Abstract
Aims
It has been increasingly recognized that only distal lower order roots turn over actively within the <2 mm fine root system of trees. This study aimed to estimate fine root production and turnover rate based on lower order fine roots and their relations to soil variables in mangroves.
Methods
We conducted sequential coring in five natural mangrove forests at Dongzhai Bay, China. Annual fine root production and turnover rate were calculated based on the seasonal variations of the biomass and necromass of lower order roots or the whole fine root system.
Results
Annual fine root production and turnover rate ranged between 571 and 2838 g m−2 and 1.46–5.96 yr−1, respectively, estimated with lower order roots, and they were increased by 0–30 % and reduced by 13–48 %, respectively, estimated with the whole fine root system. Annual fine root production was 1–3.5 times higher than aboveground litter production and was positively related to soil carbon, nitrogen and phosphorus concentrations. Fine root turnover rate was negatively related to soil salinity.
Conclusions
Mangrove fine root turnover plays a more important role than aboveground litter production in soil C accumulation. Sites with higher soil nutrients and lower salinity favor fine root production and turnover, and thus favor soil C accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root turnover is the process by which short-lived fine roots frequently produce, die and transfer carbon (C) to the soil. Fine root production and turnover represent one of the major pathways of soil C fluxes in forests, and fine root biomass and turnover rates are commonly utilized to parameterize terrestrial biogeochemical models (Strand et al. 2008; Smithwick et al. 2014; McCormack et al. 2015a). Mangroves are among the most C-rich forests in the tropics, with 2–4 times higher C density than other major forest domains in the world (Donato et al. 2011; Mcleod et al. 2011). Theoretical and empirical studies suggest that root production may contribute more to soil organic matter than aboveground litter fall in mangroves due to the slow root decomposition rate and the export of aboveground litter from mangroves by tides (Chen and Twilley 1999; Middleton and McKee 2001). In addition, root production and accumulation of belowground biomass and necromass also contribute to soil volume and consequently affect the ability of a coastal forest to keep pace with sea level rise (Mckee et al. 2007; Krauss et al. 2014). Extensive studies have been conducted on fine root dynamics in terrestrial forests in recent decades (reviewed by Yuan and Chen 2010 and Finér et al. 2011). However, fine root production and turnover in mangroves remain poorly understood due to the logistical difficulties of working in mangroves and the intensive labor required in fine root processing (Adame et al. 2014; Cormier et al. 2015).
Fine roots were traditionally defined as roots <2 mm in diameter, which was somewhat arbitrary but easy to apply. By this definition, all roots <2 mm were assumed to be ephemeral and contribute equally to soil C accumulation (Jackson et al. 1997). However, it has been increasingly recognized that the traditional definition of fine roots is problematic for trees because it lumps together static and dynamic root populations that cycle C at significantly different rates (Pregitzer et al. 2002; McCormack et al. 2015b). Root branching order was proposed as a more accurate indication of root function than size (Pregitzer et al. 2002). Within the <2 mm fine root system, distal tips of roots (first 1–2 or first 1–3 orders depending on tree species) may be classed together as ephemeral root modules with high rates of respiration, uptake and turnover, while higher order roots with secondary development have limited uptake capacity, turn over infrequently and function more for transport and storage (Guo et al. 2008a, 2008b; Xia et al. 2010). The arbitrary cutoff at diameter of 2 mm can result in biased estimates of fine root turnover rate, and it is important to assess turnover rates for the most distal, fast-cycling fine roots separately from more proximal, perennial fine roots (Smithwick et al. 2014; McCormack et al. 2015a). Root-order or functional group based studies on fine root production and turnover rate have been increasing in the past decade in terrestrial forests (Guo et al. 2008a; Sun et al. 2012; McCormack et al. 2015b). However, no studies on mangroves so far have considered root orders or separated the <2 mm fine root pool into ephemeral versus perennial compartments.
Fine root production and turnover rate are controlled by a complex set of factors that function at different scales in terrestrial forests. At the global and biome scales, fine root production and turnover rate are correlated with latitude, mean annual air temperature, precipitation and soil nutrient status (Gill and Jackson 2000; Yuan and Chen 2010; Finér et al. 2011). At a more local level, they have responded to several environmental factors such as soil temperature and soil nutritional or moisture status, and stand characteristics such as species composition, stand age, aboveground productivity and fine root biomass (Pregitzer et al. 2000; Hendricks et al. 2006). Due to the few studies on mangrove fine root dynamics, the factors controlling mangrove fine root production and turnover rate are even more ambiguous. For a specific study site, soil salinity and nutrients have been proposed to be important influencing factors, but the response directions contradict among studies. For example, some studies showed that higher fine root production was associated with higher nutrient availability (McKee et al. 2007; Castañeda-Moya et al. 2011; Adame et al. 2014). However, greater root productivity was related to phosphorus limitation in Cormier et al. (2015). Higher interstitial salinity was associated with higher root production in Adame et al. (2014), but root production decreases as salinity increases in Ball (2002). Besides, mangrove fine root dynamics were affected by inundation regime and soil temperature although the mechanisms were not fully understood (Poungparn et al. 2016).
In this study, we aimed to estimate the annual fine root production and turnover rate based on ephemeral root clusters (lower order fine roots) in five natural mangrove forests with distinctly different stand characteristics and edaphic conditions, and compare these estimates to those based on the whole <2 mm fine root system. We hypothesized that (1) biomass of lower order roots would vary among seasons and contribute primarily to fine root necromass because they are ephemeral and sensitive to seasonal climate changes; (2) fine root turnover rate would be underestimated based on the whole fine root system compared to that based on lower order roots, because only lower orders roots turn over actively; and (3) fine root production and turnover rate would be related to stand variables and soil nutrients and salinity.
Materials and methods
Study site
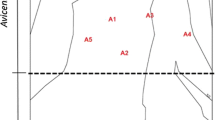
The study was carried out at Dongzhai Harbor National Natural Reserve (N 19°55′, E 110°36′), Hainan Island, China (Fig. 1). The reserve covers an area of 3337 ha, and has one of the most extensive mangrove areas and most mangrove species (25 in total including nine introduced species) in China. Mangrove forests in the reserve comprise mainly of naturally occurring mono-species stands of Ceriops tagal, Bruguiera sexangula, Rhizophora stylosa, Avicennia marina and Kandelia obovata, and mix-species communities. R. stylosa and A. marina forests mostly occur along the seashore of the outer part of the bay; B. sexangula and K. obovata forests are mostly found along rivers that run into the bay; mix-species forests occur mainly along the seashore of the inner part of the bay; and C. tagal forests are distributed extensively over the whole range of the bay, and both along riverside and seashore. The study area is characterized by a tropical monsoon climate. The mean annual air temperature is 23.5 °C, with a maximum of 28.4 °C in July and a minimum of 17.1 °C in January. The mean annual rainfall is 1676 mm, with a rainy season between May and October. The tides are irregularly semi-diurnal, with an average range of about 0.89 m.
To gain a broad range of environmental conditions, two riverine forests and three seashore forests were selected for study (Fig. 1). All of these forests are mono-species with ages >60 yr., and almost no understory vegetation occurs. The riverine forests (C. tagal and B. sexangula) are located along a river which runs into the bay, 1.5 km and 3.6 km away from the river mouth, respectively, and these forests are flooded by a mixture of marine and fresh water. The seashore forests (C. tagal, R. stylosa and A. marina) are located at the interior zone along the seashore of the outer part of the bay, and these forests are mainly flooded by marine water. The riverine forests are expected to have relatively lower soil salinity and higher nutrient contents than seashore forests due to the input of fresh water and terrigenous nutrients from the river. Stand characteristics in five forests are shown in Table 1. C. tagal stands in this area manifest as dense shrubs while plants of other stands present as typical trees. Four 10 m × 10 m plots (> 50 m apart from each other) were randomly set up in each forest for sequential root coring and soil sampling.
Sequential root coring, soil sampling and litter collection
Sequential root coring was conducted at low tide in January (winter), April (spring), July (summer) and October (autumn) of 2015. At each sampling time, three soil cores were randomly taken from each plot with a steel corer of 5 cm in diameter and 1 m in depth. Each soil core was separated into five segments (0–20 cm, 20–40 cm, 40–60 cm, 60–80 cm and 80–100 cm). Three core segments of the same soil depth from each plot were pooled as a composite sample for subsequent root separation. There were 100 samples in total (5 forests × 4 plots × 5 soil depths) at each sampling time.
Soil samples of 0–20 cm and 20–40 cm layers (where 80 % of fine root biomass was located, see results) were taken in May 2015 with a steel corer (5 cm in diameter). Three soil cores of the same soil layer were taken from each plot and pooled as a composite sample. There were 40 soil samples in total (5 forests × 4 plots × 2 layers). The soil samples were dried in the air for subsequent analysis.
In each plot, three litter traps (1.5 mm mesh) were randomly set up to collect litter. The traps were sized 0.52 m × 0.37 m in the two dense C. tagal forests, and 1 m × 1 m in the other three forests. Traps were hung 30 cm above ground in the seashore C. tagal because the plants were short and branched as low as 35 cm aboveground. As the seashore C. tagal stand was located at a relatively high elevation, the flooding depth rarely exceeded 30 cm, and therefore litter traps were not flooded by tides for most of time. Traps were hung 1–1.3 m aboveground in the other forests to avoid tide influences. Litter was collected biweekly from February 2015 to January 2016. After collection, litter from three traps of each plot were pooled and dried at 65 °C to constant weight, and the dry weight was recorded.
Separation of fine roots into different functional groups
After root core sampling, the cores were put in a 0.25 mm meshed bag and washed with tap water. All materials remained in the bag after washing were collected and diffused in a basin of water, and then stones, sand and half-decomposed plant debris that quickly (in five seconds) sank to the bottom of the basin were discarded. Coarse roots (> 2 mm in diameter) in the remained roots were picked out by hand, and then fine roots (< 2 mm) were collected with a 0.25 mm meshed sieve. Fine roots were separated into live (biomass) and dead (necromass) fractions with 11 % and 6 % solutions of colloidal silicate (Ludox® TM, Sigma-Aldrich Inc., USA) following Robertson and Dixon (1993). Colloidal silicate is a water suspension of silicon dioxide micro-particulates. The method relies on live fine roots having lower specific gravity than dead roots and therefore live roots float on the top and dead roots sink to the bottom of the solution. Further, we separated fine root biomass and necromass into first two order roots (ephemeral absorptive group) and higher order roots (perennial transport group) according to the functional classification of tree fine roots (Xia et al. 2010; Smithwick et al. 2014; McCormack et al. 2015b). In this classification, the first order was defined as the distal order, and second-order roots begin at the junctions of two first order roots, and so on (Pregitzer et al. 2002). The separation between first two orders and higher orders was also feasible for detached fine root segments because root diameter increases with root order and the diameters of first two order roots were obviously smaller than higher order roots for a given species. For example, the mean diameters of the second root order were 0.30 mm, 0.37 mm, 0.34 mm and 0.26 mm for C. tagal, B. sexangula, R. stylosa and A. marina, respectively, and the mean diameters of the third root order were 0.63 mm, 0.61 mm, 0.83 mm and 0.42 mm, respectively (Xiong et al. unpublished data).
Calculation of fine root production and turnover rate
Annual fine root production was calculated by summing up all calculated productions between each pair of consecutive seasons throughout a full year (January–April, April–July, July–October and October–January) with the Decision-Matrix method (Fairley and Alexander 1985; Ostonen et al. 2005; Brunner et al. 2013). The production between two consecutive seasons is calculated either by adding the differences in biomass and necromass, by adding only the differences in biomass, or by equalling production to zero depending on the relative changes of biomass and necromass (Brunner et al. 2013). Since root sampling was not conducted in January 2016, the fine root production during October to January was calculated with the biomass and necromass between October 2015 and January 2015. Fine root turnover rate was calculated by dividing annual production by annual mean biomass (Brunner et al. 2013).
Two sets of estimates were carried out for both annual fine root production and turnover rate, one set based on the biomass and necromass of lower order roots, and the other set based on the biomass and necromass of the whole <2 mm fine root system.
Soil analyses
Soil bulk density was measured as the dry weight of the soil sample divided by its volume. Soil C and nitrogen (N) were measured with an elemental analyzer (Elementar vario MAX CNS, Germany). Soil pH was measured with a 1: 2.5 (w / v) ratio of soil to deionized water using a pH meter. Interstitial water was extracted from the ground at a depth of 30 cm using a syringe and an acrylic tube and from which salinity was measured using an YSI-ProPlus multiprobe sensor (YSI Incorporated, Ohio, USA) (Adame et al. 2014). Soil total phosphorus (P) was determined following the standard method of the Chinese Ecosystem Research Network (Liu 1996). Briefly, 0.2 g of dry soil was digested in the mixture of HF and HClO4 thoroughly, and then dissolved in deionized water and read at 700 nm using the colorimetric method from the reaction of ortophosphates with ammonium molybdate, potassium antimony tartrate and ascorbic acid.
Statistical analyses
Differences of fine root biomass, fractions of first two orders in fine root biomass and necromass among seasons and forest types were determined by two-way ANOVAs. Differences of soil properties, annual fine root production and turnover rate among forests were determined by one-way ANOVAs. Log or square root transformations were conducted prior to analysis in order to meet ANOVA requirements for homogeneity of variances. Pearson’s correlations were performed to test the relationships between annual fine root production or turnover rate and stand or soil variables. All statistical analyses were performed using SPSS software (version 16.0, SPSS Inc., Chicago, USA) at a significance level of 0.05.
Results
Soil properties
Soil properties were significantly different among forests, and the patterns were similar for 0–20 cm and 20–40 cm soil layers (p < 0.05 for all measured properties; Table 2). Soil C and N concentrations were higher in the two riverine forests (C. tagal and B. sexangula) than in the other three seashore forests. In contrast, soil bulk density was higher in seashore than riverine forests. Soil P concentration was higher in riverine C. tagal, B. sexangula and R. stylosa forests than in seashore C. tagal and A. marina forests. Soil interstitial water salinity was highest in the two C. tagal forests (riverine and seashore), intermediate in R. stylosa and A. marina forests, and lowest in the B. sexangula forest (Table 2). Soil C, N and P concentrations were higher in the 0–20 cm layer than in the 20–40 cm layer while bulk density was higher in the 20–40 cm layer.
Variation of fine root biomass and necromass among seasons and forests and along soil depth
The biomass of first two order fine roots varied significantly among seasons and forests, but without a consistent seasonal pattern among forests (p < 0.001; Fig. 2a). However, biomass of higher order fine roots and coarse roots did not show significant seasonal variations (Fig. 2b and c). The whole <2 mm fine root biomass ranged between 243 and 1869 g m−2 and also showed significant seasonal variations (p < 0.001; data not shown) due to the seasonal variations of first two order roots. First two order roots accounted for 64 %, 43 %, 60 %, 82 % and 62 % of fine root biomass on average of four sampling times in riverine C. tagal, seashore C. tagal, B. sexangula, R. stylosa and A. marina forest, respectively (p < 0.001; Fig. 3).
Fine root necromass was lower, equal to or higher than fine root biomass, with the fine root necromass: biomass ratio ranging between 0.6–5 and varying among seasons and forests (p < 0.001; Fig. 4). First two order roots accounted for 83–98 % of fine root necromass on average of four sampling times, varying among forests (p < 0.001; Fig. 5).
Despite the large difference of fine root biomass among forest types, the distribution patterns of fine root biomass along soil depth were similar in five forests. Fine root biomass decreased with soil depth increasing, with the largest portion distributed in the upper 20 cm soil layer (Fig. 6). On average, around 50 % of fine root biomass was distributed in 0–20 cm layer, 80 % in 0–40 cm layer, and 90 % in 0–60 cm layer (Fig. 6).
Fine root production and turnover rate
Annual fine root production ranged between 571 and 2838 g m−2 calculated based on first two order roots and was 1–1.3 times higher (576–3011 g m−2 ) based on the whole fine root system (Tables 3 and 4). Annual fine root production was positively related to fine root biomass and the C, N and P concentrations in the 0–40 cm layer (p < 0.01; Table 5), but not related to other stand or soil variables (data not shown). Annual fine root production was 3.5, 1.3, 3.3, 1.9 and 1.0 times higher than annual aboveground litter production in riverine C. tagal, seashore C. tagal, B. sexangula, R. stylosa and A. marina forest, respectively (data not shown).
Fine root turnover rate ranged from 1.46 y−1 to 5.96 y−1 calculated with first two order roots and was reduced by 13–48 % (0.76–5.91 y−1) when calculated with the whole fine root system (Table 6). The percent reduction of turnover rate based on whole fine root system versus lower order roots was negatively related to the proportion of lower order roots in fine root biomass across five forests (p < 0.05; R2 = 0.825; Fig. 7). Fine root turnover rate was negatively related to soil interstitial salinity and fine root biomass (p < 0.05 or 0.01; Table 5), but not related to other stand or soil variables (data not shown).
Discussion
Seasonal variation of lower order roots and their contribution to fine root turnover in mangroves
Consistent with our first hypothesis, biomass of lower order fine roots (distal first two orders) showed significant seasonal variations, while biomass of higher order fine roots and coarse roots (> 2 mm) kept relatively stable across seasons (Fig. 2). This result was consistent with those found in terrestrial forests that fine root responses to soil environmental changes were diameter dependent, with the very fine roots (< 0.5 mm, distal roots) being more dynamic and short-lived than roots of 0.5–2 mm (mostly higher orders) (Montagnoli et al. 2012, 2014). The seasonal dynamics of fine root biomass should be related to the seasonal changes in soil environmental conditions, because fine root growth and mortality adjust regularly to adapt to changing soil temperature, moisture and nutrients in terrestrial forests (López et al. 2001; Maeght et al. 2015; Jiang et al. 2016). In mangroves, Poungparn et al. (2016) found that variations of fine root production across seasons and forest zones were mainly driven by soil temperature and inundation period. The seasonality of fine root biomass suggests that root sampling in different seasons is necessary to capture the complete dynamics of fine root biomass in mangroves.
The seasonal dynamics of lower order roots together with the high proportions they accounted for in fine root necromass suggest that lower order roots represent the fast-cycling unit in mangrove fine root systems and they are the major contributor to fine root turnover. Lower order roots accounted for 43–82 % of fine root biomass but accounted for higher proportions (83–98 %) in fine root necromass (Figs. 3 and 5). The higher proportions of lower order roots in necromass than in biomass should be due to the ephemeral property and faster turnover rate of lower order roots than higher order roots. In contrast, higher order roots did not show seasonal variations, similar to coarse roots, which might reflect their perennial characteristics. Higher order roots within the fine root system of terrestrial trees usually turn over on timescales of years to a decade and contribute little to fine root turnover (Xia et al. 2010; McCormack et al. 2015b). Our study was the first attempt to separate different fine root fractions in mangroves, and the results provided some evidence that the functional divergence within mangrove fine root system was similar to that in terrestrial trees.
Fine root production and turnover rate estimated with lower order roots versus the whole fine root system
As predicted in our second hypothesis, fine root turnover rate was lower when calculated with the whole fine root system compared to that estimated by lower order roots, which was because only lower order roots turned over actively and showed significant seasonal changes. Annual fine root production was also different between two sets of estimates. Nevertheless, the differences were small (less than two folds) compared to the great differences among forests (Tables 3-4 and 6) and among studies (more than 10 folds) (i.e., this study; Adame et al. 2014; Cormier et al. 2015). The high proportions of lower order roots in fine root biomass may explain for the small differences between the two sets of estimates. Indeed, the difference between two sets of turnover rates was negatively related to the proportion of lower order roots in fine root biomass across forest types (Fig. 7). Lower order roots accounted for 43–82 % of fine root biomass in our study, which were generally higher than those previously reported (10–58 %) for terrestrial trees (reviewed in McCormack et al. 2015b). Therefore, the inclusion of a relatively small portion of perennial higher order roots lowered the estimate of fine root turnover rate only moderately. Since fine root processing (especially the separation of different root orders) is labor intensive, the tradeoffs between precision and ease of sample processing have always been considered. Therefore, our result of the comparison between two sets of estimates provides a basis for determining fine root processing strategy in future studies.
It should be noted that the annual fine root productions estimated in our study (571–3011 g m−2) were higher than previously reported (18–1146 g m−2) in mangroves (i.e., McKee and Faulkner 2000; Cahoon et al. 2003; Sánchez 2005; Castañeda-Moya et al. 2011; Adame et al. 2014; Cormier et al. 2015). The difference might be due partly to different spatial climatic conditions, soil nutrient conditions or tree species. Also, the difference might be due partly to the different methods used to calculate root production among studies. Sequential coring was used in our study while in-growth bag method was used in previous studies. In-growth core method gives underestimates relative to sequential coring method due to too short durations (≤ 1 y) to allow for a recovery to the steady state after disturbance (Finér et al. 2011). Due to the higher estimates of annual fine root production, fine root turnover rates were also higher in our study (0.76–5.96 yr.−1) than previously reported in mangroves (0.04–0.6 yr.−1) (Castañeda-Moya et al. 2011; Adame et al. 2014; Cormier et al. 2015). The turnover rates calculated in our study were within the range of 0.15–9.6 yr.−1 used in ecosystem models parameterized for terrestrial forests (McCormack et al. 2015a). Given that there still lacks a consensus as to which method represents the best to estimate fine root production (Addo-Danso et al. 2016), comparative studies on the same forests with multiple methods are recommended for yielding realistic estimates of mangrove fine root production and turnover rate.
Factors influencing fine root production and turnover rate
Annual fine root production was positively related to soil nutrients such as N and P concentrations, suggesting that mangrove fine root production is nutrient limited at our study site. The result is not surprising given that mangroves are commonly recognized as oligotrophic ecosystems that are faced with N and P limitations globally (Feller et al. 2003; Reef et al. 2010). Nutrient increase was found to be related to increased fine root productivity (McKee et al. 2007; Adame et al. 2014). Stand characteristics might affect fine root productivity mainly through fine root biomass because fine root biomass was the only stand variable related to annual fine root production (Table 5). By comparing the riverine and seashore C. tagal forests we believe that soil characteristics played a more important role than species composition in controlling fine root production at our study site, because the annual fine root production in the two C. tagal forests represented the higher and lower end of the range, respectively (Tables 3 and 4), corresponding to their remarkably different soil C and nutrient concentrations and bulk density (Table 2).
According to the cost-benefit hypothesis (Eissenstat et al. 2000) and the conceptual model proposed by McCormack and Guo (2014), favorable conditions should increase fine root lifespan (decrease turnover rate) and stresses should decrease lifespan (increase turnover rate) for a given species. In contrast to this model, fine root turnover rate was negatively related to soil salinity across forests in our study (Table 5), suggesting that fine root lifespan was higher at sites with higher salinity stress. Consistent with our result, Cormier et al. (2015) found the highest and lowest mangrove fine root turnover rates at sites with the lowest and highest soil salinity, respectively. The mechanism was unclear of the negative relationship between turnover rate and salinity and merits further study. Species composition and stand characteristics may have played a role in influencing fine root turnover rate given that the two scrub and dense C. tagal forests had lower turnover rates than other forests (Table 6). Tree fine root lifespan is highly variable (95–336 days) across species within a single site, which are linked to plant functional traits (McCormack et al. 2012). However, the effects of species and salinity could not be separated under our experimental settings.
Mangrove C allocation between fine root production and aboveground litter production
Fine root production (together with subsequent mortality) and aboveground litter production represent the major pathways of plant C input to soil in forests (Freschet et al. 2013; Leppälammi-Kujansuu et al. 2014). The C allocation between fine root production and aboveground litter production (dominated by leaf litter production) has important implications for soil C accumulation, especially in mangroves. In contrast to the fast decomposition rate of leaf litter, roots decay slowly and accumulate over time (Silver and Miya 2001; Xiong et al. 2013), and this differential pattern is more obvious in mangroves due to the anaerobic conditions that depress microbial activities and root decomposition (Middleton and McKee 2001; McKee et al. 2007). Moreover, due to the openness of mangrove ecosystems, roughly one-half of aboveground litter production is exported to adjacent waters by tidal activities (Robertson et al. 1992; Dittmar et al. 2001). Therefore, a higher proportion of root C is retained in the soil than aboveground litter C, and thus higher allocation to root than aboveground litter production favors soil C enrichment.
In our study, annual fine root production was 1–3.5 times higher than aboveground litter production in five studied mangrove forests. Similarly, Sánchez (2005) found higher root production than aboveground litter production in mangroves. However, in terrestrial forests, the ratio of root litter: aboveground litter production was close to 1 (0.7–1.3) (Freschet et al. 2013; Leppälammi-Kujansuu et al. 2014), and the ratio was even lower (0.49) on grasslands (Freschet et al. 2013). The results suggest that mangroves invest much more C belowground than other ecosystems, possibly due to unfavorable environmental conditions such as high salinity and low nutrient availability (Lovelock 2008; Donato et al. 2011). Correspondingly, annual fine root production was positively related to soil C concentration (Table 5), which supports the important role of fine root production and turnover in soil C accumulation in mangroves.
Conclusions
We found that mangroves allocated a larger portion of C to fine root production than aboveground litter production, suggesting that fine root turnover plays a more important role than aboveground litter production in soil C accumulation in mangroves. By separating the whole <2 mm fine root pool into groups of first two orders and higher orders, we confirmed that the functional differentiation within the <2 mm fine root system also exists in mangrove trees, as in terrestrial trees. By comparing the estimates of fine root turnover rate based on lower order roots versus the whole <2 mm fine root system, we showed that fine root turnover rate would be underestimated by pooling the ephemeral lower order roots with perennial higher order roots as a whole. Nevertheless, the underestimation of fine root turnover rate was moderate due to the high proportions of lower order roots in fine root biomass in mangroves. The positive relation between annual fine root production and soil nutrients and the negative relation between fine root turnover rate and soil interstitial salinity suggest that sites with higher soil nutrients and lower salinity favor fine root production and turnover, and thus may favor soil C accumulation.
References
Adame MF, Teutli C, Santini NS, Caamal JP, Zaldívar-Jiménez A, Hernández R, Herrera-Silveira JA (2014) Root biomass and production of mangroves surrounding a karstic oligotrophic coastal lagoon. Wetlands 34:479–488
Addo-Danso SD, Prescott CE, Smith AR (2016) Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: a review. For Ecol Manag 359:332–351
Ball MC (2002) Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees 16:126–139
Brunner I, Bakker MR, Björk RG, Hirano Y, Lukac M, Aranda X, Børja I, Eldhuset TD, Helmisaari HS, Jourdan C, Konôpka B, López BC, Miguel Pérez C, Persson H, Ostonen I (2013) Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362:357–372
Cahoon DR, Hensel P, Rybczyz J, McKee KL, Proffitt CE, Perez BC (2003) Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after hurricane Mitch. J Ecol 91:1093–1105
Castañeda-Moya E, Twilley RR, Rivera-Monroy VH, Marx BD, Coronado-Molina C, Ewe SML (2011) Patterns of root dynamics in mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. Ecosystems 14:1178–1195
Chen R, Twilley RR (1999) A simulation model of organic matter and nutrient accumulation in mangrove wetland soils. Biogeochemistry 44:93–118
Comley BWT, McGuinness KA (2005) Above- and below-ground biomass, and allometry of four common northern Australian mangroves. Aust J Bot 53:431–436
Cormier N, Twilley RR, Ewel KC, Krauss KW (2015) Fine root productivity varies along nitrogen and phosphorus gradients in high-rainfall mangrove forests of Micronesia. Hydrobiologia 750:69–87
Dittmar T, Lara RJ, Kattner G (2001) River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Mar Chem 73:253–271
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geos 4:293–297
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Fairley RI, Alexander IJ (1985) Methods of calculating fine root production in forests. In: Fitter AH, Atkinson D, Read DJ (eds) Ecological interactions in soil: plants, microbes and animals. Blackwell, Oxford, pp. 37–42
Feller IC, Whigham DF, McKee KL, Lovelock CE (2003) Nitrogen limitation of growth and nutrient dynamics in a disturbed mangrove forest, Indian River lagoon, Florida. Oecologia 134:405–414
Finér L, Ohashi M, Noguchi K, Hirano Y (2011) Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For Ecol Manag 262:2008–2023
Freschet GT, Cornwell WK, Wardle DA, Elumeeva TG, Liu W, Jackson BG, Onipchenko VG, Soudzilovskaia NA, Tao J, Cornelissen JHC (2013) Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. J Ecol 101:943–952
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL, Cadamuro L (1998) Structure above-ground biomass and dynamics of mangrove ecosystems: new data from French Guiana. Oecologia 115:39–53
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Guo D, Li H, Mitchell RJ, Han W, Hendricks JJ, Fahey TJ, Hendrick RL (2008a) Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol 177:443–456
Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z (2008b) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Hendricks JJ, Hendrick RL, Wilson CA, Mitchell RJ, Pecot SD, Guo D (2006) Assessing the patterns and controls of fine root dynamics: an empirical test and methodological review. J Ecol 94:40–57
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Nat Acad Sci USA 94:7362–7366
Jiang H, Bai Y, Du H, Hu Y, Rao Y, Chen C, Cai Y (2016) The spatial and seasonal variation characteristics of fine roots indifferent plant configuration modes in new reclamation saline soil ofhumid climate in China. Ecol Engin 86:231–238
Krauss KW, McKee KL, Lovelock CE, Cahoon DR, Saintilan N, Reef R, Chen L (2014) How mangrove forests adjust to rising sea level. New Phytol 202:19–34
Kusmana C, Sabiham S, Abe K, Watanabe H (1992) An estimation of above ground tree biomass of a mangrove forest in East Sumatra, Indonesia. Tropics 1:243–257
Leppälammi-Kujansuu J, Aro L, Salemaa M, Hansson K, Kleja DB, Helmisaari H-S (2014) Fine root longevity and carbon input into soil from below- and aboveground litter in climatically contrasting forests. For Ecol Manag 326:79–90
Liu G (1996) Analysis of soil physical and chemical properties and description of soil profiles. China Standard, Beijing in Chinese
López B, Sabaté S, Gracia CA (2001) Annual and seasonal changes in fine root biomass of a Quercus ilex L. Forest. Plant Soil 230:125–134
Lovelock CE (2008) Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 11:342–354
Maeght JL, Gonkhamdee S, Clément C, Ayutthaya SIN, Stokes A, Pierret A (2015) Seasonal patterns of fine root production and turnover in a mature rubber tree (Hevea brasiliensis Müll.Arg.) stand-differentiation with soil depth and implications for soil carbon stocks. Front. Plant Sci 6:1022
McCormack LM, Guo D (2014) Impacts of environmental factors on fine root lifespan. Front Plant Sci 5:205
McCormack LM, Adams TS, Smithwick EA, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195:823–831
McCormack LM, Crisfield E, Raczka B, Schnekenburger F, Eissenstat DM, Smithwick EAH (2015a) Sensitivity of four ecological models to adjustments in fine root turnover rate. Ecol Model 297:107–117
McCormack LM, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari H-S, Hobbie EA, Iversen CM, Jackson RB, Leppälammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015b) Redefining fine roots improves understanding of belowground contributions to terrestrial biosphere processes. New Phytol 207:505–518
McKee KL, Faulkner PL (2000) Restoration of biochemical function in mangrove forests. Rest Ecol 8:247–259
McKee KL, Cahoon DR, Feller IC (2007) Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob Ecol Biogeogr 16:545–556
Mcleod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
Middleton BA, McKee KL (2001) Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J Ecol 89:818–828
Montagnoli A, Terzaghi M, Di Iorio A, Scippa GS, Chiatante D (2012) Fine-root morphological and growth traits in a Turkeyoak stand in relation to seasonal changes in soil moisture in the southern Apennines, Italy. Ecol Res 27:1015–1025
Montagnoli A, Di Iorio A, Terzaghi M, Trupiano D, Scippa GS, Chiatante D (2014) Influence of soil temperature and water content on fine-root seasonal growth of European beech natural forest in southern alps, Italy. Eur J For Res 133:957–968
Ostonen I, Lõhmus K, Pajuste K (2005) Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: comparison of soil core and ingrowth core methods. For Ecol Managem 212:264–277
Poungparn S, Charoenphonphakdi T, Sangtiean T, Patanaponpaiboon P (2016) Fine root production in three zones of secondary mangrove forest in eastern Thailand. Trees 30:467–474
Pregitzer KS, King JS, Burton AJ, Brown SE (2000) Responses of tree fine roots to temperature. New Phytol 147: 105–115
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine north American trees. Ecol Monog 72:293–309
Reef R, Feller IC, Lovelock CE (2010) Nutrition of mangroves. Tree Physiol 30:1148–1160
Robertson AI, Dixon P (1993) Separating live and dead fine roots using colloidal silica: an example from mangrove forests. Plant Soil 157:151–154
Robertson AI, Alongi DM, Boto KG (1992) Food chains and carbon fluxes. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems, Coastal and Estuarine Series No, vol 41. American Geophysical Union, Washington, pp. 293–326
Sánchez BG (2005) Belowground productivity of mangrove forests in Southest Florida. Dissertation, Louisiana State University
Silver WL, Miya RK (2001) Global patterns of root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Slim FJ, Gwada PM, Kodjo M, Hemminga MA (1996) Biomass and litterfall of Ceriops tagal and Rhizophora mucronata in the mangrove forest of Gazi Bay, Kenya. Mar Freshw Res 47:999–1007
Smithwick EAH, Lucash MS, McCormack ML, Sivandran G (2014) Improving the representation of roots in terrestrial models. Ecol Model 291:193–204
Strand AE, Pritchard SG, McCormack ML, Davis MA, Oren R (2008) Irreconcilable differences: fine-root life spans and soil carbon persistence. Science 319:456–458
Sun J, Gu J, Wang Z (2012) Discrepancy in fine root turnover estimates between diameter-based and branch-order-based approaches: a case study in two temperate tree species. J For Res 23:575–581
Xia M, Guo D, Pregitzer KS (2010) Ephemeral root modules in Fraxinus mandshurrica. New Phytol 188:1065–1074
Xiong Y, Fan P, Fu S, Zeng H, Guo D (2013) Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 363:19–31
Yuan ZY, Chen HYH (2010) Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses. Crit Rev Plant Sci 29:204–221
Acknowledgments
We thank Jianhai Chen for his assistance in the field. We thank Dr. Jiacun Gu for constructive suggestions on manuscript preparation and Drs. Deliang Kong and Donna Devlin for helpful comments on an earlier draft of the manuscript. Thanks to two anonymous reviewers for their constructive comments that greatly improved the manuscript. This work was supported by the Chinese Academy of Forestry (RITFYWZX2015-03), the Forestry Administration of Guangdong (2014KJCX021-01) and the State Forestry Administration of China (2016-LYPT-DW-130).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alexia Stokes.
Rights and permissions
About this article
Cite this article
Xiong, Y., Liu, X., Guan, W. et al. Fine root functional group based estimates of fine root production and turnover rate in natural mangrove forests. Plant Soil 413, 83–95 (2017). https://doi.org/10.1007/s11104-016-3082-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3082-z