Abstract
Aims
Recently, much attention has been paid to the plant-mediated effects of aboveground herbivory on soil ecosystems. However, studies about the herbivore-induced effects of invasive plants on soil ecosystem are still lacking. In this study, we aimed to examine the soil biota and nutrient availability to an invasive plant under aboveground herbivory stress, and compare the soil responses with a native plant.
Methods
We subjected an invasive plant (Spartina alterniflora) and a native plant (Phragmites australis) to herbivory by caterpillars of native moth Laelia coenosa, and measured soil microbes, nematodes, inorganic nitrogen (N), plant biomass and N content.
Results
Soil microbial biomass, nematode abundance, ammonium N concentrations and N mineralization rates were significantly stimulated by herbivory of the invasive S. alterniflora. Besides, the stimulation of bacteria: fungi ratio, abundance of bacterivorous nematodes, and ammonium N availability were significantly higher for S. alterniflora than for P. australis.
Conclusions
In general, aboveground insect herbivory of the invasive S. alterniflora enhanced the abundance of soil biota, and the soil N availability. The greater soil responses associated with S. alterniflora suggest stronger positive soil feedback than those with the native P. australis, which might facilitate the invasive plant to successfully invade its new range under biotic stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-mediated multi-trophic interactions between aboveground and belowground biota have received great attention over the past decades (Bardgett and Wardle 2003; van Dam and Heil 2011; Johnson et al. 2012; Pineda et al. 2015). There is increasing evidence that foliar herbivores not only influence the aboveground parts of plants, but also exert indirect effects on soil organisms across trophic levels (Mikola et al. 2001; Bardgett and Wardle 2003; Wardle et al. 2004; Campos-Herrera et al. 2013), which in turn influence soil nutrient mineralization and dynamics, and feedback to plant growth and productivity (Hamilton and Frank 2001; Hamilton et al. 2008; Medina-Roldán et al. 2012). Several mechanisms have been proposed to explain the effects of aboveground herbivores on belowground biota, including root exudation (Hamilton and Frank 2001), litter production (Chapman et al. 2003), and plant community changes (Bardgett and Wardle 2003). These mechanisms interact to link the above- and belowground biota. In addition, the plant–soil interactions are context dependent, varying among plant species (Mikola et al. 2001; Ayres et al. 2004) and with soil fertility (Stark et al. 2002; Sankaran and Augustine 2004). Therefore, mixed effects of aboveground herbivory on soil ecosystems have been reported in previous studies (Stark et al. 2000; Wardle et al. 2001).
Plant invasions have been one of the greatest challenges for most ecosystems in the world and are a major threat to biodiversity in the invaded ecosystems (Strayer et al. 2006; Pejchar and Mooney 2009). They modify ecosystem structure and functioning and cause substantial ecological damage to the invaded ecosystems through altering native communities, trophic interactions, and nutrient availability and cycling (Weidenhamer and Callaway 2010; Vilà et al. 2011). ‘Enemy release’ of invasive plants, escaping from natural enemies in their home ranges, has been recognized as an important mechanism accounting for successful invasions (Keane and Crawley 2002), but invasive plants often encounter generalist herbivores in their introduced ranges (Keane and Crawley 2002; Bezemer et al. 2014). Previous studies have found that invasive plants and native plants respond to local herbivore attack by excreting different biochemical defence compounds (Thelen et al. 2005; Russell et al. 2007). However, little is known about whether invasive and native plants involve different belowground responses when they suffer from aboveground herbivory, and whether these different responses further influence ecosystem functioning or feedback to invasive plants. To our knowledge, belowground responses of invasive plants to herbivory pressure have not been reported yet.
Spartina alterniflora is a wetland halophyte native to the Atlantic and Gulf coasts of North America, and has become one of the most aggressive invasive plants in estuaries and coasts in the world including the Pacific coast of North America, Europe, New Zealand, and China (Chen et al. 2004; Li et al. 2009). In China, S. alterniflora was first introduced in 1979 for erosion control and dike protection in coastal regions of China, and since then it has expanded very rapidly and become a dominant invasive plant in the saltmarshes along the east coast from Tianjin (39°11′ N) to Beihai (21°29′ N) (Li et al. 2009). In the invaded areas, S. alterniflora excludes native plant species, alters the local soil food web components and nutrient cycling such as C, N and P, and exerts strong influence on native saltmarshes (Chen et al. 2007; Peng et al. 2011; Li et al. 2014).
In this study, we aimed to explore the soil biota and nitrogen (N) responses to an invasive plant (S. alterniflora) under aboveground herbivory. Besides, we also wanted to detect whether invasive and native plants induce different belowground responses to aboveground herbivore attack. Here, we selected Phragmites australis as a native species to compare with invasive S. alterniflora. Both S. alterniflora and P. australis belong to the family Gramineae. Phragmites australis, a native brackish species, occupies similar tidal zones and coexists with S. alterniflora in many places along the Chinese coast (Li et al. 2009).
Spartina alterniflora escaped from its natural enemies such as Prokelisia marginata and Littoraria irrorata that prevail in its home range, but suffers from some herbivorous generalists such as Laelia coenosa in its invaded areas in China. Laelia coenosa (Lymantriidae, Lepidoptera) is a generalist herbivorous insect with its larvae feeding on a wide range of host plants mainly including Gramineae species (Ma et al. 2015). It is common and widespread in China, particularly in the middle and lower reaches of the Yangtze River. Laelia coenosa generally produces three generations per year and each generation includes seven to nine instars. In the Yangtze estuarine saltmarshes, L. coenosa is one of the native generalist insects feeding both on S. alterniflora and P. australis (Ju et al. 2016). In the present study, L. coenosa was therefore selected as the herbivore to examine the effects of aboveground herbivory on soil systems mediated by invasive plants.
Among soil fauna, nematodes are usually considered as key components of soil micro-food webs and important regulators of soil nutrient cycling (Ferris 2010). By feeding on microorganisms and regulating their activities and dynamics, nematodes influence the immobilization and mineralization of soil nutrients and affect N availability to plants (Bonkowski et al. 2000). Previous studies have revealed that soil microbes, nematodes, and N dynamics are all sensitive to aboveground herbivory because of the changes in carbon input to soil through plants and the bottom-up trophic effects of soil food web. For instance, Hamilton and Frank (2001) and Hamilton et al. (2008) have reported that the defoliation of Poa pratensis facilitates the rhizospheric microbial populations, consequently increasing soil inorganic N, plant N uptake, and leaf N content. In a subarctic mountain birch forest, Kaukonen et al. (2013) have found that outbreaks of moth herbivory increase the bacteria: fungi ratio, resulting in enhanced soil ammonium content and resource turnover. Both Mikola et al. (2001) and Ilmarinen et al. (2009) have detected a shift of soil food web structure after defoliation, including altered composition of nematodes, which in turn enhances the soil inorganic N concentration.
In this study, we conducted a glasshouse experiment with two plant species (S. alterniflora and P. australis), which were subjected to L. coenosa herbivory, to characterize the responses of soil subsystems to aboveground herbivores through quantifying soil microbial biomass, nematode density and composition, soil inorganic N concentration, and N mineralization. We hypothesized that (1) the invasive plant S. alterniflora would induce changes in soil food web components and N availability due to herbivorous insect attack, and (2) the soil response of invasive S. alterniflora would be different from that of the native P. australis.
Materials and methods
Material preparation and experimental design
The experiment was a 2 × 2 factorial design including two levels of insect herbivory (with or without L. coenosa) and two plant species (an exotic plant S. alterniflora or a native plant P. australis). Thus, there were four treatments with six replicates, giving a total of 24 pots.
The ramets of S. alterniflora and P. australis were collected from saltmarshes of Chongming Island, Shanghai (31°25′ to 31°38’N, 121°50′ to 122°05’E) in March 2014. After 2-week acclimation in glasshouse, based on the growth condition of plant materials, we chose 12 ramets of similar size for each species and removed attached rhizomes and leaves to maintain homogeneity of experimental plants. The average height of S. alterniflora for the experiment was 21.5 ± 1.28 cm and that of P. australis was 25.8 ± 1.69 cm. The ramets were then transplanted into PVC pots (diameter 15 cm, height 20 cm) with one plant per pot. The pots were maintained in a glasshouse on the Fudan University campus in Shanghai (31°17’N, 121°29’E). During the preparation and experimental period (from March to August), midday temperatures in the glasshouse ranged from 25 to 35 °C without supplementary lighting. All pots were located randomly in the glasshouse and re-arranged twice a week.
Each pot was filled with 1.5 kg sandy clay loam soil. The soil was collected from a mixed S. alterniflora and P. australis community at Dongtan, Chongming Island, where the experimental plant materials were collected. Before use, the soil was sieved to remove roots and carefully homogenized. The soil had an average of 60% (w/v) water content, 20 g/kg organic materials, 8.5 mg/kg inorganic N (nitrate and ammonium), and pH of 7.9. During the experiment, the soil in all pots was kept submerged under a 1 to 2 cm water layer with 5‰(w/v) NaCl solution.
Cocoons of L. coenosa were collected from Imperata cylindrica at Dongtan, Chongming Island in May 2014. We did this to avoid any possible pre-adaptations of the insect to either of S. alterniflora and P. australis. After emergence and mating, L. coenosa eggs were hatched in a laboratory incubator. Larvae from a single colony were chosen for experimental use.
On 20 June 2014, fourth instar larvae of L. coenosa were added at a density of three larvae per plant to each of six pots of each plant species as ‘L. coenosa present’ treatment. This density is similar to the naturally-occurring level in the field at Dongtan, Chongming Island. PVC cages with gauze (diameter = 20 cm, height = 1.5 m) were applied to all pots to prevent larvae escaping. L. coenosa larvae fed on the plants for about 30 days and started to cocoon.
Sampling and measurements
On 24 July, after all L. coenosa cocoons were removed from the test plants, all the shoots were cut at the soil level. Root–soil column was removed from each pot and roots were gently hand-picked from the soil. Soil in the whole pot was considered as rhizospheric soil because all the pots were almost fully filled with roots. The soil from each pot was mixed thoroughly; one-half of each sample was retained for analysis of soil inorganic N, microbial C, and phospholipid fatty acids (PLFA) and the remainder for extraction of nematodes.
Leaves and roots were oven dried at 65 °C to constant weight and weighed. Subsamples of leaves and roots were ground to determine the carbon and nitrogen concentrations with an elemental analyser (VARIO EL3 Series, Elementar, Germany). Plant N content difference between the insect herbivory present and absent treatments represents the difference in plant N uptake efficiency.
Soil inorganic N (ammonium N (NH4 +-N) and nitrate-N (NO3 −-N)) were determined from fresh soil samples. Moist soil (10 g dry weight equivalent) was shaken with 100 mL 2 M KCl for 1 h, and suspensions were then filtered through quantitative filter papers. NO3 −-N was determined with the copperized cadmium reduction method (Gal et al. 2004) and NH4 +-N was determined with the indophenol-blue colorimetric method (Brzezinski 1987; Kodama et al. 2015).
Potential net N mineralization rate for a period of 40 days was derived by subtracting final incubation KCl extractable inorganic N concentrations from N concentrations in initial solutions. We added 3 to 5 mL water to soil (10 g dry weight equivalent) in glass flasks and maintained the water content at about 70% (w/v). Soil samples were incubated for 40 days in an incubator at 35 ± 2 °C, and KCl extractable inorganic N was then determined following previously described methods (Warning and Bremner 1964; Stanford and Smith 1972).
Soil microbial biomass C (MBC) was determined by an improved chloroform fumigation incubation method (Vance et al. 1987). Two replicates of 8 g fresh soil sample were placed in 50-mL plastic centrifuge tubes; one of them was fumigated for 24 h by adding 0.5 mL ethanol-free chloroform. Both fumigated and non-fumigated soils were extracted by shaking with 40 mL 0.5 M K2SO4 for 30 min and the suspensions were heated at 100 °C in a water-bath for 60 min to remove organic C from chloroform. MBC was analyzed by a TOC analyzer (TOC-L, Shimadzu, Japan).
Phospholipid fatty acids (PLFA) were extracted following the method of Bossio and Scow (1998). We extracted the lipids from 8 g freeze-dried soil using 23 mL of a chloroform: methanol: phosphate buffer mixture (6:12:5 v/v). We separated the lipid phase from the water phase after adding 12 mL of phosphate buffer (0.05 M, pH 7.4) and 12 mL of chloroform. The lipids were separated into glycol-, neutral-, and phospholipids using pre-packed silica columns and eluting the different fractions with 5 mL chloroform, 5 mL acetone, and 5 mL methanol, respectively. The phospholipids were methylated and fatty acid methyl esters extracted to hexane, then vacuum dried and redissolved in 400 μL hexane with internal standard (19:0 methylnonadecaonate). PLFAs were analyzed on a capillary gas chromatograph with a flame ionization detector (Agilent 6890A GC with 7693 series injector).
Nematodes were extracted from 150 g (dry weight equivalent) of soil sample by the colloidal silica (Ludox-TM) flotation method (de Jonge and Bouwman 1977), and then fixed in 4% formaldehyde solution. The total abundance of nematodes was counted and each nematode was dehydrated in anhydrous glycerol, prepared on slides. We randomly selected 100 specimens that were identified to genus level under the microscope (Nikon E200). Nematodes were assigned to five trophic functional groups: algal feeder, bacterial feeder, plant feeder, omnivore, and carnivore (Yeates et al. 1993), and the percentage of each group was calculated. There were no fungal-feeding nematodes in any sample.
Data analysis
The data were natural-log or squared-root transformed to achieve normality where necessary. A two-way ANOVA analysis with plant biomass as covariate was carried out to examine the main and interactive effects of L. coenosa and plant species on soil biotic and abiotic properties. A post-hoc LSD test was performed if significant differences among treatments were found.
The relative increasing or decreasing proportion of each response index between L. coenosa present and absent treatments was calculated to represent the effect size of insect herbivory (Eq. 1). t-test was further performed to compare the different effect size between two plant species.
Statistical significance was set to P < 0.05. All statistical tests were performed using SPSS Statistics 19 (SPSS Inc., Chicago, America) and STATISTICA 8.0 (StatSoft Inc., Tulsa, OK, America).
Results
Plant biomass
Herbivory by L. coenosa larvae affected both plant leaf (F = 15.12, d.f. = 1, P = 0.001) and root biomass of both S. alterniflora and P. australis (F = 13.4, d.f. = 1, P = 0.002) (Fig. 1). Leaf biomass significantly decreased by 42% in native P. australis and 25% in invasive S. alterniflora under insect herbivory (Fig. 1a); similarly the root biomass was reduced by 36 and 32%, respectively (Fig. 1b). The t-test showed no significant difference in relative effect size of insect herbivory on plant biomass between S. alterniflora and P. australis.
Plant biomass of Phragmites australis and Spartina alterniflora without (open bars) and with (filled bars) herbivory treatment. a leaf biomass, b root biomass. The vertical bars represent the standard errors. Significance (Herbivory, Plant, the interaction of Herbivory and Plant) is indicated with ns (not significant), ** (P < 0.01) or *** (P < 0.001)
Microbial biomass and PLFA composition
Insect herbivory significantly increased soil MBC of both plant species (Fig. 2a, F = 16.78, d.f. = 1, P = 0.001). MBC of the invasive plant (S. alterniflora) increased by over 60% in response to insect herbivory, and that of the native plant (P. australis) by 50%.
Soil microbial biomass and community composition in rhizospheric soils of Phragmites australis and Spartina alterniflora without (open bars) and with (filled bars) herbivory treatment. a microbial biomass carbon (MBC), b proportion of bacterial phospholipid fatty acids (PLFA), c proportion of fungal PLFA, and d bacteria: fungi ratio. The vertical bars represent the standard errors. Significance (Herbivory, Plant, the interaction of Herbivory and Plant) is indicated with ns (not significant), * (P < 0.05),** (P < 0.01) or *** (P < 0.001)
PLFA analysis showed that the proportions of bacterial PLFA significantly increased by 6% in P. australis and 11% in S. alterniflora after herbivore attack (Fig. 2b, F = 14.01, d.f. = 1, P = 0.001) whereas those of fungal PLFAs decreased by 25 and 46% in P. australis and S. alterniflora, respectively (Fig. 2c). This produced a significant rise in bacteria : fungi PLFA ratio for both P. australis (P < 0.05) and S. alterniflora (P < 0.001). The interactive effects of herbivory and plant species was only found on bacteria : fungi PLFA ratio (F = 4.86, d.f. = 1, P = 0.04). In the presence of L. coenosa, the bacteria : fungi PLFA ratio was almost double that without insect herbivory for S. alterniflora, but was only 39% higher for P. australis (Fig. 2d). The effect sizes of insect herbivory on bacterial PLFA (P = 0.05) and bacteria: fungi PLFA (P = 0.04) for S. alterniflora were significantly higher than for P. australis.
Nematode community
The infestation with L. coenosa stimulated an increase in the total abundance of nematodes (Fig. 3a; F = 12.69, d.f. = 1, P = 0.002). When the insect herbivores were present, total soil nematode abundances increased by 30 and 25% in S. alterniflora and P. australis pots, respectively.
The nematode abundance of each trophic group in rhizospheric soils of Phragmites australis and Spartina alterniflora without (open bars) and with (filled bars) herbivory treatment. a total nematode abundance, b algal feeders, c plant feeders, d bacterial feeders, e carnivores, and f omnivores. The vertical bars represent the standard errors. Significance (Herbivory, Plant, the interaction of Herbivory and Plant) is indicated with ns (not significant), ** (P < 0.01) or *** (P < 0.001)
In our experiment, 29 soil nematode genera were identified, which belonged to 20 families and 6 orders. Numbers of nematode genera were not significantly different between the two plant species in the experiment. However, the herbivory of L. coenosa had significant effects on bacterivores (F = 25.90, d.f. = 1, P < 0.001) and carnivores (F = 17.81, d.f. = 1, P < 0.001) of the nematode trophic groups for both S. alterniflora and P. australis. Abundances of nematode bacterivores increased by 43% in S. alterniflora and 37% in P. australis when the plants were subject to aboveground insect herbivory (Fig. 3d). Spartina alterniflora with herbivory treatment had twice as many carnivores as without insect herbivory, while the increase for P. australis was 80% (Fig. 3e). No significant interactive effects of herbivory and plant species was found on the abundance of nematodes. But the effect size of insect herbivory on abundance of nematode bacterivores for S. alterniflora was significantly higher than that for P. australis (P = 0.03).
Soil inorganic N availability
The NH4 +-N content (F = 26.38, d.f. = 1, P < 0.001) and its mineralization rate (F = 17.01, d.f. = 1, P < 0.001) were affected by insect herbivory (Figs. 4a and b). The interactive effects of herbivory and plant species was found on NH4 +-N content (F = 5.70, d.f. = 1, P = 0.03). For the invasive plant S. alterniflora, the total concentration and the mineralization rate of soil NH4 +-N after aboveground insect herbivory were doubled by herbivory, as shown by their respective increases of 140 and 130%. For the native plant P. australis, no significant change was found for the total NH4 +-N content after aboveground insect herbivory, while the mineralization rate of soil NH4 +-N increased by 75%. There was no significant difference in NO3 −-N concentration and mineralization rate between herbivory and no-herbivory treatments for either of the two plant species, even though we detected an increasing trend of overall NO3 −-N concentration (Figs. 4c and d). In addition, t-tests on effect size showed that the magnitudes of increase in NH4 +-N (P = 0.03) and its mineralization rate (P = 0.01) for S. alterniflora were significantly greater than for P. australis.
Soil inorganic nitrogen (N) availability in rhizospheric soils of Phragmites australis and Spartina alterniflora without (open bars) and with (filled bars) herbivory (filled bars) treatment. a soil ammonium-N (NH4 +-N) content, b NH4 +-N mineralization rate, c nitrate-N (NO3 −-N) content, and d NO3 −-N mineralization rate. The vertical bars represent the standard errors. Significance (Herbivory, Plant, the interaction of Herbivory and Plant) is indicated with ns (not significant), * (P < 0.05) or *** (P < 0.001)
Plant nutrient content
The C: N ratio of plant leaf was influenced by plant species (F = 8.18, d.f. = 1, P = 0.01), while insect herbivory and plant species had interactive effect on it (F = 4.73, d.f. = 1, P = 0.04) (Fig. 5b). The C: N ratio of S. alterniflora leaf reduced remarkably by 9%, after insect herbivory (Figs. 5b; P < 0.05), while no obvious change was detected in leaf of P. australis. Besides, the leaf N content was also strongly affected by plant species (F = 17.47, d.f. = 1, P < 0.001), while insect herbivory and plant species had interactive effect on it (F = 6.75, d.f. = 1, P = 0.02). The N content of leaf significantly increased by 13% in S. alterniflora (Fig. 5a; P = 0.04). However, no change in N concentration was induced by herbivory in both leaves and roots of P. australis. The t-tests of effect sizes showed that the increase of leaf N (P = 0.01) and decrease of leaf C: N ratio (P = 0.02) were significantly greater for S. alterniflora than that for P. australis.
Plant nitrogen (N) content and carbon : nitrogen (C : N) ratio of Phragmites australis and Spartina alterniflora without (open bars) and with (filled bars) herbivory treatment. a leaf N, b leaf C : N, c root N, d root C: N. The vertical bars represent the standard errors. Significance (Herbivory, Plant, the interaction of Herbivory and Plant) is indicated with ns (not significant), * (P < 0.05) or *** (P < 0.001)
Discussion
Positive soil responses of Spartina alterniflora to insect herbivory
In this study, PLFA analysis revealed a significant increase in soil bacterial PLFA abundance when the invasive plant S. alterniflora was attacked by insects. Stimulation of bacterial activity could be the result of increased plant root deposition (release of root C-compounds) providing C compounds that were more labile and easily used by bacteria (Bardgett et al. 1996; Kaukonen et al. 2013). By using a 13C pulse-chasing method, Hamilton and Frank (2001) and Hamilton et al. (2008) found that herbivore defoliation stimulated root exudation both in a greenhouse and in grassland and then increased accumulation of microbial biomass. In a meta-analysis, Zhang et al. (2015) also indicated that insect/pathogen attacks significantly increased MBC by 14%, which was possibly related to a 38.6% increase in dissolved organic C. In this study, although we detected a significant increase in soil bacterial biomass after herbivory for S. alterniflora, a significant decrease in root biomass in response to defoliation was observed. This is consistent with many similar findings in short-term pot experiments, because many newly grown roots were short lived under insect grazing (Frank et al. 2002; Ziter and MacDougall 2013). Our results suggest that although the root biomass of S. alterniflora decreased, root production might not be the case. Enhanced root turnover rate and short-lived roots induced by herbivory might produce a greater amount of exudates and more root litter, together leading to an increase in soil bacterial abundance or biomass under insect grazing. Because we did not measure the root exudation and root mortality directly, and with the limited replication numbers, further experiments are required to confirm this hypothesis.
It has been reported that aboveground herbivory affects different trophic groups of the soil food web through an upward cascading effect (Mikola et al. 2001; Wardle et al. 2004). This view was confirmed in our study because, in accordance with the increase in bacterial abundance, both the abundance of bacterial-feeding and predatory nematodes significantly increased when the insect herbivores attacked S. alterniflora. The consistency in abundances of microbes and nematodes can be explained by the trophic bottom-up control of the soil micro-food web following manipulation of the resource base (Wardle and Yeates 1993; Wardle et al. 1998). Therefore, L. coenosa herbivory might have induced an enhanced energy flow upwards through the food chain from soil microbes to bacterial feeding nematodes, and to predatory nematodes at a high trophic level.
Herbivory has been reported to cause a shift from fungal-based energy channels to bacterial-based channels in the decomposer food web, with the latter being usually considered to represent more active and rapid cycling of soil nutrients (Bardgett et al. 1996; Bardgett et al. 1998). For instance, Kaukonen et al. (2013) have demonstrated that massive moth herbivory increases the bacteria: fungi ratio and enchytraeids in mountain birch forests, which consequently enhances the resource turnover rate. As an increase in the bacteria: fungi PLFA ratio and bacterial-feeding nematodes was also observed in this study, insect herbivory on S. alterniflora might prioritize the bacterial-based energy channel in soil nutrient cycling. Soil biota included in the micro-food web, such as microbes and nematodes, are critical in regulating soil ecosystem process such as N mineralization and cycling (Bonkowski et al. 2000; Ferris 2010). Stimulated soil biota after defoliation by herbivores have been found to account for the increases in soil N availability including NH4 + and NO3 − concentrations (Hamilton and Frank 2001; Mikola et al. 2001) and their mineralization rates (Ayres et al. 2004; Hamilton et al. 2008). Our study revealed that the soil NH4 +-N concentration and its potential mineralization rate were more than doubled in S. alterniflora with insect herbivory than those without herbivory. These results suggested an enhanced inorganic N availability under insect herbivory in soils of invasive S. alterniflora.
Greater soil response of invasive S. alterniflora than native P. australis
This study found that, under insect herbivory, invasive S. alterniflora enhanced its soil bacteria: fungi ratio by 100% and bacterivorous nematode abundance by 43%, and native P. australis increased the bacteria: fungi ratio and bacterivorous nematodes by 39 and 37%, respectively. Many other studies have reported that invasive plants strongly influence soil biota in introduced areas, such as soil microbes (Vilà et al. 2011), nematodes (Chen et al. 2007), or higher trophic groups of soil food web components (Yeates and Williams 2001). However, to best of our knowledge, no studies have reported the effects of invasive plants on soil biota under herbivory pressure. Our study is the first to demonstrate that an invasive plant stimulates a greater abundance of soil bacteria and animals of other higher trophic level under aboveground herbivory than its co-occurring native plants.
Invasive plant species can cause quite different physiological responses to defoliation, as compared to native plants (Joshi and Vrieling 2005). In a series of experiments in the greenhouse and in the field, Siemann and his colleagues have observed that the invasive populations of Sapium sebiferum has lower resistance and higher tolerance than native ones under herbivory stress (Rogers and Siemann 2002, 2004; Siemann and Rogers 2003). This is probably because invasive plants tend to allocate more resources for growth and reproduction and reduce the production of expensive defences such as toxic secondary metabolites after novel herbivore attack in their introduced range (Joshi and Vrieling 2005; Doorduin and Vrieling 2011). Little information, however, is available on the differences in secondary metabolites and root exudation between invasive S. alterniflora and native P. australis after herbivore attack. Liang et al. (2012) compared the soils from invasive S. alterniflora and another plant Scirpus mariqueter native to China, and detected heneicosanoic acid, hexadecane, octadecane, sulfuric acid, and diethyl ester only in S. alterniflora, but not in Sc. mariqueter. Therefore, we speculate that amounts and components of the root exudation were different between invasive S. alterniflora and native P. australis, which is likely to account for the different strength of their belowground biotic responses to insect defoliation.
Actually, some work has been done to compare the effects of invasive S. alterniflora and native P. australis on soil communities and ecosystems in the absence of herbivory disturbance, which provides some hints to our results. For example, Li et al. (2009) have detected that, the invasive S. alterniflora has changed the abundance of some macrobenthonic invertebrates compared with native S. mariqueter. Moreover, the aboveground plant N and soil inorganic N pools in S. alterniflora saltmarshes have also been reported significantly higher than those in native P. australis marshes (Peng et al. 2011). Chen et al. (2007) have found the invasion of S. alterniflora stimulates the growth of bacterial nematodes and supports more abundant nematodes than native P. australis. These changes may result in a different nematode community structure and a faster nutrient cycling than native plant species. Together with their results, this study indicates that S. alterniflora might lead to a further more activated soil biotic community if it is subjected to herbivory stress.
Soil biota can be important drivers for plant invasion, either hampering or promoting invasion success (Reinhart and Callaway 2006; Inderjit and van der Putten 2010). Parepa et al. (2013) found that soil biota facilitates knotweed invasion and suggest that the invasive plants could obtain benefits from underground. They have also found that such effect is dependent on chemical context such as activated C addition (Parepa et al. 2013). In addition to abiotic conditions such as chemical properties, invasive plants may also encounter biotic disturbance such as herbivores’ defoliation. Our study found that, under defoliation disturbance, the invasive S. alterniflora might benefit more from the stimulated soil community than the native P. australis. Insect herbivory increased the total concentration of soil NH4 +-N by 140% and its mineralization rate by 130% in the invasive S. alterniflora soil. For native P. australis, however, the increasing ratio of NH4 +-N mineralization rate was much lower than in the invasive S. alterniflora soil, and no significant change was detected in NH4 +-N content. This could be related to the beneficial functions of soil biota, because microbial-feeding nematodes can not only maintain higher bacterial abundance and activity, but also release inorganic N that is bound to bacteria and make it available to plants (Bonkowski et al. 2000; Lenoir et al. 2007).
Previous studies have demonstrated that increased soil N availability can further influence plant N uptake, resulting in elevated plant shoot N (Hamilton and Frank 2001) or root N content (Ilmarinen et al. 2009). In our study, the soil NH4 concentrations significantly increased and the NO3 concentrations showed an increasing tendency in response to herbivory for both S. alterniflora and P. australis. However, the leaf N content of S. alterniflora significantly increased by 13%, and C: N ratio decreased by 9% after herbivory, whereas such changes were not detected in P. australis. Enhanced quality of roots implies a higher N uptake efficiency of plants, which may compensate for the effects of root mass reduction after leaf damage (Mikola et al. 2001). Increased N concentrations in leaves may imply higher chlorophyll contents and enhanced rates of photosynthesis (Ziter and MacDougall 2013). Therefore, generally, it is likely that although S. alterniflora and P. australis both induced positive soil biota and N availability responses to aboveground herbivory, the invasive plant (S. alterniflora) benefited more by gaining greater N uptake and compensatory growth than the native plant (P. australis), consequently exerting potentially stronger competitive effects on the native P. australis.
The higher nutrient concentrations in invasive plant leaves might attract more herbivores, resulting in greater consumer pressure on native plants (Russell et al. 2007). As described by Bezemer et al. (2014), invasive plants can induce an increase in the shared herbivores that may exert an increased negative effect on the native plants. Orrock et al. (2015) have also reported that in the forest of Missouri, USA, apparent competition helped the exotic Amur honeysuckle (Lonicera maackii) to suppress native plants through the small-mammal consumer. Ju et al. (2016) have reported that S. alterniflora increased L. coenosa’s abundance through its extended phenology, compared to the native host, P. australis. In our study, the significantly enhanced leaf N concentration of S. alterniflora induced by soil responses to herbivory stress can be likely to attract more L. coenosa, which may in turn exert more feeding pressure on native P. australis.
Conclusions
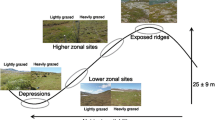
Plant–soil feedbacks describe the ways that plant species affect soil biota, which in turn alter the relative performance of plant species. In this study, generally, we demonstrated the altered soil biota and N availability of an invasive plant (Spartina alterniflora), and also found a greater soil response of invasive S. alterniflora than that of a native plant (P. australis) to aboveground herbivory (Fig. 6). This was reflected by the significantly higher bacteria: fungi ratio, abundance of bacterivorous nematodes, ammonium N availability in soils conditioned by S. alterniflora when the plants were subjected to insect defoliation than those conditioned by P. australis. The increased soil inorganic N availability would further influence the nutrient uptake of plants, leading to a more prominent increase in plant tissue nutrient concentration for S. alterniflora than for P. australis. This suggests that foliar feeding might trigger a greater photosynthesis rate for S. alterniflora, which would serve as a compensatory response to foliar loss. Higher leaf N concentration might potentially attract more herbivores that feed on the native plants. Thus, the invasive S. alterniflora may indirectly affect P. australis through apparent competition by intensifying herbivore pressure. Our results suggest that aboveground herbivores and belowground biota interact across the soil interface and multi-trophic levels, which potentially lead to better performance and an elevated competitive ability of invasive plants. This study finds that the plant–soil feedbacks in the presence of biotic disturbance differ in extent between an invasive plant and a native plant, the question of whether such a pattern is common for invasive plants is still open to further investigation.
Overview of the effects of aboveground insect herbivory on soil systems by comparing the invasive Spartina alterniflora and native Phragmites australis. (1) Generally, insect herbivory induced a greater positive soil responses for S. alterniflora than for P. australis in terms of the increasing soil microbial biomass, nematode abundance and inorganic N availability, (2) herbivory-induced soil response had positive feedback effect on N content of S. alterniflora, but no effect on P. australis, and (3) the enhanced N content of invasive S. alterniflora may lead to a greater herbivory pressure on native P. australis
References
Ayres E, Heath J, Possell M, Black HIJ, Kerstiens G, Bardgett RD (2004) Tree physiological responses to above-ground herbivory directly modify below-ground processes of soil carbon and nitrogen cycling. Ecol Lett 7:469–479
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Hobbs PJ, Frostegard A (1996) Changes in soil fungal: bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol Fertil Soils 22:261–264
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 14:1867–1878
Bezemer TM, Harvey JA, Cronin JT (2014) Response of native insect communities to invasive plants. Annu Rev Entomol 59:119–141
Bonkowski M, Griffiths B, Scrimgeour C (2000) Substrate heterogeneity and microfauna in soil organic 'hotspots' as determinants of nitrogen capture and growth of ryegrass. Appl Soil Ecol 14:37–53
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Brzezinski MA (1987) Colorimetric determination of nanomolar concentrations of ammonium in seawater using solvent-extraction. Mar Chem 20:277–288
Campos-Herrera R, Ali JG, Diaz BM, Duncan LW (2013) Analyzing spatial patterns linked to the ecology of herbivores and their natural enemies in the soil. Front Plant Sci 4:378
Chapman SK, Hart SC, Cobb NS, Whitham TG, Koch GW (2003) Insect herbivory increases litter quality and decomposition: an extension of the acceleration hypothesis. Ecology 84:2867–2876
Chen Z, Li B, Zhong Y, Chen J (2004) Local competitive effects of introduced Spartina alterniflora on Scirpus mariqueter at Dongtan of Chongming Island, the Yangtze River estuary and their potential ecological consequences. Hydrobiologia 528:99–106
Chen H, Li B, Hu J, Chen J, Wu J (2007) Effects of Spartina alterniflora invasion on benthic nematode communities in the Yangtze estuary. Mar Ecol Prog Ser 336:99–110
de Jonge VN, Bouwman LA (1977) A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM. Mar Biol 42:143–148
Doorduin LJ, Vrieling K (2011) A review of the phytochemical support for the shifting defence hypothesis. Phytochem Rev 10:99–106
Ferris H (2010) Contribution of nematodes to the structure and function of the soil food web. J Nematol 42:63–67
Frank DA, Kuns MM, Guido DR (2002) Consumer control of grassland plant production. Ecology 83:602–606
Gal C, Frenzel W, Moller JR (2004) Re-examination of the cadmium reduction method and optimisation of conditions for the determination of nitrate by flow injection analysis. Microchim Acta 146:155–164
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Hamilton EW, Frank DA, Hinchey PM, Murray TR (2008) Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol Biochem 40:2865–2873
Ilmarinen K, Mikola J, Nissinen K, Vestberg M (2009) Role of soil organisms in the maintenance of species-rich seminatural grasslands through mowing. Restor Ecol 17:78–88
Inderjit, van der Putten WH (2010) Impacts of soil microbial communities on exotic plant invasions. Trends Ecol Evol 25:512–519
Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW, Koricheva J (2012) Aboveground-belowground herbivore interactions: a meta-analysis. Ecology 93:2208–2215
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Ju RT, Chen YY, Gao L, Li B (2016) The extended phenology of Spartina invasion alters a native herbivorous insect's abundance and diet in a Chinese salt marsh. Biol Invasions 18:2229–2236
Kaukonen M, Ruotsalainen AL, Wali PR, Mannisto MK, Setala H, Saravesi K, Huusko K, Markkola A (2013) Moth herbivory enhances resource turnover in subarctic mountain birch forests? Ecology 94:267–272
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kodama T, Ichikawa T, Hidaka K, Furuya K (2015) A highly sensitive and large concentration range colorimetric continuous flow analysis for ammonium concentration. J Oceanogr 71:65–75
Lenoir L, Persson T, Bengtsson J, Wallander H, Wirén A (2007) Bottom–up or top–down control in forest soil microcosms? Effects of soil fauna on fungal biomass and C/N mineralisation. Biol Fertil Soils 43:281–294
Li B, Liao CZ, Zhang XD, Chen HL, Wang Q, Chen ZY, Gan XJ, Wu JH, Zhao B, Ma ZJ, Cheng X, Jiang LF, Chen JK (2009) Spartina alterniflora Invasions in the Yangtze River estuary, China: an overview of current status and ecosystem effects. Ecol Eng 35:511–520
Li H, Zhang X, Zheng R, Li X, Elmer WH, Wolfe LM, Li B (2014) Indirect effects of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native saltmarsh plants in China. J Ecol 102:1112–1119
Liang X, Zheng H, He CQ, Xu QY, Zhan YW, Lei YR, Du W, Yang JN (2012) Allelopathic effcts of invasive Spartina alterniflora root exudates in soil on the offspring (seeds) of Scirpus mariqueter. Allelopath J 29:251–262
Ma D, Ju R, Li B (2015) Preference of Laelia coenosa for native and introduced populations of invasive Spartina alterniflora. Biodivers Sci 23:101–108
Medina-Roldán E, Paz-Ferreiro J, Bardgett RD (2012) Grazing-induced effects on soil properties modify plant competitive interactions in semi-natural mountain grasslands. Oecologia 170:159–169
Mikola J, Yeates GW, Barker GM, Wardle DA, Bonner KI (2001) Effects of defoliation intensity on soil food-web properties in an experimental grassland community. Oikos 92:333–343
Orrock JL, Dutra HP, Marquis RJ, Barber N (2015) Apparent competition and native consumers exacerbate the strong competitive effect of an exotic plant species. Ecology 96:1052–1061
Parepa M, Schaffner U, Bossdorf O (2013) Help from under ground: soil biota facilitate knotweed invasion. Ecosphere 4:t31
Pejchar L, Mooney HA (2009) Invasive species, ecosystem services and human well-being. Trends Ecol Evol 24:497–504
Peng RH, Fang CM, Li B, Chen JK (2011) Spartina alterniflora Invasion increases soil inorganic nitrogen pools through interactions with tidal subsidies in the Yangtze estuary, China. Oecologia 165:797–807
Pineda A, Soler R, Pozo MJ, Rasmann S, Turlings TCJ (2015) Editorial: above-belowground interactions involving plants, microbes and insects. Front Plant Sci 6:318
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Rogers WE, Siemann E (2002) Effects of simulated herbivory and resource availability on native and invasive exotic tree seedlings. Basic Appl Ecol 3:297–307
Rogers WE, Siemann E (2004) Invasive ecotypes tolerate herbivory more effectively than native ecotypes of the Chinese tallow tree Sapium sebiferum. J Appl Ecol 41:561–570
Russell FL, Louda SM, Rand TA, Kachman SD (2007) Variation in herbivore-mediated indirect effects of an invasive plant on a native plant. Ecology 88:413–423
Sankaran M, Augustine DJ (2004) Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Ecology 85:1052–1061
Siemann E, Rogers WE (2003) Reduced resistance of invasive varieties of the alien tree Sapium sebiferum to a generalist herbivore. Oecologia 135:451–457
Stanford G, Smith SJ (1972) Nitrogen mineralization potential of soil. Soil Sci Soc Am Proc 36:465–472
Stark S, Wardle DA, Ohtonen R, Helle T, Yeates GW (2000) The effect of reindeer grazing on decomposition, mineralization and soil biota in a dry oligotrophic scots pine forest. Oikos 90:301–310
Stark S, Strommer R, Tuomi J (2002) Reindeer grazing and soil microbial processes in two suboceanic and two subcontinental tundra heaths. Oikos 97:69–78
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Thelen GC, Vivanco JM, Newingham B, Good W, Bais HP, Landres P, Caesar A, Callaway RM (2005) Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol Lett 8:209–217
van Dam NM, Heil M (2011) Multitrophic interactions below and above ground: en route to the next level. J Ecol 99:77–88
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Wardle DA, Yeates GW (1993) The dual importance of competition and predation as regulatory forces in terrestrial ecosystems-evidence from decomposer food webs. Oecologia 93:303–306
Wardle DA, Verhoef HA, Clarholm M (1998) Trophic relationships in the soil microfood-web: predicting the responses to a changing global environment. Glob Chang Biol 4:713–727
Wardle DA, Barker GM, Yeates GW, Bonner KI, Ghani A (2001) Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Wardle DA, Yeates GW, Williamson WM, Bonner KI, Barker GM (2004) Linking aboveground and belowground communities: the indirect influence of aphid species identity and diversity on a three trophic level soil food web. Oikos 107:283–294
Warning SA, Bremner JM (1964) Ammonium production in soil under waterlogged conditons as an index of nitrogen avaliabliity. Nature 201:951–952
Weidenhamer JD, Callaway RM (2010) Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J Chem Ecol 36:59–69
Yeates GW, Williams PA (2001) Influence of three invasive weeds and site factors on soil microfauna in New Zealand. Pedobiologia 45:367–383
Yeates GW, Bongers T, Degoede R, Freckman DW, Georgieva SS (1993) Feeding-habits in soil nematode families and genera- an outline for soil ecologists. J Nematol 25:315–331
Zhang B, Zhou X, Zhou L, Ju R (2015) A global synthesis of below-ground carbon responses to biotic disturbance: a meta-analysis. Glob Ecol Biogeogr 24:126–138
Ziter C, MacDougall AS (2013) Nutrients and defoliation increase soil carbon inputs in grassland. Ecology 94:106–116
Acknowledgements
We would like to thank Professor Anthony John Davy for his constructive comments on an early version of this manuscript. We also thank Ding Ma for assistance with preparing insect materials, Sikai Wang and Zaichao Yang for help with collecting plant and soil materials. This research was financially supported by National Basic Research Program of China (Grant No. 2013CB430404) and the National Natural Science Foundation of China (31670544).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Zhou, J., Ju, R., Li, B. et al. Responses of soil biota and nitrogen availability to an invasive plant under aboveground herbivory. Plant Soil 415, 479–491 (2017). https://doi.org/10.1007/s11104-017-3179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3179-z