Abstract
Aims
Soil organic nitrogen (N) turnover is significantly influenced by elevated N deposition, precipitation and human-caused disturbances, but the underlying mechanism remains unclear. Identifying the relationships among the soil organic N fractions and N-mineralizing enzymes activities may advance our knowledge of the dynamics of soil organic N.
Methods
A field experiment was conducted in a semi-arid steppe and an abandoned cropland in northern China to investigate the effects of elevated N deposition and precipitation on soil organic N fractions and their relationships with N-mineralizing enzymes, i.e., protease, amidase, urease and N-acetyl-β-D-glucosaminidase (NAG) activities.
Results
The concentrations of N in various fractions were consistently lower in the abandoned cropland compared with the steppe. Nitrogen addition consistently decreased amino acid N content and activities of urease, protease and amidase in both sites but increased amino sugar N content and NAG activity in the steppe. Water addition decreased hydrolysable ammonium N content but increased amino sugar N content and activities of protease and NAG in both sites. Furthermore, urease and NAG activities were significantly positively correlated with the proportions of amino acid N and amino sugar N and, explained significant proportions of the variations in soil organic N fractions in the steppe. However, soil organic carbon (C), rather than N-mineralizing enzymes, explained greatest proportion of the variations in soil organic N fractions in the abandoned cropland.
Conclusions
The concurrent increase of N deposition and precipitation could promote the recovery of soil N (and C) losses in the abandoned cropland resulting from previous agriculture. Furthermore, in the steppe where NH4 + was available at relative high concentrations, enzymatic mineralization was the dominant route involved in potential soil organic N turnover. However, the direct route may be favored over the enzymatic mineralization route with decreasing availability of C relative to N in the abandoned cropland, which is driven by the need for C. These findings confirmed that the forms of N available, and the relative availability of C and N determine N uptake pathways both through enzymatic mineralization route and direct uptake route in the semi-arid grasslands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grasslands are the dominant landscape in China and cover over 40% of the nation’s land surface, playing a vital role in serving environmental health and regional economy (Kang et al. 2007). As a main limiting factor for plant growth and net primary productivity, soil nitrogen (N) availability and its responses to elevated atmospheric N deposition, precipitation, and land use changes, are crucial for grassland ecosystem functions and stability (Xu et al. 2015a, b). As over 90% of soil N is present in organic forms, the mineralization rate of the soil organic N pool primarily determines soil N availability, as well as ecosystem N cycling (Stevenson 1982). There is however a lack of information on soil organic N turnover in grassland ecosystems under global climate changes.
The depolymerization and mineralization of organic N in soils is a sequence of microbial enzymatic processes (Mengel 1996). Most important enzymes involved are protease for the depolymerization of protein, amidase and urease for releasing ammonia from linear amides, N-acetyl-β-D-glucosaminidase (NAG) for the degradation of chitin and other β-1,4-linked glucosamine polymers (Mengel 1996; Kandeler et al. 2011). N-mineralizing enzyme activities therefore, have been widely used to predict N mineralization in response to changes in management practices or environmental conditions (e.g., cultivation, fertilization and rain events) (Kandeler et al. 2011; Landi et al. 2011). For example, it has been shown that intensive cultivation systems generally decreases protease and urease activities compared with undisturbed soils (Caravaca et al. 2002; Landi et al. 2011), which was contributed to the decreased microbial activities and soil organic matter content through tillage and soil disturbance. Other studies have reported that environmental change like N deposition or N addition can result in both increase or decrease of soil urease activities (Ajwa 1999; Saiya-Corka et al. 2002). Additionally, Bell and Henry (2011) reported that extracellular enzyme activities increase in response to water addition, which may be accounted for by immobilization of enzymes by soil organic matter. However, as organic N substrates in various fractions differ in their potentials for degradation or mineralization (Mengel 1996; Qiu et al. 2012), mechanisms in organic N mineralization will not be properly elucidated until more attention is paid to different organic N fractions involved in N turnover.
According to the classic methods (Stevenson 1982), soil organic N can be divided into total hydrolysable N and acid insoluble N, with total hydrolysable N comprising hydrolysable ammonium N, amino acid N, amino sugar N and hydrolysable unidentified N. Numerous studies have been conducted to investigate the dynamic patterns in the soil organic N pools in response to changes of environment conditions or land use, most of them are carried out in agricultural ecosystems (Xu et al. 2003; Nannipieri and Eldor 2009; Spargo et al. 2012; Lü et al. 2013). For example, Gonzalez-Prieto and Carballas (1991) and Gonzalez-Prieto et al. (1997) have reported that cultivation decrease the proportion of the soil N as amino acid N compared to adjacent virgin soils, they concluded that amino acid N could be considered as an active N pools potentially available for plants. Under intensively managed systems, N derived from fertilizer was mainly transformed into amino acid N or amino sugar N (Xu et al. 2003; Lü et al. 2013). It has been reported that water availability can significantly influenced the relative abundance of different organic N pools in protected field (Ji et al. 2007). Few studies, however, have elucidated the relative abundance of different soil organic N fractions in response to environmental and land use changes in semi-arid grasslands. Given the important interactions between organic N substrates of different composition and N-mineralizing enzymes (Sinsabaugh 1994), studies that link soil organic N fractions with N-mineralizing enzymes activities will therefore shed light on the underlying mechanisms of soil organic N mineralization turnover.
In Inner Mongolia, grasslands are the dominant landscape and accounts for 78% of total grassland area in China (Kang et al. 2007). However, grasslands in this area have experienced serious land degeneration and desertification since 1950s because of over grazing and intensive farming, more than 2 million ha of steppe were converted to farmland until 1990s (Wei and Shuang 2001). Policies of returning cultivated land to grasslands since the end of last century have been imposed to prevent grasslands from further degradation. As a result, the natural steppe fenced after overgrazing and abandoned cropland succeeding after farmland abandonment are most widely distributed grassland types in northern China (Xu et al. 2015a). This has provided an opportunity to study the potential organic N turnover mechanisms of the two grassland types following disturbances. Given the atmospheric N deposition (Liu et al. 2013) and summer precipitation (Sun and Ding 2010) in this area are predicted to increase in the coming decades, studying the relationships between soil organic N fractions and N-mineralizing enzymes will greatly improve our knowledge of soil organic N turnover in response to changing environmental conditions, and help to improve practices in management and restoration of degraded grasslands.
In this study, a field experiment was conducted in a semi-arid steppe and an adjacent abandoned cropland under experimentally-elevated N deposition and precipitation to investigate (a) the effects of N and water additions on soil organic N fractions in semi-arid grasslands and (b) the relationships between soil organic N fractions and N-mineralizing enzymes activities in the steppe and abandoned cropland.
Materials and methods
Study sites and experimental design
The study sites were located in the typical temperate grassland in Duolun county (116° 17′ E, 42° 02′ N, elevation 1324 m a.s.l.), Inner Mongolia in northern China. This area is located in an agro-pastoral ecotone with a semi-arid continental monsoon climate. Mean annual precipitation is 385.5 mm and mean annual temperature is 2.1 °C, with mean monthly temperatures ranging from −17.5 °C in January to 18.9 °C in July (Xu et al. 2015b). The soil type in this area is classified as Haplic Calcisol according to the FAO classification (FAO/ISRIC/ISSS 1998).
A steppe and an adjacent abandoned cropland were selected for the present study. Both grassland systems were used for livestock (mainly including sheep, cattles, horses and donkeys) grazing freely before the abandoned cropland was converted to farmland in early 1980s (Xu et al. 2016). During the period of farming, Sesamum indicum L., Avena chinensis, Triticum aestivum L., and Fagopyrum sagittatum Gilib were common crops in the abandoned cropland, the land was plowed and about 90 kg ha−1 diammonium phosphate fertilizers were applied every year. The steppe was free grazed until it was fenced in 2000, and the abandoned cropland was abandoned and fenced in the same year. Both grasslands have not been used in any form since 2000. At the beginning of the present experiment, the dominant plant species were Artemisia frigida, Agropyron cristatum, and Stipa krylovii in the steppe, and Agropyron cristatum and Artemisia scoparia in the abandoned cropland (Xu et al. 2010).
In April 2005, seven blocks (each 107 m × 8 m) were set up within each of the two grassland systems using a split-plot experimental design. Each block was divided into two main plots with water management (ambient precipitation and water addition) as treatments. The ambient precipitation plot without fertilizer inputs served as control. The water addition plots received a simulated precipitation of 15 mm weekly by sprinkling irrigation from June to August. A total of 180 mm water was added during each growing season from 2005 to 2013. Increased precipitation of similar magnitude has occurred during some of the previous years (e.g., 1979) and is expected to occur more frequently in the future under scenarios of global change (Sun and Ding 2010). Each main plot was divided into six subplots (each 8 m × 8 m separated by 1 m wide corridors) with two N treatments (ambient N vs. N addition with 100 kg N ha−1 year−1) randomly assigned. While the other four subplots were different levels of N and phosphorus additions treatments which were not considered in this study, more detailed information about the experimental design has been reported by Zhang and Han (2008). Nitrogen (in the form of urea) was applied twice a year, half of which was applied in early May and the other half in late June. The amount of N addition is comparable to the estimated mean atmospheric N deposition rate in northern China (about 83 kg N ha−1 year−1) (He et al. 2007). Each treatment was replicated seven times.
Soil sampling
Soil samples were collected in August 2013. Five randomly placed soil cores (0–10 cm depth) were taken from each subplot using a soil auger (3 cm in diameter) to form one composite sample. After removing visible plant residues and stones, each sample was homogenized and passed a 2-mm sieve. A portion of fresh soil samples were stored at 4 °C before the laboratory analysis of soil microbial biomass C (Cmic), N (Nmic) and enzyme activity assays. The rest of the soil samples were air-dried and ground for soil organic N fractionation. All the analyses were performed within two weeks after soil sampling.
Soil properties analyses
Methods for the analysis of soil organic C (SOC), total soil C (Ctot) and N (Ntot) were described in detail in previous publication (Tian et al. 2016). Briefly, SOC was determined by chemical oxidation using K2Cr2O7 solution, Ctot and Ntot were determined using a Vario MACRO cube analyzer (Elementar Analysensysteme Vario MACRO cube, German). Soil Cmic and Nmic were determined using the chloroform fumigation extraction method (Joergensen 1996). Briefly, 10 g of field-moist soil was fumigated at room temperate with ethanol-free chloroform for 24 h. The fumigated and a non-fumigated control sample were both extracted with 40 ml 0.5 M K2SO4 and shaken at 150 rpm for 1 h before filtering. The Cmic was calculated as the difference in extractable C concentrations between fumigated and non-fumigated samples divided by an efficiency factor of 0.45. The Nmic was calculated as the difference in extractable N concentrations between fumigated and non-fumigated samples divided by an efficiency factor of 0.54. Extractable C and N were determined by a C and N analyzer (VarioTOC Analyzer, Elementar, Germany). Inorganic N (sum of NO3 − and NH4 +) was extracted by 1 M KCl (Mulvaney 1996), and determined using a colorimetrically method by an AutoAnalyser III continuous Flow Analyzer (Bran & Luebbe, Norderstedt, Germany). Soil organic N was calculated as the difference between Ntot and inorganic N.
Soil organic N fractionation
Organic N fractions were measured by hydrolyzing the soil sample with 6 M HCl in an autoclave at 15 lb./in2 for 6 h (Stevenson 1982). Total hydrolysable N was determined by steam distillation with 10 M NaOH after Kjeldahl digestion of the acid hydrolysate. Amino acid N was determined by steam distillation of the hydrolysate with phosphate–borate buffer and, 5 M NaOH, ninhydrin powder was added to convert the amino N to ammonium N. Hydrolysable ammonium N was measured by steam distillation with 3.5% (w/v) MgO. Amino sugar N was calculated as the difference between the amounts of N liberated by steam distillation of the hydrolysate with phosphate–borate at pH 11.2 and the amounts of hydrolysable ammonium N. Hydrolysable unidentified N was calculated as the difference between total hydrolysable N and the N accounted for as (ammonium + amino acid + amino sugar)-N. Acid insoluble N was calculated as the difference between total soil N and total hydrolysable N. The distribution of N (%) in various fractions was calculated as the proportion of Ntot.
Soil N-mineralizing enzyme assays
Soil protease, amidase and urease activities were determined using colorimetrical methods as described by Landi et al. (2011) and Kandeler et al. (2011). For protease activity measurement, field-moist soil was incubated with sodium caseinate, and 0.1 M Tris (hydroxymethyl) amino methane buffer (pH 8.1) for 2 h at 50 °C. The released tyrosine was spectrophotometrically determined at 700 nm, and the activity of protease was expressed as μg tyrosine g−1 soil h−1 (Landi et al. 2011). Urease activity was determined by incubating field-moist soil with buffered urea solution (0.72 M) for 2 h at 37 °C (Kandeler et al. 2011). Amidase activity was measured based on determination of the ammonium released by amidase after soil was incubated with 0.5 M formamide substrate and 0.1 M sodium borate buffer (pH 8.5) for 2 h at 37 °C (Kandeler et al. 2011). The released ammonium was determined using colorimetric method at 660 nm. The activity of amidase and urease were expressed as μg NH4 + g−1 soil h−1 (Kandeler et al. 2011).
The activity of N-acetyl-β-D-glucosaminidase (NAG) was measured by a fluorimetric microplate assay using 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide as substrate, enzyme activities were determined as the rate of release of 4-methylumbelliferone (MUB) from the MUB-labeled substrate (4-MUB-N-acetyl-β-D-glucosaminide) (Marx et al. 2001). An aqueous soil suspension (soil:water =1:100, 1 mM NaN3 was included to prevent microbial activity), 50 μl, was pipetted into a 96-well microplate together with 50 μl of acetate buffer (pH 5.0) and 100 μl of substrate solution, giving a final substrate concentration of 100 μM. Microplates were incubated for 1.5 h at 30 °C and the released MUB was determined immediately on a fluorimetric plate-reader (Tecan Infinite 200 PRO, TECAN Group Ltd., Mannedorf, Switzerland), with excitation at 360 nm and emission at 450 nm. The activity of NAG was expressed as nmol MU g−1 soil h−1.
All measured enzyme activities were also expressed on a soil Cmic and soil organic N basis to obtain two different specific activity indices (Fig. S1 and S2 in the supplementary materials).
Statistical analysis
ANOVAs with split-plot design were executed separately in the steppe and abandoned cropland to determine the effects of N, water addition and their interactions on soil chemical and biological characteristics. In all the split-plot ANOVAs, water was the main plot factor, N was the subplot factor, and block was treated as a random effect. Multiple comparisons with Duncan test at the P = 0.05 level were performed to evaluate the differences among the experimental treatments. The correlation of soil parameters was based on the Pearson correlation coefficients. All statistical analyses mentioned above were conducted with SPSS 16.0 (SPSS, Chicago, IL, USA). Redundancy analysis (RDA) was selected to study the relationships between soil organic N fractions and soil environmental parameters using CANOCO software, because the lengths of gradient was less than 3 in the detrended correspondence analysis (DCA) (ter Braak 1988). The soil organic N variables were standardized [Log transformation Y′ = log (A × Y + B), A = 1, B = 1] before RDA. A Monte Carlo permutation test (999 permutations) was used to test the significance of first and second axes.
Results
Soil properties
The steppe showed higher Cmic, Nmic, Ctot, Ntot, SOC and NH4 + contents, as well as (C:N)tot ratio, compared with the abandoned cropland. Nitrogen addition resulted in significant decrease of Cmic and Nmic contents in both sites, no significant effects of N addition on Ctot, Ntot, SOC, NH4 + contents and (C:N)tot ratio were observed in both sites. Water addition significant increased Cmic content in the steppe, while Cmic for the water addition treatment was also higher than control in the abandoned cropland, the difference between them was not significant. Combined additions of N and water significantly decreased Nmic content but increased the (C:N)mic ratios in both sites, and significantly increased Ctot and Ntot contents in the abandoned cropland (Table 1).
Soil organic N fractions
The concentrations of Ntot and N in various fractions (ammonium N, amino acid N, amino sugar N, unidentified N and insoluble N) were consistently higher in the steppe compared with the abandoned cropland (Tables 1 and 2). The proportions of total hydrolysable N to Ntot were consistently lower in the steppe (74–82%) compared with the abandoned cropland (82–86%) (Fig. 1). Nitrogen addition decreased the concentrations and proportions of amino acid N in both sites (28% and 14% decrease for the steppe and abandoned cropland, respectively), although differences were only significant for the steppe. Also in the steppe, N addition significantly increased the concentrations and proportions of amino sugar N and hydrolysable unidentified N (Table 2; Fig. 1). Furthermore, water addition significantly decreased the proportions of hydrolysable ammonium N but increased the concentrations and proportions of amino sugar N both in the steppe and the abandoned cropland (Table 2; Fig. 1). Combined N and water additions significantly increased unidentified N in steppe and acid insoluble N contents in both sites, no significant effects of solely N or water addition on acid insoluble N contents were observed (Table 2; Fig. 1).
Soil N-mineralizing enzyme activities
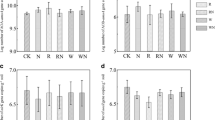
Nitrogen addition significantly decreased urease activities in both sites, however, increased NAG activities in the steppe. Although the protease and amidase activities for the N addition treatment were lower compared with control, the differences between them were not significant. Water addition resulted in significant increase of protease and NAG activities in both sites, and amidase activities in the steppe (Fig. 2).
Soil enzyme activities of protease, amidase, urease and N-acetyl glucosaminidase (NAG), in the steppe and abandoned cropland under N and water (W) additions. N: N addition, W: water addition, NW: N plus water additions. Upper cases and lower cases indicate significant difference (P < 0.05) of enzyme activities between treatments in the steppe and abandoned cropland soils, respectively. Error bars indicate standard deviation
Relationships between soil organic N fractions and related soil properties
The proportions of total organic N and hydrolysable ammonium N showed significant positive and negative correlations with all N-mineralizing enzyme (except urease and amidase in the abandoned cropland) activities, respectively. In the steppe, urease activity was significantly positively correlated with the proportions of amino acid N (P < 0.05) and insoluble N (P < 0.01), but significantly negatively correlated with the proportion of hydrolysable unidentified N (P < 0.05); NAG activity was significantly positively correlated with the proportion of amino sugar N (P < 0.05). However, no significant relationships between soil organic N fractions and N-mineralizing enzyme activities were observed in the abandoned cropland (Table 3).
Results from RDA revealed that soil NAG and urease activities explained significant proportions of the variations in organic N fractions in the steppe (P < 0.01) (Table 4; Fig. 3). In contrast, SOC content explained greatest proportion of the variations in organic N fractions in the abandoned cropland (P < 0.01), while other parameters did not (Table 4; Fig. 3). The first two axes explained 68.1 and 52.2% of the total species-environment variation in the steppe and the abandoned cropland, respectively (Fig. 3).
Redundancy analysis (RDA) between soil N fractions and soil environmental parameters under nitrogen (N) and water (W) additions in the (a) steppe and (b) abandoned cropland. N: N addition, W: water addition, NW: N plus water additions. AIN: acid insoluble N, HUN: hydrolysable unknown N, AA: amino acid N, AS: amino sugar N, NAG: N-acetyl-β-D-glucosaminidase, SOC: soil organic C
Discussion
Soil organic N fractions as affected by N and water additions
Previous cultivation causes markedly losses of N in various fractions ranging from 5% to 42% in the abandoned cropland. These losses can be caused by decreased plant inputs (crop removal) and accelerated decomposition of organic matter and probable erosion associated with agriculture (Standish et al. 2006). It is believed that cultivation could greatly decrease the total soil porosity due to breakup of soil aggregates, which influences the physical protection of soil organic matter and results in an increase in mineralization of soil organic matter and consequent losses of organic C and N (Balesdent et al. 1988; Evrendilek et al. 2004). However, despite the fact that the two grassland systems experienced the same situations (no land use and no removal of plant biomass) for 13 years, the abandoned cropland organic C and N pools were still not “recovered” from agricultural use. This could be explained by the fact that the residual effects of deteriorating soil structure and probable erosion can be recognized even decades after abandonment of agriculture (Standish et al. 2006). These legacies are not surprising given that the study conducted in a chronosequence of abandoned croplands suggested that recovery of soil C and N to 95% of the pre-agricultural levels is predicted to require centuries (Knops and Tilman 2000). However, it is important to note that combined N and water additions significantly increased Ntot and Ctot contents in the abandoned cropland, suggesting that concurrent increase of N deposition and precipitation in this area could promote the recovery of soil C and N pools from the residual effects of previous cultivation. This could be attributed to the stimulated root production and consequently accelerated root residue incorporation into soil organic matter (Xu et al. 2016).
The amino acid concentrations in soil were controlled by uptake and release both by microorganisms and plants (Stevenson 1982). The decrease of amino acid N content under N addition can be attributed, partly at least, to the decreased Cmic (Table 1). Because a large proportion of amino acids in soils appear to be those present in soil microorganisms (Stevenson 1982) and, the synthesis and dynamics of amino acids in soil-plant system are closely associated with microbial metabolism (Schulten and Schnitzer 1998). In addition, the decrease of amino acid N can in part be explained by marked increases in above ground productivity by N addition (Ren et al. 2017), which may result in decrease of soil amino acid N by plant uptake. The uptake of amino acid by plants has been shown to be independent of inorganic N concentration in soil, which may significantly contribute to total N taken up by plants even in situation where fertilizer inputs are high (Jones and Darrah 1994). Furthermore, the decreased soil pH under N addition may also accelerate amino acid turnover (Table 1), as Jones and Kielland (2002) reported that amino acid turnover increased 4-fold with a drop in soil acidity of less than half a pH unit.
It is generally assumed that amino sugars in soils are derived primarily from microorganisms, and most of them come from fungi cell walls whereas little occurs in bacterial cell walls or other tissues (Olk 2008; Nannipieri and Eldor 2009). As an indicator of soil microbial community structure, the (C:N)mic ratio is often used to describe the relative contributions of bacterial and fungal cell populations to the soil microbial biomass. A high (C:N)mic ratio indicates a higher proportion of fungi in microbial biomass, whereas a low value suggests that bacteria may predominate in the microbial population (Campbell et al. 1991; Moore et al. 2000). And the (C:N)mic ratios of selected microorganisms frequently isolated from soils, and cultivated under optimal conditions, range from 7 to 12 in fungi and from 3 to 6 in bacteria (Anderson and Domsch 1980). In this study, the significantly increased amino sugar N content in the steppe by N addition may be attributed to stimulating soil fungal populations as indicated by significantly increased (C:N)mic ratio, the effect of which was non-significant in the abandoned cropland (Table 1). The stimulated fungal populations here can be explained by markedly increased above ground biomass by N addition (64% of increase in the steppe and 41% of increase in the abandoned cropland, unpublished data), as enhanced plant inputs can stimulate the fungal population and increase the fungal:bacterial ratio in soil microbial biomass (Brant et al. 2006). Similarly, the consistently increased concentrations and proportion of amino sugar N by water addition in both sites may also be attributed to the stimulating fungal populations (Table 1).
Hydrolysable ammonium N is the fraction in soil hydrolysates originated dominantly from decomposition of organic compounds as well some derived from exchangeable and clay fixed NH4 +, which serves as a fast released and available pool of N for plant and microorganisms (Qiu et al. 2012; Lü et al. 2013). Therefore, the significantly decreased proportion of N as ammonium N under water addition (Fig. 1), could be attributed to the enhanced immobilization or uptake of mineral N by microorganisms and plants, as both soil microbial biomass and above ground biomass were significantly enhanced by water addition in both sites (Table 1) (Xu et al. 2010). Acid-insoluble N is believed to occur as a structural component of humic substances (Nannipieri and Eldor 2009), the most important sources of which are senescent materials as above and below ground detritus (Horwath 2007). The increased concentrations and proportions of acid-insoluble N by combined additions of N and water, therefore, could be explained by the consistent increase of both above ground biomass and below ground root production in both sites (Xu et al. 2015b; Xu et al. 2016). Hydrolysable unidentified N was calculated by subtracting ammonium N, amino acid N and amino sugar N from total hydrolysable N, little information is available on the nature of this fraction, the biological significance of the hydrolysable unidentified N was therefore not discussed in this study.
The potential N-mineralizing enzyme activities as affected by N and water additions
It is well established that protease, amidase, urease and NAG in soils come largely from microorganisms (Kandeler et al. 2011; Landi et al. 2011), the consistent increase of these enzyme activities (except urease) under water addition can therefore be attributed to the increased Cmic. In contrast, N addition significantly decreased Cmic in both sites, which consequently decreased protease, amidase and urease activities (Table 1, Fig. 2). However, despite the decrease of Cmic in the steppe, the relative abundance of fungi in soil microbial biomass may be stimulated by enhanced plant inputs under N addition (Brant et al. 2006), which is indicated by significantly increased (C:N)mic ratio (Table 1) (Campbell et al. 1991; Moore et al. 2000). It is widely assumed that NAG activities were mainly expressed by a diverse group of fungi (Miller et al. 1998; Muruganandam et al. 2009), the increased activities of NAG under N addition can therefore be explained by the stimulated relative abundance of fungi in soils.
Potential mechanisms involved in soil organic N turnover in the steppe and abandoned cropland
Most of the organic N in soils are in the form of polymers, which first have to be degraded into smaller units, the small organic molecules (e.g., amino acid, amino sugar etc.) can then be taken up directly (direct route), or mineralized further by extracellular enzymes and the N taken up in the mineral N (enzymatic mineralization route) (Nannipieri and Eldor 2009). In this study, the relationships between soil organic N fractions and extracellular N-mineralizing enzymes activities differed markedly between land use systems, suggesting differential potential mechanisms involved in organic N turnover. In the steppe, urease and NAG activities were significantly positively correlated with the proportions of amino acid N and amino sugar N, and explained great proportions of the variations in soil organic N fractions (Tables 3 and 4; Fig. 3). These results suggested that extracellular N-mineralizing enzymes were actively involved in potential turnover of soil organic N in the steppe. However, in the abandoned cropland, the fact that soil extracellular N-mineralizing enzymes activities were not related to organic N fractions and SOC explained greatest proportion of the variations in soil organic N fractions, suggested that the potential organic N turnover may be more C-limited compared with the steppe (Tables 3 and 4; Fig. 3).
Ammonium is considered as preferred source of N for microorganisms and plants (Merrick and Edwards 1995; Näsholm et al. 2009). Therefore, in the steppe where NH4 + is available at relative high concentrations (32% higher for the control compared with the abandoned cropland, Table 1), the transcription of genes encoding for enzymes systems required for the direct utilization of alternative N sources, such as organic N molecules, may be repressed (Magasanik 1993). The fact that extracellular N-mineralizing enzymes were actively involved in potential turnover of soil organic N, suggested that the enzymatic mineralization route may be favored over the direct route under these circumstances (Geisseler et al. 2009, 2010) (Tables 3 and 4; Fig. 3). In contrast, in the abandoned cropland where the NH4 + availability is lower, systems for the direct uptake of organic N molecules may be de-repressed and becomes relative more important. Furthermore, conversion of steppe to cultivated cropland causes long-term loss of soil organic matter, the proportionally greater loss of C than that of N, i.e., relative lower (C:N)tot ratios (Table 1), suggested that C may became limiting. When C becomes limiting relative to N, low molecular weight N-containing substrates, such as amino acid and amino sugar, can be taken up directly by microorganisms as C sources with intracellular deamination (Nannipieri and Eldor 2009; Geisseler et al. 2010). Additionally, when most of the available C in soils is in the form of molecules also contains N, such as amino acid and amino sugar, the direct route may even be important when inorganic N is available at high concentrations because microorganisms have to meet their C demand by taking up organic N molecules (Geisseler et al. 2010). The relative higher ratios of amino acid N and amino sugar N to SOC in the abandoned cropland (Table 2), also suggested that the direct route might be favored over the enzymatic mineralization route. Therefore, our results suggested that the relative importance of the dominant processes involved in organic N turnover is not only dependent on N availability, but also on the source of C and relative availability of C and N (Geisseler et al. 2010; Yang et al. 2016).
Conclusions
Using the long-term manipulation experiment in the semi-arid grassland, our results suggested that N addition decreased soil amino acid N, while water addition increased amino sugar N in soils. Conversion of grassland to cropland causes long-term losses of organic C and N that are not re-stablished even 13 years after abandonment. However, it is important to note that concurrent increase of N deposition and precipitation could promote the recovery of soil C and N pools from previous cultivation. Furthermore, our findings highlight that the dominant processes involved in organic N turnover differed between land use systems. In the undisturbed steppe where NH4 + was preferred as N source, enzymatic mineralization was the dominant route involved in potential organic N turnover. However, the direct route may be favored over the enzymatic mineralization route in the abandoned cropland with decreasing availability of C relative to N. These findings confirmed that the forms of N available, and the relative availability of C and N determine N uptake pathways both through enzymatic mineralization route and direct route in the semi-arid grasslands.
References
Ajwa HA (1999) Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31:769–777
Anderson JPE, Domsch KH (1980) Quantities of plant nutrients in the microbial biomass of selected soils. Soil Sci 130:211–216
Balesdent J, Wagner GH, Mariotti A (1988) Soil organic-matter turnover in long-term field experiments as revealed by carbon-13 natural abundance. Soil Sci Soc Am J 52:118–124
Bell TH, Henry HAL (2011) Fine scale variability in soil extracellular enzyme activity is insensitive to rain events and temperature in a mesic system. Pedobiologia 54:141–146
ter Braak CJFt (1988) CANOCO: a FORTRAN program for canonical community ordination by (partial) (detrended) (canonical) correspondence analysis, principla components analysis and redundancy analysis (version 2.1). Groep Landbouwwiskunde, Wageningen
Brant JB, Sulzman EW, Myrold DD (2006) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Campbell CA, Biederbeck VO, Zentner RP, Lafond GP (1991) Effect of crop rotations and culturalptactices on soil organic matter, miciobial biomass and respiration in a thin Black Chernozem. Can J Soil Sci 71:363–376
Caravaca F, Masciandaro G, Ceccanti B (2002) Land use in relation to soil chemical and biochemical properties in a semiarid Mediterranean environment. Soil Till Res 68:23–30
Evrendilek F, Celik I, Kilic S (2004) Changes in soil organic carbon and other physical soil properties along ajacent Mediterranean forest, grassland, and cropland ecosystems in Turkey. J Arid Environ 59:743–752
FAO/ISRIC/ISSS (1998) World reference base for soil resources. FAO, Rome
Geisseler D, Horwath WR, Doane TA (2009) Significance of organic nitrogen uptake from plant residues by soil microorganisms as affected by carbon and nitrogen availability. Soil Biol Biochem 41:1281–1288
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms – a review. Soil Biol Biochem 42:2058–2067
Gonzalez-Prieto SJ, Carballas T (1991) Composition of organic N in temperate humid region soils (NW Spain). Soil Biol Biochem 23:887–895
Gonzalez-Prieto SJ, Jocteur-Monrozier L, Hétier JM, Carballas T (1997) Changes in the soil organic N fractions of a tropical Alfisol fertilized with 15N-urea and cropped to maize or pasture. Plant Soil 195:151–160
He C-E, Liu X, Fangmeier A, Zhang F (2007) Quantifying the total airborne nitrogen input into agroecosystems in the North China Plain. Agric Ecosyst Environ 121:395–400
Horwath W (2007) Carbon cycling and formation of soil organic matter. In: Paul EA (ed) Soil microbiology, ecology, and biochemistry, 3rd edn. Academic, Amsterdam, pp 303–340
Ji J, Yulong Z, Yuling Z, Yu N, Yu B (2007) Effect of different irrigation methods on soil organic matter and nitrogen forms in protected field. Chinese J Soil Sci 6:1105–1109
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EC value. Soil Biol Biochem 28:25–31
Jones DL, Darrah PR (1994) Amino-acid influx at the soil-root interface of Zea mays L. and its implications in the rhizosphere. Plant Soil 163:1–12
Jones DL, Kielland K (2002) Soil amino acid turnover dominates the nitrogen flux in permafrost-dominated taiga forest soils. Soil Biol Biochem 34:209–219
Kandeler E, Poll C, Frankenberger WT, Tabatabai MA (2011) Nitrogen cycle enzymes. In: Dick RP (ed) Methods of soil enzymology. Soil Science Society of America, Madison, pp 211–245
Kang L, Han X, Zhang Z, Sun OJ (2007) Grassland ecosystems in China: review of current knowledge and research advancement. Phil Trans Roy Soc Lond B Biol Sci 362:997–1008
Knops JMH, Tilman D (2000) Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology 81:88–98
Landi L, Renella G, Giagnoni L, Nannipieri P (2011) Activities of proteolytic enzymes. In: Dick RP (ed) Methods of soil enzymology. Soil Science Society of America, Madison, pp 247–260
Liu X, Zhang Y, Han W, Tang A, Shen J, Cui Z, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang F (2013) Enhanced nitrogen deposition over China. Nature 494:459–462
Lü H, He H, Zhao J, Zhang W, Xie H, Hu G, Liu X, Wu Y, Zhang X (2013) Dynamics of fertilizer-derived organic nitrogen fractions in an arable soil during a growing season. Plant Soil 373:595–607
Magasanik B (1993) The regulation of nitrogen-utilization in enteric bacteria. J Cell Biochem 51:34–40
Marx MC, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640
Mengel K (1996) Turnover of organic nitrogen in soils and its availability to crops. Plant Soil 181:83–93
Merrick MJ, Edwards RA (1995) Nitrogen control in bacteria. Microbiol Rev 59:604–622
Miller M, Palojärvi A, Rangger A, Reeslev M, Kjøller A (1998) The use of fluorogenic substrates to measure fungal presence and activity in soil. Appl Environ Microb 64:613–617
Moore JM, Klose S, Tabatabai MA (2000) Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol Fertil Soils 31:200–210
Mulvaney RL (1996) Nitrogen—inorganic forms. In: Sparks DL, Page AL, Helmke PA, Loeppert RH (eds) Methods of soil analysis part 3—chemical methods. Soil Science Society of America, American Society of Agronomy, Madison, pp 1123–1184
Muruganandam S, Israel DW, Robarge WP (2009) Activities of nitrogen-mineralization enzymes associated with soil aggregate size fractions of three tillage systems. Soil Sci Soc Am J 73:751–759
Nannipieri P, Eldor P (2009) The chemical and functional characterization of soil N and its biotic components. Soil Biol Biochem 41:2357–2369
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Olk DC (2008) Organic forms of soil nitrogen. In: Schepers JS, Raun WR (eds) Nitrogen in Agricultural Systems. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, pp 57–100
Qiu S-J, Peng P-Q, Li L, He P, Liu Q, Wu J-S, Christie P, Ju X-T (2012) Effects of applied urea and straw on various nitrogen fractions in two Chinese paddy soils with differing clay mineralogy. Biol Fertil Soils 48:161–172
Ren H, Xu Z, Isbell F, Huang J, Han X, Wan S, Chen S, Wang R, Zeng D, Jiang Y, Fang Y (2017) Exacerbated nitrogen limitation ends transient stimulation of grassland productivity by increased precipitation. Ecological monographs.(accept)
Saiya-Corka KR, Sinsabaugha RL, Zakb DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Schulten HR, Schnitzer M (1998) The chemistry of soil organic nitrogen: a review. Biol Fertil Soils 26:1–15
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fertil Soils 17:69–74
Spargo JT, Cavigelli MA, Alley MM, Maul JE, Buyer JS, Sequeira CH, Follett RF (2012) Changes in soil organic carbon and nitrogen fractions with duration of no-tillage management. Soil Sci Soc Am J 76:1624–1633
Standish RJ, Cramer VA, Hobbs RJ, Kobryn HT (2006) Legacy of land-use evident in soils of Western Australia's wheatbelt. Plant Soil 280:189–207
Stevenson FJ (1982) Organic forms of soil nitrogen. In: Stevenson FJ (ed) Nitrogen in Agriculture Soil. ASA-CSSA-SSSA, Madison, pp 67–122
Sun Y, Ding Y (2010) A projection of future changes in summer precipitation and monsoon in East Asia. Sci China Earth Sc 53:284–300
Tian J, Wei K, Condron LM, Chen Z, Xu Z, Chen L (2016) Impact of land use and nutrient addition on phosphatase activities and their relationships with organic phosphorus turnover in semi-arid grassland soils. Biol Fertil Soils 52:675–683
Wei Z, Shuang Q (2001) The problem and control strategy on the eco-environmental degradation of Inner Mogolia grassland. Inner Mogolia Prataculture 1:24–27
Xu YC, Shen QR, Ran W (2003) Content and distribution of forms of organic N in soil and particle size fractions after long-term fertilization. Chemosphere 50:739–745
Xu Z, Wan S, Zhu G, Ren H, Han X (2010) The influence of historical land use and water availability on grassland restoration. Restor Ecol 18:217–225
Xu Z, Ren H, Cai J, Wang R, He P, Li M-H, Lewis B, Han X, Jiang Y (2015a) Antithetical effects of nitrogen and water availability on community similarity of semiarid grasslands: evidence from a nine-year manipulation experiment. Plant Soil 397:357–369
Xu Z, Ren H, Li M-H, van Ruijven J, Han X, Wan S, Li H, Yu Q, Jiang Y, Jiang L (2015b) Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J Ecol 103:1308–1316
Xu Z, Ren H, Li M-H, Brunner I, Yin J, Liu H, Kong D, Lü X-T, Sun T, Cai J, Wang R, Zhang Y, He P, Han X, Wan S, Jiang Y (2016) Experimentally increased water and nitrogen affect root production and vertical allocation of an old-field grassland. Plant Soil:1–12
Yang L, Zhang L, Geisseler D, Wu Z, Gong P, Xue Y, Yu C, Juan Y, Horwath WR (2016) Available C and N affect the utilization of glycine by soil microorganisms. Geoderma 283:32–38
Zhang J, Han X (2008) N2O emission from the semi-arid ecosystem under mineral fertilizer (urea and superphosphate) and increased precipitation in northern China. Atmos Environ 42:291–302
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (grant numbers 41171241, 41201290, 41371251, and 31370009), the National Key Technology R&D Program of China (grant number 2012BAD14B04), and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB15010403). We also thank Jiangping Cai and Shan Yang for providing soil organic matter data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Klaus Butterbach.
Electronic supplementary material
Fig. S1
Specific activities of protease, amidase, urease and N-acetyl glucosaminidase (NAG) normalized by Cmic in the steppe and abandoned cropland under N and water (W) additions. N: N addition, W: water addition, NW: N plus water additions. Upper cases and lower cases indicate significant difference (P < 0.05) of enzyme activities between treatments in the steppe and abandoned cropland soils, respectively. Error bars indicate standard deviation. (DOCX 202 kb)
Fig. S2
Specific activities of protease, amidase, urease and N-acetyl glucosaminidase (NAG) normalized by soil organic N (ON) in the steppe and abandoned cropland under N and water (W) additions. N: N addition, W: water addition, NW: N plus water additions. Upper cases and lower cases indicate significant difference (P < 0.05) of enzyme activities between treatments in the steppe and abandoned cropland soils, respectively. Error bars indicate standard deviation. (DOCX 204 kb)
Rights and permissions
About this article
Cite this article
Tian, J., Wei, K., Condron, L.M. et al. Effects of elevated nitrogen and precipitation on soil organic nitrogen fractions and nitrogen-mineralizing enzymes in semi-arid steppe and abandoned cropland. Plant Soil 417, 217–229 (2017). https://doi.org/10.1007/s11104-017-3253-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3253-6