Abstract
Background and Aims
Information on soil seed bank processes is crucial for understanding vegetation dynamics. Despite the documented importance of soil seed banks in many ecosystems, their role is not fully understood in some sensitive habitats, such as the alpine meadows of the Tibetan Plateau.
Methods
We studied the seasonal dynamics of the germinable soil seed bank under four disturbance intensities in an alpine meadow on the Tibetan Plateau as well as seed size distribution relative to disturbance intensity. Composition of the seed bank was compared with that of the standing vegetation.
Results
Density of buried seeds increased with disturbance intensity, but species richness and species diversity decreased. Seed density and species richness of the seed bank varied seasonally in all layers (0–2, 2–7, 7–12 cm) and the whole (0–12 cm). The species composition of seed bank was not significantly influenced by season. There was no trend in seed size distribution as disturbance increased. Seasonal seed bank turnover rates increased with increase in disturbance. The result of the NMDS showed that species composition of seed bank and vegetation exhibited a fairly uniform pattern in each season.
Conclusions
Although as a whole the species composition of the vegetation and seed bank showed a relatively low degree of similarity in each season, similarity was highest in the most disturbed habitat. There was no alteration in species composition of seed bank regardless of disturbance intensity, but seed density decreased as disturbance increased. Disturbances in alpine plant communities might increase persistence of regeneration niches. Regeneration from the seed bank together with vegetative reproduction contributed to aboveground vegetation in highly disturbed habitats. Clonal species played an important role in regeneration of vegetation in slightly disturbed areas, where there was little contribution of ruderals from soil seed banks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
So far, much research has been conducted on disturbance effects on plant communities (e.g. Pickett and White 1985; Huston and Smith 1987). However, effects on soil seed banks have received little attention (Bueno et al. 2011) although seed banks strongly influence the resilience (van Andel and Grootjans 2005) and restorability (Norbert and Annette 2004; Klimkowska et al. 2009) of plant communities. Because buried seeds form a reservoir of individuals capable of replacing vegetation following disturbance, information on soil seed bank processes is crucial for evaluating how to manage species with seed banks for conservation (Satterthwaite et al. 2007) and understanding vegetation dynamics, particularly in ecosystems experiencing frequent disturbance (Scott et al. 2010) such as alpine meadows. If seed density and species richness in the soil seed bank are sufficient and if seeds of target species have survived in the soil, successful restoration might be possible in degraded habitat (Jalili et al. 2003). Despite the documented importance of soil seed banks in many ecosystems, their role is not fully understood in some sensitive habitats (Funes et al. 2003), such as the alpine meadows of the Tibetan Plateau (Ma et al. 2010a, b).
Recent studies have shown that intensive grazing in the high Arctic tundra deplete seed banks (Cooper 2006). Bueno et al. (2011) also found lower quantities of seeds in disturbed alpine grasslands. The highest number of species was found at intermediate disturbance intensities, and it decreased drastically with high disturbance intensity (Levassor et al. 1990). Decreases in seed density may be related to reduced seed set due to livestock eating plant reproductive parts (Cooper and Wookey 2003), high mowing frequency (Mitlacher et al. 2002), or heavy grazing intensity (Sternberg et al. 2003). In fact, high grazing pressure reduced the investment in sexual reproduction in many reindeer forage species (Bråthen and Junttila 2006). Despite these results, other investigators have predicted that the density of buried seeds would decline with decreasing intensity of disturbance (e.g. Matus et al. 2005; Michaela and Wolfgang 2009). Kalamees et al (2012) found high seed densities in the soil seed banks of grazed sites. Species subjected to frequent disturbance tended to expend a large portion of their resources on the development of survival mechanisms. Thus, large seed banks would occur in communities subject to recurrent, large-scale disturbances; where the life spans of most species are shorter than the average disturbance interval and recruitment is confined to the immediate post-disturbance period (Pierce and Cowling 1991). In addition, large seed banks are created by potentially limiting the number of propagules that actually contribute to the existing population, i.e. seeds do not germinate. Our first of three hypotheses is that the Tibetan alpine meadow seed bank density increases with increasing disturbance intensity.
There is conflicting evidence, however, concerning the potential of soil seed banks to contribute to grassland restoration. The role of seeds in the recovery process from large disturbances in alpine grasslands might be only secondary (Bossuyt and Honnay 2008). This is likely because grassland plant species do not produce many seeds (Bossuyt and Honnay 2008) and seeds are dispersed over a relatively short-distance (Körner 1999). Moreover, this may also be related to a lack of suitable niches in a dense vegetation canopy in less disturbed habitats, preventing seed germination and /or establishment. However, an alternative proposal has been made. Moore (1980) suggested a high similarity between the vegetation and the seed bank for frequently disturbed communities. Some studies show that with increasing disturbance intensity the difference between the seed bank and the aboveground vegetation increases (e.g., Michaela and Wolfgang 2009). The ability of a plant community to regenerate from the soil seed bank dramatically decreases with decreasing disturbance intensity (e.g. Michaela and Wolfgang 2009), because disturbance-adapted species are characterized by high seed accumulation capacity and high seed bank persistence. In this study, we examined the similarity in species composition of the seed bank and standing vegetation along a disturbance gradient. Our second hypothesis was that similarity of seed bank and vegetation increases with increasing disturbance intensity and most likely that soil seed banks together with the clonal perennials contribute to aboveground vegetation in seriously disturbed habitats.
In addition, in continuously grazed sites, the average seed mass of species present in seed bank samples is generally smaller than that of less disturbed sites (Kalamees et al. 2012). In perennial grasslands, gap colonizers are characterized by having relatively rapid vegetative spread (Kotanen 1997) and a relatively small seed mass (Kalamees and Zobel 2002). Eriksson and Eriksson (1997) have also shown that gaps are relatively more important for the regeneration of grassland species with smaller seeds. Thirdly, we hypothesized that soils in seriously disturbed alpine meadow habitats would contain seeds with smaller mean mass than those in nondisturbed habitats.
Material and methods
Study sites
The study was conducted in an alpine meadow in Maqu, Gansu, China on the eastern Tibetan Plateau (N35°58′, E101°53′, 3500 m. a.s.l.). The average annual temperature is 1.2 °C, ranging from −10 °C in January to 11.7 °C in July. Precipitation is 620 mm per year (over the last 35 years) and is mainly distributed during the short, cool summer. The area has 2580 h of sunshine and more than 270 frost days per year. The soil type is alpine meadow, and parent materials are from a variety of sources including glacial, alluvial, residual, and residual slope deposits (Chen and Wang 1999). The community type is mainly alpine meadow and is dominated by many monocotyledons, primarily Poaceae and Cyperaceae and by various dicotyledons, such as Ranunculaceae, Polygonaceae, Saxifragaceae, Asteraceae, Scrophulariaceae, Gentianaceae, and Fabaceae (Ma et al. 2010a, b).
Seed bank sampling and assessments of vegetation composition were carried out in four meadow habitats. These habitats, referred to as C, F, L, and S, represent a gradient of increasing grazing disturbance intensity (Table 1) and were 500–1000 m apart. C-ve, F-ve, L-ve, S-ve represent vegetation in habitats C, F, L, and S, respectively, and C-sb, F-sb, L-sb, and S-sb represent the soil seed bank. Germination studies of seeds in soil samples were conducted in the Research Station of Alpine Meadow and Wetland Ecosystems of Lanzhou University (Hezuo Branch Station) (N34°55′, E102°53′), Gansu, China, located on the eastern Tibetan plateau at an elevation of 2900 m above sea level. The average temperature there is 2.0 °C and precipitation is 557.8 mm.
Soil seed bank sampling
Soil samples were collected three times: in May 2005 and May 2006, before seed germination in the field, and in July 2005 after the spring germination flush but before dispersal of the current season seeds. Ten randomly selected replicate sites (2 m × 2 m in May, 2005; 20 m × 20 m in July, 2005 and May, 2006) were established in each habitat, each with the same slope and exposure. In each of 10 plots (0.4 m × 1 m in May, 2005; 5 m × 5 m in July, 2005 and May, 2006), randomly distributed in each site, 10 cylindrical soil cores (3.6 cm diameter) were taken randomly. The soil cores were separated into three layers, shallow (0–2 cm deep), mid (2–7 cm deep), and deep (7–12 cm deep). For each plot, the ten cores from each depth were pooled. Overall, there were 30 samples from each site (10 samples for each layer), and 300 soil samples for each habitat type.
Treatment of soil samples and maintenance of seed trays
The soil samples were stored for 15 days until they could be processed. The seed bank was sampled by the concentration method followed by seedling emergence as described by Ter Heerdt et al (1996) and Ma et al. (2009, 2011, 2012). That is, samples were sieved, after which they were washed through a coarse (4 mm mesh) and then a fine (0.2 mm mesh) sieve to remove debris, root fragments, and coarse and fine soil material. Visual inspection of the coarse particles retained by the wide mesh sieve revealed that no large seeds were also removed. The concentrated samples were spread evenly on a layer of sterile sand (sterilized at 140 °C for 24 h) in sterile plastic germination trays (width 30 cm). Depth of the soil layer was less than 2 cm. Thirty control trays with only sterilized sand were set alongside the experimental trays and used to determine if there were any airborne seeds or contaminants from other samples. Trays were watered regularly. Emerging seedlings were removed, and those that could not be identified immediately were grown separately until identification was possible. Soil samples were carefully turned over following cessation of the initial germination flush to facilitate the emergence of new seedlings. After 5 months when no more seedlings emerged for several consecutive weeks, sampling was stopped. Subsequent sifting and careful inspection found that no seeds remained.
Seed mass
Seeds of 138 of the 155 species found in the seed bank were collected in Maqu from August to October in 2005 at the start of natural dispersal. Seeds of a given species were pooled, mixed well, and three subsamples of 100 seeds selected. Each subsample was placed into an envelope. Seed mass was defined as the mass of the embryo and endosperm, plus the seed coat; dispersal structures were not included. The average mass of the three subsamples was used as the seed mass variable.
Above-ground vegetation sampling
Vegetation sampling was performed during the peak of the summer growing season (July, 2005 and 2006). On the basis of species-area curve on the eastern alpine meadow of Tibetan plateau, we calculated that the minimum sampling area was 50 × 50 cm. Thus, we used 50 cm × 50 cm quadrats placed randomly within each of the four habitats to collect soil seed bank samples. In 2005, five quadrats were used in each habitat, and in 2006 10 quadrats were used in each habitat. We recorded the presence and cover of all species within each quadrat. Cover was estimated using the Braun-Blanquet scale (Westhoff and Van der Maarel 1978). Clonal species were examined by presence of ramets or tillers and species with sexual reproduction were examined by presence of seedlings (Harper 1977).
Data analysis
To estimate seasonal variation in the seed bank, seed bank depletion (e.g. Funes et al. 2003) and accumulation were calculated as:
Species richness in aboveground vegetation was analysed using a two-factor repeated measures ANOVA (factors disturbance level and sampling time) to test for general effects and their interactions and using post-hoc tests to reveal the factor-level differences. Seed bank density and species richness in soil seed bank were analysed using three-factor repeated-measures ANOVA (factors disturbance level, sampling time, and soil depth) to test for general effects and their interactions and using post-hoc tests to reveal the factor-level differences. The differences of proportion of life form in vegetation and soil seed bank, seed mass, seed bank depletion and accumulation in different disturbance intensities were compared by ANOVA and Tukey range test. To meet the requirement of variance homogeneity, the data were log-transformed prior to analysis. All ANOVA tests were conducted with a SPSS 13.0 program.
Species composition similarity among different vegetation and seed banks in three seasons (May, July, 2005; May, 2006) was analyzed using a nonmetric multidimensional scaling (NMDS), a nonparametric ordination technique that represents a similarity matrix in a multidimensional space and preserves the ordering of relationships among the original items (Legendre and Legendre 1998). Similarity between the seed bank and the vegetation within each of the four habitats was tested using a NMDS, and data for each season were analyzed separately. Ordination was made using the R-program for Windows version 2.0.7, applying package VEGAN by Jari Oksanen. NMDS is considered the best method for graphical representation of floristic relationships (Clarke 1993). We calculated similarity matrices using the Bray Curtis coefficient. All ordinations were based on frequency data.
Results
Aboveground vegetation
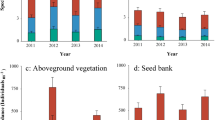
We recorded 79 species, belonging to 21 families in 2005, and 15.4 % of the species were annuals, 2.6 % biennials, and 82.1 % perennial herbs. In 2006, 62 species were recorded, belonging to 17 families, and 9.7 % of the species were annuals, 4.8 % biennials, and 85.5 % perennial herbs. The proportion of perennial species decreased with disturbance intensity (Fig. 1). Species richness per quadrat differed significantly along a disturbance gradient both in 2005 and 2006 (Table 2, Fig. 2), with the lowest species richness in the most disturbed habitat, S. In contrast, species richness per quadrat did not differ significantly between two seasons (Table 2). There was no interaction between disturbance level and season.
The proportion of life forms changes (per quadrat, mean ± SE, n = 10) in vegetation and seed bank in different seasons along a disturbance gradient in the Tibetan Plateau. Letters indicate significant differences (ANOVA, Tukey range test) in proportion of lifeform among disturbance types within one date. Black bar: annual and biennial species, gray bar: perennial species. Abbreviations of sites names: C: Control meadow site, F: Fenced site, L: Largely disturbed site, S: Seriously disturbed site (disturbance C < F < L < S)
Species richness (per quadrat, mean ± SE, n = 10) changes in vegetation along a disturbance gradient in the Tibetan Plateau. Letters indicate significant differences (ANOVA, Tukey range test) in species richness among disturbance types within a date. Abbreviations of sites names: C: Control meadow site, F: Fenced site, L: Largely disturbed site, S: Seriously disturbed site (disturbance C < F < L < S)
The plant communities in habitat S from 2005 to 2006 were dominated by clonal species, such as Potentilla anserine,Elymus dahuricus, and Poa pratensis. Plant cover of Potentilla anserine was 52.0 ± 22.8 % in 2005 and 46.1 ± 12.6 % in 2006. The plant communities in habitat F from 2005 to 2006 were dominated by clonal species, such as Elymus dahuricus, Koeleria cristata, Kobresia graminifolia, Poa poophagorum, Kobresia capillifolia, and Stipa aliena. For example, plant cover of these clonal species was 56.0 ± 4.4 % in 2005 and 59.8 ± 3.5 % in 2006. The dominate clonal species in habitat S from 2005 to 2006 were Kobresia graminifolia, Elymus dahuricus, Poa poophagorum, Stipa aliena, Roegneria nutans, Kobresia capillifolia with their cover being 63.9 ± 6.0 % in 2005 and 47.9 ± 2.8 % in 2006.
The results of NMDS showed that the first Dim separated habitat S-ve from other habitats in both 2005 and 2006, whereas C-ve, F-ve, and L-ve overlapped with each other along Dim 1 (Fig. 3).
Two-dimensional nonmetric multidimensional scaling (NMDS) ordination of seed banks and vegetation along a disturbance gradient, and seed banks and vegetation in different seasons in alpine meadows of the Tibetan Plateau. (Stress value = 0.10 in May, 2005; Stress value = 0.11 in July, 2005 and May, 2006; Stress value = 0.15 in vegetation change; Stress value = 0.17 in seed bank change). Ordination was based on species frequency data. Different marks represent different vegetation and seed bank types. There were 10 quadrats in each seed bank, 5 plots for the vegetation in 2005, and 10 plots for the vegetation in 2006. The location of ordination points within each diagram indicates the degree of similarity between each one. Abbreviations of sites names: C: Control meadow site, F: Fenced site, L: Largely disturbed site, S: Seriously disturbed site (disturbance C < F < L < S)
Soil seed bank
Visual inspection of soil samples after germination had ceased revealed very few nongerminated seeds, indicating an accurate estimation of the seed bank. No seedlings were recorded in the control trays, indicating that there were no airborne seed contaminants. Overall, 155 species emerged from the three seasonal sets of seed bank samples. Three species could only be identified to the family level (Compositae sp. Gramineae sp. and Polygonaceae sp.) and two only to genus (Saussurea sp. and Pedicularis sp.).
Altogether 17531 seedlings from 87 species emerged from the May 2005 soil samples, and 19.6 % of them were annuals, 5.7 % biennials, and 74.7 % perennial herbs. In the July, 2005 samples, 14195 seedlings from 130 species were recorded with 26.4 % of them being annuals, 5.4 % biennials, and 68.2 % perennial herbs. A total of 24098 seedlings from 112 species were recorded from May 2006 samples, and 26.6 % of them were annuals, 3.7 % biennials, and 69.7 % perennial herbs. The proportion of perennial species in the seed bank showed a decreasing trend with disturbance gradient in each season (Fig. 1).
Seed density and species richness change
Mean seed density and species richness per plot differed significantly among the four habitats, different seasons and soil depths (Table 2, Fig. 4). Both for seed density and species richness, there was a significant interaction between disturbance level—season, disturbance level—soil depth, and season—soil depth (Table 2). Three way interactions were not detected (Table 2).
Mean species richness (per plot) (top two rows) and seed density m−2 (±SE) (bottom two rows) (±SE) at four soil depths classes along a disturbance gradient of Tibetan Plateau. Letters indicate significant differences (ANOVA, Tukey range test) among disturbance types within a date. Abbreviations of sites names: C: Control meadow site, F: Fenced site, L: Largely disturbed site, S: Seriously disturbed site (disturbance C < F < L < S)
The highest density was recorded in the most disturbed site (S), while the lowest density was recorded for the control site (C), and increasing from habitat C to habitat S regardless of season, separate layer, or all layers combined (Table 2, Fig. 4). The highest seed densities were found in surface samples (0–2 cm), and decreased with depth (Fig. 4). The species richness per plot did not differ significantly with layer or total for all layers combined along the disturbance gradient, and it showed an increase trend in 2–7 cm and 0–12 cm with increase in disturbance; the lowest soil depth (7–12 cm) showed no trend (Table 2, Fig. 4). The Shannon-Wiener Index per plot differed significantly among the four habitats (Fig. 5) and was highest in habitat F and lowest in habitat S. The species diversity index decreased with an increase in disturbance intensity.
The Shannon-wiener index per plot (n = 10) found in soil seed bank along a disturbance gradient. Different letters indicate significant differences, indicated by different superscripts assessed by a Tukey-test after one way ANOVA on differences between disturbance types for a respective sampling time. Abbreviations of sites names: C: Control meadow site, F: Fenced site, L: Largely disturbed site, S: Seriously disturbed site (disturbance C < F < L < S)
Seasonal seed bank dynamics
Both seed bank density and species richness differed significantly in each depth layer and total for all layers among seasons, except seed density in the mid-layer (2–7 cm) (Table 3). The NMDS results showed that the seed bank communities were similar to each other with disturbance intensity in each season and among different seasons (Fig. 3).
Depletion and accumulation
Seasonal depletion in seed bank density differed significantly along the disturbance gradient (F = 8.268, p < 0.001), increasing with disturbance; however, accumulation did not differ (F = 1.178, p > 0.05). The highest accumulation (46.9 ± 16.2 %) and depletion (27.9 ± 15.2 %) occurred in habitat S (Fig. 6).
Seed bank depletion (May, 2005 to July, 2005) and accumulation (July, 2005 to May, 2006) (mean ± SE, n = 10) in alpine meadow along a disturbance gradient in Tibetan Plateau. Letters indicate significant differences (ANOVA, Tukey range test) in depletion among disturbance types within a date. Abbreviations of sites names: C: Control meadow site, F: Fenced site, L: Largely disturbed site, S: Seriously disturbed site (disturbance C < F < L < S)
Seed mass
For the 138 species examined, mass per seed ranged from 0.0018 mg (Juncus effusus) to 4.5354 mg (Cynoglossum zeylanicum). Seed mass did not differ significantly along the disturbance gradient in each season (F = 0.374, p > 0.05 in May, 2005; F = 0.026, p > 0.05 in July, 2005; F = 0.593, p > 0.05 in May, 2006). There was also no trend in seed size distribution with disturbance or with soil depth.
Relationship of soil seed bank and vegetation
In general, species composition of seed bank and vegetation exhibited a fairly uniform pattern in each season (Fig. 3). In vegetation communities, S-ve was different from the other three communities that clustered together. The major vegetation changes in habitat S were not reflected in the seed bank. The seed bank communities overlapped with each other, except S-sb that only has a little difference with other seed bank groups. Among the four seed bank and four vegetation communities, C-ve, L-ve, and F-ve were the most different along dim 1 and 2 axes; however, community S-sb was very close to community S-ve relative to the other seed bank communities and their corresponding vegetation communities. Aboveground plant community of habitat S strongly reflected its seed bank composition (Fig. 3).
Discussion
Soil seed bank changes with disturbance intensity and season
Many alpine areas are known to be seed limited in the soil seed bank (e.g. Turnbull et al. 2000; Eskelinen and Virtanen 2005). However, we found a relatively high number of seeds in the seed bank in the investigated alpine meadows (Fig. 4). The seed densities (3069–6105 m−2 in May, 2005, 2991–4297 m−2 in July, 2005, and 4563–8355 m−2 in May, 2006) are similar to those from our previous Tibetan Plateau studies (Ma et al. 2010a, b), and larger than other alpine meadow, arctic meadows and high-altitude meadows (e.g. Funes et al. 2003; Bossuyt et al. 2007; Bueno et al. 2011). Large amounts of seeds occur at such sites because cold climates could contribute to the maintenance of many seeds in the soil (Cavieres and Arroyo 2000; Ma et al. 2010a, b).
Density of seeds in the soil increased with disturbance intensity, a result consistent with our first hypothesis. This may be due to the interaction of several factors. There is selection for persistence-related traits (k-strategy) under less disturbed conditions (e.g., Diaz et al. 2004) and a strong selection for reproductive traits (r-strategy, Grime 1979) under disturbed conditions. The high relative contribution of ruderal strategists in the seed banks can be seen as a function of their rapid growth, high seed production, and seed bank persistence, which are adaptations to disturbed habitats (Thompson et al. 1997; Fenner and Thompson 2005; Wellstein et al. 2007). We found that the proportions of annuals and biennials (often r-strategists) in the vegetation increased with disturbance, and they were relatively high in the most disturbed habitat (S) (Fig. 1). Hence, we think competitive strategies in habitats C and F are gradually replaced by ruderal strategies with increasing disturbance intensity.
In some cases, species from disturbed sites invest predominantly in generative propagation and produce large amounts of seeds (e.g. Bakker and Berendse 1999). Increased disturbance may enhance seed production at the community level (Klimkowska et al. 2009). Thus, ruderal species generally make a higher contribution to the seed bank because of high seed production. Our results also indicate that disturbance favours high seed production (e.g. Grime 2002).
The dominant seed bank species in disturbed habitat S were Plantago asiatica, Artemisia desertorum, and Poa annua, which together made up 70.7 % of the seedlings recorded in May, 2005, 55.2 % in July, 2005, and 56.1 % in May, 2006. Therefore, seed density to a very large extent depends on the distribution and seed production of these species. However, they occurred only sporadically in the aboveground vegetation, indicating that their seeds have persistent seed banks. There is selection for more persistent seeds under conditions of disturbance (Thompson et al. 1998). This is supported by our data, which show that density of buried seeds increased as disturbance increased.
Overall, species richness and diversity showed a decreased trend along the disturbance gradient (Figs. 4 and 5). We know that seed bank size is determined by the balance between seed input and output (Fenner 1985), and that floristic composition is determined by the current species composition and by vegetation history (Grandin and Rydin 1998). Our results also showed that species richness of the vegetation decreased significantly along the disturbance gradient (Fig. 2). Species richness, however, showed no clear trend in the deepest soil depth (7–12 cm) with increased disturbance. This was due to a larger number of long-lived seeds. The species composition of this layer, made up of persistent seed bank species, is not influenced by seed input to the surface layers. Although the aboveground vegetation changed with the disturbance intensity, the persistent seed bank, varied little.
The greatest seed bank accumulation and depletion occurred in the most disturbed habitat (S), and each increased with increase in disturbance (Fig. 6). Thus, similarity between soil seed bank and vegetation increased along the disturbance gradient, and seed bank turnover rates increased with an increase in disturbance.
The result of NMDS showed that the seed bank groups were close to each other with disturbance intensity in each season (Fig. 3. May, 2005; July, 2005; May, 2006) and among seasons (Fig. 3. Soil seed bank change). Although seed bank density and species richness had an obvious seasonal change (Table 3), there was no difference in seed bank species composition under different disturbance intensities and among different seasons (Fig. 3).
In a previous study, we found persistence to be the most frequent seed bank strategy of Tibetan Plateau alpine meadow species (Ma et al. 2010b). In the present study, the most widely distributed and dominant species in the seed banks were Plantago asiatica, Potentilla fragarioides, Artemisia desertorum, Chenopodium album, and Hypecoum leptocarpum, and they accounted for 64.9 % of the seedlings recorded in May, 2005, 50.3 % in July, 2005, and 47.7 % in May, 2006. Although the transient part of seed bank changed every year, the persistent seed bank did not vary with change in the disturbance intensity. Hence, we think the seed bank was not significantly influenced by season.
Some studies have shown that small-seeded species are more dependent on disturbance for establishment than large-seeded species and that large seed size permitted seedling establishment in closed vegetation (Burke and Grime 1996). Thus, small seed size was identified as a characteristic functional trait of gap colonizers (Kalamees and Zobel 2002). In contrast, Lavorel et al. (1999) found no association between seed size and colonization. Our results support the latter opinion as we found no trend in seed size distribution with disturbance in seed bank; consequently, our third hypothesis with regard to seed size was not supported. Seed mass ranged from 0.0018 mg (Juncus effusus) to 4.5354 mg (Cynoglossum zeylanicum), but this is a smaller range of sizes than recorded in other studies (Leishman and Westoby 1998; Moles et al. 2000). The limited range of seed size in Tibetan Plateau vegetation may be due to evolutionary and ecological constraints including glaciation history, disturbance regimes, and predation pressure on larger seeds.
Role of soil seed bank
Similarity between species composition of vegetation and seed bank as a whole was low, thus agreeing with results from other alpine studies (e.g. Arroyo et al. 1999; Ma et al. 2010a, b). Indeed, this low similarity between vegetation and seed banks seems to be a characteristic of alpine environments which have a relatively short growing season and where there is less reliance on seed banks for reproduction in favour of clonal strategies (Körner 1999; Bueno et al. 2011). The species composition of the seed bank and extant vegetation was very different from each other in C and F, the least disturbed habitats. Thus, the seed bank would play a minor role in regeneration of the vegetation of C and F, which are dominated by clonal species of Poaceae (e.g., Elymus dahuricus, Poa poophagorum, Roegneria nutans, Festuca ovina, Stipa aliena, and Poa pratensis) and Cyperaceae (e.g., Kobresia graminifolia, Kobresia capillifolia, Kobresia humilis, and Scirpus pumilus). The C and F communities of the control and fenced meadows often regenerate vegetatively forming very stable communities, and lack of similarity in these undisturbed habitats is partly due to low seed input/production by the clonal species. Also, there is little opportunity for ruderals to become established in the closed canopy of these plant communities that are dominated by clonal species.
It is probable that the role of the seed bank in alpine plant community regeneration from large disturbances is relative low. This may be due to the following reason. Firstly, many species in alpine area do not produce many seeds (Bossuyt and Honnay 2008) and have relatively short-distance seed dispersal (Körner 1999). Secondly, large disturbances may not represent a chance for colonization from seeds (Bueno et al. 2011). In addition, clonal strategies prevail among the dominant species in alpine grasslands (Körner 1999), which limits establishment from the seed bank. However, we found that the soil seed bank has the potential to play an important regeneration role in the most seriously disturbed habitat (S) (Fig. 3), and this situation was apparent in each season. This result is consistent with our second hypothesis. Similarly, others have observed a clear increase in the similarity between vegetation and soil seed bank under conditions of increasing disturbance (Bekker et al. 2000). In seriously disturbed habitat (S), cattle activities such as grazing (removing biomass) and trampling affect vegetation dynamics differently at a very fine scale (Kohler et al. 2004). Seeds at the soil surface receive more light and greater temperature fluctuation than those in the soil. The increase in light in disturbed sites increases the probabilities of seedling emergence and seedling establishment (Fenner 2000). In disturbed areas, there is more space for the establishment of opportunistic, annual species that produce large amounts of seeds. Hence, disturbances in alpine plant communities assure the presence of regeneration niches. There is a tradeoff, however, in the maintenance of sustainable meadows and deterioration associated with overgrazing and lack of regeneration potential.
Disturbances may change the nature of community seed banks from long-term persistent to short-term persistent or transient seed banks, because of increased exposure of seeds to germination conditions (Bueno et al. 2011). In large disturbances, where the lateral expansion of dominant grasses is insufficient to recover bare ground efficiently (Kotanen 1997), soil seed banks seem to play a more determinant role in recovery (Thompson et al. 1998). Moreover, fertilization from urine and dung stimulate plant growth and reproduction; with dung there also is the possible addition of seeds from new species (e.g. Bakker and Olff 2003).
The dominant vegetation species in the seriously disturbed habitat S were Potentilla anserine, Elymus dahuricus, and Poa pratensis. Although these perennial species might not produce seeds in highly degraded sites, they persisted in the vegetation because they are able to spread clonally after disturbances. An especially good colonizer, Potentilla anserina had significantly larger plant cover in habitat S (52.0 ± 22.8 % in 2005 and 46.1 ± 12.6 % in 2006) than in the less disturbed habitats. This rhizomatous species quickly colonized trampled and mowed plots.
Our results show that soil seed banks together with the clonal growth make a contribution to aboveground vegetation in seriously disturbed alpine meadow habitats. Further, the overall high species richness and high seed bank densities in habitat S suggest that the soil seed bank can potentially play an important role in disturbed habitat restoration.
Abbreviations
- NMDS:
-
Nonmetric multidimensional scaling
References
Arroyo MTK, Cavieres LA, Castor C, Humaña AM (1999) Persistent seed bank and standing vegetation in high alpine site in the central Chilean Andes. Oecologia 119:126–132
Bakker JP, Berendse F (1999) Constraints in the restoration of ecological diversity in grassland and heathland communities. Trends Ecol Evol 14:63–68
Bakker ES, Olff H (2003) Impact of different-sized herbivores on recruitment opportunities for subordinate herbs in grasslands. J Veg Sci 14:465–474
Bekker RM, Verweij GL, Bakker JP, Fresco LFM (2000) Soil seed bank dynamics in hay field succession. J Ecol 88:594–607
Bossuyt B, Honnay O (2008) Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J Veg Sci 19:875–884
Bossuyt B, Cosyns E, Hoffmann M (2007) The role of soil seed banks in the restoration of dry acidic dune grassland after burning of Ulex europaeus. Appl Veg Sci 10:131–138
Bråthen KA, Junttila O (2006) Infertile times: response to damage in genets of the clonal sedge Carex bigelowii. Plant Ecol 187:83–95
Bueno CG, Reiné R, Alados CL, Gómez-García D (2011) Effects of large wild boar disturbances on alpine soil seed banks. Basic Appl Ecol 12:125–133
Burke MJW, Grime JP (1996) An experimental study of plant community invisibility. Ecology 77:776–790
Cavieres LA, Arroyo MTK (2000) Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae): altitudinal variation in the Mediterranean Andes of Central Chile. Plant Ecol 149:1–8
Chen LZ, Wang ZW (1999) The impacts of human alteration on ecosystem and diversity. Scientific Technology Press, Zhejiang, China
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australia J Ecol 18:117–143
Cooper EJ (2006) Reindeer grazing reduces seed and propagule bank in the high Arctic. Can J Bot 84:1740–1752
Cooper EJ, Wookey PA (2003) Floral herbivory of Dryas octopetala by Svalbard reindeer. Arct Antarct Alp Res 35:369–376
Diaz S, Hodgson JG, Thompson K et al (2004) The plant traits that drive ecosystems: evidence from three continents. J Veg Sci 15:295–304
Eriksson A, Eriksson O (1997) Seedling recruitment in semi-natural pastures: the effects of disturbance, seed size, phenology and seed bank. Nord J Bot 17:468–482
Eskelinen A, Virtanen R (2005) Local and regional processes in low-productive mountain plant communities: the roles of seed and microsite limitation in relation to grazing. Oikos 110:360–368
Fenner M (1985) Seed ecology. Chapman and Hall, London, UK
Fenner M (2000) The ecology of regeneration in plant community, 2nd edn. CABI Publisher, London
Fenner M, Thompson K (2005) The ecology of seeds. Cambridge University Press, Cambridge UK
Funes G, Basconcelo S, Díaz S, Cabido M (2003) Seed bank dynamics in tall-tussock grasslands along an altitudinal gradient. J Veg Sci 14(2):253–258
Grandin U, Rydin H (1998) Attributes of the seed bank after a century of primary succession on islands in Lake Hjalmaren, Sweden. J Ecol 86:293–303
Grime JP (1979) Plant strategies and vegetation processes. John Wiley, Chichester, UK
Grime JP (2002) Declining plant diversity: empty niches or functional shifts? J Veg Sci 13:457–460
Harper JL (1977) Population biology of plants. Academic, London
Huston M, Smith T (1987) Plant succession: life history and competition. Am Nat 130:168–198
Jalili A, Hamzeh’ee B, Asri Y et al (2003) Soil seed banks in the Arasbaran protected area of Iran and their significance for conservation management. Biol Conserv 109:425–431
Kalamees R, Zobel M (2002) The role of the seed bank in gap regeneration in a calcareous grassland community. Ecology 83:1017–1025
Kalamees R, Püssa K, Zobel K, Zobel M (2012) Restoration potential of the persistent soil seed bank in successional calcareous (alvar) grasslands in Estonia. Appl Veg Sci 15:208–218
Klimkowska A, van Diggelen R, den Held S, Brienen R, Verbeek S, Vegelin K (2009) Seed production in fens and fen meadows along a disturbance gradient. Appl Veg Sci 12:304–315
Kohler F, Gillet F, Gobat JM, Buttler A (2004) Seasonal vegetation changes in mountain pastures due to simulated effects of cattle grazing. J Veg Sci 15:143–150
Körner C (1999) Alpine plant life. Functional plant ecology of high mountain ecosystems. Springer, Berlin
Kotanen P (1997) Effects of gap area and shape on recolonization by grassland plants with differing reproductive strategies. Can J Bot 75:352–361
Lavorel S, Rochette C, Lebreton JD (1999) Functional groups for response to disturbance in Mediterranean old fields. Oikos 84:480–498
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, New York
Leishman MR, Westoby M (1998) Seed size and shape are not related to persistence in soil in Australia in the same way as in Britain. Funct Ecol 12:480–485
Levassor C, Ortega M, Peco B (1990) Seed bank dynamics of Mediterranean pastures subjected to mechanical disturbance. J Veg Sci 1:339–344
Ma M, Du G, Zhou X (2009) Role of the soil seed bank during succession in a subalpine meadow on the Tibetan plateau. Arct Antarct Alp Res 41(4):469–477
Ma M, Zhou X, Du G (2010a) Role of soil seed bank along a disturbance gradient in an alpine meadow on the Tibet plateau. Flora 205(2):128–134
Ma M, Zhou X, Wang G, Ma Z, Du G (2010b) Seasonal dynamics in alpine meadow seed banks along an altitudinal gradient on the Tibetan Plateau. Plant Soil 336:291–302
Ma M, Zhou X, Du G (2011) Soil seed bank dynamics in alpine wetland succession on the Tibetan Plateau. Plant Soil 346:19–28
Ma M, Zhou X, Ma Z, Du G (2012) Composition of the soil seed bank and vegetation changes after wetland drying and soil salinization on the Tibetan Plateau. Ecol Eng 44:18–24
Matus G, Papp M, Tothmeresz B (2005) Impact of management on vegetation dynamics and seed bank formation of inland dune grassland in Hungary. Flora 200:296–306
Michaela D, Wolfgang S (2009) The relationship between soil seed bank, above-ground vegetation and disturbance intensity on old-field successional permanent plots. Appl Veg Sci 12:415–428
Mitlacher K, Poschlod P, Rosén E, Bakker JP (2002) Restoration of wooded meadows - a comparative analysis along a chronosequence on öland (Sweden). Appl Veg Sci 5:63–73
Moles AT, Hodson DW, Webb CJ (2000) Seed size and shape and persistence in the soil in the New Zealand flora. Oikos 89:541–545
Moore PD (1980) Soil seed banks. Nature 284:123–124
Norbert H, Annette O (2004) Assessing soil seed bank persistence in flood-meadows: The search for reliable traits. J Veg Sci 15:93–100
Pickett STA, White PS (1985) The ecology of natural disturbance and patch dynamics. Academic, San Diego, CA, US
Pierce SM, Cowling RM (1991) Disturbance regimes as determinants of seed banks in coastal dune vegetation of the southeastern Cape. J Veg Sci 2:403–412
Satterthwaite WH, Holl KD, Hayes GF, Barber AL (2007) Seed banks in plant conservation: case study of Santa Cruz tarplant restoration. Biol Conserv 135:57–66
Scott K, Stterfield S, Douglas M, Andersen A (2010) Soil seed bank confer resilience to savanna grass-layer plants during seasonal disturbance. Acta Oecol 36:202–210
Sternberg M, Gutman M, Perevolotsky A, Kigel J (2003) Effects of grazing on soil seed bank dynamics: an approach with functional groups. J Veg Sci 14:375–386
ter Heerdt GNJ, Verweij GL, Bakker RM, Bakker JP (1996) An improved method for seed bank analysis: seedling emergence after removing the soil by sieving. Funct Ecol 10:144–151
Thompson K, Bakker JP, Bekker RM (1997) The soil seed banks of North West Europe: methodology, density and longevity. Cambridge University Press, Cambridge, UK
Thompson K, Bakker JP, Bekker RM, Hodgson JG (1998) Ecological correlates of seed persistence in soil in the north-west European flora. J Ecol 86:163–169
Turnbull LA, Crawley MJ, Rees M (2000) Are plant populations seed-limited? A review of seed sowing experiments. Oikos 88:225–238
van Andel J, Grootjans AP (2005) Concepts in restoration ecology. In: van Andel J, Aronson J (eds) Restoration ecology. Blackwell Publishing, Oxford, pp 16–30
Wellstein C, Otte A, Waldhardt R (2007) Seed bank diversity in mesic grasslands in relation to vegetation type, management and site conditions. J Veg Sci 18:153–162
Westhoff V, Van Der Maarel E (1978) The Braun Blanquet approach. In: Whittaker RH (ed) Classification of plant communities. Junk, The Hague, pp 297–399
Acknowledgements
We are grateful to Prof. Mary A. Leck and Prof. Carol C. Baskin for carefully editing an earlier draft of the manuscript. The study was funded by the Natural Science Foundation of China (Grant No. 40930533 and 41101527), the Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20110211120026), and the Fundamental Research Funds for the Central Universities (Grant No. lzujbky-2011-41).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeffrey Walck.
Rights and permissions
About this article
Cite this article
Ma, M., Zhou, X. & Du, G. Effects of disturbance intensity on seasonal dynamics of alpine meadow soil seed banks on the Tibetan Plateau. Plant Soil 369, 283–295 (2013). https://doi.org/10.1007/s11104-012-1560-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1560-5