Abstract
A major question in soil ecology is whether soil food webs are regulated by resources or by predators, i.e. bottom–up (donor) or top–down controlled. We tested the hypothesis that meso- and macrofaunal soil predators can regulate fungivore populations and, thereby cause a top–down cascade effect on fungal biomass and decomposition/mineralisation processes in boreal forest soils. The study was performed as a microcosm experiment with two contrasting soils (humus layers), one poor and one rich in N, and with different combinations of fungivore and predator soil fauna added to “defaunated” soil. In comparison with control microcosms lacking mesofauna (but with nematodes and protozoans), the presence of a diverse Collembola and Oribatida fungivore community significantly reduced the FDA-active fungal biomass or tended to reduce the ergosterol fraction of the fungal biomass in the N-poor humus, but no clear effect could be detected in the N-rich humus. Fungivores as well as fungivores plus predators (a predator community consisting of gamasids, spiders and beetles or a subset thereof) reduced C mineralisation and increased net N mineralisation in both soils. The presence of predators (particularly gamasid mites) reduced collembolan numbers and alleviated the negative effect of fungivores on fungal biomass in the N-poor soil. In the N-rich soil, the presence of predators increased fungal biomass (ergosterol) in relation to the “defaunated” soil. Therefore, a top–down trophic cascade could be detected in the N-poor humus but not in the N-rich humus. Our results suggest that the degree of top–down control in soil fauna communities depends on resource quality and soil fertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decomposition and mineralisation processes are controlled by environmental factors, such as temperature and substrate quality, and by the structure of the decomposer community (Swift et al. 1979; Sulkava et al. 1996; Bradford et al. 2002; Smith and Bradford 2003). Detritus-based food webs are often thought to be donor-controlled (Polis and Strong 1996), i.e. biomasses at higher trophic levels are determined by lower trophic levels but not vice versa. However, it has been suggested that soil food webs are not exclusively donor-controlled, but sometimes regulated by ‘top–down’ mechanisms (Bengtsson et al. 1995).
Microbivorous soil animals can affect decomposition rates by regulating the microbial community and mobilising nutrients from soil organic matter (Faber and Verhoef 1991; Setälä et al. 1991; Kandeler et al. 1999; Hedlund and Sjögren Öhrn 2000; Leps et al. 2001; Liiri et al. 2002; Villenave et al. 2004). Clarholm (1985) suggested that protozoa can regulate bacterial biomass and Moore et al. (1988) found that microarthropods could regulate densities of organisms at lower trophic levels. Even predators have a potential to indirectly affect microbes by controlling the microbivore community. Trophic cascades in detritus-based food webs, where predators indirectly affect microbes and the rate of decomposition, have been reported (Santos et al. 1981; Wyman 1998; Hedlund and Sjögren Öhrn 2000). Laakso and Setälä (1999) showed that nematode predators can decrease the abundance of the microbivorous nematodes, and that this can cascade down to the base of the detrital food web and affect the productivity of microbes and nutrient dynamics.
Patterns in carbon (C) and nitrogen (N) mineralisation are theoretically expected to differ, depending on whether a soil system is dominated by top–down or bottom–up interactions (Zheng et al. 1997, 1999). In a bottom–up system, C and N mineralisation will increase as trophic levels are added to the system. In a top–down system, the effects of adding trophic levels on C and N mineralisation are more complex. Both increases and decreases are possible, depending on microbial properties and trophic complexity (Zheng et al. 1997, 1999).
Most studies on trophic cascades in soil have been carried out with a limited number of faunal species. This could limit the applicability of these studies to more diverse natural systems. Polis and Strong (1996) argued that complexity in food webs would attenuate top–down control of lower trophic levels. Thus, strong interactions between trophic levels would only be found in species-poor and non-heterogeneous systems (Van de Koppel et al. 2005).
Some studies have investigated the effects of faunal composition on N mineralisation and decomposition by using litterbags or soil cores with different mesh sizes, thus manipulating the soil community composition into groups of different body size. For instance, Kandeler et al. (1999) found that mesofauna, compared to microfauna, could enhance microbial biomass and mineralisation processes in humus material. Smith and Bradford (2003) showed that the decomposition rate of litter increased with increasing faunal body size. However, these studies do not allow examination of the trophic relations between fungi and groups of predators or fungivores with similar body size.
The prime aim of the study was to determine the effect of one (decomposer microorganisms), two (decomposer microorganisms and fungivores) and three (microorganisms, fungivores and predatory soil fauna) trophic levels on C and N mineralisation and fungal biomass. Another aim was to find out whether a complete community of predators affects C and N mineralisation more than single groups of predators. Our main hypothesis was that a trophic cascade would occur, i.e., that a complete fungivore community without predators would reduce the fungal biomass more than a fungivore community preyed upon by predators. We also hypothesised that fungivore grazing on fungi would increase C and N mineralisation. The study was made with two contrasting mor humus types to find out whether soil conditions influenced the responses. The faunal populations introduced to the microcosms were similar to those found in the field soils, both in total numbers and in the proportion between species.
Materials and methods
Materials and pre-treatments
Soil samples were taken in October 1992 from the FH layer (Oe + Oa horizons) of an untreated area and a fertilised area in a 40-year-old stand of Scots pine (Pinus sylvestris L.) in the Jädraås experimental forest (60°49′ N, 16°30′ E, 185 m elevation) in central Sweden. The soil is an arenosol overlying sandy sediments. The humus form is a mor. The fertilised treatment consisted of daily applications of a liquid solution of micro- and macronutrients from May to September from 1974 to 1990. During this period, a total of 1,740 kg N ha−1 was applied, but from 1991 onwards, no fertiliser was added. The treatment altered both the ground vegetation and the soil properties. The dwarf shrubs Calluna vulgaris (L.) Hull. and Vaccinium vitis-idaea L., the moss Pleurozium schreberi (Brid.) Mitt. and Cladonia/Cladina lichens were common in the untreated area, while Chamaenerion angustifolium (L.), Rubus idaeus L. and the moss Brachythecium oedipodium (Mitt.) Jaeg. were dominant in the treated area.
Humus layer samples were taken from four N-poor non-fertilised and four fertilised plots. After sampling, the samples were pooled into one bulk of N-poor material (0 humus, pH 4.1, C/N 33.0) and one bulk of N-rich material (N humus, pH 4.4, C/N 28.6). Thus, the field experiment was used to obtain contrasting substrates as regards the N content. Exchangeable \({\text{NH}}^{{\text{ + }}}_{{\text{4}}} {\text{ - N}}\) and \({\text{NO}}^{{\text{ - }}}_{{\text{3}}} {\text{ - N}}\) was 25 and 0.5 μg g−1 OM (organic matter), respectively, in both materials, indicating low initial levels of inorganic N also in the fertilised plots.
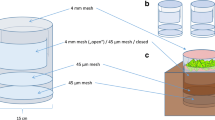
Both materials were sieved through a 5-mm mesh and, after sieving, were frozen at −20°C for 3 days to kill all soil fauna except for microfauna (nematodes, rotifers, protozoans and tardigrades) and microarthropod eggs (incomplete mortality). After thawing, the humus fresh weight/dry weight ratios were determined after drying the samples at 105°C for 24 h. Loss on ignition (LOI) was determined after combustion at 550°C for 3 h and was considered as equal to the OM content. Total C and N were measured on ground material using a flash combustion reactor (Carlo Erba NA 1500 Elemental Analyzer). Portions corresponding to 16 g dry weight of humus material were placed in plastic containers (50 cm2 surface area, 466-ml volume) forming an artificial humus layer (mostly consisting of F layer materials, i.e., fragmented litter) of 3-cm depth.
To ensure that the ‘background’ microbial, protozoan, nematode and tardigrade communities were similar in all containers, 15 g (fresh weight) of fresh litter and humus layers were thoroughly mixed (Braun mixer) in 1,000 ml of tap water for 2 min. After sedimentation of coarse soil particles, 5 ml of the humus suspension was added to each experimental container (microcosm). The suspension taken from the untreated humus was added to the containers with untreated humus, and that taken from the fertilised humus was added to the containers with that kind of humus. Thus, the effects of the experimental additions of soil fauna were investigated against a more-or-less constant background of bacterial and microfaunal activity.
Addition of soil fauna
The experimental setup for both the unfertilised N-poor (0) and the fertilised (N) humus is shown in Table 1. Each treatment contained ten replicates, five to be sampled on day 50 and five on day 100.
To collect the animals, litter and humus layer blocks (400-cm2 surface area) from the two field treatments were placed in Tullgren funnels for 3 days. The arthropods extracted were collected in jars with tap water. Floating collembolans could be collected from the water surface with a spoon, whereas sunk arthropods could be sucked up from the bottom of the jar by a pipette. The field density of soil arthropods is in the order of 60,000 collembolans and 400,000 oribatids m−2 at Jädraås (Persson et al. 1980), and, therefore, corresponding numbers, 300±100 collembolans and 2,000±1,000 oribatids, were added to each microcosm containing “fungivores”. The relative proportion of individual species varied depending on the outcome of the extractions. About 20 Collembola and 30 Oribatida species were added to the microcosms. Most of these species were found after 50 and 100 days of incubation (Appendices 1 and 2). Thus, a mixture of macro- and microphytophages, as well as epi- and hypogeic species, was added to mimic a natural microarthropod community. To avoid addition of potential predators to the fungivore (0F and NF) microcosms, we did not include any prostigmatid, astigmatid or mesostigmatid mites.
The microcosms with fungivores and predators (0P and NP) were supplied with the fungivore groups mentioned above and also a mixture of mesostigmatid mites, beetles and spiders (Tables 1 and 2). Because the 0 and N humus contained slightly different species and numbers of predators, the added predators differed to some extent between the humus materials. Small mesostigmatids, such as Parazercon sarekensis and Trachytes aegrota, were added to the mixed predator microcosms in proportion to their occurrence in the extraction jars.
The collembolans were determined according to Fjellberg (1980, 1998), oribatid mites according to Willmann (1931), Balogh (1972) and Gilyarov and Krivolutsky (1975) and mesostigmatid mites according to Karg (1993).
The animal additions lasted for 16 days, during which period the microcosms not being handled in the laboratory were kept at 4–5°C. When all animals had been added, the water content of the samples was kept at 50% of water-holding capacity (WHC). One hundred percent WHC was defined as the water content obtained when an inundated soil was allowed to drain for 12 h in a 3-cm-high cylinder. To allow gas exchange, the microcosms had a lid with a 5-mm diameter aperture. The microcosms were placed in a dark room with a constant temperature of 15°C. At that time (day 0) background samples were taken for determination of the chemical properties reported above.
Destructive samplings
After 50 and 100 days, samples of the humus layer in the microcosms were collected for determination of (1) dry-to-fresh-weight ratio and LOI (see above), (2) pH (H2O), (3) meso- and macroarthropods, (4) ergosterol, (5) FDA-active fungal biomass and (6) inorganic N. Also, nematodes and tardigrades were sampled, but the results on the latter organisms have been reported earlier (Hyvönen and Persson 1996).
The pH was determined with a glass electrode in the supernatant after shaking the sample in distilled water for 2 h and sedimentation in an open flask for 24 h. The fresh soil:water proportions were 1:1 by volume (about 1:10 by dry matter to water for the humus material).
On day 50 meso- and macroarthropods were extracted from subsamples of 4 g fresh weight (approximately 1 g dry weight) in Macfadyen high gradient canister extractors, and the animals were collected and preserved in jars with 80% ethanol. Because the number of animals extracted from the small samples by this method was not sufficient to allow analyses at the species level, we extracted soil animals by the Tullgren method on day 100 (16–20 g fresh weight).
Samples (about 2 g fresh weight) for ergosterol (a component of fungal biomass) determination were, because of time constraints, only taken from the C, F and P treatments, and were processed according to Nylund and Wallander (1992) with modifications as described by Kårén and Nylund (1996). Samples (about 3 g fresh weight) for determination of FDA-active fungal biomass from all treatments were processed according to the fluorescein diacetate (FDA) method (Söderström 1977).
Inorganic N (\({\text{NH}}^{{\text{ + }}}_{{\text{4}}} {\text{ - N + NO}}^{{\text{ - }}}_{{\text{3}}} {\text{ - N}}\)) was extracted from 8 g fresh weight samples with 100-ml 1 M KCl solution for 1 h in a rotary shaker. Because no leaching could occur, inorganic N accumulated in the system, and the accumulation rate was considered as the net N mineralisation rate. Net nitrification was calculated in the same manner as net N mineralisation (daily mean accumulation of \({\text{NO}}^{{\text{ - }}}_{{\text{3}}} {\text{ - N}}\)). \({\text{NO}}^{{\text{ - }}}_{{\text{2}}} \) was included in the estimates of \({\text{NO}}^{{\text{ - }}}_{{\text{3}}} .\) Net mineralisation rates were expressed per gram of OM.
To determine CO2 evolution from the humus material, the microcosms were periodically closed with airtight lids with a rubber septum. Background gas samples were taken from the headspace with a syringe and were injected into a gas chromatograph (Hewlett Packard 5890, HP Company, Avondale, PA, USA) after 15 min. The measurement was repeated when an appropriate amount of CO2 had accumulated in the containers, normally after about 120 min. The mass of C evolved per container and hour was calculated according to Persson et al. (1989) and Persson and Wirén (1993), taking the pH-dependent solubility of CO2 in the soil water into account. CO2 measurements were performed once a week during the first month and every fourth week thereafter.
Statistical analyses
Exclusion of samples and treatments
Originally, we had intended the experiment to last for 200 days. However, the data from day 200 had to be rejected because microarthropod eggs had vigorously hatched between day 100 and 200, and the control treatments 0C and NC could no longer be considered as fungivore-free controls. Furthermore, we found that the proportion of collembolan species in the B treatment (fungivores plus beetles) deviated greatly from that in the other treatments, probably due to inaccuracies during the inoculation process. The B treatment had high collembolan numbers (more than 20 individuals per gram) because of large populations of juvenile Isotoma notabilis and/or Anurida pygmaea, indicating vigorous hatching of these species. This did not occur in the other treatments, and, therefore, this treatment (0B and NB) was excluded from the statistical analyses.
Animals
We first examined the hypothesis that a full-predator community had an effect on fungivores by two-way ANOVAs with faunal treatment (F vs P), and soil treatment as factors and the total abundance of Collembola and Oribatida as dependent variables. This was done to avoid confounding the results for the full-predator treatment by the treatments with only subsets of the predator community (see below). Because the extraction method for animals differed between 50 and 100 days, we did separate analyses for 50 and 100 days.
To examine the effects of the predator groups on fungivores in more detail, we then performed ANOVAs with the full set of predation treatments (F, P, G, S). We also tested each of the predator groups against the F treatment. When appropriate, Tukey–Kramer tests were used to examine pairwise differences between treatments.
To analyse the composition of fungivores in the different treatments (C, F, P, G, S), principal component analysis (PCA) on STATVIEW II for Macintosh was carried out on species numbers extracted on the second sampling occasion (100 days). Differences between the treatments were examined with an ANOVA on the unrotated factor scores. We did not do this for the first sampling occasion, because the small sample sizes made estimates of individual species abundances less precise. The C treatment (no mesofauna) was included to analyse if the defaunation had been effective, despite the fact that some eggs had survived and hatched by day 100.
Fungi and N mineralisation
Soil C/N ratio and fungal biomass differed between the untreated and the fertilised area. Therefore, differences in fungal biomass and N mineralisation were tested separately for each soil treatment. To address our main hypothesis that the presence or absence of predators through a trophic cascade affected fungal biomass, we first examined differences in N mineralisation and fungal biomass measured as ergosterol and FDA-active fungal biomass, using a two-way analysis of variance with the factor arthropod addition at three levels (C, F and P) and the factor time at two levels (50 and 100 days). Differences between all the predator treatments in FDA-active fungal biomass were subsequently tested using a two-way analysis of variance with the factor arthropod addition at five levels (C, F, P, G and S) and the factor time at two levels (50 and 100 days). Differences between all the predator treatments in N mineralisation were tested as described above. All ANOVAs were followed by Tukey–Kramer tests and least square means.
Results
General
The community of collembolans and oribatid mites added to the microcosms was intended to be as equal as possible for each microcosm. The total number of added collembolans and oribatid mites ranged from 12 to 25 and 60 to 180 individuals per gram dry weight, respectively, at the start of the experiment. However, the species composition and the proportions of dominant species varied among replicates already at the start of the experiment. Consequently, the mean numbers of fungivore species varied between the 0 humus (Appendix 1) and the N humus (Appendix 2) after 50 and 100 days.
Predator effects on fungivores
In both humus types, the full-predator community (P) had significantly (p<0.05) depressed the abundance of Collembola after 50 days, whereas the total density of oribatids had increased compared to the fungivore only (F) treatment (Fig. 1). After 100 days, there was still a tendency for decreased Collembola densities in the predator treatment, while no effect could be seen among the Oribatida.
When the full set of treatments was examined (C, F, P, G and S, but excluding the B treatment), there was no significant difference after 50 days between the predator treatments as regards total Collembola abundances, but the total abundance of Oribatida differed between the treatments, being higher in the P and G than in the F and S treatments (not shown in figures or tables). After 100 days, there were no significant differences between the predator treatments in any of the two groups examined.
In the PCA on fungivore species composition on day 100, the first three axes together explained about 50% of the total variation in fungivore composition. The first axis (23%) mainly described the difference between the control treatment and the other treatments (Fig. 2a). This result shows that the defaunation had been, to a large extent, effective.
Results from a PCA on species composition of Collembola and Oribatida in the different treatments. Plot scores (microcosm) in a along PC1 and PC2 for different treatments and soils. Species scores in b along PC1 and PC2 (b). Species codes in b refer to names in Appendices 1 and 2. Open symbols denote Oribatida and closed symbols Collembola
The second PCA axis (16%) mainly described differences in species composition between the predator treatments (p<0.0001; Fig. 2a). Along this axis, most Collembola species scored high values and most Oribatida species low values (Fig. 2b). The third axis (11%) mainly described the difference between the two soil treatments (p<0.0001), but also to some extent differences between predator treatments (p<0.017).
Fungivore and predator effects on fungal biomass
Both fungal estimates, ergosterol content and FDA-stained fungi, indicated higher amounts in the 0 humus than in the N humus, and both estimates indicated a marked decrease over time (p<0.003 in all cases).
In the unfertilised humus (0), addition of fungivores tended to reduce the ergosterol content (Fig. 3a), but the decline was not significant. Addition of predators did not have a significant effect. In the N humus, addition of predators (NP) increased the ergosterol content in relation to both the NC and NF treatments on day 50 (Fig. 3b; p<0.02).
In the 0 humus, addition of fungivores decreased the FDA content significantly (p<0.01), while the addition of predators caused an increase of FDA content, compared with the fungivore treatment (ANOVA: treatment, p<0.002, time, p<0.0001, treatment × time, p<0.028). The significant interaction indicates a difference in the effect of predator addition between the two samplings (Fig. 4a). When including all predator treatments in the analyses, the addition of a mixed community of predators (0P) or gamasid mites (0G) resulted in a significantly higher FDA-active fungal biomass (p<0.001) than in the 0F treatment, while the addition of spiders (0S) resembled the effect of 0F.
FDA-stained fungi (mean±1 SE) in microcosms with additions of no fauna (C, closed symbols), fungivores (F, open squares), fungivores plus predators (P, open triangles), fungivores plus gamasids (G, crosses), fungivores plus spiders (S, diamonds) and fungivores plus beetles (B, small dots) in 0 and N humus
In the N humus, no significant effect of fungivores or mixed predators on FDA-active fungal biomass could be detected (Fig. 4b). There was also a lack of significant effects of individual predator groups on FDA estimates (p=0.16).
N mineralisation
The accumulation of inorganic N (net N mineralisation) followed the same pattern in the 0 humus and the N humus with a lower accumulation rate during the first 50 days than between day 50 and 100 (p<0.001; Fig. 5). More inorganic N was accumulated in the N humus than in the 0 humus, and in both humus materials almost all inorganic N (\({\text{NH}}^{{\text{ + }}}_{{\text{4}}} {\text{ - N + NO}}^{{\text{ - }}}_{{\text{3}}} {\text{ - N}}\)) was accumulated as \({\text{NH}}^{{\text{ + }}}_{{\text{4}}} {\text{ - N}}.\) The \({\text{NO}}^{{\text{ - }}}_{{\text{3}}} {\text{ - N}}\) concentrations were generally lower than 1% of total inorganic N.
The addition of fungivores (F) and fungivores and predators (P) significantly (p<0.01 in all cases) increased net N mineralisation in comparison with the control materials (0C and NC) in both the 0 and N humus (Fig. 5). In the 0 humus, addition of fungivores (0F) increased net N mineralisation by 50% after 50 days and 15% after 100 days in relation to the control (p<0.0001). In the N humus, the corresponding values were 97% after 50 days and 26% after 100 days (p<0.0001). The predator treatments (P, G, S, B) also increased the net N mineralisation in relation to the control, and had a tendency (not significant at p=0.05) to increase net N mineralisation even more than the F treatment after 100 days.
C mineralisation
Cumulative C mineralisation was significantly higher in the 0 humus than in the N humus samples (Fig. 6), and at the end of the incubation 15% more CO2–C had accumulated in the 0C than the NC humus.
The addition of fungivores (F) and fungivores plus a complete set of predators (P) reduced C mineralisation by, on average, 5–6% in the 0 humus and 12% in the N humus in relation to microcosms with no fauna added (Fig. 6). The addition of selected groups of predators did not change C mineralisation in relation to the addition of fungivores only (not shown in Fig. 6).
Discussion
Our main hypothesis was that a trophic cascade would occur, with a decrease in fungal biomass in the fungivore treatment and a subsequent increase when predators were added. In the 0 humus, we found that the fungivore community without predators reduced FDA, as well as ergosterol estimates, compared to the control, and that addition of a full-predator community, as well as gamasids only, partly counteracted this effect on FDA-active fungal biomass. However, no similar effects of fungivore and predator additions could be found in the N-rich humus. In contrast, the addition of both fungivores and predators increased the amount of ergosterol.
The addition of predators affected the fungivore communities in both humus types. The abundance of Collembola decreased, while Oribatida numbers increased when the full-predator community was added, and, in the PCA, clear differences in fungivore species composition related to the predator treatments were found.
We also hypothesised that fungivore grazing on fungi would increase C and N mineralisation. While N mineralisation indeed increased with the addition of fungivores as well as predators, this was not found for C mineralisation. In fact, C mineralisation rate decreased with faunal addition in our experiment.
Methodological aspects
The community of collembolans and oribatid mites, considered in this paper as fungivores, added to the microcosms was intended to be as equal as possible for each microcosm. Because of the enormous amount of fungivores needed to start the experiment (about 20,000 collembolans and 150,000 oribatid mites), we had no time to examine, identify or add the same numbers of species to each microcosm. These initial differences in the fungivore community probably obscured the effects of predators on the fungivores. In addition, we found it necessary to delete one of the predator treatments (the beetle-only treatment, B) because of its highly deviant species composition, making it likely that this treatment suffered from methodological artefacts. However, in the other treatments (F, P, G, S), our results are biologically reasonable, and different analyses, such as PCA and total abundances of fungivore groups, provide a similar general picture.
Microcosms are useful for investigating interactions between soil organisms and the effect of soil animals and microbes on mineralisation processes because they allow for a greater degree of control, replication and repeatability than is achievable in open-field experiments (Kareiva 1989). However, the use of microcosms has some restrictions. Firstly, experimental duration is short, relative to the time scales of many ecological processes, and exchanges of abiotic and biotic components are often reduced (Petersen and Hastings 2001). In our experiment, litterfall did not occur. This might have affected the survival of oribatid species that are dependent on fresh plant or litter material (Siepel and de Ruiter-Dijkman 1993). The number of oribatids was dramatically lower on day 50 than at the start of the experiment and juveniles were only observed on day 100. Another possible explanation of the low number of oribatids after 50 days is that predominantly adult oribatids were added to the microcosms. Natural mortality of adult oribatids, in addition to a time lag between egg laying and hatching of juveniles, may explain the low number of oribatids and lack of juveniles on day 50.
Our microcosms did not contain plant roots. The lack of N uptake by plant roots allowed us to estimate net N mineralisation by measuring the accumulation of inorganic N. On the other hand, the lack of connection to plant roots probably caused mycorrhizal fungal hyphae to die and decay. In our study, fungal biomass, measured as ergosterol, decreased during the incubation, most likely because of degradation of ectomycorrhizal fungi deprived of their energy supply (Bååth et al. 2004).
Effects on fungivores
The number of collembolans was higher in the fungivore (F) treatment than in the treatment with mixed predators (P) or gamasid mites (G) after 50 days (Fig. 1a; Appendices 1 and 2). This was in accordance with the hypothesis that predators should reduce fungivores. Gamasid mites (present in both P and G treatments) are known to prey upon collembolans (Karg 1993, 1994; Koehler 1999). Spiders feed on prey on the humus layer surface, and the beetles (mainly cantharid larvae) were generally too big to feed on collembolans and mites. Hence, the effects of these groups were minor. Oribatid mites were more numerous in the microcosms with predators than in those lacking predators (Fig. 1b), which is in accordance with the observation that the larger gamasid mites mainly prey on Collembola. Competition from collembolans might be responsible for a reduction in oribatid numbers in the F treatments. Although we do not believe that under field conditions competition from collembolans is a stronger factor than predation, especially as oribatids normally have five to ten times higher densities than collembolans in the mor humus used, competitive interactions might still have influenced the faunal composition in the microcosms lacking predators.
Effects on fungal biomass
The effects of fungivores on fungal biomass measured as ergosterol content and on FDA-active fungal hyphae were different. Approximately 50% of the ergosterol originates from mycorrhizal hyphae (Bååth et al. 2004), while a larger proportion of FDA-active hyphae originates from saprophytic fungi (Söderström and Erland 1986). The presence of fungivores tended to decrease the ergosterol content in the 0 humus, but not significantly at the p<0.05 level. The FDA-active fungal biomass was reduced significantly when fungivores were present. The addition of gamasids as the third trophic level in the G and P treatments resulted in higher FDA estimates in the 0 humus, which can be explained by predation on the fungal-feeding collembolans. This may have reduced the grazing pressure on the first trophic level (Hedlund and Sjögren Öhrn 2000). The addition of spiders (S) and beetles (B) resembled the effect of fungivores alone (0F). Thus, the hypothesis that active predators (in the P and G treatments) will increase the biomass of (saprophytic) fungi through a top–down effect was supported in the 0 humus. However, the abundance of Oribatida increased when the full-predator group was added. Increased numbers of oribatid mites did not affect the fungal biomass negatively.
However, in the N-humus, our main hypothesis was not supported. Fungal estimates were higher (ergosterol) or not affected (FDA) in the presence of predators (NP), and the presence of fungivores (NF) had no detectable effect on any of the fungal estimates. The different responses of fungal biomass to soil animals in the two humus materials suggest that the effects of soil fauna on fungal biomass can depend on the substrate quality. The composition of the fungal communities depends on resource quality, including N content (Frankland 1998) and soil moisture (Klamer and Hedlund 2004). In the present study, the two humus types differed considerably in C/N ratio and might have been inhabited by different fungal communities with distinct metabolic properties and respiration rates. This could also have resulted in different interactions with soil fauna.
Fungal mycelia can be stimulated to grow by low population densities of fungivores, but might be reduced at high population densities (Van der Drift and Jansen 1977). Hanlon (1981) showed that grazing by Collembola on senescent fungal colonies did not stimulate fungal growth if growing on nutrient-poor substrates, whereas, the fungal respiration increased if the fungi grew on nutrient-rich substrates. We do not have the detailed data on the fungal community and the specific responses to grazing to draw firm conclusions about the responses of fungi in this experiment.
Effects on C and N mineralisation
The presence of fungivores (F) and fungivores plus predators (P) tended to reduce C mineralisation in both N-poor and N-rich humus. This is in contrast to the studies reported by, for example, Seastedt (1984), who, in a review, found that most authors have reported that microarthropods stimulate microbial activity. Most of these studies concerned litter decomposition, whereas, our study was made on already fragmented litter or humus. It is possible that the number of bacteria decreased when fungivores or fungivores and predators were added. The decrease in the abundance of bacterial feeding nematodes in the F and P treatments observed by Hyvönen and Persson (1996) indicates that this might have occurred. Another possibility is that the fungi that were growing in these treatments had a higher metabolic efficiency than the fungal community in the control. However, our data are not sufficiently detailed to explain the C mineralisation results.
More inorganic N was accumulated in systems with two or three trophic levels than in the system with microbes and microfauna alone. A stimulating effect of soil mesofauna on net N mineralisation is often reported in the literature (e.g. Persson 1989; Setälä and Huhta 1992; Setälä 1995). However, the addition of a third trophic level did not further increase net N accumulation than with fungivores alone. This is in agreement with other studies that have shown decreasing strength of cascading effects towards the bottom of terrestrial food webs (Laakso and Setälä 1999; Schmitz et al. 2000).
The dominant form of inorganic N was \({\text{NH}}^{{\text{ + }}}_{{\text{4}}} ,\) and practically no \({\text{NO}}^{{\text{ - }}}_{{\text{3}}} \) was detected, even at the end of the incubation. This result was expected for the N-poor humus, in which net nitrification was probably inhibited by low pH (4.1), high C/N ratio (33) and low populations of ammonia-oxidising bacteria (Persson et al. 2000). In the N-rich humus, where pH was higher (4.4), C/N lower (29), and net N mineralisation higher (Fig. 5) than in the N-poor humus, the conditions seemed more favourable for net nitrification, but only 0.1–1% of mineralised N at day 100 comprised of \({\text{NO}}^{{\text{ - }}}_{{\text{3}}} {\text{ - N}}.\) However, acid-sensitive nitrifiers seem to dominate in boreal forests, and net nitrification is seldom occurring at pH and C/N levels found in the N-rich humus (Rudebeck and Persson 1998, Persson et al. 2000).
Top–down vs bottom–up control and food web complexity
As mentioned before, different responses of fungal biomass and N mineralisation to increased trophic complexity in the two humus materials may also suggest that the interactions between soil organisms can depend on differences in the substrate quality. Valiela et al. (2004) proposed that, in aquatic environments, species composition is controlled by a mix of top–down and bottom–up forces, mediated by external physical constrains. Top–down control is more likely to be important where nutrient supplies are relatively low. Top–down control is also most likely to be important where producers are most palatable or susceptible to grazing. Possibly the soil system is controlled by a mixture of top–down and bottom–up (donor-control) forces. In the present study, differences in nutrient content and fungal composition might explain why we found a trophic cascade in the 0-humus, while interactions between organisms in the N-humus were more affected by resource quality. Also, the results on C mineralisation are not consistent with the increase in decomposition rate with the number of trophic levels expected from a purely donor-controlled system (Zheng et al. 1997).
Most studies of trophic cascades have investigated interactions between dominant species or subsets of full communities (Schmitz et al. 2000). One of the explanations of the lack of cascades in terrestrial systems is that many species are linked in a network of interaction pathways (Strong 1992; Schmitz et al. 2000). Soil animals are quite flexible in their diet, and some species might belong to several trophic levels (Scheu 2002). Furthermore, different responses of prey species to predation can reduce the strength of cascading effects in communities (Polis and Strong 1996). Prey is almost never uniformly edible to a single consumer (Polis et al. 2000). If some species within a trophic level are less palatable, or more difficult to capture than others, the biomass of these species may increase with increasing resource availability even though the biomass of the other species within the same level is controlled by predation (Osenberg and Mittelbach 1996). This would add heterogeneity within trophic levels and dampen trophic cascades (Persson et al. 1995). Finke and Denno (2004) showed that enhanced predator density and diversity promoted intra-guild interactions among predators and weakened cascading effects on basal resources.
In contrast, our study suggests that also a diverse predator community can, under some circumstances, be able to affect basal resources in soil food webs, as well as influence C and nutrient mineralisation. Several other field and laboratory studies suggest that soil ecosystems should not be considered as entirely regulated by bottom up forces (e.g. Sohlenius 1990; Bengtsson et al. 1998; Laakso and Setälä 1999), but it still remains to be examined how the degree of top–down regulation depends on environmental factors such as soil fertility.
References
Bååth E, Nilsson LO, Göransson H, Wallander H (2004) Can the extent of degradation of soil fungal mycelium during soil incubation be used to estimate ectomycorrhizal biomass in soil? Soil Biol Biochem 36:2105–2109
Balogh J (1972) The oribatid genera of the world. Akadémiai kiadó, Budapest
Bengtsson J, Zheng DW, Ågren GI, Persson T (1995). Food webs in soil: an interface between population and ecosystem ecology. In: Jones CG, Lawton JH (eds) Linking species and ecosystems. Chapman and Hall, London, pp 159–165
Bengtsson J, Lundkvist H, Saetre P, Sohlenius B, Solbreck B (1998) Effects of organic matter removal on the soil food web: forestry practices meet ecological theory. Appl Soil Ecol 9:137–143
Bradford MA, Jones TH, Bardgett RD, Black HIJ, Boag B, Bonkowski M, Cook R, Eggers T, Gange AC, Grayston SJ, Kandeler E, McCaig AE, Newington JE, Prosser JI, Setälä H, Staddon PL, Tordoff GM, Tscherko D, Lawton JH (2002) Impacts of soil faunal community composition on model grassland ecosystems. Science 298:615–618
Clarholm M (1985) Possible roles of roots, bacteria, protozoa and fungi in supplying nitrogen to plants. In: Fitter AH, Atkinson D, Read DJ, Usher MB (eds) Ecological interactions in soils. Blackwell Scientific, Oxford, pp 355–366
Faber JH, Verhoef HA (1991) Functional differences between closely related soil arthropods with respect to decomposition processes in the presence or absence of pine roots. Soil Biol Biochem 23:15–24
Finke DL, Denno RF (2004) Predator diversity dampens trophic cascades. Nature 429:407–409
Fjellberg A (1980) Identification keys to Norwegian collembola. The Norwegian Entomological Society, Ås
Fjellberg A (1998) The collembola of Fennoscandia and Denmark. I: Poduromorpha and onychiuridae. Fauna ent Scand 35:184
Frankland JC (1998) Fungal succession—unravelling the unpredictable. Mycol Res 102:1–15
Gilyarov MS, Krivolutsky DA (1975) A key to soil-inhabiting mites. Sarcoptiformes. Izdatel´stvo Nauka, Moscow (In Russian)
Hanlon RDG (1981) Influence of grazing by collembola on the activity of senescent fungal colonies grown on media of different nutrient concentration. Oikos 36:362–367
Hedlund K, Sjögren Öhrn M (2000) Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88:585–591
Hyvönen R, Persson T (1996) Effects of fungivorous and predatory arthropods on nematodes and tardigrades in microcosms with coniferous forest soil. Biol Fertil Soils 21:121–127
Kandeler E, Kampichler C, Joergensen RG, Mölter K (1999) Effects of mesofauna in a spruce forest on soil microbial communities and N cycling in field mesocosms. Soil Biol Biochem 31:1783–1792
Kareiva P (1989) Renewing the dialogue between theory and experiments in population ecology. In: Roughgarden J, May RM, Levin SA (eds) Perspectives in ecological theory. Princeton University Press, Princeton, New Jersey
Kårén O, Nylund JE (1996) Effects of N-free fertilization on ectomycorrhiza community structure in Norway spruce stands in southern Sweden. Plant Soil 181:295–305
Karg W (1993) Acari (Acarina), Milben Parasitiformes (Anactinochaeta), Cohors Gamasina Leach, Raubmilben. Die Tierwelt Deutschlands 59
Karg W (1994) Raubmilben, nützliche Regulatoren im Naturhaushalt. Neue Brehm Bücherei 624:206
Klamer M, Hedlund K (2004) Fungal diversity in set-aside agricultural soil investigated using terminal-restriction fragment length polymorphism. Soil Biol Biochem 36:983–988
Koehler HH (1999) Predatory mites (Gamasina, Mesostigmata). Agric Ecosyst Environ 74:395–410
Laakso J, Setälä H (1999) Population- and ecosystem-level effects of predation on microbial-feeding nematodes. Oecologia 120:279–286
Leps J, Brown VK, Diaz Len TA, Gormsen D, Hedlund K, Kailová J, Korthals GW, Mortimer SR, Rodriguez-Burrueco C, Roy J, Santa Regina I, Van Dijk C, Van der Putten WH (2001) Separating the chance effect from other diversity effects in the functioning of plant communities. Oikos 92:123–134
Liiri M, Setälä H, Haimi J, Pennanen T, Fritze H (2002). Soil processes are not influenced by the functional complexity of soil decomposer food webs under disturbance. Soil Biol Biochem 34:1009–1020
Moore JC, Walter DE, Hunt HW (1988) Arthropod regulation of micro- and mesobiota in below-ground detrital food webs. Ann Rev Entom 33:419–439
Nylund J-E, Wallander H (1992) Ergosterol analysis as a means of quantifying mycorrhizal biomass. In: Norris JR, Read DJ, Varma AK (eds) Methods Microbiol 24:77–88
Osenberg CW, Mittelbach GG (1996) The relative importance of resource limitation and predator limitation in food web chains. In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman & Hall, New York, pp 134–148
Persson T (1989) Role of soil animals in C and N mineralization. Plant Soil 115:241–245
Persson T, Wirén A (1993) Effects of experimental acidification on C and N mineralization in forest soils. Agric Ecosyst Environ 47:159–174
Persson T, Bååth E, Clarholm M, Lundkvist H, Söderström, BE, Sohlenius B (1980) Trophic structure, biomass dynamics and carbon metabolism of soil organisms in a Scots pine forest. Ecol Bull (Stockholm) 32:419–459
Persson T, Lundkvist H, Wirén A, Hyvönen R, Wessén B (1989) Effects of acidification and liming on carbon and nitrogen mineralization and soil organisms in mor humus. Water Air Soil Pollut 45:77–96
Persson L, Bengtsson J, Menge B, Power M (1995) Productivity and consumer regulation—concepts, patterns and mechanisms. In: Polis G, Winemiller K (eds) Food webs: patterns and processes. Chapman & Hall, London, pp 396–434
Persson T, Rudebeck A, Jussy JH, Colin-Belgrand M, Priemé A, Dambrine E, Karlsson PS, Sjöberg RM (2000) Soil nitrogen turnover—mineralisation, nitrification and denitrification in European forest soils. In: Schulze E-D (ed) Carbon and nitrogen cycling in European forest ecosystems. Ecol Stud 142:297–331
Petersen JE, Hastings A (2001) Dimensional approaches to scaling experimental ecosystems: designing mousetraps to catch elephants. Am Nat 157:324–333
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
Polis GA, Sears ALW, Huxel GR, Strong DR, Maron J (2000) When is a trophic cascade a trophic cascade? TREE 15:473–475
Rudebeck A, Persson T (1998) Nitrification in organic and mineral soil layers in coniferous forests in response to acidity. Environ Pollut 102:377–383
Santos PF, Phillips J, Whitford WG (1981) The role of mites and nematodes in early stages of buried litter decomposition in a desert. Ecology 62:664–669
Scheu S (2002) The soil food web: structure and perspectives. Eur J Soil Biol 38:11–20
Schmitz OJ, Hambäck PA, Beckerman AP (2000) Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am Nat 155:141–153
Seastedt TR (1984) The role of microarthropods in decomposition and mineralization processes. Annu Rev Entomol 29:25–46
Setälä H (1995) Growth of birch and pine seedlings in relation to grazing by soil fauna on ectomycorrhizal fungi. Ecology 76:1844–1851
Setälä H, Huhta V (1992) Soil fauna increase Betula pendula growth: laboratory experiments with coniferous forest floor. Ecology 72:665–671
Setälä H, Tyynismaa M, Martikainen E, Huhta V (1991) Mineralisation of C, N and P in relation to decomposer community structure in coniferous forest soils. Pedobiologia 35:285–296
Siepel H, de Ruiter-Dijkman EM (1993) Feeding guilds of oribatid mites based on their carbohydrase activities. Soil Biol Biochem 25:1491–1497
Smith VC, Bradford MA (2003) Litter quality impacts on grassland litter decomposition are differently dependent on soil fauna across time. Appl Soil Ecol 24:197–203
Söderström BE (1977) Vital staining of fungi in pure culture and in soil with fluorescein diacetate. Soil Biol Biochem 9:59–63
Söderström B, Erland S (1986) Isolation of fluorescein diacetate stained hyphae from soil by micromanipulation. Trans Br Mycol Soc 86:465–468
Sohlenius B (1990) Influence of cropping system and nitrogen input on soil fauna and microorganisms in a Swedish arable soil. Biol Fertil Soils 9:168–173
Strong DS (1992) Are trophic cascades all wet? Differentiation and donor control in speciose ecosystems. Ecology 73:747–754
Sulkava P, Huhta V, Laakso J (1996) Impact of soil faunal structure on decomposition and N-mineralisation in relation to temperature and moisture in forest soil. Pedobiologia 40:505–513
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in ecology, vol 5. Blackwell, Oxford, pp 372
Valiela I, Rutecki D, Fox S (2004) Salt marshes: biological controls of food webs in a diminishing environment. J Exp Mar Biol Ecol 300:131–159
Van der Drift J, Jansen E (1977) Grazing of springtails on hyphal mats and its influence on fungal growth and respiration. Ecol Bull (Stockholm) 25:203–209
Van de Koppel J, Bardgett RD, Bengtsson J, Rodriguez-Barrueco C, Rietkerk M, Wassen M, Wolters V (2005) The effects of spatial scale on trophic interactions. Ecosystems 8:801–809
Villenave C, Ekschmitt K, Nazaret S, Bongers T (2004) Interactions between nematodes and microbial communities in a tropical soil following manipulation of soil food web. Soil Biol Biochem 36:2033–2043
Willmann C (1931) Moosmilben oder Oribatiden (Oribatei). Die Tierwelt Deutschlands 22:70–196
Wyman RL (1998) Experimental assessment of salamanders as predators of detrital food webs: effects on invertebrates, decomposition and carbon cycle. Biodivers Conserv 7:641–650
Zheng DW, Bengtsson J, Ågren GI (1997) Soil food webs and ecosystem processes: decomposition in donor-control and Lotka–Volterra systems. Am Nat 149:125–148
Zheng DW, Ågren GI, Bengtsson J (1999) How do soil organisms affect total organic nitrogen storage in soils and substrate nitrogen to carbon ratio? A theoretical analysis. Oikos 86:430–442
Acknowledgements
Grants for the study were received from the Swedish Council for Forestry and Agricultural Research, SJFR (TP), Swedish Natural Science Research Council, NFR (JB) and the Erasmus program (LL).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Mean number (no. g−1 dry weight) of “fungivores” (collembolans and oribatids) in control soil (0) obtained after 50 and 100 days in the following five treatments: F=fungivores, P=fungivores + predators, G=fungivores + gamasid mites, S=fungivores + spiders, B=fungivores + beetles. *The estimates of F on day 50 were based on much smaller samples than the other treatments (4 g vs 20 g) resulting in uneven species composition. Values for 50 days for Collembola have been corrected for differences in extraction efficiency, and are not the same as in Fig. 1.
Appendix 2
Mean number (no. g−1 dry weight) of “fungivores” (collembolans and oribatids) in fertilised soil (N) obtained after 50 and 100 days. See “Appendix 1” for details.
Rights and permissions
About this article
Cite this article
Lenoir, L., Persson, T., Bengtsson, J. et al. Bottom–up or top–down control in forest soil microcosms? Effects of soil fauna on fungal biomass and C/N mineralisation. Biol Fertil Soils 43, 281–294 (2007). https://doi.org/10.1007/s00374-006-0103-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0103-8