Abstract
Background and aims
Soil seed banks are an important source of seedling recruits in grassland ecosystems, particularly following large-scale disturbance. Nonetheless, the relative importance of above-ground plant communities versus below-ground processes in maintaining these seed banks is poorly understood.
Methods
Here we collected 1026 soil seed bank samples and sampled 171 aboveground vegetation quadrats in 57 sites representing six distinct grass-dominated vegetation types present at high elevation on the Tibetan Plateau, China. To understand processes affecting seed banks at these sites we examined the associations of soil environmental variables with community composition, density and species richness.
Results
We found significant differences in species composition between the seed bank and standing vegetation in each of the vegetation types, whereas soil seed banks were much more similar to each other. Nonetheless, seed bank composition was significantly associated with soil moisture, pH, and available nitrogen (all p < 0.05). Seed density was significantly negatively correlated with soil pH and positively correlated with soil moisture and soil organic matter. Multiple regression analysis showed that for seed bank density a model including pH alone had the lowest AIC value.
Conclusions
Soil conditions influence not only seed inputs but also potentially seed survival in the soil, this study present a framework which supply a plausible explanation on effect of soil environment factors (chemical and physical factors) in the formation of species composition of soil seed bank. Future work should isolate the direct and indirect effects of soil chemistry on seed persistence using seed burial experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil seed banks represent a critical source of recruits for many plant populations that occupy temporally variable environments (Venable and Brown 1988). For some species, seed persistence has been shown to be influenced by biotic (e.g. Schafer and Kotanen 2003, 2004; Dalling et al. 2011; Mordecai 2012) or abiotic conditions in the soil (e.g. Pakeman et al. 2012; Abedi et al. 2014; Basto et al. 2015a). Determining how these environmental factors influence seed survival and germination is therefore critical to understanding the population and community dynamics of seed bank forming species, and has implications for how seed banks are managed in restoration ecology (Khurana and Singh 2001).
In this study, we have proposed a conceptual model of the direct and indirect effect of soil environmental factors on seed survival and species composition of soil seed bank through have direct effects on aboveground plant community and belowground soil microbes (Fig. 1). Soil seed bank is a balance of seed input and seed output (Fenner 1985). Plant communities have a direct effect (seed input) on soil seed bank through seed dispersal. Soil microbes also have a direct effect to soil seed bank through pathogens invasion. Soil seed bank experiences a complicated ecological process in the soil. However, this process is often neglected. What role does soil environment play during soil seed bank formation and function is an unresolved scientific issue.
Abiotic environmental conditions in the soil may not only have a direct impact on ungerminated seeds, but also influence seed survival via effects on seed predators and pathogens (Fig. 1). In particular, fungal pathogens have been shown to reduce seed survival in many habitats (e.g. Dalling et al. 1998; Schafer and Kotanen 2003, 2004; O’Hanlon-Manners and Kotanen 2006), with the activity of soil fungi linked to soil moisture and other soil characteristics (Blaney and Kotanen 2001; Schafer and Kotanen 2004; Mordecai 2012). As a consequence, wetter habitats may have lower seed survival at least in part due to the effect of fungal pathogens, while arid habitats have longer-lived seed banks (Mordecai 2012; Kaiser and Pirhofer-Walzl 2015).
Previous work has also shown that soil chemical variables are often correlated with soil microbial communities across space (e.g. Nilsson et al. 2007; Lauber et al. 2008). In particular, the composition of fungal communities can be sensitive to variability in soil nutrient availability (Lauber et al. 2008). In contrast, the composition of soil bacterial communities is more strongly related to soil pH (Fierer and Jackson 2006; Hartman et al. 2008; Lauber et al. 2009), potentially reflecting a narrower optimal pH range for bacterial than fungal growth (Rousk et al. 2010). As yet, however, the effects of soil pH on grassland seed bank richness and composition has received little attention (Basto et al. 2015a), although recent work in acidic and calcareous grasslands in the UK suggests that seed persistence, as well as seed bank richness and density decrease with increasing pH and is potentially mediated by pathogens (Basto et al. 2015b). Little is known about how soil environmental factors potentially affect the species composition, density and richness of soil seed banks across the biomes.
On the Tibetan plateau diverse grassland and meadow ecosystems have been recognized that reflect variation in elevation, moisture regime and soil properties (Qi et al. 2015). However, no study currently exists to identify common trends in above-ground vegetation and seed bank composition across these different vegetation types. While the effects of environmental factors on the seed bank of species present in above-ground vegetation (transient seed bank) may be primarily determined via effects on local seed production (Fig. 1), environmental effects on species absent from aboveground vegetation (persistent seed bank) may instead primarily represent direct effects on seeds mediated over long periods by the soil physical and chemical environment, and by the action of seed predators and pathogens.
The objectives of this paper were therefore: (1) To determine how the density, richness and compositional similarity of seed banks compares to aboveground communities across six Tibetan plateau vegetation types, and (2) To determine how seed bank density, richness and species composition varies in relation to soil moisture and soil chemical variables (pH, nitrogen and phosphorus availability, and soil organic carbon). Specifically, we hypothesized, that the richness and density of the persistent seed bank would be negatively affected by increasing soil moisture and pH reflecting increasing losses of seeds under conditions that favor seed infection and decay by soil microbial communities.
Materials and methods
Study sites
The study was carried out in the northeastern part of Tibetan Plateau in Gansu Province, P.R China (101°06′-103°33′E, 33°22′-35°24′N, about 37,000 km2, altitude 2339–4038 m a.s.l). We chose six vegetation types (Table S1 in Appendix S1, Table 1), classified as AM (alpine meadow, 27 sites), ASM (alpine scrub meadow, 8 sites), SM (swamp meadow, 7 sites), SAM (subalpine meadow, 6 sites), UG (upland grassland, 5 sites), and FEM (forest edge meadow, 4 sites). The different vegetation types have been distinguished based on species composition, and the identity of dominant species (Wu 1980). To examine the characteristics of the soil seed bank community, a germination experiment was conducted in the Research Station of Alpine Meadow and Wetland Ecosystems of Lanzhou University (Hezuo Branch), Gansu Province of China (N34°55′, E102°53′, altitude 2900 m a.s.l), which was also located on the northeastern part of Tibetan Plateau. The mean annual temperature is 2.0 °C and the mean annual precipitation is 560 mm.
Soil seed bank sampling
To sample the persistent seed bank, soil samples were collected in August 2009, after the spring germination flush, but before dispersal of the current season’s seeds. Randomly selected replicate sites were established in each vegetation type. Within each site, three 100 m × 100 m plots at least 1000 m apart were selected at random. Each plot was considered an independent spatial replicate in each site. We randomly chose three subplots (10 m × 10 m) in each plot, and 10 cylindrical soil cores (3.6 cm diameter) were taken randomly from each subplot for a total sample area of 0.092 m2 and the total volume was 0.0092 m3 per site. The soil cores were divided into two fractions: the upper layer (0–5 cm depth), and lower layer (5–10 cm depth). Further, the 10 cores from each depth were then pooled to generate one representative sample per subplot. In total, there were 18 samples from each site, 57 sites and 1026 soil samples. All the soil samples were transported to the Hezuo Research Station and placed under a north-facing window of direct exposure at ambient room temperatures for 15 days to allow them to dry. Then, samples were kept dry in a dark storeroom of Hezuo Research Station with no heat until May 2010.
Characterization of the germinable soil seed bank
We used the seedling emergence method to evaluate the germinable species composition of the seed bank (e.g. ter Heerdt et al. 1996). To reduce sample volume, each sample was sieved to remove plant fragments, coarse debris, and stones, then sieved through a coarse (4 mm mesh), and then a fine (0.2 mm mesh) sieve (Ma et al. 2013b). Material retained on the 0.2 mm mesh was spread on to plastic germination trays (30 × 30 cm) filled with a 15 cm deep layer of sand previously sterilized at 140 °C for 24 h. Twenty control trays containing sterilized sand were set up alongside the experimental trays to check for contamination from wind-dispersed seeds. Trays were watered and monitored several times a week. Emerging seedlings were noted and removed once they could be identified. The seedlings that could not be identified were grown separately until identification was possible. We turned over the soil regularly to facilitate the germination of seeds from soil samples. After 5 months (May 10th – Oct 10th), the germination trial was ended as no more seedlings emerged after three consecutive weeks.
Plant community sampling
Aboveground plant communities were surveyed in August 2009, at the peak of plant development. Three 50 cm × 50 cm quadrats, corresponding to the location of soil seed bank samples within each plot, were used to record the richness, composition and cover of the aboveground plant community. In total, 171 quadrats were examined. The Braun-Blanquet scale was used to estimate cover (Ma et al. 2013a, b).
Analysis of soil properties
Three additional soil samples were collected from each subplot where aboveground plant communities were sampled. In August 2009, three cores (3.6 cm in diameter × 0–15 cm deep) were mixed to generate a single soil sample in each subplot, resulting in nine composite soil samples from each site. Overall, 513 composite soil samples [3 subplots × 3 plots ×57 sites = 513 mixed soil samples] were used to analyze soil characteristics. For this study, soil moisture, soil pH, total nitrogen (N), soil available N (sum of nitrate and ammonium), total phosphorus (P), available P, and soil organic matter (SOM) were measured. After removal of roots, each composite soil sample was sieved to <2 mm and split into two sub-samples. One sub-sample was kept at 4 °C for analyses of soil available N (NH4 +-N and NO3 —N), and available phosphorus measurements within 1 month of collection. The other sub-sample was air-dried at room temperature and ground to <0.15 mm for soil pH, SOM, total N, and total P.
Soil moisture before air drying was obtained by the oven-drying method. Soil pH was measured using a pH meter with a glass electrode (soil/KCl ratio 1:2.5). Soil organic matter was measured by the K2Cr2O7 method using the modified Kjeldahl wet digestion procedure of Miller and Keeney (1982). Total nitrogen was determined by the Kjeldahl method (Institute of Soil Science, Academia Sinica 1978). Total P was determined after digestion by HClO4 – H2SO4 (Parkinson and Allen 2008) using molybdenum-blue colorimetry. For soil available N (NH4 +-N and NO3 −-N), fresh soil equivalent to 10 g dry mass was extracted with 2 M KCl (soil:solution ratio of 1:5) and determined using a Flow Solution® IV Analyzer (OI Analytical Corporation, USA). Soil available phosphorus was extracted by the Bray method (Bray and Kurtz 1945).
Data analysis
One-way analysis of variance (ANOVA) was used to compare species richness, abundance, and proportion of life forms (annual, biennial and perennial) across vegetation types in aboveground vegetation, and seed density, species richness, and proportion of life forms in the seed bank. Post-hoc comparisons between vegetation types were made using the LSD test. Data for each soil depth (0–5 cm, 5–10 cm) and the whole seed bank sample (0–10 cm) were analyzed separately. We examined the relationship between aboveground species richness and abundance with soil pH by calculating Pearson correlation coefficients.
To explore the relationship between each environmental factor (soil moisture, pH, total and available N, total and available P, and soil organic matter) and seed bank characteristics (species richness and seed density) we used linear regression. Independent variables except soil pH were log-transformed prior to these analyses. In this step, we confirmed which soil environmental factors significantly correlated with seed bank characteristics. We further used multiple linear regression analysis (using backward elimination) to explore the combined effects of soil environmental factors on seed bank richness and density. Dependent (seed bank) variables were seed bank richness and density. Independent (soil environmental factors) variables included in multiple regressions were those that were significantly correlated with seed bank characteristics in one or more univariate analyses. Data were examined for normality and homogeneity of variance prior to analysis, and all independent variables (except soil pH), seed density in soil seed bank and species abundance in plant community data were log-transformed. Aikake’s information criterion (AIC) was used for model selection. All ANOVA tests and linear regressions were performed with a SPSS 16.0.
The similarity of species composition among seed banks, aboveground vegetation, and between seed bank and aboveground vegetation across the different vegetation types was visualized using non-metric multidimensional scaling (NMDS). We calculate similarity matrices using the Bray Curtis coefficient (Kindt and Coe 2005). The final dataset contained 489 species and consisted of 114 sites (one per site; 57 seed bank and 57 above-ground community sites). We pooled all the seed bank and the vegetation samples per site. All ordinations and Bray Curtis dissimilarity indices were based on relative abundance data. PERMANOVA (Permutational multivariate analysis of variance) using Bray-Curtis dissimilarities was used to statistically assess differences in species composition among seed banks, aboveground plant communities, and between seed banks and aboveground vegetation across the six vegetation types. Finally, canonical correspondence analysis (CCA) was used to examine the relationship between soil environmental factors and species composition of the aboveground vegetation and seed bank. A Monte Carlo permutation (999 permutations) was used to identify axes with significant eigenvalues and species-environment correlations. CCA, NMDS and PERMANOVA were conducted using the package vegan (Oksanen et al. 2007) in the R 3.0.1 (www.r-project.org).
Results
Variation in aboveground vegetation and seed banks across sites
The standing plant communities sampled at the 57 sites consisted of 396 species, belonging to 47 families, of which 11.3% were annuals, 2.4% were biennials, and 86.3% were perennials. Three species could only be identified to genus level (one Artemisia sp. and two Pedicularis sp), seven to family level (Caryophyllaceae sp., Chenopodiaceae sp., Compositae sp., Brassicaceae sp., Orchidaceae sp., Polygonaceae sp., and Ranunculaceae sp), and five remained unknown. These 15 species are not included in life form tallies. The proportion of annual, biennial and perennial species did not differ across the six vegetation types.
No seedlings emerged in the seed bank control trays. During the study, a total of 11,726 seedlings of 150 species, belonging to 26 families, germinated from the soil samples. Of these species, 19.4% were annuals, 2.3% biennials, and 78.3% perennials. Seven species could only be identified to the genus level (Arenaria sp., Cerastium sp., Draba sp., Pedicularis sp., Poa sp., Potentilla sp., and Saussurea sp), three to family level (one Ranunculaceae and two Poaceae), and 11 were unidentified. These 21 species are not included in life form tallies. The proportion of species in different life forms did not differ across vegetation types, but the proportion of annuals and biennials in the seed bank (18.96 ± 0.67%; mean ± 1 SE) was significantly higher than in aboveground vegetation (9.45 ± 0.56%) (F = 119.11, p < 0.001).
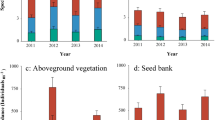
Species richness per quadrat differed significantly among the different vegetation types (Fig. 2), with the highest species richness in ASM, AM and SAM, and the lowest in SM, FEM and UG. Plant community abundance, i.e. the total number of individuals per quadrat (0.25m2), differed significantly among vegetation types (Fig. 2). The highest abundances were found in ASM, AM and SM, the lowest in UG. Species richness (r = −0.28, p = 0.034) and abundance (r = −0.49, p < 0.001) of aboveground vegetation was significantly negatively correlated with soil pH.
Seed bank species richness differed significantly among the six vegetation types only in the 0–5 cm soil layer (Fig. 3). Seed density also only differed significantly among vegetation types in the 0–5 cm soil layer. The lowest seed density was in UG (747 ± 87 seeds m−2), and there were no differences among other five vegetation types (969 ± 150–1623 ± 184 seeds m−2; Fig. 3).
Variation in species richness (per plot) and density (per m−2) of soil seed banks among vegetation types. Seed density comparisons were carried out using log-transformed data. Letters indicate significant differences (ANOVA, LSD test) in species richness (left) and seed density (right) among vegetation types in 0-5 cm soil layer. ASM alpine scrub meadow, AM alpine meadow, SAM subalpine meadow, SM swamp meadow, FEM forest edge meadow, UM upland grassland
Aboveground vegetation composition varied among six vegetation types (r 2 = 0.240, p = 0.001) (Fig. 4a). Pairwise comparisons of vegetation types were all significant except for AM and ASM, FEM and SAM, FEM and UG, and SAM and UG (Table 2). There was strong separation of aboveground vegetation of the different vegetation types along the first two axes of the NMDS, with the exception of AM and ASM communities, where there was no clear difference in community composition (Fig. 4b). Overall, species composition of seed bank showed a significant difference among six vegetation types (r2 = 0.162, p = 0.001). However, based on the observed clustering in the NMDS ordination, and PERMANOVA pairwise comparison (Fig. 4b, Table 2), the seed bank samples were much more similar across vegetation types than the aboveground vegetation. Most significant differences were attributable to the ASM and AM types (Table 2).
Two-dimensional nonmetric multidimensional scaling (NMDS) ordination of species composition of the six vegetation types based on abundance data for a aboveground vegetation (Stress value = 0.19), b seed banks (Stress value = 0.24), and c seed bank (sb) and aboveground vegetation (ve) combined (Stress value = 0.17) based on sampling 57 plant communities. The Ordination was based on relative abundance data. Abbreviations as in Table 1
Similarity of species composition between the seed bank and aboveground vegetation
In total there were 104 species in common between the standing vegetation and soil seed bank. Overall, similarity between the species composition of the seed bank and aboveground vegetation was low in each of six vegetation types (Fig. 4). Also, based on the result of PERMANOVA, similarity between seed bank and aboveground vegetation showed a significant difference in each of six vegetation types (Table 2). The NMDS showed that SAM-ve, AM-ve and ASM-ve grouped more closely to their seed bank groups than did FEM-ve, UG-ve, and SM-ve (Fig. 4c).
Relationship between soil environmental factors and community composition of aboveground vegetation and seed banks
Overall, a relatively small proportion of the variance in aboveground vegetation and seed bank community composition (18.8% in vegetation and 15.4% in seed bank) was accounted for in the CCA, with 10% (vegetation) and 7.6% (seed bank) explained by the first two axes. Nonetheless, there was a significant relationship between community composition and environmental variables (standing vegetation p = 0.001; seed bank p = 0.009). Three variables for standing vegetation: soil moisture (p = 0.001), pH (p = 0.037) and total nitrogen (p = 0.024), and three variables for seed bank composition: soil moisture (p = 0.005), pH (p = 0.035), total soil available nitrogen (p = 0.008) were significantly correlated with either of the first two ordination axes (Table 3).
Relationship between soil environmental factors and species richness and seed density in the soil seed bank
Seed density was significantly negatively correlated with soil pH and positively correlated with soil moisture and soil organic matter (Fig. 5). Environmental variables soil pH, soil moisture and SOM were also correlated with each other. Soil pH was negatively correlated with soil moisture (r = −0.648, p < 0.001) and SOC (r = −0.464, p < 0.001), soil moisture was significantly positively correlated with SOM (r = 0.429, p < 0.001).
Multiple regression analysis of seed bank responses to environmental variables showed that for seed bank density a model including pH alone had the lowest AIC value (Table 4).For seed bank richness, none of the environmental variables were significant predictors (Table 4).
Discussion
Composition of soil seed banks in the eastern Tibetan Plateau
We found that the species composition of the soil seed bank differed significantly among the six vegetation types, reflecting differences in aboveground community composition. Nonetheless, the seed bank samples were much more similar across vegetation types than were the aboveground samples, indicated by their strong clustering in the NMDS ordination and PERMANOVA pairwise comparison. Several mechanisms might contribute to this. Firstly, the aboveground vegetation was dominated by perennials, which contribute little to the seed bank in late successional grassland ecosystems on the Tibetan Plateau (Ma et al. 2009, 2013b). Secondly, in previous studies in alpine areas on the Tibetan Plateau, we found that the soil seed banks were dominated by species characteristic of early successional communities. These species produce long-lived seeds that have the potential to persist in the soil through succession (Ma et al. 2009, 2011, 2013b).
Similarity between soil seed bank and plant community across vegetation types
This study is the first report that identifies the trends in similarity between seed bank and aboveground vegetation across different vegetation types on the Tibetan Plateau, with significance for the restoration of different vegetation types in the region. We found that similarity in species composition between the seed bank and aboveground vegetation differed significantly (Table 2) and uniformly low in all six vegetation types (Fig. 4), consistent with results from other alpine studies (e.g. Arroyo et al. 1999). The low similarity may in part reflect the relatively short growing season, resulting in a greater reliance on clonal regeneration than seedling recruitment (Körner 1999; Bueno et al. 2011). In addition, previous research on grasslands dominated by perennial grasses has also found low similarities between the seed bank and the standing vegetation (Peco et al. 1998; Arroyo et al. 1999, Edwards and Crawley 1999). In this study, we found perennial species dominated the aboveground vegetation in the all six vegetation types. These species rely almost exclusively on vegetative reproduction and contribute little to the seed bank (e.g. Ma et al. 2010a, b). Finally, the contribution of seed banks to the aboveground plant community decreases through succession on the Tibetan plateau (Ma et al. 2009, 2013b), and in other vegetation types (Grandin 2001); the plant communities of each of six vegetation types in this study all represent mature plant communities.
Relationship between soil environmental factors and seed banks
We found that three correlated variables: soil moisture, soil pH, and total soil available N were significantly associated with the composition of soil seed banks, while soil moisture, soil pH, total N were associated with the composition of aboveground vegetation (Table 3). This suggests that both aboveground vegetation and seed bank communities are structured by soil moisture and soil pH. Additional effect of total soil inorganic N on seed bank composition suggests that environmental effects on seed banks may not only influence seed inputs, but potentially also mediate seed survival.
In addition to effects on seed bank community composition, soil pH was negatively correlated with seed bank density (Table 4, Fig. 5). To date, contrasting effects of pH on seed bank density, richness and persistence have been reported (Erenler et al. 2010, Pakeman et al. 2012, Basto et al. 2015b). While Basto et al. (2015b) found that seed density in acidic and calcareous grasslands in England decreased with soil pH (range 3.5 to 7). Using a seed burial experiment, Pakeman et al. (2012) found seed survival was lower in soils with a lower pH (3.7–5.4), which they suggested may be due to toxicity from aluminum or other metals. The following mechanisms might account for the negative relationships of seed bank density with pH observed here.
First, soil pH directly affects the growth and survival of species in the aboveground plant community due to effects on the availability of essential mineral nutrients, and on soil microbial communities (e.g. Stephenson and Rechcigl 1991). Grime (1973) showed that the highest species richness in aboveground vegetation in European grassland occurs at a soil pH range from 6.1 to 6.5, where most plant nutrients are in their most available state, while species richness decreases with both increasing acidity and alkalinity. In this study, soil pH ranged from 5.6 to 8.5 across the 57 sites, with a mean soil pH value of 6.8. Decreasing richness in the aboveground plant community was therefore associated with increased alkalinity. Second, Basto et al. (2015b) attributed the negative effect of increasing soil pH on seed density to a reduction in the abundance of grasses with increasing pH. However, using seed burial experiments Basto et al. (2015b) also found that fungicide increased seed persistence but only when pH was >5.6. This suggests that seed persistence could decrease at high pH in part due to increasing losses to fungal pathogens. In addition, decomposition processes at high pH may also have a negative effect on seed persistence (Bekker et al. 1998). In grasslands bacterial growth also increases at higher pH (Fernández-Calvinõ et al. 2011). Hence, moderately acidic soils could slow the decomposition rate, and be more conducive to seed persistence in the soil.
While pH is one of the main drivers of species composition and diversity of grassland ecosystems (Kalusová et al. 2009; Michalcová et al. 2011), it frequently correlates with other soil environmental factors that may be responsible for relationships with seed bank characteristics(Valkó et al. 2014). Often, lower pH soils are associated with higher soil moisture content and increased soil organic matter accumulation related to reduced rates of decomposition (Brady and Weil 2008). However, in this study, the more alkaline soils had both higher moisture and organic matter contents, resulting in positive correlations with seed bank density and richness (Fig. 5). We found that SOM correlated with seed bank density. Consequently, a combination of environmental conditions may determine seed accumulation in the soil. Although soil seed bank dynamics are controlled by complex interactive factors, this study suggests hypotheses for the effect of soil environment factors. Here we suggest that soil pH has two effects on seed bank density: first by acting as an environmental filter that determines the composition of the past above-ground vegetation that contributed to the seed bank, and second by filtering the soil microbial community that influences seed survival in the soil.
Generally, wetter sites have lower seed survival, while arid sites may have longer-lived seed banks (Mordecai 2012, Kaiser and Pirhofer-Walzl 2015). Seeds susceptible to high soil moisture are thought to suffer from deleterious fungi and bacteria that are favored under these conditions (Blaney and Kotanen 2001; Schafer and Kotanen 2003). Here, we found a positive relationship between moisture and seed density, suggesting that effects were mediate through responses of aboveground vegetation. Consistent with this pattern, the above-ground plant community richness also increased with soil moisture. Our results indicated that soil moisture has an indirect effect to seed survival via influences on the aboveground vegetation that produces seed banks. Much research found fungal pathogens could reduce seed survival in many habitats (e.g. Dalling et al. 1998, Schafer and Kotanen 2003, 2004, O’Hanlon-Manners and Kotanen 2006), while soil moisture influence the activity of soil fungi (Schafer and Kotanen 2004; Mordecai 2012). Soil moisture has an indirect effect on seed bank density through soil fungi pathogens. We think the potential mechanism is the rates of decomposition tend to be low in cool alpine environments and therefore rates of seed loss from decay are expected to be low (McGraw and Vavrek 1989). In this study, although soil biotic community hypothesis has been addressed in our conceptual model (Fig. 1), we don’t have any soil biotic data. Hence, it will be further explored in subsequent research.
References
Abedi M, Bartelheimer M, Poschlod P (2014) Effects of substrate type, moisture and its interactions on soil seed survival of three Rumex species. Plant Soil 374:485–495
Arroyo MTK, Cavieres LA, Castor C, Humana AM (1999) Persistent soil seed bank and standing vegetation at a high alpine site in the central Chilean Andes. Oecologia 119:126–132
Basto S, Thompson K, Phoenix G, Sloan V, Leake J, Rees M (2015a) Long-term nitrogen deposition depletes grassland seed banks. Nat Commun 6:6185
Basto S, Thompson K, Rees M (2015b) The effect of soil pH on persistence of seeds of grassland species in soil. Plant Ecol 216:1163–1175
Bekker RM, Knevel IC, Tallowin JBR, Troost EML, Bakker JP (1998) Soil nutrient input effects on seed longevity: a burial experiment with fen meadow species. Funct Ecol 12:673–682
Blaney CS, Kotanen PM (2001) Effects of fungal pathogens on seeds of native and exotic plants: a test using congeneric pairs. J Appl Ecol 38:1104–1113
Brady NC, Weil RR (2008) The nature and properties of soils. Fourteenth Edition. Prentice Hall, New Jersey 965 pp
Bray RH, Kurtz LT (1945) Determination of total organic, and available forms of phosphorus in soils. Soil Sci 59:39–45
Bueno CG, Reiné R, Alados CL, Gómez-García D (2011) Effects of large wild boar disturbances on alpine soil seed banks. Basic Appl Ecol 12:125–133
Dalling JW, Swaine MD, Garwood NC (1998) Dispersal patterns and seed bank dynamics of pioneer trees in moist tropical forest. Ecology 79:564–578
Dalling JW, Davis AS, Schutte BJ, Arnold AE (2011) Seed survival in soil: interacting effects of predation dormancy and the soil microbial community. J Ecol 99:89–95
Edwards GR, Crawley MJ (1999) Herbivores, seed banks and seedling recruit- ment in mesic grassland. J Ecol 87:423–435
Erenler HE, Ashton PA, Gillman MP, Ollerton J (2010) Factors determining specie richness of soil seed banks in lowland ancient woodlands. Biodivers Conserv 19(6):1631–1648
Fenner M (1985) Seed ecology. Chapman and Hall, London
Fernández-Calvinõ D, Rousk J, Brookes PC, Bååth E (2011) Bacterial pH-optima for growth track soil pH, but are higher than expected at low pH. Soil Biol Biochem 43:1569–1575
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. P Natl Acad Sci USA 103:626–631
Grandin U (2001) Short-term and long-term variation in seed bank/vegetation relations along an environmental and successional gradient. Ecography 24:731–741
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347
Hartman WH, Richardson CJ, Vilgalys R, Bruland GL (2008) Environmental and anthropogenic control of bacterial communities in wetland soils. P Natl Acad Sci USA 105:17842–17847
Kaiser T, Pirhofer-Walzl K (2015) Does the soil seed survival of fen-meadow species depend on the groundwater level? Plant Soil 387:219–231
Kalusová V, Le Duc MG, Gilbert JC, Lawson CS, Gowing DJG, Marrs RH (2009) Determining the important environmental variables controlling plant species community composition in mesotrophic grasslands in Great Britain. Appl Veg Sci 12:459–471
Khurana E, Singh JS (2001) Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: a review. Environ Conserv 28:39–52
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agoforestry Centre (ICRAFF), Nairobi
Körner C (1999) Alpine plant life. Functional plant ecology of high mountain ecosystems. Springer, Berlin
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community composition at the continental scale. Appl Environ Microb 75:5111–5120
Ma M, Du G, Zhou X (2009) Role of the soil seed bank during succession in a subalpine meadow on the Tibetan plateau. Arct Antarct Alp Res 41(4):469–477
Ma M, Zhou X, Du G (2010a) Role of soil seed bank along a disturbance gradient in an alpine meadow on the Tibet plateau. Flora 205(2):128–134
Ma M, Zhou X, Wang G, Ma Z, Du G (2010b) Seasonal dynamics in alpine meadow seed banks along an altitudinal gradient on the Tibetan Plateau. Plant Soil 336:291–302
Ma M, Zhou X, Du G (2011) Soil seed bank dynamics in alpine wetland succession on the Tibetan Plateau. Plant Soil 346:19–28
Ma M, Zhou X, Du G (2013a) Effects of disturbance intensity on seasonal dynamics of alpine meadow soil seed banks on the Tibetan Plateau. Plant Soil 369:283–295
Ma M, Zhou X, Qi W, Liu K, Jia P, Du G (2013b) Seasonal dynamics of the plant community and soil seed bank along a successional gradient in a subalpine meadow on the Tibetan Plateau. PLoS One 8(11):e80220
McGraw JB, Vavrek MC (1989) The role of buried seeds in arctic and alpine plant communities. In: Leck MA, Parker VT, Simpson RL (eds) Ecology of Soil Seed Banks. Academic Press, San Diego, pp 91–106
Michalcová D, Gilbert JC, Lawson CS, Gowing DJG, Marrs RH (2011) The combined effect of waterlogging, extractable P and soil pH on a-diversity: a case study on mesotrophic grasslands in the UK. Plant Ecol 212:879–888
Miller RH, Keeney DR (eds) (1982) Methods of soil analysis. Part 2: chemical and microbiological properties, 2nd edn. American Society of Agronomy, Soil Science Society of America, Madison
Mordecai EA (2012) Soil moisture and fungi affect seed survival in California grassland annual plants. PLoS One 7(6):e39083
Nilsson LO, Bååth E, Falkengren-Grerup U, Wallander H (2007) Growth of ectomycorrhizal mycelia and composition of soil microbial communities in oak forest soils along a nitrogen deposition gradient. Oecologia 153:375–384
O’Hanlon-Manners D, Kotanen P (2006) Losses of seeds of temperate trees to soil fungi: effects of habitat and host ecology. Plant Ecol 187:49–58
Oksanen J, Kindt R, Legendre P, O’Hara B (2007) Vegan: community ecology package. R package version 1:8–5 Available at: http://cran.r-project.org. Accessed 10 April 2007
Pakeman RJ, Small JL, Torvell L (2012) Edaphic factors influence the longevity of seeds in the soil. Plant Ecol 213:57–65
Parkinson JA, Allen SE (2008) A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun Soil Sci Plant Anal 6(1):1-11
Peco B, Ortega M, Levassor C (1998) Similarity between seed bank and vegetation in Mediterranean grassland: a predictive model. J Veg Sci 9:815–828
Qi W, Zhou X, Ma M, Knops JMH, Li W, Du G (2015) Elevation, moisture and shade drive the functional and phylogenetic meadow communities’ assembly in northeastern Tibetan Plateau. Community Ecol 16(1):66–75
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal 4:1340–1351
Schafer M, Kotanen PM (2003) The influence of soil moisture on losses of buried seeds to fungi. Acta Oecol 24:255–263
Schafer M, Kotanen P (2004) Impacts of naturally-occurring soil fungi on seeds of meadow plants. Plant Ecol 175:19–35
Stephenson RJ, Rechcigl JE (1991) Effects of dolomite and gypsum on weeds. Commun Soil Sci Plan 22:1569–1579
ter Heerdt GNJ, Verweij GL, Bakker RM, Bakker JP (1996) An improved method for seed bank analysis: seedling emergence after removing the soil by sieving. Funct Ecol 10:144–151
Valkó O, Tóthmérész B, Kelemen A, Simon E, Miglécz T, Lukács BA, Török T (2014) Environmental factors driving seed bank diversity in alkali grasslands. Agric Ecosyst Environ 182:80–87
Venable DL, Brown JS (1988) The selective interactions of dispersal, dormancy and seed size as adaptations for reducing risks in variable environments. Am Nat 131:360–384
Wu Z (1980) The vegetation of China. Science Press, Beijing (in Chinese)
Acknowledgements
We would like to thank Zhengkuan Gu, Wei Li for their help with field assistance. The study was funded by the Natural Science Foundation of China (41671246), the Fundamental Research Funds for the Central Universities (lzujbky-2015-k15), and project of State Key Laboratory of Grassland Agro-ecosystems of Lanzhou University (SKLGAE201706).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeffrey Walck.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Ma, M., Dalling, J.W., Ma, Z. et al. Soil environmental factors drive seed density across vegetation types on the Tibetan Plateau. Plant Soil 419, 349–361 (2017). https://doi.org/10.1007/s11104-017-3348-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3348-0