Abstract
Background and aims
Plants can develop various root traits which may contribute to their nutrient acquisition. We investigated the occurrence of five root traits among species of genus Carex to determine their frequency, mutual associations and dependence on taxonomy (subgenus) or habitat.
Methods
Root samples of 40 species were collected in their natural habitats within Russia and Belarus. They were examined microscopically to quantify the abundance of AM-fungal hyphae (H), arbuscules (A) and vesicles (V), dark septate endophyte (DSE), typical (long) root hairs (TRH), bulbous (shortened) root hairs (BRH) and dauciform roots (DR).
Results
The frequency of root traits decreased in the order TRH (100% of the species), DSE (80%), BRH (43%), A and V (25%) and DR (23%). Most species possessed 2–4 different traits, but up to five were possible, even on a single plant. Traits occurred largely independently of each other. DR were only found in subgenus Carex and mainly in grasslands. DSE and BRH were more abundant in subgenus Carex. BRH were most abundant in wetlands, TRH were shortest and thinnest in grasslands.
Conclusions
A diversity of root traits exists in the genus Carex, with variation both among and within species. The abundance and size of traits exhibits some taxonomic and ecological patterns, which differ for each trait.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can improve their nutrition through a wide range of root traits (Vance et al. 2003; Lambers et al. 2008). These include symbiotic associations with fungi, bacteria or prokaryotes, as well as specialized structures such as root hairs, cluster roots and dauciform roots (Bates and Lynch 2001; Shane and Lambers 2005; Shane et al. 2005).
Most land plants (over 80% of the species and 92% of the families) acquire part of their nutrients via symbioses with mycorrhizal fungi (Wang and Qiu 2006; Brundrett 2009), reflecting the importance of this strategy for nutrient acquisition. However, some plant taxa are usually not or only sparsely colonised by mycorrhizal fungi and do not establish a functional symbiosis due to the lack of symbiosis-specific genes (Delaux et al. 2014). These non-mycorrhizal plants include specialized life forms such as carnivorous, parasitic or aquatic plants, plants with hairy root clusters on soils with low P availability, and plants growing in arid or disturbed habitats (Brundrett 2009; Lambers et al. 2011). Thus, non-mycorrhizal roots are important in a wide range of environments. Some plant families regarded as primarily non-mycorrhizal include many widespread and abundant plant species, such as the Brassicaceae, Caryophyllaceae, Chenopodiaceae, Cyperaceae, Juncaceae and Polygonaceae (Brundrett 2009).
The obvious success of non-mycorrhizal species raises the question whether the absence of mycorrhiza promotes the development of alternative root traits (Delaux et al. 2014). In other words, are alternative root traits more frequent or on average better developed in non-mycorrhizal plants than in mycorrhizal ones?
We investigated this question for sedges of the genus Carex L. (Cyperaceae). Carex species are ideally suited for such an investigation because they are widespread and both taxonomically and ecologically highly diverse as a result of fast speciation during the past 20 million years (Spalink et al. 2016). There are about 2000 Carex species worldwide (Global Carex Group 2015), and some 450 species in Russia and adjacent states (Egorova 1999). Cyperaceae are usually regarded as non-mycorrhizal, but the frequent observation of mycorrhizal fungi in their roots, sometimes even with arbuscules (Muthukumar et al. 2004; Veselkin et al. 2014a), has caused some authors to propose a possible classification of Cyperaceae as variable non-mycorrhizal-arbuscular mycorrhizal (NM-AM) family (Brundrett 2009). Their actual root colonisation may then depend on edaphic factors and/or co-occurring host plants (Muthukumar et al. 2004; Brundrett 2009). Although occasional arbuscules do not imply mycotrophy (Brundrett 2009), some studies reported colonisation patterns or responses to soil nutrients suggesting a functional symbiosis (Meney et al. 1993; Lagrange et al. 2013).
Other root traits reported for the genus Carex include dark septate endophytes (DSE), typical root hairs (TRH), bulbous root hairs (BRH) and dauciform roots (DR). DSE are widespread fungi that colonize root tissues without inducing special structures in the host plant. They can solubilise unavailable organic and inorganic phosphates in soil, so that they may indirectly promote phosphorus uptake by plants (Barrow and Osuna 2002; Della Monica et al. 2015). TRH are common in the vast majority of terrestrial plants; they greatly increase the soil volume exploited by plant roots. BRH are short outgrowths of rhizodermis cells with a length approximately equal to the diameter (Miller et al. 1999; Ghosh et al. 2014). DR are specific to the Cyperaceae and consist in swollen lateral root axes densely covered with long root hairs (Davies et al. 1973; Shane et al. 2005). They exude carboxylates and phosphatases, similar to cluster roots (Shane et al. 2006; Playsted et al. 2006).
Some further root traits have been described only occasionally for Carex species. There is a single report of ectomycorrhizal-like structures in Carex flacca (Harrington and Mitchell 2002), although these were probably not functional (Brundrett 2009). Functional ectomycorrhiza have been for some Kobresia species (Massicotte et al. 1998; Lipson et al. 1999). The presence of bacteria inside roots of Carex has also been reported (Qin et al. 2008; Song et al. 2011).
Many studies of specialized root traits in Carex have focused on the structure, occurrence or functioning of one particular trait (e.g. Shane et al. 2005, 2006; Playsted et al. 2006; Güsewell 2017). Fewer studies have simultaneously analysed two or more traits (Miller et al. 1999; Ghosh et al. 2014; Nobis et al. 2015). Yet, it has often been assumed that some root traits are developed at the expense of others. In particular, some authors suggested that the occurrence of arbuscular mycorrhiza (AM) is negatively associated with the development of BRH (Miller et al. 1999; Ghosh et al. 2014), the development of TRH (Muthukumar et al. 1999), colonisation by DSE (Weishampel and Bedford 2006; Sieber and Grünig 2013) or the development of DR (Shane et al. 2006).
We are not aware of any simultaneous study of the occurrence of AM, DSE, TRH, BRH and DR among Carex species. We filled this gap by studying the occurrence of these root traits on plants of 40 Carex species sampled in their natural habitats in Russia and Belarus. We specifically addressed the following questions: (1) How frequent is each of these root traits among Carex species? (2) How many different root traits can be found in one species or on a single plant? (3) Is the possession of a certain root trait positively or negatively associated with the possession of other root traits? (4) Is the abundance and size of the relevant root traits related to taxonomic status (subgenus), to the species’ habitat or to a plant’s P status?
Materials and methods
Sampling
Plants were collected in 2012–2014 at five locations within the temperate and boreal zones of Russia and Belarus (Table 1). Natural vegetation ranges from pine-birch forests and steppe grasslands in the South to sparse spruce forest and shrub tundra in the North (Table 1). A total of 40 Carex species were collected. Each species was collected at one site representing its typical habitat in the region (Table S1 in Supplementary Material). Thus, in the following, interspecific differences will include differences among species-specific habitats. Species nomenclature and taxonomic classification (subgenera) follows Egorova (1999), except that subgenus Kreczetoviczia, which is not generally recognized (Global Carex Group 2015), was included in subgenus Carex. One species of the former subgenus Psyllophora (C. obtusata) is now attributed to the Caricoid clade (Spalink et al. 2016).
Five plants per species were collected (see Table S1 for a few exceptions). For tussock-forming species, we sampled one entire tussock, while for rhizomatous species we attempted to sample entire clones, but if this was not possible, we sampled at least five ramets with the associated rhizomes. Root systems of each plant were excavated carefully and washed with water directly in the field. While doing so, roots were checked for the presence or absence of dauciform roots, defined as root sections with swollen axis and a ‘brush’ of root hairs (Davies et al. 1973; Shane et al. 2005, 2006; Playsted et al. 2006). Thereafter, roots were air-dried at room temperature. All further investigations were carried out on the dried root material of each plant.
Measurements
To determine the occurrence of arbuscules (H), vesicles (V), AM fungal hyphae (H) and DSE, a subsample of 5–7 roots attached to one stem or rhizome was removed from each plant. These roots were cleared in 15% КОН at 120°С for 40 min and then stained with aniline blue for at least 1 h. Twenty 1-cm fragments of first- and second-order roots were selected following the centripetal (functional) segment scheme in Berntson (1997), and squashed on microscope slides. The presence of A, V, H and DSE was recorded microscopically (Leica DM 5000B; Leica Microsystems GmbH, Germany; ×200) in five fields of view per 1-cm root fragment (100 fields of view per plant, corresponding to 120 mm root length). DSE were identified by their dark-coloured, usually thin hyphae with septa and frequent anastomoses (Haselwandter and Read 1982; Ruotsalainen et al. 2002). Structures (A, V, H and DSE) visible in the field of view were marked as ‘present’. A field of view containing either A or V or both (i.e. at least one specialized AM structure) was noted as ‘A|V present’. DSE and A|V were scored as ‘present’ on a plant if they were observed in at least one field of view, although structures found only occasionally may not actually contribute to plant nutrition (Muthukumar et al. 2004). Similarly, DSE and A|V were scored as ‘present’ in a species if they were found in at least one plant. Finally, fields of view containing any of A, V or H (meaning that AM-fungi were present), were noted as A|V|H; this information was used to determine the colonisation level by AM fungi (see data analysis).
Root hairs were studied on another root subsample from each plant, again for twenty 1-cm fragments of first- and second-order roots (Berntson 1997). Eight photomicrographs (each with 2–3 fragments) were made per plant using a microscope Leica DM 5000B. The occurrence of root hairs was determined with the software SIAMS MesoPlant (Ltd «SIAMS», Russia). Presence or absence of hairs was recorded for 50 sections with 100 μm length per plant (2–3 sections per 1-cm root fragment). We separately recorded TRH (long and thin) and BRH, defined as roots whose length was less than double the width (Miller et al. 1999). We also measured the length and diameter of one TRH and of one BRH per 100-μm section, as well as root diameter. Finally, the remaining dried root system was checked again carefully for the presence of DR.
We checked the reliability of our sampling procedure in several ways. First, we compared the traits found on air-dried roots to those found on roots preserved more conventionally in 70% ethanol by splitting roots systems of 42 additional plant (seven species) in two halves. Each part was preserved with one method, and then examined for root traits as in the main study (without considering DR, as these were observed on fresh roots). We found TRH on 39 air-dried root halves vs. 41 ethanol-preserved root halves, BRH on 36 plants in both cases, DSE on 21 vs. 18 plants, and A|V on 3 plants in both cases. Percentages of root length with a certain traits were also similar with both preservation methods. We thus concluded that air-drying provided results comparable to ethanol preservation. We then established accumulation curves for the number of traits found on air-dried roots to verify that the 20 root fragments examined in the main study were sufficient to detect all traits present on a plant with high probability (Fig. S1).
Our sample size of five plants per species implies that a trait present in only some plants of a species may have been missed. We estimated the error introduced in this way as follows: If a trait is present in 20% of all plants of a population, the probability of missing it with 5 randomly selected plants would be (1–0.2)5 = 0.33. For multiple species, all having the trait in 20% of the plants, this trait would be missed for approximately half as many species (33%) as those for which it is found (67%). We actually observed DR in 20% of the plants for 2 species, A|V and BRH for 3 species, and DSE for 5 species, suggesting that each of these traits may have been missed for approximately 1–3 species due to having sampled only 5 plants. A trait present in 40% of the plants would be missed in the sample with a probability of 0.08, i.e. only rarely. We further note that similar sample sizes have been used in a number of recent studies (Miller et al. 1999; Muthukumar and Udaiyan 2000; Uma et al. 2010; Zemunik et al. 2015).
Since root traits are plastic, and their expression depends on a plant’s phosphorus (P) status, we analysed shoot P concentrations for three plants per species, if available. Analyses included all living vegetative parts of the collected shoots, excluding inflorescences and senesced leaves. Four species could not be analysed for lack of sufficient material. Plant material was dried at 70 °C, digested for 1 h at 420 °C with concentrated H2SO4 and K2SO4-CuSO4-Kjeltabs, then the P concentration of the digests was determined colorimetrically with the ammonium molybdate-ascorbic acid method.
Data analysis
The colonisation level by AM fungi and DSE (hereafter called ‘abundance’) was calculated for each plant as the proportion of visual fields with A|V|H and DSE, respectively. The abundance of TRH and of BRH was calculated similarly as the proportion of 100-μm segments with these structures. Mean values of the five plants per species (Table S1) were used for species-level analyses. DR roots were only recorded as present-absent at plant or species level. We further calculated mean root hair length and diameter per plant and per species for both TRH and BRH.
Taxonomic and habitat-related differences in the abundance and size of root traits were explored using species-level data, i.e. mean values of the studied plants per species (n = 39, excluding the single species of subgenus Psyllophora). The log (x + 1) transformation was applied to the abundances of A|V|H, DSE and BRH, which were mostly below 30%. The abundance of TRH was square-root (arcsine)-transformed, and size measures were log-transformed if necessary. We also analyzed the total number of root traits (0 to 5) observed in each species. We tested whether these variables differ between the two main subgenera (Carex and Vignea) and among three main habitat types (forest, grassland and wetland) with models including these two factors as fixed, additive effects. The interaction term was not included, as preliminary analyses showed that it was never significant, leading to simple models with 3 degrees of freedom for the effects and 35 df for the residuals. Habitats classified as ‘grassland’ included meadows, steppe and rocky slopes, while ‘wetland’ included mires, shorelines, floodplains and wet tundra. We used linear models for most variables but a logistic model for the presence-absence of DR and a Poisson model for the number of traits, followed by analysis of variance or deviance as appropriate. Type-II tests were used to account for the different representation of the two subgenera in the three habitats (more subgenus Carex species in wetlands).
Shoot P concentrations were compared between the three habitats and the two subgenera with two-way ANOVA based on mean values per species. P concentrations were also compared between plants with and without each root trait and between subgenera Carex and Vignea with two-way ANOVA. Where appropriate, this was followed by Tukey HSD tests comparing all group means to each other. This analysis was done at plant level because trait presence varied among plants of the same species. Shoot P concentrations were also related to the abundance and size of root traits with linear models including one of these variables (transformed as indicated above) and subgenus as predictors. All analyses were carried out using the R statistical software, version 3.1.1 (R Foundation for Statistical Computing, 2014).
Results
Frequency and number of root traits
The five root traits were differently represented among the 40 sedge species: typical root hairs (TRH) were found in all species, while dauciform roots (DR) were only found in nine of them (Figs. 1a and 2). The number of root traits found in one species ranged from one (species with only TRH) to five (in C. buxbaumii and C. rotundata). Most species had 2–4 different traits (Fig. 1b). Even a single plant individual could exhibit all five root traits, but most plants had 1–3 of them (Fig. 1c).
Frequency of root traits among 40 Carex species: (a) number of species with each individual root trait, (see Table 2 for abbreviations), (b) number of species with a certain number of root traits, and (c) number of plants with a certain number of root traits
Symbiotic associations
Structures formed by AM-fungi (A|V|H) were found in the roots of 21 species, or 53% of the 40 species investigated (Table 2). However, A|V were found only in ten (25%) of the species, and only in small numbers. The A|V|H were of Arum-type and did not have any distinct features differing from Arum-type AM in other plant groups. Hyphae were spreading in intercellular spaces; they were aseptate and stained in blue colour by aniline blue. The abundance of A|V|H was generally low (Table S1) with a maximum of 34% in C. buxbaumii growing in a peat bog. Dark septate endophytes (DSE) were found in 32 or 80% of the species (Table 2). The abundance of DSE was generally low (Table S1) with a maximum of 36% in C. obtusata. The mycelium of DSE did not show a strict localization within sedge roots, as we observed it on the root surface, in the cortex of the root, and sometimes in the stele.
In total, 33 species contained A|V and/or DSE, and nine of the ten species with A|V also had DSE. There was no relationship between the occurrence of A|V and DSE: species or individual plants with A|V were colonized by DSE as often as species or plants without A|V (Table 3). Within plants, mycelium of DSE was found on root fragments both with and without A|V.
Root hairs
Typical root hairs (TRH) were found in all 40 Carex species, while bulbous root hairs (BRH) were found in 17 species. TRH were found on 96% of the plants, but their abundance varied widely, ranging from 3% in C. atherodes to 88% in C. globularis (Table S1). BRH were found on 60 (32%) of the plant individuals. Thus, BRH were less frequent among species than TRH but tended to be more abundant in the species which had them.
There was no association between the occurrence of BRH and TRH at the level of species, individual plants (Table 3) or root fragments. Both hair types could occur alone or together with the other root type within a single root fragment. Similarly, there was no association between the occurrence of TRH or BRH on one hand, and A|V or DSE on the other hand, at the level of either species or individual plants (Table 3). All these traits could be found together or separately. AM-fungal hyphae were occasionally found inside TRH, but not in BRH, and no mycelium of DSE was detected in either hair type.
The mean length of TRH varied four-fold among the 40 species (110–426 μm), while the mean diameter varied two-fold (4–8 μm, Table S1). BRH had different shapes: circular, rectangular, square, trapezoidal, irregular. Their length (3–12 μm) and their diameter (3–11 μm) both varied four-fold among species (Table S1).
Dauciform roots
We found DR in nine species of subgenus Carex (23%, Table 2). Young DR were 1–2 mm long, while mature and old DR (Shane et al. 2006) could be 4–6 mm long. The hairs of mature DR had a similar length as the TRH of the same species but their density was 3–4 times higher. We occasionally observed AM-fungal hyphae or mycelium of DSE inside DR. DR tended to be more frequent among species with A|V and among individual plants with A|V and DSE, while being less frequent among plants with BRH (Table 3).
Taxonomic and ecological patterns
The abundance of two root traits showed a taxonomic pattern: DSE and BRH were more abundant in subgenus Carex than in subgenus Vignea (Table 4, Fig. S2). BRH were only found in wet habitats, i.e. in wetlands plus three wet forest sites (Table 4, Table S1, Fig. S2). The size of TRH was related to the habitat of the species: TRH were shortest and thinnest in grasslands (Table 4, Fig. S2). DR were only found in species of subgenus Carex (Table 4, Fig. 2a). In addition, DR were found more often in grassland species than in forest or wetland species (Fig. 2b).
Phosphorus concentrations of shoots
Shoot P concentrations mostly ranged from 1 to 3 mg g−1 with higher values in a few species of subgenus Vignea collected in wetlands (Fig. 3a, Table S1). Species of subgenus Vignea had significantly higher shoot P concentrations than species of subgenus Carex in wetlands but not in the two other habitats (Fig. 3a). Also, subgenus Vignea species had higher P concentrations in wetlands than in grasslands and forests, whereas P concentrations of subgenus Carex species did not differ significantly among the three habitat types (Fig. 3a).
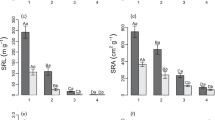
Phosphorus concentrations of plant shoots in relation to species taxonomy (subgenus) and (a) habitat, (b) presence of dauciform roots, (c) presence of dark septate endophytes (DSE), and (d) presence of arbuscules or vesicles (A|V). Bars show means ± SE, based on a total of 36 Carex species in (a) and 92 individual plants in (b) and (c). Two-way ANOVA indicated a significant subgenus × habitat interaction in (a), a significant subgenus × DSE interaction in (c), and significant main effects of both subgenus and A|V in (d). Bars with different letters are significantly different in multiple pairwise comparisons (Tukey HSD test, p < 0.05
Within subgenus Carex, shoot P concentrations did not differ significantly between plants with and without DR (Fig. 3b). Plants with DSE tended to have lower P concentrations in subgenus Carex and higher P concentrations in subgenus Vignea (Fig. 3c, significant interaction DSE × subgenus, p = 0.002). The abundance of DSE (when present) was unrelated to shoot P concentrations (p = 0.48). Regardless of subgenus, plants with A|V tended to have higher P concentrations (Fig. 3d, significant effect of A|V presence, p = 0.013). In plants with A|V, the colonisation level by AM fungi was also positively related to shoot P concentrations (p = 0.025). The presence or absence of BRH and the size of both BRH and TRH were unrelated to P concentrations (not shown).
Discussion
In this paper we simultaneously studied the occurrence of five root traits in almost 200 plant individuals of 40 Carex species, which allowed us to evaluate their frequency, abundance and patterns of co-occurrence at different levels (species, plant or root segment). Previous studies of root traits in Carex either considered fewer traits and fewer species (Miller et al. 1999; Ghosh et al. 2014), or reviewed published information (Muthukumar et al. 2004), so that patterns of co-occurrence could not be evaluated at the individual plant level. Thus, our study was more comprehensive and detailed than previous ones. Further studies might consider including even more root traits, such as ectomycorrhizal-like structures (Harrington and Mitchell 2002), associations with bacteria (Qin et al. 2008; Song et al. 2011), aerenchyma (Visser et al. 2000) or particular physiological traits, such as function at low temperatures (Edwards and Jefferies 2010) or root exudation.
We found A|V in 25% of the studied species, which is remarkably similar to the proportion of Carex species found to exhibit such structures in a single study (24%) in a review of 60 publications about mycorrhiza in Carex (Veselkin et al. 2014a). Our results also support the general statement that the abundance of AMF mycelium in roots of Carex is usually low, and that arbuscules may be absent (Miller et al. 1999; Miller 2005). Accordingly, the 25% of species forming A|V must be considered weakly mycorrhizal at best and may function like non-mycorrhizal plants. The nutritional implications of mycorrhizal colonisation in the Cyperaceae requires clarification through appropriate experiments (Muthukumar et al. 2004) or by screening for symbiosis-specific genes (Delaux et al. 2014).
The presence of DSE in 80% of the species closely matches the proportion reported by Weishampel and Bedford (2006) for Carex species in wetlands. However, these authors found colonisation rates between 17% and 48%, which is much higher than the abundance found in our study, even if we consider only wetlands. Our plants mostly had shoot P concentrations between 1 and 3 mg g−1, which are in the upper part of the range found in other studies (Güsewell and Koerselman 2002, Güsewell 2005a, b), suggesting that plant growth was at most weakly P-limited (Güsewell and Koerselman 2002). This might possibly explain the low abundance of DSE in our study. Again, the mere presence of DSE does not necessarily indicate an influence on plant nutrition, but this possibility would deserve further investigation given the high frequency of DSE among Carex species.
Although two of the species presented all five examined root traits, most species had only two or three of them, and all combinations of root traits were possible. This leads to a great diversity of root types, which might be related to different ecological strategies. A common expectation is that stress-tolerant species have a greater diversity of root traits than competitive species (Veselkin et al. 2014b). The formation of DR (similar to cluster roots) has particularly been associated with low phosphorus availability (Miller 2005; Lambers and Shane 2007) and stress tolerance (Veselkin et al. 2014b), while AM tend to be found on soils with higher phosphorus availability (Lambers et al. 2008) and may even increase after phosphorus addition (Lagrange et al. 2013). Thus, the relative rarity of species with DR and frequency of species with A|V|H in our sample may be related to relatively high shoot P concentrations. Unexpectedly (Shane et al. 2005), the occurrence of DR among plants of subgenus Carex was independent of their shoot P concentrations, presumably because the latter were never high enough to fully suppress DR formation in the absence of other suppressing factors (Güsewell 2017). The rare occurrence of DR in forests and wetlands suggests that shade and wetness also suppress DR formation when plants are not strongly P-deficient.
Our examples of multiple root traits found within one species can be extended based on data of other authors. For instance, C. flacca and C. pilulifera form ectomycorrhizal-like structures, in which the agaricoid basidiomycete Cortinarius cinnamomeus L. (Fr.) has been identified (Harrington and Mitchell 2002). Both species also form DR (Harrington and Mitchell 2002; Shane et al. 2005; Güsewell 2005a, b), and C. flacca may by forming AM too (Read et al. 1976; Wang and Qiu 2006). Finally, the species almost certainly have TRH. Consequently C. flacca exhibits at least four root traits and C. pilulifera at least three. Generally our conclusion that there is a great variety of root traits in Carex is in good agreement with results published by other authors.
Previous studies suggested the existence of negative associations between AM and the other root traits studied here (see Introduction). We could not confirm any of these negative associations when we related the presence of A|V to the other root traits. Given the low abundance of A|V in most of our species, the absence of trade-off with other root traits is not surprising. The presence and abundance of AM fungi was positively related to shoot P concentrations, hence it would in principle be conceivable that an amelioration of plant P status by AM affects the development of other root traits. However, this was not the case because the other root traits were not (DR, BRH, TRH) or weakly and inconsistently (DSE) related to shoot P concentrations.
The weak negative association found between DR and BRH reflected a reduced formation of DR at the wettest sites, where the formation of BRH was promoted. This may be a general pattern, as earlier studies suggested that DR formation tends to be inhibited by wet, anoxic conditions (Lamont 1993), while BRH formation has been associated with wetness in Carex (Miller et al. 1999). Recent speciation in northern Carex clades has partly been associated with niche partitioning regarding water level, but the role of root traits in this diversification is not yet understood.
The significant positive association between DR and DSE resulted from the combination of two patterns: On one hand, most plants (76%) of subgenus Vignea (without DR) were free of DSE. The presence or absence of DSE in Vignea plants was independent of shoot P concentration, and their low abundance suggests random colonisation. On the other hand, most plants (78%) of subgenus Carex with DR were infected by DSE. The presence of DSE was associated with slightly lower shoot P concentrations, suggesting that infection by DSE was promoted both by P deficiency and by the presence of DR. Indeed, ephemeral root structures with intense rhizodeposition, such as DR, may promote fungal infection (Laliberté et al. 2015). Dense fungal mycelium has been observed around DR in alpine sedges (Gao and Yang 2016), and DR showed the highest degree of AM fungal colonization in the sedge Lepidosperma gracile (Meney et al. 1993). The possibility that DR make roots more susceptible to colonisation by DSE and AM fungi and implications for plant nutrition would deserve further testing (Gao and Yang 2016).
Root traits were also related to taxonomy, with a confinement of DR to the subge-nus Carex, and a lower abundance of BRH and DSE in the subgenus Vignea. Although shoot P concentrations were on average higher in subgenus Vignea, this alone cannot explain the absence of DR for three reasons: First, shoot P concentrations differed mainly because of very high values in a few wetland populations of subgenus Vignea; there was no significant difference between subgenera in grasslands and forests. Second, plants with and without DR did not differ in shoot P in subgenus Carex. Third, subgenus Vignea did not form DR even under strong P deficiency in greenhouse experiments (Güsewell and Schroth 2017). Conversely, Miller et al. (1999) found no difference in BRH and DSE abundance between the subgenera Vignea and Carex. While family-level taxonomic patterns in the occurrence of roots traits are well established, patterns at the subgenus level are often poorly known. An important implication is that interspecific variation in nutrient acquisition traits may reflect the evolutionary history of species, so that associations between traits and ecological function may be elusive without consideration of phylogeny even in a comparison of congeneric species (Miller et al. 1999; Lambers et al. 2013).
In conclusion, our research has shown that a substantial diversity of root traits exists in the genus Carex. Traits can occur in any combination, and all of them can co-occur on a single plant. Both the combination of traits found on a plant and their abundance on roots varied among and within species. Our results suggest that the presence and abundance of traits follows taxonomic and ecological patterns, which differ for each trait considered. This independent regulation leads to a great diversity of root types among closely related sedge species.
References
Afonin AN, Greene SL, Dzyubenko NI, Frolov AN (eds.) (2008) Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Economic Plants and their Diseases, Pests and Weeds [Online]. Available at: http://www.agroatlas.ru
Barrow JR, Osuna P (2002) Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J Arid Environ 51:449–459
Bates TR, Lynch JP (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236:243–250
Berntson GM (1997) Topological scaling and plant root system architecture: developmental and functional hierarchies. New Phytol 135:621–634
Brundrett МC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:33–37
Davies J, Briarty LG, Rieley JO (1973) Observations on the swollen lateral roots of the Cyperaceae. New Phytol 72:167–174
Delaux P-M, Varala K, Edger PP, Coruzzi GM, Pires JC, Ané J-M (2014) Comparative phylogenomics uncovers the impact of symbiotic associations on host genome evolution. PLoS Genet 10:e1004487
Della Monica IF, Saparrat MCN, Godeas AM, Scervino JM (2015) The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecol 17:10–17
Edwards KA, Jefferies RL (2010) Nitrogen uptake by Carex aquatilis during the winter–spring transition in a low Arctic wet meadow. J Ecol 98:737–744
Egorova TV (1999) The sedges (Carex L.) of Russia and adjacent states (within the limits of the former URSS). Takhtajan AL (ed). St. Petersburg Chemical-Pharmaceutical Academy, St-Petersburg; Missouri Botanical Garden Press, Saint-Louis
Gao Q, Yang ZL (2016) Diversity and distribution patterns of root-associated fungi on herbaceous plants in alpine meadows of southwestern China. Mycologia 108:281–291
Ghosh A, Bhujel S, Maiti GG (2014) Occurrence of mycorrhizae in some species of Carex (Cyperaceae) of the Darjeeling Himalayas, India. International Journal of Life Science and Pharma Research 4:1–10
Global Carex Group (2015) Making Carex monophyletic (Cyperaceae, tribe Cariceae): a new broader circumscription. Bot J Linn Soc 179:1–42
Güsewell S (2005a) High nitrogen: phosphorus ratios reduce nutrient retention and second year growth of wetland sedges. New Phytol 166:537–550
Güsewell S (2005b) Nitrogen and phosphorus resorption efficiency of wetland graminoids with contrasting N:P ratios. Funct Ecol 19:344–354
Güsewell S (2017) Regulation of dauciform root formation and root phosphatase activities of sedges (Carex) by nitrogen and phosphorus. Plant Soil, in press.
Güsewell S, Koerselman W (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Persp Plant Ecol Evol Syst 5:37–61
Güsewell S, Schroth MH (2017) How functional is a trait? Phosphorus mobilisation through root exudates differs little between Carex species with and without specialised dauciform roots. New Phytol, accepted pending revisions
Harrington TJ, Mitchell DT (2002) Colonization of root systems of Carex flacca and C. pilulifera by Cortinarius (Dermocybe) cinnamomeus. Mycol res 106:452–459
Haselwandter K, Read DJ (1982) The significance of a root-fungus association in two Carex species of high-alpine plant communities. Oecologia 53:352–354
Lagrange A, L’Huillier L, Amir H (2013) Mycorrhizal status of Cyperaceae from new Caledonian ultramafic soils: effects of phosphorus availability on arbuscular mycorrhizal colonization of Costularia comosa under field conditions. Mycorrhiza 23:655–661
Laliberté E, Lambers H, Burgess TI, Wright SJ (2015) Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol 206:507–521
Lambers H, Shane MW (2007) Role of root clusters in phosphorus acquisition and increasing biological diversity in agriculture. In: scale and complexity in plant systems research: Gene-Plant-crop relations. Spiertz JHJ, Struik PC and van Laar HH (eds). Springer, pp 237–250.
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Lambers H, Brundrett MC, Raven JA, Hopper SD (2011) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31
Lambers H, Clements JC, Nelson MN (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J bot 100:263–288
Lamont BB (1993) Why are hairy root clusters so abundant in the most nutrient-impoverished soils of Australia? Plant Soil 155(156):269–272
Lipson DA, Schadt CW, Schmidt SK, Monson RK (1999) Ectomycorrhizal transfer of amino acid-nitrogen to the alpine sedge Kobresia myosuroides. New Phytol 142:163–167
Massicotte HB, Melville LH, Peterson RL, Luoma DL (1998) Anatomical aspects of field ectomycorrhizas on Polygonum viviparum (Polygonaceae) and Kobresia bellardii (Cyperaceae). Mycorrhiza 7:287–292
Meney KA, Dixon KW, Scheltema M, Pate JS (1993) Occurrence of vesicular mycorrhizal fungi in dryland species of Restionaceae and Cyperaceae from south-west Western Australia. Aust J bot 41:733–737
Miller RM (2005) The nonmycorrhizal root – a strategy for survival in nutrient-impoverished soils. New Phytol 165:655–658
Miller RM, Smith CI, Jastrow JD, Bever JD (1999) Mycorrhizal status of the genus Carex (Cyperaceae). Am J bot 86:547–553
Muthukumar T, Udaiyan K (2000) Arbuscular mycorrhizas of plants growing in the Western Ghats region, southern India. Mycorrhiza 9:297–313
Muthukumar T, Udaiyan K, Vasantha K, Kleiner D, Manian S (1999) Mycorrhizae in sedges as related to root character and its ecological significance. Pertanika J Trop Agric Sci 22:9–17
Muthukumar T, Udaiyan K, Shanmughavel P (2004) Mycorrhiza in sedges – an overview. Mycorrhiza 14:65–77
Nobis A, Błaszkowski J, Zubek S (2015) Arbuscular mycorrhizal fungi associations of vascular plants confined to river valleys: towards understanding the river corridor plant distribution. J Plant res 128:127–137
Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170:491–500
Qin S, Wang H-B, Chen H-H, Zhang Y-Q, Jiang C-L, Xu L-H, Li W-J (2008) Glycomyces endophyticus sp. nov., an endophytic actinomycete isolated from the root of Carex baccans Nees. Int J Syst Evol Micr 58:2525–2528
Read DJ, Koucheki HK, Hodgson J (1976) Vesicular-arbuscular mycorrhiza in natural vegetation system. New Phytol 77:641–653
Ruotsalainen AL, Väre H, Vestberg M (2002) Seasonality of root fungal colonization in low alpine herbs. Mycorrhiza 12:29–36
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125
Shane MW, Dixon KW, Lambers H (2005) The occurrence of dauciform roots amongst Western Australian reeds, rushes and sedges, and the impact of phosphorus supply on dauciform-root development in Schoenus unispiculatus (Cyperaceae). New Phytol 165:887–898
Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H (2006) Specialized ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant Cell Environ 29:1989–1999
Sieber TN, Grünig CR (2013) Fungal root endophytes. In: plant roots – the hidden half, 4th ed. Eshel A, Beeckman T (eds). CRC Press, Taylor and Francis Group, Boca Raton, pp 38-1–38-49
Song GC, Yasir M, Bibi F, Chung EJ, Jeon CO, Chung YR (2011) Nocardioides caricicola sp. nov., an endophytic bacterium isolated from a halophyte, Carex scabrifolia Steud. Int J Syst Evol Micr 61:105–109
Spalink D, Drew MT, Pace MC, Zaborsky JG, Starr JR, Cameron KM, Givnish TJ, Systma KJ (2016) Biogeography of the cosmopolitan sedges (Cyperaceae) and the area-richness correlation in plants. J Biogeogr 43:1893–1904
Uma E, Muthukumar T, Sathiyadash K, Muniappan V (2010) Mycorrhizal and dark septate fungal associations in gingers and spiral gingers. Botany 88:500–511
Vance CP, Uhde-Stone C, Allan D (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Veselkin DV, Konoplenko MA, Betekhtina AA (2014a) The ability to form mycorrhiza in the genus Carex L. (Cyperaceae): the published data analysis. Plant Life of Asian Russia 16:26–35
Veselkin DV, Konoplenko MA, Betekhtina AA (2014b) Means for soil nutrient uptake in sedges with different ecological strategies. Russ J Ecol 45:547–554
Visser EJW, Bögemann GM, van de Steeg HM, Pierik R, Blom CWPM (2000) Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol 148:93–103
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Weishampel PA, Bedford BL (2006) Wetland dicots and monocots differ in colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza 16:495–502
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:1–4
Acknowledgements
The authors are grateful to A.A. Betekhtina for advice; to N.V. Zolotareva for providing some samples; and to N.B. Kuyantseva for organizing the fieldwork. They also thank Hans Lambers and two referees for constructive comments on a first version of the manuscript. The work was supported by Act 211 Government of the Russian Federation, contract № 02.A03.21.0006; by the Ural Federal University (a grant for the young scientists, а graduate students and students, contract no. 1.2.2.2-14/99 on March 31, 2014) and by the Russian Foundation for Basic Research (project no. 16–54–00105).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 312 kb)
Rights and permissions
About this article
Cite this article
Konoplenko, M.A., Güsewell, S. & Veselkin, D.V. Taxonomic and ecological patterns in root traits of Carex (Cyperaceae). Plant Soil 420, 37–48 (2017). https://doi.org/10.1007/s11104-017-3292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3292-z