in recalcitrant forms and limited amount of N was released during the study period;only 12 % of root N and 26 % of leaf litter N was released in 2 years.
Conclusions
We conclude that the slow decomposition of lower-order roots may be a common phenomenon and is mainly driven by their high acid-insoluble fraction. Moreover, litter N, especially root N, is retained during decomposition and may not be available for immediate plant uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decomposition of dead organic matter is a major pathway of energy consumption and a primary driver of nutrient cycling in ecosystems. During the decomposition, organic compounds are converted into CO2 and inorganic nutrients, which are taken up by microbes and plants. Litter decomposition has been a topic of numerous studies since 1920s given its importance (Berg and McClaugherty 2008). More recently, decomposition has drawn increasing attention under the context of global change because plant litter plus soil organic matter (SOM) are the largest carbon (C) pool in terrestrial ecosystems and small changes in this C pool will have profound effects on atmosphere CO2 (Gholz et al. 2000; Davidson and Janssens 2006).
Far less studies on decomposition have been focused on roots than on leaf litter (Silver and Miya 2001). This is in part because leaf litter represents a large flux of C and nutrients from plants to soil. However, an increasing number of studies suggest that fine roots may represent a more important pathway of stable SOM accumulation than leaf litter. First, roots are decomposed within the soil and are more apt to physical protection in aggregates and to physical-chemical association with clay minerals, two important stabilizing mechanisms of SOM (Rasse et al. 2005; Crow et al. 2009; Prescott 2010; Kätterer et al. 2011). In contrast, leaf litter may largely decompose on the soil surface and only limited amount of material from initial litter enters the soil (Prescott 2010; Wang et al. 2010). Moreover, recent findings showed that root-derived materials were transformed to stable SOM fractions such as humin and humic to a greater extent compared with leaf litter (Bird et al. 2008; Mambelli et al. 2011). Therefore, root mortality and decomposition may be critical processes determining accumulation of stable SOM.
While studies of root mortality and decomposition are not uncommon, most studies of root mortality have not considered functional heterogeneity within the fine root system (<2 mm in diameter). Tree fine root systems have a complex branching structure, with only the smallest distal branching orders mainly serving water and nutrient uptake functions (Guo et al. 2008a, b; Valenzuela-Estrada et al. 2009). Moreover, these distal absorptive roots are the ones that turn over rapidly and may make up the majority (i.e. 70 %) of root production and turnover despite their small biomass (Wells and Eissenstat 2001; Guo et al. 2008b; Xia et al. 2010). Therefore, the decomposition of these ephemeral roots should be the primary pathway of C and nutrient input from roots to the soil.
So far, only two studies have examined root decomposition by branch order and both have shown that short-lived lower-order roots (first and second orders) decomposed more slowly than roots with slower turnover rates (i.e. third or fourth order roots with wood development) despite a lower C : N ratio in lower-order than higher-order roots (Fan and Guo 2010; Goebel et al. 2011). More evidence is clearly needed for validating the commonality of these patterns, and the potential mechanisms have yet to be identified to explain the slow decomposition of lower-order roots. One explanation is that the un-lignified, nutritious lower-order roots especially root tips are faced with a greater herbivore pressure (mainly insects and nematodes) than higher-order roots and are likely to produce more defensive secondary metabolites and thus decrease root tissue quality (Seastedt and Murray 2008). Indeed, legumes which potentially suffer from severe herbivore due to high tissue N concentration usually produce N-rich defensive chemicals such as alkaloids (Coley et al. 1985). Moreover, lower-order roots are usually heavily colonized by mycorrhizal fungi, which produce fungal chitin and increase defense-related secondly antifungal metabolites in roots, both slowing root decomposition (Langley et al. 2006; Guo et al. 2008a), although recent evidence showed that chitin may not be recalcitrant thus slow decomposition of lower order roots may not be related to fungal chitin (Fernandez and Koide (2012). Nonetheless, lower-order roots have been shown to have higher concentrations of acid-insoluble compounds (a coarse-scale but integrated measure of possibly defensive compounds) compared with higher-order roots (Hendricks et al. 2000; Guo et al. 2004; Fan and Guo 2010), consistent with the defence hypothesis.
Here we studied root and leaf litter decomposition for 2 years in a temperate and a subtropical forest in China with two coniferous and two broad-leaved tree species in each forest. We hypothesized that lower-order roots would decompose more slowly than higher-order roots and this pattern is resulted from higher acid-insoluble fractions (AIF) (some of which might be N-rich defensive compounds) in lower-than higher-order roots. The higher AIF may indicate lower C quality, thus slows down the overall decomposition process because in the early stage of decomposition when C concentration (quantity) is high, C quality may be the main control of the rate of microbial C use. We also tested the relative importance of litter C quality (as represented by AIF) vs. initial N concentrations in mediating decay rates and N release patterns by taking advantage of the unique chemistry in roots of different branch orders that lower-order roots have both higher N concentrations and higher AIF whereas in most other tissues (e.g., leaf litter) N concentrations and AIF (sometimes termed as “lignin”) are negatively related.
Materials and methods
Site description
The temperate forest site was located at Maoershan Forest Research Station (45°23′N, 127°32′E) of Northeast Forestry University, Heilongjiang, China. Mean annual, July and January temperatures were 2.8°C, 20.9°C and −19.6°C, respectively. Mean annual precipitation was 723 mm, mostly (about 477 mm) occurring from June to August. The soils are loamy Hap-Boric Luvisols that exceed 50 cm in depth and have more than 10 % of organic matter content in the upper 10 cm layer. Soil pH was between 6.0 and 6.5 in top 0–10 cm. At this site, four 21-year-old monoculture plantations were selected for our litter collection and root sampling: Pinaceae Larix gmelinii, Pinaceae Picea koraiensis, Rutaccac Phellodendron amurense and Juglandaceae Juglans mandshurica. All species were dominant species in natural forest communities in the area. Among them, Larix gmelinii and Picea koraiensis formed ectomycorrhizal (EM) associations and the two broad-leaved species were associated with arbuscular mycorrhizal (AM) fungi (Guo et al. 2008a). Detailed site information was given in Wang et al. (2006).
The subtropical study site was located at Heshan Forest Research Station (22°41′N, 112°54′E) of South China Botanical Garden, the Chinese Academy of Sciences. Mean annual, July and January temperatures were 21.7°C, 28.7°C and 13.1°C, respectively. Mean annual precipitation was 1801 mm with a dry (October to March) and wet season (April to September). The soils are Acrisols with a texture of sandy clay loam developed from sandstone. Soil pH is about 4.0 and soil organic matter content is around 2.0 % in top 0–10 cm. Monoculture plantations of Pinaceae Pinus massoniana, Taxodiaceae Cunninghamia lanceolata, Theaceae Schima wallichii and Myrtaceae Eucalyptus citriodora were selected. Despite being exotic, Eucalyptus citriodora plantation is common in this area and the other three species are dominant species in natural communities. Of the four species, Pinus massoniana and Cunninghamia lanceolata are conifers and the other two are broad-leaved species. Schima wallichii and Cunninghamia lanceolata formed AM associations whereas the other two species formed EM associations (DL Guo, unpublished data). All plantations were 24 years old and detailed site information is given in Li et al. (2001).
Root and leaf material preparation
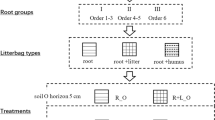
In late April and May of 2007, we sampled fine root branching segments from the litter layer and the upper mineral soil under four individual trees from each of the eight monoculture plantations listed above by excavating intact soil blocks of 30 cm × 30 cm × 20 cm (depth) and then carefully separated lateral root branches from the soil. The sampled roots were transported to the lab within 2 h and preserved at −20°C until further processing. Fine root branch orders were classified following the method of Pregitzer et al. (2002): the root tips were defined as first order; the root from which two first-order roots branched was classified as second order, and so on. For each species, fine root segments were gently washed and brushed free of soil and dissected into 5 orders (first to fifth orders). The root samples were then dried in the air and preserved at 20°C for 2 months. All fresh leaf litter was collected in October from each plantation using litter traps and then air dried for 20 days.
Litterbag incubation, harvest and analyses
An equivalent of 1.000 g dry weight of air-dried root samples or leaf litter was placed in each nylon bag (10 cm × 10 cm). The root bags were 0.1 mm meshed on both sides whereas leaf bags were 1 mm meshed on the top side and 0.1 mm meshed on the bottom side as designed in Gholz et al. (2000). The mesh of 0.1 mm in root bags would undoubtedly exclude earthworms and microarthropods, which have been reported to play an important role in leaf litter decomposition in wet tropical forests (Gonzalez and Seastedt 2001; Liu and Zou 2002; Hättenschwiler and Bracht Jørgensen 2010). However, lower-order roots often had diameters less than 0.3 mm, and a fine mesh had to be used to prevent significant loss from litterbags. To test potential influence of mesh size on decomposition rates, we incubated intact root branches consisting of first three branch orders in root litterbags with a 1 mm mesh, and we did not find significant effects of mesh size on decomposition rates. Our field observations also suggested that even a mesh size of 3 mm could not fully account for faunal effects on decomposition because all kinds of mesh appeared to constrain movement and activity of soil fauna. Thus, our study focuses mainly on microbial decomposition of litter.
Litterbags were incubated in the original plantation of each species for 2 years (only 1 year for Juglans, Schima and Eucalyptus as insufficient root mass did not allow for longer incubation time). Root litterbags were horizontally incubated at 5 cm depth of the mineral soil and leaf litterbags were incubated on the soil surface (under the litter layer if litter existed). There were 600 root litterbags and 120 leaf litterbags in total. An additional set of three replicates of each litter type for each species was assigned for initial chemistry analyses.
Litterbags were retrieved after 152 days, 259 days, 367 days, 516 days and 747 days for subtropical species, and after 203 days, 274 days, 357 days, 566 days and 725 days for temperate species. Four litterbags for each litter type of each species were collected in each harvest. After the collection, attached soil and in-growing roots were carefully removed from the litterbags and the remaining litter was dried at 65°C for 72 hours and then weighed.
Litter initial total C and N concentrations were determined with a CHN elemental analyzer (model 2400 II, PerkinElmer, USA). Acid-insoluble C fraction (AIF) was determined by sulfuric acid digestion method (Ryan et al. 1990) following extraction with chloroform-methanol (Gessner and Neumann 2005). Specifically, a milled powder sample of 0.2–0.3 g was extracted with 15 ml of chloroform-methanol (v/v = 2/1) for 2 h and then filtered. The residue after extraction was digested with 15 ml of sulfuric acid (72 %) for 3 h, then filtered through a cellulose acetate membrane (0.45 μm) with a vacumm pump and repeatedly washed with de-ionized water during filtration until pH was 7, and lastly the residue was oven-dried and weighed. The weight of the residue after acid digestion subtracted by ash in the residue was the weight of acid-insoluble fraction (AIF), and AIF concentration was calculated by dividing the weight of AIF with the ash-free weight of initial sample. AIF is considered to be comprised of lignin and other recalcitrant structures like condensed tannins, cutin and suberin, and was difficult for microbes to decompose (Ryan et al. 1990). A weight of 3–5 mg of the residue after acid digestion was also measured with the CHN elemental analyzer for N concentration to calculate the proportion of litter total N found in AIF. The proportion of litter total N found in AIF should provide an estimate of recalcitrant N because AIF is usually considered recalcitrant. Ash content in whole litter or in the residue after acid digestion was measured after combusting 15–40 mg of sample at 550°C for 4 h. All litter initial chemistry indices were expressed on an ash-free dry mass basis.
Statistical analyses
The first two root orders generally showed similar patterns of decomposition but were distinct from third to fifth orders, so we separated five root branch orders into two groups: lower-order roots, or first two branch orders (R1–2), and higher-order roots, or root order third to fifth (R3–5). Significance of litter mass remaining and initial N remaining was tested between R1–2 and R3–5 within each species with an analysis of covariance (ANCOVA) with decomposition time as the covariate. A linear model was used to determine litter decay constants (k values) of leaf litter and roots of R1–2 and R3–5 because our data were better fitted by the linear model than by negative exponential decay model. The linear model was y = 100-kt, where y is the percentage of initial mass remaining at time t (days) and k is the decay constant. Differences of k values between R1–2 and R3–5 across species were compared by paired-samples t test with k values of R1–2 and R3–5 of each species as a pair. Two-way ANOVAs were conducted to compare the differences of initial AIF%, N%, C : N ratio and the proportion of total N found in AIF between root orders and among species. Regression analyses were conducted on root and leaf litter k values and initial N remaining (after 1 or 2 years of decomposition) against initial litter chemistry. Leaf litter mass remaining, initial N remaining and decay constants were not directly compared with those of roots in statistical analysis because of their different decomposition environments.
Results
Litter decay rate and N release pattern
In five out of eight species studied here, mass remaining was significantly higher in the lower- than higher-order roots during decomposition (Eucalyptus, Schima and Juglans decomposed for 1 year and the other five species decomposed for 2 years), indicating the slower decomposition of lower-order roots (P < 0.05 or < 0.0001; Fig. 1a, c, e, f and g). Mass remaining did not differ significantly between order classes in Eucalyptus and Schima (Fig. 1b and d), and was marginally significantly lower in higher-order roots (R3–5) than in lower-order roots (R1–2) in Phellodendron (P = 0.07; Fig. 1h). Across all species, R1–2 had significantly lower decay constants (k) than R3–5 by paired-samples t test (P < 0.05) (Table 1). Leaf litter had lower mass remaining than roots throughout the experiment in all species except Picea, in which leaf litter decomposed more slowly than higher-order roots (R3–5) (Fig. 1; Table1).
Mass remaining (%) during 2 years of decomposition for roots of different branch orders and leaf litter. R1–2 refers to root orders 1 to 2, and R3–5 refers to root orders 3 to 5. Error bars represent ± SE (n = 4 for leaf, 8 for R1–2 and 12 for R3–5). ANCOVA analyses were conducted to compare the difference between root orders in each species with decomposition time as the covariate
Fraction of initial N remaining was significantly higher in R1–2 than in R3–5 in Cunninghamia and Pinus, but the opposite pattern was found in Larix and Phellodendron (P < 0.01; Fig. 2a, c, e and h). No significant difference of fraction of initial N remaining between root orders was found in the remaining four species. Unlike the consistent pattern of continuous mass loss of all litter, N was accumulated in some litter types of all species except Phellodendron after 1 year or even 2 years of decomposition (Fig. 2). Phellodendron had net N release in both roots and leaf litter. Fraction of initial N remaining was correlated with mass remaining (P < 0.001; data not shown). Additionally, N release was much lower than mass loss. Averaged across species, only 0.2 % and 12 % of root N was released after 1 and 2 years of decomposition, respectively, compared with 28 % and 39 % of root mass loss. Moreover, an average of 5.7 % and 26 % of leaf N was released after 1 and 2 years of decomposition, respectively, compared with 43 % and 58 % of leaf litter mass loss.
Fraction of initial N remaining (%) during 2 years of decomposition for roots of different branch orders and leaf litter. R1–2 refers to root orders 1 to 2, and R3–5 refers to root orders 3 to 5. Error bars represent ± SE (n = 4 for leaf, 8 for R1–2 and 12 for R3–5). ANCOVA analyses were conducted to compare the difference between root orders in each species with decomposition time as the covariate
Initial litter chemistry
N concentrations were significantly higher in lower-order roots (R1–2) than in higher-order roots (R3–5) across all species (Tables 2 and 3; P < 0.0001). Leaf litter N concentrations were lower than root N concentrations in Pinus, and were between R1–2 and R3–5 for other species. C concentrations were similar in all litter types for all species (around 51 %, data not shown), and litter C : N ratios depended largely on N concentrations and showed an opposite trend to that of N concentrations (Tables 2 and 3).
Overall, acid insoluble fraction (AIF) concentration was significantly higher in lower-order roots than in higher-order roots across species, but an interactive effect between species and root order was found as shown by the opposite pattern of Schima compared to other species (P < 0.0001; Tables 2 and 3). Leaf litter had lower AIF concentrations than roots in all species (Table 2). The proportion of total N found in AIF was 59 % on average of all litter types in all species, and was positively correlated with AIF concentration (P < 0.0001; R2 = 0.741; data not shown). Lower-order roots had higher proportions of total initial N found in AIF in Larix, Picea, Phellodendron and Pinus, but the opposite trend was found in Cunninghamia and Eucalyptus (Tables 2 and 3).
Relations between litter decay rate or N release rate and initial litter chemistry
Decay constants (k values) of lower-order roots (R1–2) were negatively related to litter initial AIF concentrations across all species (P < 0.0001; R2 = 0.874; Fig. 3), but k values of higher-order roots (R3–5) or leaf litter were not related to initial AIF concentrations (data not shown). When all roots and leaf litter were pooled, k values were negatively related to initial AIF concentrations (P < 0.0001; R2 = 0.673; Fig. 3). No significant correlations were observed between root or leaf k values and initial C : N ratio or between k values and initial N concentrations (data not shown).
Regression relation between root and leaf litter decay constants (k values) and litter initial acid-insoluble fraction (AIF) concentrations across all species. Significant correlations (P < 0.0001) were found in lower-order roots (R1–2) and in all roots combined (R1–2 and R3–5) as well as in all roots and leaf litter combined
Fraction of initial N remaining of all litter types across all species after 1 year of decomposition was not related to any of the measured initial litter chemistry (data not shown). Fraction of initial N remaining in R1–2 of Cunninghamia, Pinus, Larix, Picea and Phellodendron (litter of the other three species decomposed only for 1 year) after 2-year decomposition was positively related to litter initial AIF concentration (P < 0.01; Fig. 4a). As a significant correlation was found between AIF and the proportion of total N found in AIF of all litter types across all species (P < 0.0001; data not shown), partial correlations between fraction of initial N remaining and the proportion of total N found in AIF were conducted controlling for AIF. We found that fraction of initial N remaining in R1–2 of the above-mentioned five species after 2-year decomposition was positively related to the proportion of total N found in AIF (P < 0.05; Fig. 4b). Initial N concentration was also significantly related to fraction of initial N remaining of both root order classes of the five species after 2-year decomposition (Fig. 4c). Initial C : N ratio was not related to fraction of initial N remaining (data not shown). No significant relation was found between fraction of initial N remaining and initial litter chemistry indices in other litter types.
Linear regressions between fraction of initial N remaining in root and leaf litter after two years of decomposition and litter initial AIF concentration (a), % of total N found in AIF (b) and N concentration (c) across five species. Significant correlations (P < 0.05) were found in lower-order roots (R1–2), in R1–2 and R3–5 combined, and in all roots and leaf combined
Discussion
Slow decomposition of lower-order roots and potential mechanisms
In the fine root system, the lowest two to three orders are the root tissues with absorptive function and fast turnover rate (Guo et al. 2008a; Xia et al. 2010). Thus the mortality and decomposition of these lowest orders dictate root C and nutrient input into the soil. In five out of eight species studied here (including both conifers and broadleaf species, and both AM and EM species), we found that lower-order roots decomposed more slowly than higher-order roots (P < 0.0001; Fig. 1). This pattern is consistent with the results of Fan and Guo (2010) and Goebel et al. (2011), in which all studied AM and EM trees and all conifers and broadleaf trees showed slower decomposition rates in lower-order than higher-order roots. Thus the slow decomposition of the lower-order roots seems to be common in the tree species studied so far and no systematic differences among plant functional groups have been identified.
The slower decomposition of lower-order roots corresponds with their higher AIF concentrations in the five species we found the pattern (Table 2). By contrast, decomposition rate did not differ significantly between root order classes in Eucalyptus, Schima, and Phellodendron (Fig.1b, d, and h), corresponding to similar AIF concentrations between root order classes for these three species (Table 2). In addition, decay constant (k) of lower-order roots was tightly related to initial AIF concentration across species (P < 0.0001; Fig. 3). Thus, the mechanistic basis for slower decomposition of the lower-order roots is likely to be the higher AIF concentration, which may indicate lower energy supply from decaying roots to microbial decomposers for a given quantity of root material.
Neither initial litter N concentration nor C : N ratio was related to litter decay constant (data not shown), which was in contrast with studies showing a positive effect of litter N on litter mass loss rate (Aerts 1997; Silver and Miya 2001). This contradiction may be partly explained by the confounding effects of litter C quality and litter N concentration on decomposition because in many leaf litter or fine root decomposition studies, litter C quality (sometimes measured by acid-insoluble fractions) and N concentration are negatively correlated. For example, hardwood litters generally have lower AIF and higher N concentration whereas coniferous litters have higher AIF and lower N concentration (Silver and Miya 2001; Zhang et al. 2008) so how AIF and N concentration independently mediate decay rates cannot be tested. Moreover, the litters with lower N concentration, which might cause greater N limitation for decomposers, tend to have higher lignin and higher lignin : N ratios, which might cause greater energy-limitation (indicated by energy supply potential of C compounds) (Taylor et al. 1989; Hendricks et al. 2000; Zhang et al. 2008). As such, the effects of C quality vs. stoichiometry on litter (root) decay have not been elucidated (Hättenschwiler and Bracht Jørgensen 2010).
In our study, lower-order roots generally had lower C quality but higher N concentrations than higher-order roots, we therefore were able to show unequivocally that higher AIF fraction (which may indicate lower C quality), rather than N concentration, was the major driver of slow decay rate in lower-order roots. A recent study using the model plant system Arabidopsis thaliana, in which plants were manipulated to have low levels of lignin, cellulose, or litter N, showed that microbial degradation of structural carbohydrates such as cellulose and hemicellulose was insensitive to initial litter N in the first year of decomposition (Talbot and Treseder 2012). Therefore, how fast decaying roots (or litter) supply energy to decomposer may be the key mechanism for short-term litter decomposition (Hättenschwiler and Bracht Jørgensen 2010).
N release patterns
Across all eight species, an average of 12 % of root N and 26 % of leaf N was lost after 2 years of decomposition, compared to an average of 39 % of root mass loss and 58 % of leaf mass loss. Goebel et al. (2011) also showed that total root N content was similar to the initial levels in some litters even after 36 months of decomposition. Other studies provide further evidence for slow release N from fresh litter. An isotope tracing experiment showed that almost 80 % of 15 N label in leaf litter remained in the un-decomposed leaf material after 2 years of field decomposition (Christenson et al. 2002). Lindahl et al. (2007) reported that litter C decomposition may be decoupled from N release in the litter layer of a pine forest. Moreover, the wide-spread patterns of N immobilization (Parton et al. 2007; also occurred in our study for various litter types, Fig. 2) further suggest that much of the litter N may not be released during the first several years of decomposition. This finding deserves attention because this would change the view that litter decomposition is an important pathway for N release and plant uptake and the magnitude of N release during litter decomposition is probably much lower than previously assumed, at least in the first several years.
However, similar rates of root N release and mass loss were also found (Dornbush et al. 2002; Parton et al. 2007). These studies defined fine roots using diameter classes and did not consider root branching structure. This methodological difference may in part be responsible for the differences in the coupling of N release and mass loss between our and their studies. It was shown in our study that lower-order roots had generally higher AIF than higher-order roots (Table 2); in addition, fraction of initial N remaining of lower-order roots was positively related to initial AIF concentration and the proportion of total N found in AIF, while the relation did not hold for higher-order roots (Fig.4 a and 4b). If root mass being decomposed comprises primarily of higher-order roots, which is possible in many previous studies due to the small fraction of lower-order roots in total fine root biomass (Guo et al. 2004; Wang et al. 2006), or if root materials had low AIF concentrations, root N release rate would not be constrained by AIF concentration or AIF-associated N and thus may show a fast N release rate.
The limited N release during litter decomposition in our study and in many others may be due to strong microbial immobilization of N during litter decomposition. Litter initially has much higher C : N ratios than microbes, and re-immobilization of mineralized N or N immobilization from exogenous environments is required to meet microbial stoichiometric balance (Parton et al. 2007; Manzoni et al. 2008). Only when litter C : N ratio decreased to a critical ratio, net N mineralization would occur (Manzoni et al. 2008). Besides, there are two other possibilities explaining limited N release: (1) after labile N is released during early decomposition, much of the remaining N may be recalcitrant and does not readily release; (2) litter N may have been transformed into recalcitrant forms during microorganism metabolism (Mambelli et al. 2011). The recalcitrance of most litter N was supported by the significant relations between the fraction of initial N remaining in lower-order roots and root C quality (AIF fraction)/N quality (represented by the proportion of total N found in AIF) (Fig. 4a and b), and also by our data that the majority (59 %) of litter initial N was in AIF and the proportion of AIF-N increased as AIF concentration increased across litter types or species. In fact, the strong correlation between the fraction of initial N remaining and AIF% in lower-order roots (R2 = 0.943; Fig. 4a) suggest that the fraction of N that cannot be readily released may be boned strongly with AIF. Indeed, the data of Parton et al. (2007) showed that in all litters, highest N release rates occurred in the final stages of decomposition, during which the most recalcitrant fraction of litter was decomposed.
Implications
Similar to previous studies (Fan and Guo 2010; Goebel et al. 2011), we found that short-lived lower-order roots decomposed more slowly than higher-order roots; moreover, roots decomposed more slowly than leaf litter (Fig. 1; Table 1). This observation has important implications. Although fine root production has often been considered to equal leaf production (Jackson et al. 1997; Klopatek 2007), the relative contribution of dead roots versus leaf litter to SOM is not clear (Silver and Miya 2001; Rasse et al. 2005). Lower-order roots turn over at a much faster rate than higher-order roots (Xia et al. 2010), but lower-order roots decomposed more slowly than higher-order roots (Fan and Guo 2010; Goebel et al. 2011; this study), suggesting the disproportionate importance of lower-order roots as a major contributor to more stable organic matter. We also found that only a small amount of root and litter N was released during the first 2 years of decomposition. This suggests that root and litter N may not be the main source of immediate plant N uptake unless roots and mycorrhizal fungi can actively forage these organic N compounds. We speculate that much of root and litter N may be bonded with recalcitrant C fractions and release of recalcitrant N occurs only when recalcitrant C fractions are utilized by decomposers. We propose that more attention should be paid to the time sequence of litter decay and N mineralization to fully understand how C and N in litter are transformed into more stable SOM and why more stable SOM is able to supply the majority of plant N demand in forests.
Conclusions
Our study provided further evidence that lower-order roots of trees in general decompose more slowly than higher-order roots and roots decompose more slowly than leaf litter in the first 2 years. These patterns can be explained by higher recalcitrant C fractions in lower-order than higher-order roots and higher recalcitrant C fractions in roots than in leaf litter. By contrast, stoichiometry-related indices such as initial N concentration and C : N ratio were not related to decomposition rate. Together with recent findings, our results suggest that during early to middle stage of litter decomposition when C quantity (concentration) is high, the rate of C decomposition is mainly determined by C quality.
C and N quality was also a better predictor of root N release rate during decomposition than initial N concentration or C:N ratio. Most of root and litter initial N was in recalcitrant forms and was retained during decomposition, particularly in litters with high proportions of recalcitrant C and N whereas rapid N release occurred only in litters with high C and N quality.
Our findings that short-lived lower-order roots decomposed more slowly than long-lived higher-order roots as well as leaf litter suggest that lower-order roots should be an important contributor to stable SOM.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Berg B, McClaugherty C (2008) Plant litter–eecomposition, humus formation, carbon sequestration (2nd edition). Springer-Verlag Berlin, Heidelberg, Germany
Bird JA, Kleber M, Torn MS (2008) 13C and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org Geochem 39:465–477
Christenson LM, Lovett GM, Mitchell MJ, Groffman PM (2002) The fate of nitrogen in Gypsy moth frass deposited to an oak forest floor. Oecologia 131:444–452
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Crow SE, Lajtha K, Filley TR, Swanston CW, Bowden RD, Caldwell BA (2009) Sources of plant-derived carbon and stability of organic matter in soil: implications for global change. Global Change Biol 15:2003–2019
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Dornbush ME, Isenhart TM, Raich JW (2002) Quantifying fine-root decomposition: an alternative to buried litterbags. Ecology 83:2985–2990
Fan P, Guo D (2010) Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163:509–515
Fernandez CW, Koide RT (2012) The role of chitin in the decomposition of ectomycorrhizal fungal litter. Ecology 93:24–28
Gessner MO, Neumann PTM (2005) Total lipids. In: Graca MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition: a practical guide. Springer, Dordrecht, The Netherlands, pp 91–95
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biol 6:751–765
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat DM (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monog 81:89–102
Gonzalez G, Seastedt TR (2001) Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 82:955–964
Guo DL, Mitchell RJ, Hendricks JJ (2004) Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 140:450–457
Guo DL, Xia M, Wei X, Chang W, Shi W, Wang Z (2008a) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Guo DL, Mitchell RJ, Withington JM, Fan PP, Hendricks JJ (2008b) Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: root branch order predominates. J Ecol 96:737–745
Hättenschwiler S, Bracht Jørgensen H (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hendricks JJ, Aber JD, NadelhoVer KJ, Hallett RD (2000) Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 3:57–69
Jackson RB, Mooney HA, Schulze E-D (1997) A global budget for fine root biomass, surface area, and nutrient contents. PNAS 94:7362–7366
Kätterer T, Anders Bolinder M, Andren O, Kirchmann H, Menichetti L (2011) Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric Ecosyst Environ 141:184–192
Klopatek JM (2007) Litterfall and fine root biomass contribution to nutrient dynamics in second- and old-growth Douglas-fir ecosystems. Plant Soil 294:157–167
Langley JA, Chapman SK, Hungate BA (2006) Ectomycorrhizal colonization slows root decomposition: the post-mortem fungal legacy. Ecol Lett 9:955–959
Li Z, Peng S, Rae DJ, Zhou G (2001) Litter decomposition and nitrogen mineralization of soils in subtropical plantation forests of southern China, with special attention to comparisons between legumes and non-legumes. Plant Soil 229:105–116
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620
Liu ZG, Zou XM (2002) Exotic earthworms accelerate plant litter decomposition in a Puerto Rican pasture and a wet forest. Ecol Appl 12:1406–1417
Mambelli S, Bird JA, Gleixner G, Dawson TE, Torn MS (2011) Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Org Geochem 42:1099–1108
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science 321:684–686
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine North American trees. Ecol Monog 72:293–309
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 269:341–356
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of forest litter. Can J For Res 20:166–171
Seastedt TR, Murray PJ (2008) Root herbivory in grassland ecosystems. In: Johnson SN, Murray PJ (eds) Root feeders: an ecosystem perspective. Cromwell Press, Trowbridge, pp 54–67
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Talbot JM, Treseder KK (2012) Interactions between lignin, cellouse, and nitrogen drive litter chemistry-decay relationships. Ecology (in press) doi: http://dx.doi.org/10.1890/11-0843.1
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay-rates-a microcosm test. Ecology 70:97–104
Valenzuela-Estrada LR, Richards JH, Diaz A, Eissenstat DM (2009) Patterns of nocturnal rehydration in root tissues of Vaccinium corymbosum L. under severe drought conditions. J Exp Bot 60:1241–1247
Wang H, Liu S, Mo J (2010) Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 335:289–298
Wang ZQ, Guo DL, Wang X, Gu J, Mei L (2006) Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 288:151–171
Wells CE, Eissenstat DM (2001) Marked differences in survivorship among apple roots of different diameters. Ecology 82:882–889
Xia M, Guo D, Pregitzer KS (2010) Ephemeral root modules in Fraxinus mandshurica. New Phytol 188:1065–1074
Zhang P, Tian X, He X, Song F, Ren L, Jiang P (2008) Effect of litter quality on its decomposition in broadleaf and coniferous forest. Eur J Soil Biol 44:392–399
Acknowledgements
We thank Mengxue Xia and Zhengxia Chen for assistance in the field and lab. Thanks to Drs. Timothy R. Seastedt and Weixin Cheng for constructive comments on earlier drafts of the manuscript. This research was funded by the Natural Science Foundation of China (NSFC grants 30870418 and 40971030) and by National Basic Research Program of China (No. 2010CB950602).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Johannes Lehmann.
Rights and permissions
About this article
Cite this article
Xiong, Y., Fan, P., Fu, S. et al. Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 363, 19–31 (2013). https://doi.org/10.1007/s11104-012-1290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1290-8