Abstract
Background and aims
Carpobrotus spp. are amongst the most impactful and widespread plant invaders of Mediterranean habitats. Despite the negative ecological impacts on soil and vegetation that have been documented, information is still limited about the effect by Carpobrotus on soil microbial communities. We aimed to assess the changes in the floristic, soil and microbial parameters following the invasion by Carpobrotus cfr. acinaciformis within an insular Mediterranean ecosystem.
Methods
Within three study areas a paired-site approach, comparing an invaded vs. a non-invaded plot, was established. Within each plot biodiversity indexes, C and N soil content, pH and microbial biomass and structure (bacterial and fungal) were assessed.
Results
Invaded plots showed a decrease of α-species richness and diversity. The least represented plant species in invaded plots were those related to grassland habitats. In all invaded soils, a significant increase of carbon and nitrogen content and a significant decrease of pH were registered. Carpobrotus significantly increased bacterial and fungal biomass and altered soil microbial structure, particularly favoring fungal growth.
Conclusions
Carpobrotus may deeply impact edaphic properties and microbial communities and, in turn, these strong modifications probably increase its invasive potential and its ability to overcome native species, by preventing their natural regeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing distribution of invasive alien plants is one of the main factors of ecosystem degradation and of biodiversity loss at global scale (Mack et al. 2000). Some invasive species are particularly able to quickly spread, may replace whole plant communities and habitats over wide areas and therefore are denominated transformer species (Pyšek et al. 2004) or ecosystem engineers (Molinari et al. 2007). Apart from threatening biodiversity, such species can deeply modify chemical, physical and biological soil characteristics, soil erosion rate and water runoff, microclimatic substrate conditions, water content along soil profile, ecosystem food webs, nutrients cycle as well as organic matter decomposition (Vilà et al. 2011; Nanko et al. 2015). This explains why many experimental studies and research have been addressed to identify such taxa, to assess their impacts and to understand the reasons for their relevant invasive success. Some species belonging to the genus Carpobrotus fall within this category of introduced plants. Carpobrotus spp. are well-known mat-forming succulent invaders of Mediterranean-type ecosystems, where they compete aggressively with native plants, strongly hindering their growth and establishment and deeply changing the characters of whole ecosystems over large areas (D’Antonio and Mahall 1991; Albert 1995; Suehs et al. 2004; Vilà et al. 2006). The two most widespread naturalized species of the genus, Carpobrotus edulis (L.) Nebr and C. acinaciformis (L.) L. Bolus, are both native to coastal areas of South Africa (Malan and Notten 2006); however, the identification of the two species has been complicated by the frequent hybridization events that raise a hybrid complex named C. affine acinaciformis (Delipetrou 2006; Andreu et al. 2010). Although the introduction of Carpobrotus spp. in Europe is relatively ancient, dating back to the 17th century (Fournier 1952), the naturalization and the subsequent spread in the Mediterranean Basin began between the end of the 19th century and the beginning of the 20th century (Lojacono-Pojero 1891; Sanz-Elorza et al. 2004). Nowadays C. edulis, C. acinaciformis and their hybrids forms are considered a major threat to biological diversity and ecosystem functioning of Mediterranean islands (Brundu 2013), where they are undergoing a number of control and eradication programs in order to protect native biodiversity (Brunel et al. 2013).
If the spread of Carpobrotus spp. in the Mediterranean has been undoubtedly favoured by their extensive use for the stabilization of sand dunes in many coastal areas (La Mantia 2011), a number of biological traits are underlying their outstanding invasive potential. Traveset et al. (2008), for instance, highlighted the remarkable plasticity of Carpobrotus aff. acinaciformis with respect to light availability, because it grows well in full light conditions, but tolerates partial shading. Carpobrotus spp. are capable of rooting at nodes even without a direct contact with the ground, radially spreading with growth rates equal to 1 m per year in the native range (Wisura and Glen 1993), and exceeding 30 cm per year in the Mediterranean (Traveset et al. 2008; Passetti et al. 2012). Sexual reproduction is equally effective. The flowers are frequently visited by native pollinators (Bartomeus et al. 2008), seed production is particularly abundant, reaching an average of 1000 seeds per fruit (Carta et al. 2004; Bartomeus and Vilà 2009), and a high proportion of seeds is stored in the soil seed bank and in litter (Chenot et al. 2014). Seed dispersal is then carried out mainly by small mammals such as rats and rabbits, whose ingestion causes an increase in the germination percentage and therefore the chance for new individuals to become established (D’Antonio 1990a; Bourgeois et al. 2005; Novoa et al. 2012).
In invaded Mediterranean ecosystems, Carpobrotus spp. possess many unique traits, typically different from those of native Mediterranean species, such as life form, size, spatial distribution and litterfall dynamics; these differences are expected to bring about important changes in the quality of soil microsites as well as in resident plant communities. Such marked differences with the local biota may be important for the widespread presence and the recognized negative ecological impacts displayed by Carpobrotus spp. in many Mediterranean island ecosystems, which generally do not host Carpobrotus-like species (Heywood 1995). Phylogenetic relatedness between native and introduced species has been recently considered a valuable tool to forecast which introduced species are more likely to have higher ecological effects on recipient ecosystems. For instance, introduced grass species less closely related to native grasses were found to be those having the most relevant impacts and tending to spread much further in California grasslands (Strauss et al. 2006). From an ecological point of view this could be explained, for instance, by the reduced likelihood that parasites as well phytophagous insects may specialize and attack host plants far different from native species. Even the plant-mediated introduction of a new biochemical process, which reflects the adaption and evolution in a given habitat, may have catastrophic consequences for invaded ecosystem functioning. Myrica faya Ait. is such an emblematic case in this respect because it has been capable of quadrupling the nitrogen content of the recent lava flows in Hawaii, where normally no nitrogen-fixing native species occurs (Vitousek et al. 1987).
Much effort has been either devoted to understanding the biological reasons for Carpobrotus spp. invasive success (D’Antonio 1990b, 1993; D’Antonio et al. 1993; Novoa et al. 2012) and to assessing the ecological consequences associated with their invasion on floristic richness and diversity (Carta et al. 2004; Vilà et al. 2006; Andreu et al. 2010; Novoa et al. 2013; Chenot et al. 2014; Fried et al. 2014), on soil properties (Vilà et al. 2006; Conser and Connor 2009; de la Peña et al. 2010; Santoro et al. 2011; Novoa et al. 2013, 2014) or on both (Vilà et al. 2006; Novoa et al. 2013). In all previous references, Carpobrotus spp. were found to exert a strong impact on the composition, diversity and dynamics of plant communities, and generally lead to a significant decrease of species richness as well as diversity of invaded habitats. Recently, a number of works have focused on the impact of invasive alien species on local soil characteristics (Heneghan et al. 2006; Rout and Chrzanowski 2009; Osunkoya and Perrett 2011; Perkins et al. 2011; De Marco et al. 2013), and such effects are considered particularly important in determining the invasive success of Carpobrotus spp. as well (Vilà et al. 2006; Novoa et al. 2014). For instance, Novoa et al. (2014) found a large variation in soil pH, enzymatic activity, nutrient, salinity and moisture content in a dune ecosystem invaded by Carpobrotus edulis. Novoa et al. (2013) showed that these effects influence the function of soil microbial communities and constrain the establishment of native plants in dune communities. Increasing importance has been given to the role played by soil microbial communities (van Grunsven et al. 2009; de la Peña et al. 2010), to the changes of these communities as a result of the invasive establishment and to the plant-soil feedback mechanisms that may enhance the competitive ability of this invasive species with respect to native species (de la Peña et al. 2010). In addition, Carpobrotus litter is well known to exert a strong allelopathic effect on native species by reducing their germination rate, growth and establishment, and such effect is highly species-specific (Novoa et al. 2012; Novoa and González 2014). One of the most important consequences of Carpobrotus invasion is the significant alteration of soil nutrients dynamics that lead in most cases to an increase in the organic matter and nitrogen content and a decrease in pH, with clear long lasting ecological consequences (Vilà et al. 2006; Conser and Connor 2009; de la Peña et al. 2010; Santoro et al. 2011; Novoa et al. 2013, 2014). These changes, combined with shifts in microbial communities assemblage, make soils much more conducive to the establishment of the alien species and generally adverse for germination and/or growth of native species (de la Peña et al. 2010; Conser and Connor 2009; Novoa et al. 2013; Novoa and González 2014). The intensity with which invasive alien species are capable of altering the physical and chemical characteristics of soils appears to be strongly dependent on the identity of the invaded habitat (Forey et al. 2009; Maestre et al. 2009; Santoro et al. 2011; Fried et al. 2014); however, time since invasion could be considerably important as it is recognized that impacts may change over time (Dostál et al. 2013; Marchante et al. 2015). Although the invasive process by Carpobrotus spp. in the Mediterranean has been documented, information is still limited whether or not and how soil microbial communities are affected. More in detail, the impact on the structure of soil microbes, for instance on bacteria/fungi ratio, has never been assessed, even though it is well recognized that soil microbes are important in affecting plant performance as well as the competitive interactions between co-occurring species. With this regard, the phospholipid fatty acid analysis (PLFA) is a promising tool as fatty acids are considered valuable bioindicators of soil quality and they could be used for the development of soil quality indices (Kaur et al. 2005). The knowledge of the overall ecological impacts on soils by Carpobrotus, and its characterization in terms of type and magnitude, may play a key role in developing an effective strategy to control this invasive species. In fact, the germination, growth and establishment strategies of plants, as well as their competitive dynamics in the field, are deeply influenced and regulated by physical, chemical and biological characteristics of soil. This is particularly true for sensitive species of fragile ecosystems such as coastal habitats of Linosa island (La Mantia et al. 2009). Many precious species of dune ecosystems, for instance, are strictly linked to low nutrient as well salinity levels in soils (Santoro et al. 2012; Novoa et al. 2013), whereas ruderal, opportunistic and/or nitrophilous species are enhanced by nutrient enrichment (Marchante et al. 2015). The altered edaphic conditions due to Carpobrotus invasion, may affect the success of sowing and/or planting interventions of some native species in eradicated areas, compromising the chance for native habitat restoration and thus having clear management implications (Ruffino et al. 2014). The aim of the research was to assess the changes in the content of soil carbon and nitrogen, in the floristic richness and diversity, and in the microbial communities structure following the invasion by Carpobrotus cfr. acinaciformis at Linosa, an insular Mediterranean ecosystem.
Materials and methods
Study area and vegetation surveyed
Our survey was conducted in Linosa, a 5.45 km2 island of the Pelagie Archipelago where Carpobrotus cfr. acinaciformis is widespread. This alien species has successfully invaded coastal habitats and seminatural grasslands, among the richest in endemic species and species of particular conservation value. In fact, 36 out of 74 of the vascular plants and mosses of main biogeographic and/or conservation interest occurring on Linosa island, and listed within the SCI Management Plan (La Mantia et al. 2009), are more or less exclusively linked to annual therophytic grasslands. They include: Astragalus peregrinus subsp. warionis (Gand.) Maire, Avena saxatilis (Lojac.) Rocha Afonso, Bellium minutum (L.) L., Catapodium hemipoa subsp. occidentale (Paunero) H. Scholz & S. Scholz, Erodium neuradifolium var. linosae (Sommier) Brullo, Logfia lojaconoi (Brullo) C. Brullo & Brullo, Lotus halophilus Boiss. & Spruner., Lotus peregrinus L., Reichardia tingitana (L.) Roth, Serapias parviflora Parl.
The three following native vegetation types were taken into account:
-
A).
Patchy vegetation near the coast with discontinuous coastal sub-halophilous scrub with Lycium intricatum Boiss. and Senecio cineraria subsp. bicolor (Willd.) Arcang. (which is ascribed to the phytosociological class Crithmo-Limonietea and corresponds to the EU habitat 5320 ‘Low formations of Euphorbia close to cliffs’) and annual and perennial salt-tolerant herbs (mainly Daucus gingidium L. and Euphorbia pinea L.) in the gaps, which can be referred to the priority EU habitat 6220 (Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea);
-
B).
Patchy vegetation with dwarf annual herbs linked to acidic and sandy soils (phytosociological class Tuberarietea guttatae and priority habitat 6220 ‘Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea’) and annual and biennial sub-nitrophilous plants typical to fallow areas (e.g. Brassica fruticulosa Cirillo, Lupinus cosentinii Guss., etc.);
-
C).
Patchy vegetation dominated by few grasses and sub-shrubs (Hyparrhenia hirta (L.) Stapf, Dactylis hispanica Roth., Phagnalon saxatile (L.) Cass.) typical to the Lygeo-Stipetea phytosociological class and to the priority EU habitat 6220 (‘Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea’), encroached by some shrubs typical to the local thermo-xerophilous maquis communities like Pistacia lentiscus L. and Periploca angustifolia Labill. (class Quercetea ilicis, order Quercetalia calliprini, alliance Periplocion angustifoliae, referred to the EU habitat 5330 ‘Thermo-Mediterranean and pre-desert scrub’).
The island is characterized by a markedly Mediterranean climate, with a dry season lasting for about 6 months, concentrated in the hottest period of the year, followed by a cool and wet winter season (La Mantia et al. 2009). As concerns bioclimate, Linosa lies within the infra-mediterranean belt, according to the climatic indices proposed by Rivas-Martínez et al. (1999). In particular, during the study year (2014) the rainfall was 308.4 mm (http://www.infoclimat.fr/climatologie/annee/2014/lampedusa-e-linosa-linosa/valeurs/MNWSCL078.html), resulting in a dry year, respect to the annual average of about 458 mm (Duro et al. 1997). The average annual temperature is 20.2 °C, with an annual thermal excursion equal to 13 °C (Duro et al. 1997). According to Fierotti (1988), the soil types occurring in Linosa island are Typic Xerorthents, Lithic Xerorthents and Andic Xerochrepts.
Study design
Three study areas differing in habitat characteristics and in time since Carpobrotus cfr. acinaciformis invasion were investigated (Fig. 1; Table 1; additional data are given in Online Resource 1): (A) invaded 20 years before; (B) invaded 15 years before and (C) invaded 8 years before. Field surveys were carried out during May 2014, when most of the local annual species are still present or at least recognizable. Based on the previous surveys carried out in the surroundings of the three study areas (La Mantia et al. 2009), very few or no species with autumn or winter life-cycle might be missing.
Within each area an invaded plot (I) was compared to a non-invaded (NI) neighboring plot according to a paired-site method (Conteh 1999; Novara et al. 2012). The invaded plot was characterized by a cover value by Carpobrotus cfr. acinaciformis higher than 80 %. The non-invaded plot was covered by native vegetation, it was no more than 5 m far away from the paired invaded plot and was characterized by the same microclimatic conditions (aspect, slope, etc.). Time since invasion was assessed by means of available aerial orthophotos (sources: Regione Siciliana ATA2008 and IT2000), with the integration of field observations carried out during the preparation of the management plan of “Isola di Linosa” SIC ITA040001 (La Mantia et al. 2009) and during field surveys carried out in 2014 within LIFE11+ NAT/IT/000093 “Pelagic Birds” project (available at http://www.pelagicbirds.eu/).
Phytosociological surveys
Phytosociological relevés (Braun-Blanquet 1932) were carried out on May 2014 in the three study areas and within three 50 m2 wide (5 × 10 m) paired invaded vs. non-invaded plots. Within each plot the following physical and biological parameters were registered: altitude (m a.s.l.), mean slope (°), outcropping rock (%), litter thickness (cm), average height of shrub layer (m), average height of herb layer (m), total vegetation cover (%), shrub layer cover (%), herb layer cover (%) and taxonomic diversity (i.e. number of infrageneric taxa). Moreover, life forms spectrum (i.e. the percentage rate of each life form sensu Raunkiær 1934), phytosociological spectrum (i.e. the percentage rate of each phytosociological class), Shannon diversity index (H’: Shannon and Weaver 1949) and Evenness index (J) were calculated after converting Braun-Blanquet values according to Tüxen and Ellenberg (1937). Furthermore, the same cover values were used to estimate the Relative Impact (RI) on α-species richness, Shannon and Evenness index, and on life forms as well. The RI expresses the variation in a given parameter as a consequence of the invasion process and it is computed, according to Vilà et al. (2006), with the following formula:
in which a is the estimated parameter within non invaded (NI) and invaded (I) areas. RI is a number ranging from −1 to +1; a positive and a negative value of RI indicate a decrease and an increase of the considered parameter, respectively. The Jaccard dissimilarity index (DJ) was also computed as follows:
where
a is the number of species occurring in either invaded and non-invaded plots, b is the number of species occurring only in the invaded plots and c is the number of species occurring only in the non-invaded plots (Fried et al. 2014). Such index expresses the magnitude of the variation in invaded areas based on plant species identity.
Soil sampling and analysis
To assess the differences in soil parameters after Carpobrotus cfr. acinaciformis invasion we sampled soil at paired sites. Soil cores (20 cm deep × 10 cm diameter) were collected in May 2014, with six replicates for each invaded and uninvaded plot (total 36 samples). We used criteria adopted for collection by Conteh (1999), also applied in several research studies (Chan et al. 2010; Kucharik et al. 2003; Murphy et al. 2003; Novara et al. 2012). The soil samples were air dried, gently sieved at 2-mm, ground to a fine powder, treated with HCl 2:1 to remove carbonates and then analysed for C and N content using a CHN-Elemental Analyzer. SOC content was first expressed as a percentage (g of C per 100 g of dry soil × 100) and then converted to Mg per hectare based on bulk density (BD) and soil depth according to:
where BD is bulk density (Mg m−3), Cconc is carbon concentration (g/100 g), D is depth thickness (m), and CFcoarse is a correction factor [1 - (gravel % + stone %)/100].
Soil bulk density was measured using the tube core method (Baruah and Barthakur 1997), based on the volume of the collected sample and the weight of dry soil in the sample. Soil pH and electrical conductivity (EC) were measured in saturated extracts and soil texture by sedimentation method. We also computed an average index of SOC content change from each invaded (I) vs. non-invaded (NI) plots as follows:
where CNI = SOC content in the non-invaded soil and CI = SOC content in the invaded soil.
Analogously, an average index of Nitrogen content change from each non-invaded (NI) and invaded (I) plot was calculated as follows:
where NNI = Nitrogen content in the non-invaded soil and NI = Nitrogen content in the invaded soil. Student t test was performed to test differences between invaded and non-invaded plot in each paired-site for comparing SOC, N content, microbial biomass and community structure. Data analysis was carried out using the SPSS statistical package (IBM SPSS statistics 2013).
Fatty acids analysis
Fatty acid methyl esters (FAMEs) were extracted by mild alkaline methanolysis method according to Schutter and Dick (2000) and they were detected on a gas chromatograph (Thermo Scientific FOCUS™ GC) equipped with a flame ionization detector and a fused-silica capillary column Mega-10 (50 m × 0.32 mm I.D.; film thickness 0.25 μm). The GC temperature progression was: initial isotherm at 115 °C for 5 min, increase at a rate of 1.5 °C per minute from 115 to 230 °C, and final isotherm at 230 °C for 2 min. Both injection port and detector were set up at 250 °C and helium at 1 mL min−1 in a constant flow mode was used as carrier. Nonadecanoic acid methyl ester (19:0; cat no. N-5377, Sigma-Aldrich Co.) was used as an internal standard for quantification of FAMEs. The identification of the peaks was based on comparison of retention times to known standards (Supelco Bacterial Acid Methyl Esters mix cat no. 47080-U and Supelco 37 Component FAME mix cat no. 47885-U). The amount of detected fatty acids (FAs) was expressed as nmol g−1 of dry soil (105 °C, 24 h). The total amount of PLFAs with a chain length ranging from 14 to 20 carbon atoms was used to estimate the total microbial biomass (Bailey et al. 2002). The FAs i15:0, a15:0, 15:0, i16:0, i17:0, 17:0, cy17:0, 18:1ω7, cy19:0 were used to represent bacterial biomass while 18:2ω6,9 for fungal biomass (Frostegård and Bååth 1996). The FAs i15:0, a15:0, i16:0, i17:0 were chosen to represent Gram-positive (G+) bacteria while 18:1ω7, cy17:0 and cy19:0 for Gram-negative (G-) bacteria (Zelles 1997; Zogg et al. 1997).
Results
Phytosociological surveys
Biological forms did not occur in the same way in Carpobrotus cfr. acinaciformis-invaded areas. In particular, therophytes (T) and hemicryptophytes (H) were the least commonly observed life forms, although showing a different pattern (Table 2). In invaded plots, both the presence (number of species) and the abundance (cover values) of T were higher in areas A and B, while in area C, despite lacking a difference in species number, a higher cover value was found. The opposite trend was detected in chamaephytes (Ch), whose number was higher in both areas A and B, whereas the cover values were lower in areas A and especially C. In invaded plots, the least represented phytosociological classes were those mainly including perennial and annual grassland species, that is Stipo-Trachynietea distachyae (S-T) and Lygeo-Stipetea (L-S). S-T occurred only in non-invaded plots B and C, while L-S occurred in non-invaded plot B and was less represented in the invaded than in the non-invaded plot C.
In terms of relative impact, invaded plots showed lower α-species richness, diversity and evenness (Table 3). The Jaccard index, too, indicated considerable differences in plant species identity between invaded and non-invaded plots. However, areas A and B have proven to be much more differentiated from invaded areas than area C, having the highest values for all the investigated parameters. Despite this, a large difference in life forms was also found within area C. For what concerns plant species of particular conservation value, listed in the regional Red List (Conti et al. 1997), the impact of Carpobrotus cfr. acinaciformis seemed to be strongly selective so that some species have been considerably more affected than others (see additional data in Online Resource 1). Rumex bucephalophorus L. was the most affected species, occurring only in the three non-invaded plots. Periploca angustifolia and Phagnalon saxatile subsp. saxatile were found either in invaded and non-invaded plots, but they reached a higher cover value in non-invaded ones. Furthermore, Phagnalon saxatile subsp. saxatile occurred only in the early phases of invasion process (area C), suggesting Carpobrotus cfr. acinaciformis may exert a direct effect on its persistence in a long-term perspective. By contrast, no effect was found against Senecio cineraria subsp. bicolor and Trigonella maritima Poir., both occurring in one area with the same cover value, and in Lycium intricatum that occurred only in one invaded plot.
Soil carbon and nitrogen properties
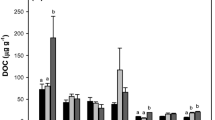
Soils of the three areas were characterized by different texture showing silt values ranging from 21.0 % (area A) to 7.5 % (area B) (Table 4). The evidence of texture on both soil carbon and nitrogen determined great differences among areas. Within the three study areas, invasion process significantly increased soil fertility, reduced soil reaction, and increased C/N ratio (Table 4). Soil carbon and nitrogen stocks (Fig. 2) showed, as well as carbon and nitrogen content, different values in the three areas. The differences between invaded and non-invaded plots tended to be higher in the areas with younger time since invasion and lower in the oldest. As a consequence, carbon and nitrogen indexes were negatively correlated with time since invasion (Fig. 3). Both indexes showed a similar pattern, being significantly higher 8 years after invasion had started.
Fatty acids
Soil microbial biomass ranged from 96.2 to 488.6 nmol FAs kg−1 in non invaded soils and from 241.8 to 787.0 nmol FAs kg−1 in invaded ones; it was always significantly higher in invaded plots. Bacteria, bacteria gram + and fungi followed the same pattern, being significantly more abundant in invaded plots (Table 5). Bacteria gram- were significantly increased within invaded plots in areas A and C, but significantly reduced within invaded plots in area B. Soil colonization by Carpobrotus cfr. acinaciformis also changed the microbial community structure. Indeed, bacterial mass was relatively less increased than fungal mass so that the bacteria/fungi ratio was significantly lower in invaded plots. The gram+/gram- ratio was significantly higher within invaded plots in area A, but significantly lower within invaded plots in areas B and C.
Discussion
The knowledge of the ecological impacts caused by invasive alien plant species represents one crucial step if we are to develop appropriate and successful management and control strategies. On the other hand, it is widely recognized that such impacts may deeply vary both in time and space, as invasion is a process involving the dynamic interaction between an introduced species and the recipient ecosystem (Shackleton and Gambiza 2008; Vilà et al. 2011). While it is acknowledged that the ecological shifts resulting from the spread of invasive species are strongly influenced by the identity of the target habitat, there is still limited knowledge about how impacts change over time (Strayer et al. 2006; Flory and D’Antonio 2015; Marchante et al. 2015). Furthermore, each ecological parameter may be affected to a different extent by invasion and to take into account these differences may provide valuable information on the prevailing impacts (e.g. Vilà et al. 2006), and hence on the ecosystem characters more influenced by a certain invasive species. Although a number of studies has investigated the impacts on soil and vegetation by plant invasions (e.g. Jandová et al. 2014), little research has been addressed to assess the consequences of plant invasion on soil, vegetation and microbial communities at the same time. In this respect, Carpobrotus cfr. acinaciformis is an excellent case study, due to its ability to completely modify Mediterranean habitats and landscapes, altering ecosystem structure and functioning, as well as biodiversity pattern.
The decrease in α-species richness and diversity in Carpobrotus-invaded habitats is a commonly observed effect within insular Mediterranean ecosystems (e.g. Vilà et al. 2006). For instance, the average Shannon index in selected bibliography was 2.67 and 1.90 in non-invaded and invaded sites, respectively (Vilà et al. 2006; Andreu et al. 2010; Novoa et al. 2013; Fried et al. 2014). Our study areas were relatively poorer but showed a similar trend, as Shannon index ranged between 1.25 and 2.03 in non-invaded areas, and between 0.82 and 1.25 in invaded ones (Table 3). Overall, the plant species associated with annual and perennial grasslands (therophytes and hemicryptophytes) were found to be the least represented in Carpobrotus cfr. acinaciformis-invaded areas. For instance, Rumex bucephalophorus, an annual species of particular conservation value (Conti et al. 1997), occurred exclusively within non-invaded plots. Other research has confirmed the sharp decrease of therophytes, and generally also of hemicryptophytes, in Carpobrotus-invaded habitats (Vilà et al. 2006; Fried et al. 2014). This is especially worrying because of the large presence of endangered or rare native species within these life forms in the Mediterranean flora (Heywood 1995). Chamaephytes seem to be differently affected by Carpobrotus cfr. acinaciformis invasion, having been observed either an increase or a decrease of their presence and/or abundance in invaded sites, suggesting the existence of a strongly site-specific response (Vilà et al. 2006; Fried et al. 2014). It is also noteworthy to point out that some native species such as Periploca angustifolia, Senecio cineraria subsp. bicolor and Lycium intricatum seem to be quite resistant to invasion by Carpobrotus cfr. acinaciformis at the adult stage, but their natural regeneration is severely impacted and seriously hindered by invasive establishment. Interestingly, a similar pattern was found in coastal dunes invaded by Carpobrotus edulis in Spain, where adult native plants were not affected by the invasive presence while the establishment of their seedlings in competition with the invasive species was seriously hindered (Novoa and González 2014). These competitive relationships have been put in relation with the altered soil conditions in terms of fertility, reaction and microbial community.
As concerns the impacts on the flora and vegetation, we may argue that a key role may be played by belowground modifications, in terms of chemical properties and microbial assemblage. Apart from direct mechanisms of competition (e.g. for water resources, D’Antonio and Mahall 1991), Carpobrotus spp. may compete via indirect mechanisms involving changes in microbial assemblages (de la Peña et al. 2010). In fact, we found significant effects on soil parameters, already 8 years after the start of the invasion, and especially starting from low soil nutrient content, as observed in other invasive species (Dassonville et al. 2008). Our results are consistent with other studies showing a clear increase in soil fertility as a consequence of Carpobrotus spp. invasion, especially in terms of C content (Vilà et al. 2006; Conser and Connor 2009; de la Peña et al. 2010; Santoro et al. 2011; Novoa et al. 2013, 2014). Accordingly, a positive value of C index is generally associated to Carpobrotus spp. invasion, whereas just three cases, never statistically significant, showed an opposite trend. Overall, in invaded soils of Linosa island N and C reached 0.25 % and 1.97 %, respectively (Tab. 4); values not so far from those registered in islands maquis stands, equal to 0.36 % and 3.21 %, respectively (Schiere 2000). In our study areas, C index decreased in relation to time since invasion (Fig. 3); the mean value was 0.62, lower than 1.05, the overall mean value found in the aforementioned bibliography.
A similar pattern is found taking into account N index, which was positive in most of the examined cases, whereas it was negative just in four, but statistically significant in a single case (Novoa et al. 2014). Overall, mean N index in the selected bibliography was 0.47 (Vilà et al. 2006; de la Peña et al. 2010; Santoro et al. 2011; Novoa et al. 2013, 2014), a value higher than that found in our study (mean value = 0.28) (Fig. 3). These results indicate that on average Carpobrotus spp. cause about a doubling in soil carbon content and an increase of almost 50 % in nitrogen content in invaded soils. Several studies reported also the acidification of soil after the invasion by Carpobrotus spp. (Vilà et al. 2006; Conser and Connor 2009; de la Peña et al. 2010; Santoro et al. 2011; Novoa et al. 2013, 2014); such effect may, in turn, affect the availability of some elements, such as Ca and Mg (D’Antonio and Haubensak 1998), and especially phosphorous, which generally is increasingly available in invaded areas (Novoa et al. 2014). Also in this case the effect is less marked in our study areas, where on average the pH was reduced by 0.21 compared to an overall average in consulted literature of 0.50. Both time since invasion and the peculiar characteristics of the habitats of Linosa could have determined this difference. Some of the previous studies have generally reported that Carpobrotus spp. were introduced in the beginning of the 20th century (e.g. Novoa et al. 2013) while in our case the invasion occurred much earlier, not more than 20 years before.
Soil microbes are essential for soil and ecosystem functioning (Jia et al. 2005; deVries and Shade 2013), particularly for organic matter decomposition and nutrient cycling (Laudicina et al. 2015), but also for the maintenance of plant diversity as plant-plant relationship are often soil microbes-regulated via direct and indirect mechanisms (e.g. van der Heijden et al. 2008). However, they have only recently become a focus of study in restoration ecology and in invasion biology as well (e.g. van der Putten et al. 2007). The functionality of soil microbial communities strongly depends on the dominant plant species and its conditioning on edaphic parameters (Kara and Bolat 2008). An invasive species, due to its ability to dominate plant communities, is expected to bring out deep changes in nutrient cycling processes, especially when it considerably differs from native species (e.g. in life history strategy, litter accumulation, leaf traits, nitrogen fixation activity, ecc.) as in the case of Carpobrotus spp. in the Mediterranean basin (Strauss et al. 2006).
The increase in soil microbial biomass after invasion by Carpobrotus cfr. acinaciformis is consistent with the observed increase in total organic C and N, being the greatest in the area A. Such results agree with Laudicina et al. (2012) who reported that microbial biomass generally increased by increasing total organic content (TOC), the latter being the food source of the former. Such findings suggest that colonisation by Carpobrotus cfr. acinaciformis improves the soil environment so hosting a greater amount of microorganisms. However, the effect of colonisation was not uniform among the investigated groups of soil microorganisms. In fact, in invaded soils the decrease of the bacteria/fungi ratio was registered, and it was mainly due to the greater relative increase of fungal biomass with respect to bacterial biomass instead of a decrease of bacterial one. The dominance of either fungi or bacteria in a soil depends on many factors among which soil reaction (Bååth and Anderson 2003), availability of organic C, quality of the plant residues (e.g. C/N ratio), as well as soil moisture and temperature (Strickland and Rousk 2010). Conser and Connor (2009) found that Carpobrotus edulis lowers soil reaction and increases organic matter content due to the recalcitrance of its tissue to decomposition. Actually, in our study areas, soil reaction decreased following colonization and the organic C content increased, but such shifts were not so consistent to justify the observed variation of the bacteria/fungi ratio (Strickland and Rousk 2010). On the other hand, the greater relative increase of the fungal biomass could be also ascribed to a cover effect by Carpobrotus cfr. acinaciformis. In fact, Carpobrotus cfr. acinaciformis is likely to determine an increase and a decrease of soil moisture and temperature, respectively; these altered microclimatic conditions may favor the fungal growth (Killham 1994). The shift in bacteria/fungi ratio could have affected the carbon use efficiency by microorganisms with fungi being more efficient in utilizing organic C substrates than bacteria (Laudicina et al. 2012). Overall, such findings have a great importance from an environmental point of view as the lower bacteria/fungi ratio has been associated to sites having a greater potential in C sequestrating (Jastrow et al. 2007). The gram+/gram- ratio was also affected by Carpobrotus cfr. acinaciformis colonisation, although no clear trend emerged comparing invaded and non-invaded plots. Such finding, however, may be of great importance also from an environmental point of view, since bacterial groups also have different C use efficiency (Fierer et al. 2003) and hence they could play a key role in affecting soil C sequestration. Furthermore, gram+/gram- ratio has a strong influence in the resistance and resilience of microbial communities to external disturbances (deVries and Shade 2013). However, further research is needed to understand how this shift in microbial community structure affects the carbon use efficiency and the growth performance of co-occurring native plants, therefore having the potential to modify competitive interactions.
Our results indicate, for the first time, that Carpobrotus cfr. acinaciformis may impact aboveground as well as belowground community diversity and structure and resources pool; moreover, a residence time of just 8 years was sufficient to modify soil and vegetation characteristics.
The management implications of such deep ecological changes are particularly relevant because Carpobrotus cfr. acinaciformis, being able to modify nutrient pools, is suspected to leave behind invasion legacies in the invaded ecosystem (Corbin and D’Antonio 2012). This means that some soil conditions, altered by invasion, may persist for a given time even after the removal of the invasive species. For instance, soils invaded by leguminous species, such as Robinia pseudoacacia L. (Von Holle et al. 2013) or Lupinus arboreus Sims (Maron and Jefferies 2001), have maintained higher N content than non-invaded areas at least for 14 and 5 years after eradication, respectively. The residual effects on soil by Carpobrotus spp. have been assessed comparing uninvaded sites (control), invaded sites (altered condition) and restored or treated sites, from which the alien species had been removed. Soil nutrient content and pH were found to be still altered 12 and 18 months after Carpobrotus edulis removal (Novoa et al. 2013; D’Antonio 1990b). By contrast, other soil parameters such as salinity, Na+ and Mg++, were approaching pre-invasion levels and seemed to quickly recover (Novoa et al. 2013).
A substrate so deep altered compared to the pre-invasion status could be not more suitable to host local plant species and could seriously compromise the chances or the required time for the native habitat restoration. Many native species experienced reduced germination, growth, development and reproduction in soils previously occupied by Carpobrotus (Conser and Connor 2009; de la Peña et al. 2010; Novoa et al. 2013; Novoa and González 2014). The effect, however, is strongly species-specific (de la Peña et al. 2010; Novoa et al. 2013; Novoa and González 2014). An alteration of interspecific competitive dynamics post-removal may occur, with ruderal and opportunistic species, which may take advantage of soil enrichment, being favored over rare and/or endangered native species (Novoa et al. 2013). One year after the eradication, the recovery by native species was quite slow both in some coastal habitats in Sardinia (Italy) and Pontevedra (Spain) (Carta et al. 2004; Novoa et al. 2013), whereas rapid recolonization processes by local species were observed in Andalucia (Andreu et al. 2010). In the first two cases, habitat restoration planning may not solely rely on eradication interventions but soil amelioration via physical or chemical means and a constant and long-term monitoring of treated areas should also be considered (Ruffino et al. 2014). Therefore, our research provide further evidence of the complicated management of this invasive species, because the changes induced in the soil and belowground communities are much more slow and difficult to recover (Molinari et al. 2007) and they are very likely contributing to the invasive success of Carpobrotus cfr. acinaciformis in Mediterranean ecosystems.
Conclusions
Carpobrotus cfr. acinaciformis is very different from co-occurring native species in our study areas and its invasion significantly increased soil fertility, microbial biomass, decreased soil pH and altered the microbial community structure by favoring the growth of fungi.
It is likely that these altered conditions, or some of them, may persist over a large temporal scale, and the long-term consequences of the invasion may thus affect and/or prevent the establishment of endemic or endangered native plant species of Linosa. In fact, the components of native flora most at risk from extinction are represented by therophytes, which typically grow in Linosean soils characterized by low levels of carbon and nitrogen content.
These results do provide further evidence that Carpobrotus cfr. acinaciformis may be quite rightly defined an ecosystem engineer, due to its great ability to change the characters of the invaded ecosystem, either at aboveground and belowground level. Furthermore, we found that a residence time of 8 years may be enough to determine significant impacts in vegetation, soil parameters as well as in the structure of microbial assemblages.
The general “benefits” provided by Carpobrotus cfr. acinaciformis, especially on soil fertility sensu lato, do not have to divert the attention from native species and local biodiversity improvement and conservation. In fact, the high competitive ability of Carpobrotus cfr. acinaciformis is known to drastically reduce biodiversity in the Mediterranean areas and we think that the enhancement of carbon and nitrogen sequestration is not sufficient to counterbalance the negative impacts on species and habitats associated to its uncontrolled spread. Studies on the relationship between time since invasion, soil fertility evolution, the dynamics of vegetation and soil microorganisms, also in the case of Carpobrotus cfr. acinaciformis removal, are worthy to be further investigated.
References
Albert ME (1995) Morphological variation and habitat association within the Carpobrotus species complex in coastal California. MS thesis, University of California, Berkeley
Andreu J, Manzano-Piedras E, Bartomeus I, Dana ED, Vilà M (2010) Vegetation response after removal of the invasive Carpobrotus hybrid complex in Andalucía, Spain. Ecol Restor 28(4):440–448
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Bailey VL, Peacock AD, Smith JL, Bolten JH (2002) Relationships between soil microbial biomass determined by chloroform fumigation-extraction, substrate-induced respiration, and phospholipid fatty acid analysis. Soil Biol Biochem 34:1385–1389
Bartomeus I, Vilà M (2009) Breeding system and pollen limitation of two supergeneralist alien plants invading Mediterranean shrublands. Aust J Bot 57:1–8
Bartomeus I, Vilà M, Santamaría L (2008) Contrasting effects of invasive plants in plant-pollinator networks. Oecologia 155(4):761–770
Baruah TC, Barthakur HP (1997) A text book of soil analysis. Vikas Publishing house PVT LTD, New Delhi
Bourgeois K, Suehs CM, Vidal É, Médail F (2005) Invasional meltdown potential: Facilitation between introduced plans and mammals on French Mediterranean islands. Ecoscience 12:248–256
Braun-Blanquet J (1932) Plant sociology. Springer, Wien
Brundu G (2013) Invasive Alien Plants in Protected Areas in Mediterranean Islands: Knowledge gaps and main threats. In: Foxcroft LC et al. (eds). Plant Invasions in Protected Areas: Patterns, Problems and Challenges, Invading Nature - Springer Series in Invasion Ecology 7, Dordrecht, pp 395–422
Brunel S, Brundu G, Fried G (2013) Eradication and control of invasive alien plants in the Mediterranean basin: towards better coordination to enhance existing initiatives. EPPO Bull 43(2):290–308
Carta L, Manca M, Brundu G (2004) Removal of Carpobrotus acinaciformis (L.) L. Bolus from environmental sensitive areas in Sardinia, Italy. In: Arianoutsou M, Papanastasis VP (eds) Proceedings of the 10th MEDECOS – international conference on ecology, conservation and management. Millpress Science Publishers, Rotterdam, pp 1–4
Chan KY, Oates A, Li GD, Conyers MK, Prangnell RJ, Poile G, Liu DL, Barchia IM (2010) Soil carbon stocks under different pastures and pasture management in the higher rainfall areas of south-eastern Australia. Aust J Soil Res 48:7–15
Chenot J, Affre L, Passetti A, Buisson E (2014) Consequences of iceplant (Carpobrotus) invasion on the vegetation and seed bank structure on a Mediterranean island: response elements for their local eradication. Acta Bot Gall 161(3):301–308
Conser C, Connor EF (2009) Assessing the residual effects of Carpobrotus edulis invasion, implications for restoration. Biol Invasions 11:349–358
Conteh A (1999) Evaluation of the paired site approach to estimating changes in soil carbon. Discussion Paper 3, of Appendix 6 to Technical Report No2. Australian Greenhouse Office, Camberra
Conti F, Manzi A, Pedrotti F (1997) Liste Rosse Regionali delle Piante d’Italia. W.W.F., Società Botanica Italiana, Camerino, 139 pp
Corbin JD, D’Antonio CM (2012) Gone but not forgotten? Invasive plants’ legacies on community and ecosystem properties. Invasive Plant Sci Manag 5(1):117–124. doi:10.1614/IPSM-D-11-00005.1
D’Antonio CM (1990a) Invasion and dominance of coastal plant communities by the introduced succulent, Carpobrotus edulis. Ph.D dissertation. Santa Barbara, CA, University of California, 212 pp
D’Antonio CM (1990b) Seed production and dispersal in the non native, invasive succulent Carpobrotus edulis (Aizoaceae) in coastal strand communities of central California. J Appl Ecol 27:693–702
D’Antonio CM (1993) Mechanisms controlling invasion of coastal plant communities by the alien succulent Carpobrotus edulis. Ecology 74:83–95
D’Antonio CM, Haubensak K (1998) Community and ecosystem impacts of introduced species. Fremontia 26:13–18
D’Antonio CM, Mahall BE (1991) Root profiles and competition between the invasive, exotic perennial, Carpobrotus edulis, and two native shrub species in California coastal scrub. Am J Bot 78:885–894
D’Antonio CM, Odion DC, Tyler CM (1993) Invasion of maritime chaparral by the introduced succulent Carpobrotus edulis: the roles of fire and herbivory. Oecologia 95:14–21
Dassonville N, Vanderhoeven S, Vanparys V, Hayez M, Gruber W, Meerts P (2008) Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 157(1):131–140. doi:10.1007/s00442-008-1054-6
de la Peña E, de Clercq N, Bonte D, Roiloa S, Rodríguez-Echeverría S, Freitas H (2010) Plant-soil feedback as a mechanism of invasion by Carpobrotus edulis. Biol Invasions 12(10):3637–3648
De Marco A, Arena C, Giordano M, Virzo De Santo A (2013) Impact of the invasive tree black locust on soil properties of Mediterranean stone pine-holm oak forests. Plant Soil 372(1):473–486
Delipetrou P (2006) Carpobrotus edulis. Delivering Alien Invasive Species Inventories for Europe, pp 1–4. DAISIE database: http://www.europe-aliens.org/pdf/Carpobrotus_edulis.pdf
deVries F, Shade A (2013) Controls on soil microbial community stability under climate change. Front Microbiol 4:265. doi:10.3389/fmicb.2013.00265, 16 pp
Dostál P, Müllerová J, Pyšek P, Pergl J, Klinerová T, Vilá M (2013) The impact of an invasive plant changes over time. Ecol Lett 16(10):1277–1284
Duro A, Piccione V, Scalia C, Zampino D (1997) Precipitazioni e temperature medie mensili in Sicilia relative al sessantennio 1926–1985. In: Piccione V, Antonelli C (a cura di). Atti 5° Workshop Progetto Strategico “Clima Ambiente e Territorio nel Mezzogiorno” (Amalfi, SA, 28–30 aprile 1993 Collana Progetto Strategico “Clima, Ambiente e Territorio nel Mezzogiorno”, C.N.R, Roma), pp 17–103
Fierer N, Allen AS, Schimel JP, Holden PA (2003) Controls on microbial CO2 production: a comparison of surface and subsurface horizons. Glob Chang Biol 9:1322–1332
Fierotti G (1988) Carta dei suoli della Sicilia (scala 1:250.000). Regione Siciliana, Assessorato Territorio e Ambiente. Università degli studi di Palermo, Facoltà di Agraria. Istituto di Agronomia Generale, Cattedra di Pedologia
Flory SL, D’Antonio CM (2015) Taking the long view on the ecological effects of plant invasions. Am J Bot 102(6):817–818
Forey E, Lortie CJ, Michalet R (2009) Spatial patterns of association at local and regional scales in coastal sand dune communities. J Veg Sci 20:916–925
Fournier P (1952) Dicotylédones. Vol. 2 of Flore illustrée des Jardins et des Parcs: Arbres, Arbustes et Fleurs de Pleine Terre. P. Lechevalier, Paris
Fried G, Laitung B, Pierre C, Chagué N, Panetta FD (2014) Impact of invasive plants in Mediterranean habitats: disentangling the effects of characteristics of invaders and recipient communities. Biol Invasions 16(8):1639–1658. doi:10.1007/s10530-013-0597-6
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Heneghan L, Fatemi F, Umek L, Grady K, Fagen K, Workman M (2006) The invasive shrub european buckthorn (Rhamnus cathartica L.) alters soil properties in midwestern U.S. woodlands. Appl Soil Ecol 32(1):142–148. doi:10.1016/j.apsoil.2005.03.009
Heywood VH (1995) The Mediterranean flora in the context of world biodiversity. Ecol Medit 21(1–2):11–18. http://ecologia-mediterranea.univ-avignon.fr/uploads/media/Ecologia_mediterranea_1995-21_1-2__02.pdf
IBM Corp. Released (2013) IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp
Jandová K, Klinerová T, Müllerová J, Pyšek P, Pergl J, Cajthaml T, Dostál P (2014) Long-term impact of Heracleum mantegazzianum invasion on soil chemical and biological characteristics. Soil Biol Biochem 68:270–278. doi:10.1016/j.soilbio.2013.10.014
Jastrow JD, Amonette JE, Bailey VL (2007) Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim Chang 80:5–23
Jia GM, Cao J, Wang G (2005) Influence of land management on soil nutrients and microbial biomass in the central Loess Plateau, northwest China. Land Degrad Dev 16(5):455–462. doi:10.1002/ldr.673
Kara Ö, Bolat İ (2008) Soil microbial biomass C and N changes in relation to forest conversion in the northwestern Turkey. Land Degrad Dev 19(4):421–428. doi:10.1002/ldr.850
Kaur A, Chaudhary A, Kaur A, Choudhary R, Kaushik R (2005) Phospholipid fatty acid – A bioindicator of environment monitoring and assessment in soil ecosystem. Curr Sci 89(7):1103–1112
Killham K (1994) Soil ecology. Cambridge University Press, UK
Kucharik CJ, Roth JA, Nabielski RT (2003) Statistical assessment of a paired-site approach for verification of carbon and nitrogen sequestration on Wisconsin conservation reserve program land. J Soil Water Conserv 1:58–66
La Mantia T (2011) I rimboschimenti delle dune. In: Ientile R, Rühl J, La Mantia T, Massa B (a cura di). I cambiamenti nell’ecosistema della Riserva Naturale di Vendicari e gli effetti sull’avifauna, Edizioni Danaus, Palermo, pp 97–109
La Mantia T, Pasta S, Rühl J (2009) Flora e vegetazione, habitat comunitari, uso del suolo. Quadro conoscitivo e proposte gestionali relative agli aspetti floristici, vegetazionali e agro-forestali. Piano di Gestione “Isole Pelagie” SIC ITA040002 “Isole di Lampedusa e Lampione” e ZPS ITA040013 “Arcipelago delle Pelagie. Area marina e terrestre”, Parte I – Fase conoscitiva. Pp 1–353. http://www.artasicilia.eu/old_site/web/pdg_definitivi/definitivi/pdg_isole_pelagie/1_relazioni/ispl_relazione_pdg_conoscitiva.pdf
Laudicina VA, Dennis PG, Palazzolo E, Badalucco L (2012) Key biochemical attributes to assess soil ecosystem sustainability. In: Malik A, Grohmann E (eds) Environmental protection strategies for sustainable development. Springer, The Netherlands, pp 193–228
Laudicina VA, Novara A, Barbera V, Egli M, Badalucco L (2015) Long-term tillage and cropping system effects on chemical and biochemical characteristics of soil organic matter in a Mediterranean semiarid environment. Land Degrad Dev 26(1):45–53. doi:10.1002/ldr.2293
Lojacono-Pojero M (1891) Flora Sicula o descrizione delle piante spontanee o indigenate in Sicilia, Vol I(2). Forni Ed., Palermo, pp 1–311
Mack R, Simberloff D, Lonsdale W, Evans H, Clout M, Bazzaz F (2000) Biotic invasions: Causes, epidemiology, global consequences and control. Ecol Appl 10:689–710
Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009) Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol 97:199–205
Malan C, Notten A (2006) Carpobrotus edulis (L.) L. Bolus. S Africa National Biodiversity Institute - Kirstenbosch. http://www.plantzafrica.com/plantcd/carpobed.htm
Marchante H, Marchante E, Freitas H, Hoffmann JH (2015) Temporal changes in the impacts on plant communities of an invasive alien tree, Acacia longifolia. Plant Ecol 216(11):1481–1498. doi:10.1007/s11258-015-0530-4
Maron JL, Jefferies RL (2001) Restoring enriched grasslands: effects of mowing on species richness, productivity, and nitrogen retention. Ecol Appl 11:1088–1100
Molinari N, D’Antonio C, Thomson G (2007) Carpobrotus as a case study of the complexities of species impacts. In: Cuddington K, Byers JE, Wilson WG, Hastings A (eds) Ecosystem engineers - plants to Protists. Theoretical ecology series 4(C). Academic Press, San Diego, pp 139–162
Murphy B, Rawson A, Ravenscroft L, Rankin M, Millard R (2003) Paired Site Sampling for Soil Carbon Estimation – New South Wales. National Carbon Accounting System Technical Report No34
Nanko K, Giambelluca TW, Sutherland RA, Mudd RG, Nullet MA, Ziegler AD (2015) Erosion potential under Miconia calvescens stands on the island of Hawaii. Land Degrad Dev 26(3):218–226. doi:10.1002/ldr.2200
Novara A, La Mantia T, Barbera V, Gristina L (2012) Paired-site approach for studying soil organic carbon dynamics in a Mediterranean semiarid environment. Catena 89(1):1–7
Novoa A, González L (2014) Impacts of Carpobrotus edulis (L.) N.E.Br. on the germination, establishment and survival of native plants: A clue for assessing its competitive strength. PLoS One 9(9), e107557
Novoa A, González L, Moravcová L, Pyšek P (2012) Effects of soil characteristics, allelopathy and frugivory on establishment of the invasive plant Carpobrotus edulis and a co-occurring native, Malcolmia littorea. PLoS One 7(12), e53166
Novoa A, González L, Moravcová L, Pyšek P (2013) Constraints to native plant species establishment in coastal dune communities invaded by Carpobrotus edulis: Implications for restoration. Biol Conserv 164:1–9
Novoa A, Rodríguez R, Richardson D, González L (2014) Soil quality: a key factor in understanding plant invasion? The case of Carpobrotus edulis (L.) N.E.Br. Biol Invasions 16(2):429–443
Osunkoya OO, Perrett C (2011) Lantana camara L. (Verbenaceae) invasion effects on soil physico-chemical properties. Biol Fertil Soils 47(3):349–355
Passetti A, Aboucaya A, Buisson E, Gauthier J, Médail F, Pascal M, Ponel P, Vidal É (2012) Restauration écologique de la Réserve intégrale de l’île de Bagaud (Parc national de Port-Cros, Var, France) et «état zéro» des suivis scientifiques: synthèse méthodologique. Sci Rep Port-Cros Natl Park 26:149–171
Perkins LB, Johnson DW, Nowak RS (2011) Plant-induced changes in soil nutrient dynamics by native and invasive grass species. Plant Soil 345(1–2):365–374
Pyšek P, Richardson DM, Rejmánek M, Webster GL, Williamson M, Kirschner J (2004) Alien plants in checklist and floras: towards better communication between taxonomists and ecologists. Taxon 53(1):131–143
Raunkiær C (1934) The life form of plants and statistical plant geography. Oxford University Press, Oxford
Rivas-Martínez S, Fernández-Gonzáles F, Loidi J (1999) Checklist of plant communities of Iberian Peninsula, Balearic and Canary Islands to suballiance level. Itinera Geobot 13:353–451
Rout ME, Chrzanowski TH (2009) The invasive Sorghum halepense harbors endophytic N2-fixing bacteria and alters soil biogeochemistry. Plant Soil 315(1–2):163–172
Ruffino L, Krebs E, Passetti A, Aboucaya A, Affre L, Fourcy D, Lorvelec O, Barcelo A, Berville L, Bigeard N, Brousset L, De Méringo H, Gillet P, Le Quilliec P, Limouzin Y, Médail F, Meunier J-Y, Pascal M, Pascal M, Ponel P, Rifflet F, Santelli C, Buisson E, Vidal E (2014) Eradications as scientific experiments: progress in simultaneous eradications of two major invasive taxa from a Mediterranean island. Pest Manag Sci 71(2):189–198. doi:10.1002/ps.3786
Santoro R, Jucker T, Carranza ML, Acosta A (2011) Assessing the effects of Carpobrotus invasion on coastal dune soils. Does the nature of the invaded habitat matter? Community Ecol 12:234–240
Santoro R, Jucker T, Carboni M, Acosta ATR, Adler P (2012) Patterns of plant community assembly in invaded and non-invaded communities along a natural environmental gradient. J Veg Sci 23(3):483–494
Sanz-Elorza M, Dana ED, Sobrino E (2004) Atlas de las plantas alóctonas invasoras en España. Madrid: Dirección General para laBiodiversidad, Ministerio de Medio Ambiente
Schiere M (2000) Soil fertility on Linosa. Quantifying the changes in Soil Organic Matter on the semi-arid Mediterranean island of Linosa. MSc. thesis, University of Wageningen, Netherlands, IATA-CNR, Italy, pp 1–127
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64:1659–1668
Shackleton CM, Gambiza J (2008) Social and ecological trade offs in combating land degradation: The case of invasion by a woody shrub (Euryops floribundus) at Macubeni, South Africa. Land Degrad Dev 19(4):454–464. doi:10.1002/ldr.849
Shannon C, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana, pp 1–117
Strauss SY, Webb CO, Salamin N (2006) Exotic taxa less related to native species are more invasive. Proc Natl Acad Sci U S A 103(15):5841–5845. doi:10.1073/pnas.0508073103
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21(11):645–651
Strickland MS, Rousk J (2010) Considering fungal: bacterial dominance in soils – Methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395
Suehs CM, Affre L, Médail F (2004) Invasion dynamics of two alien Carpobrotus (Aizoaceae) taxa on a Mediterranean island: II. Reproductive strategies. Heredity 92:550–556
Traveset A, Moragues E, Valladares F (2008) Spreading of the invasive Carpobrotus aff. acinaciformis in Mediterranean ecosystems: the advantage of performing in different light environments. Appl Veg Sci 11:45–54
Tüxen R, Ellenberg H (1937) Der systematische und ökologische Gruppenwert. Ein Beitrag zur Begriffsbildung und Methodik der Pflanzensoziologie. Mitt Flor-Soz Arbeitsgem 3:171–184
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
van der Putten WH, Klironomos JN, Wardle DA (2007) Microbial ecology of biological invasions. ISME J 1:28–37
van Grunsven RHA, Bos F, Ripley BS, Suehs CM, Veenendaal EM (2009) Release from soil pathogens plays an important role in the success of invasive Carpobrotus in the Mediterranean. S Afr J Bot 75:172–175
Vilà M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, Lambdon P, Manca M, Médail F, Moragues E, Traveset A, Troumbis AY, Hulme PE (2006) Local and regional assessment of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33:853–861
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14(7):702–708
Vitousek PM, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA (1987) Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 238(4828):802–804
Von Holle B, Neill C, Largay EF, Budreski KA, Ozimec B, Clark SA, Lee K (2013) Ecosystem legacy of the introduced N2-fixing tree Robinia pseudoacacia in a coastal forest. Oecologia 172(3):915–924. doi:10.1007/s00442-012-2543-1
Wisura W, Glen HF (1993) The South African species of Carpobrotus (Aizoaceae). Contrib Bolus Herb 15:76–107
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61:475–481
Acknowledgments
This research was funded within the MIUR-PRIN project “Climate change mitigation strategies in tree crops and forestry in Italy (CARBOTREES)”. A special thank to Maurizio Sajeva, who improved the quality of the manuscript. We are particularly grateful to Francesco Vaccari for his valuable support during bibliographic research and to Silvio Fici for his valuable suggestions. We are indebted to Dario Francaviglia for material support during laboratory activities, to Pasquale Giardina for logistic support and technical assistance in the field work, and to Giovanna Sala for its help in different phases of the work. Some activities were conducted within the Project LIFE11 NAT/IT/000093 PELAGIC BIRDS, with the contribution of the LIFE financial instrument of the European Union.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeff R. Powell.
An erratum to this article is available at http://dx.doi.org/10.1007/s11104-016-3076-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 32.6 kb)
Rights and permissions
About this article
Cite this article
Badalamenti, E., Gristina, L., Laudicina, V.A. et al. The impact of Carpobrotus cfr. acinaciformis (L.) L. Bolus on soil nutrients, microbial communities structure and native plant communities in Mediterranean ecosystems. Plant Soil 409, 19–34 (2016). https://doi.org/10.1007/s11104-016-2924-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2924-z