Abstract
The structural organization of mutualism networks, typified by interspecific positive interactions, is important to maintain community diversity. However, there is little information available about the effect of introduced species on the structure of such networks. We compared uninvaded and invaded ecological communities, to examine how two species of invasive plants with large and showy flowers (Carpobrotus affine acinaciformis and Opuntia stricta) affect the structure of Mediterranean plant–pollinator networks. To attribute differences in pollination to the direct presence of the invasive species, areas were surveyed that contained similar native plant species cover, diversity and floral composition, with or without the invaders. Both invasive plant species received significantly more pollinator visits than any native species and invaders interacted strongly with pollinators. Overall, the pollinator community richness was similar in invaded and uninvaded plots, and only a few generalist pollinators visited invasive species exclusively. Invasive plants acted as pollination super generalists. The two species studied were visited by 43% and 31% of the total insect taxa in the community, respectively, suggesting they play a central role in the plant–pollinator networks. Carpobrotus and Opuntia had contrasting effects on pollinator visitation rates to native plants: Carpobrotus facilitated the visit of pollinators to native species, whereas Opuntia competed for pollinators with native species, increasing the nestedness of the plant–pollinator network. These results indicate that the introduction of a new species to a community can have important consequences for the structure of the plant–pollinator network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The establishment and spread of introduced alien species modifies the structure and stability of ecological communities (Richardson and Pyšek 2000). The most well-known effects of invasive plant species on communities involve direct competition for nutrients, light, space or water, and the consequent loss of plant species diversity (Lodge 1993; Levine et al. 2003). In contrast, there is little information available about their effects on other trophic levels such as the bottom-up relationships. However, there is some evidence to suggest that invasive plants could cause disruptions to mutualistic interactions such as between plant and pollinators (Traveset and Richardson 2006).
A few studies on pollinator competition prompted by invasive plants have examined specific plant–pollinator interactions, in which visitation rates or diversity of pollinators were compared for particular native species present in invaded and uninvaded areas. Results of specific plant–pollinator interaction studies have yielded inconsistent results and so we cannot generalize that alien plants always affect the pollination success of native plants (Bjerknes et al. 2007). For example, the invader Lythrum salicaria reduces pollinator visitation and the seed set of its native congener Lythrum alatum under experimental conditions (Brown et al. 2002). Similarly, Impatiens glandulifera halves the seed set of the native Stachys palustris through pollinator competition (Chittka and Schürkens 2001). Although Carpobrotus spp. have no effect on the pollination of the rare plant Dithyrea maritime in southern California (Aigner 2004), in the Balearic Islands they have a competitive effect (i.e., fewer visits) on Lotus cytisoides, but a facilitative effect (i.e., more visits) on Cistus salviifolius and Anthyllis cytisoides, and no effect on Cistus monspeliensis (Traveset and Moragues 2005).
Although the above examples demonstrate some pollination changes regarding a focal plant species in the presence of a plant invader, it is important to understand how these changes affect the entire community’s plant–pollinator interaction network. Mutualistic networks are extremely important for the diversity and stability of the community because they are involved in coevolution processes where multiple species rely on each other to succeed (Jordano 1987; Bascompte and Jordano 2007). Some animal-pollinated invasive plants may become integrated into plant–pollinator networks of the recipient community (Memmott and Wasser 2002) and may even be visited by endemic super generalist pollinators (Olesen et al. 2002). It is, therefore, essential to understand how invasive plants affect not only single plant–pollinator interactions but the whole plant–pollinator network. Only one study has examined how visitation rates differ between invaded and uninvaded sites. Lopezaraiza-Mikel et al. (2007) compared sites invaded by I. glandulifera with sites where I. glandulifera was experimentally removed. They found that the invader acted as a magnet species, invaded sites being more visited than those in which the invader was removed from the community.
More recent work has explored the inherent structure of plant–pollinator networks. Plant–pollinator networks are not randomly organized; they have a nested structure (i.e., nestedness) meaning that there is a core of generalist plant and pollinator species, and an asymmetric dependence between them. Some specialized pollinators visit generalist plant species and vice versa, specific plant species are visited by generalist pollinators (Bascompte et al. 2003, 2006). The characteristics of plant–pollinator networks differ along environmental gradients such as humidity, insularity or latitude (Olesen and Jordano 2002; Ollerton and Cranmer 2002; Devoto et al. 2005). However, the effect of biotic controls on plant–pollinator networks, such as the consequences of adding a new plant species (e.g., due to biological invasions) to the community have been far less explored. Moreover, no study has empirically investigated how network nestedness changes after a new species is introduced to a community.
We present here results of a field replicated comparative study on the effects of plant invasions on plant–pollinator interaction networks. Our hypothesis is that an introduced generalist invasive plant will establish many interactions with resident pollinators and consequently some interactions with native plant species will be lost. In principle this would increase network nestedness as a greater number of pollinator species interact with fewer plant species in the community. To test this hypothesis, we studied Carpobrotus affine acinaciformis and Opuntia stricta, which have invaded Mediterranean coastal communities. Both species have showy, large flowers but contrasting phenologies that could result in different effects on plant–pollinator networks. Carpobrotus flowers in spring, when most native species flower. In contrast, Opuntia flowers in early summer, when a few late-flowering natives bloom. Mediterranean communities tend to have more flowers than insects in early spring, whereas in late spring there are more insects than flowers. Thus, in early spring one would expect plants to compete for pollinators, whereas in summer pollinators would compete for flowers (Shmida and Dafni 1989; Petanidou and Lamborn 2005). We expected Carpobrotus to have a higher impact on the pollination network than Opuntia. Specifically, we asked:
-
1.
Which pollinators visit invasive plants, as compared to native plants?
-
2.
Do pollinator diversity, composition and visitation rates differ between invaded and uninvaded communities?
-
3.
Does the structure of the plant–pollinator network (i.e., composition, strength of plant species, and nestedness of plant–pollinator interactions) change between invaded and uninvaded communities?
Materials and methods
Study area and study species
Our study area was in coastal Mediterranean shrublands in the Natural Park of Cap de Creus (Catalonia, northeastern Spain). This area is characterized by cool wet winters and warm dry summers. Mean temperatures of the coldest (January) and hottest (August) months in 2005 were 6°C and 23°C, respectively, and the annual precipitation was 450 mm (http://www.meteocat.com). Our study involved a pollinator survey of the native flora and the two most abundant invasive plants in the park.
Carpobrotus (Aizoaceae) are crawling succulent herbs with fast clonal growth (Vilà and D’Antonio 1998). They are native to South Africa and are present in almost all Mediterranean regions of the world. They were introduced in Spain for gardening and soil fixation at the beginning of the twentieth century. In the study area Carpobrotus may be hybrids between C. edulis and C. acinaciformis. We will therefore follow Suehs et al. (2004) nomenclature and refer to them as the hybrid complex Carpobrotus affine acinaciformis. These putative hybrids have flowers that are solitary and 8–10 cm in diameter, range in color from yellow to purple and have a generalist pollination system (Vilà and D’Antonio 1998). Flowering lasts from March to June (Sanz-Elorza et al. 2006).
Opuntia stricta (Cactaceae) is a succulent perennial spiny plant introduced to Spain from Central America in the sixteenth century. It grows on sunny sandstone hillsides and is commonly cultivated as an ornamental. It can reach 1.5 m high and has big yellow flowers (5–10 cm in diameter). Flowering takes place from June to July (Sanz-Elorza et al. 2006).
Field sampling
In spring 2005, we delineated three sites per study species and established two paired 50 x 50-m plots in each site, one invaded and the other uninvaded separated on average by 300 m. Paired sites were at least 3 km apart from each other. To exclude possible indirect effects of the invasive plants on pollinator presence due to changes in plant species diversity (Kunin 1997) we chose areas that exhibited initial stages of invasion and selected pairs of plots with similar plant species diversity, dominant species and vegetation cover. Plant species diversity was calculated using the Shannon index (Sh = −Σ|Pi log10 Pi|; where Pi is the frequency of occupation of species i). Plant community cover was determined by the point-intercept method in four parallel 50-m transects (Table 1). Variation in plant species richness was not significant (invaded vs. uninvaded: 10.3 ± 0.7 vs. 12 ± 2.3, t = −2.5, P = 0.13 for Carpobrotus sites; 10.3 ± 1.3 vs. 10.3 ± 0.8, t = 0.1, P = 0.9 for Opuntia sites). Field work was conducted on sunny days with little wind and with temperatures higher than 15°C. Sites were sampled every 2 weeks during the entire flowering period of both species: 6 times from April to May for Carpobrotus sites and 4 times from June to early July for Opuntia sites.
Within each site, insect pollinator counts were made along two parallel 50-m permanent transects. To avoid oversampling of the most abundant plant species we limited our observations to a total of six observation areas per flowering species. In each observation area we focused only on one flowering species. The observation areas were approximately 30 x 30 cm and were located along transects at 2-m intervals. To reduce any temporal bias in observations along transects, we started each day’s sampling at a different random initial point along each transect. In each observation areas, we counted the number of flowers, and recorded and identified all insects that visited the flowers within 1 min of observation. We captured unknown visitors during a maximum of 3 additional minutes for later identification. Voucher specimens were deposited at the CREAF. We only recorded an insect as a visitor if it touched the reproductive organs of the flower.
Our sampling protocol allowed us to standardize the time spent observing each plant species compared to classic transect walks. Each species was sampled for a total of 36 min per site. In total, each site was surveyed for pollinators for more than 6 h.
To ascertain whether differences in pollinator activity were due to differences in flower abundance, we estimated the number of flowers of each plant species in the vicinity of each transect. Each sampling day when pollinator sampling was finished, we determined the total number of flowers or inflorescences (flower units hereafter) per plant species in 1-m2 areas located at 1-m intervals along the transects. The total number of flower units in paired invaded and uninvaded plots did not differ for either species (Wilcoxon test, paired comparisons: for Carpobrotus, P = 0.3–0.5; for Opuntia, P = 0.2–0.5). We therefore assumed pollinator activity in invaded and uninvaded plots was independent of differences in native floral abundance.
Plant-pollination network analysis
With the data collected we constructed a plant–pollinator interaction network for each of the 12 plots. Networks were represented as two-dimensional matrices, in which rows represented the plants and columns the pollinator species. We constructed two matrices per plot, one in which cell values represented the presence or absence of the interaction and another in which cells were the frequency of visits observed for each plant–pollinator interaction.
To test whether the two invasive species received a number of visits comparable to average visitation on a particular native species within each invaded plot, we compared the visitation frequency received by the invader with a normal distribution fitted to the number of visits to native species, with a Z-statistic.
To explore whether invaded and uninvaded plots differed in pollinator assemblage, we calculated the Sørensen similarity index [S = (2 × C)/(2C + A + B); where C is the number of pollinator species that were common in both plots, A is the number of pollinators only present in invaded plots, and B is the number of pollinators present only in uninvaded plots]. S ranges from 1 (identical assemblages) to 0 (maximally dissimilar assemblages).
Differences between invaded and uninvaded plots in pollinator species richness, frequency of visits of the main pollinator orders, and frequency of visits to native plants were compared with paired t-tests. To account for the limited power associated with the low sample size (n = 3 networks per treatment), we also report marginally significant differences (α = 0.1). The limited sample size is largely justified by the considerable effort involved in the simultaneous characterization of several whole plant–pollinator networks in an area where a reference pollinator collection does not exist. Statistical analyses were carried out using STATISTICA (StatSoft 2001).
We tested whether invasion influenced pollinator visitation rates (i.e., number of visits per flower) to native species with a generalized linear mixed model [GLMM; PROC MIXED in SAS (SAS Institute 2001)]. The model was visitation rate = presence of the invader (yes or no) + plot + plant family + plant species nested within family + error. We considered family and plot random factors, and species and presence or absence of the invader fixed factors. In this way, we could test whether the invaders affected the visitation rates of native plant species while accounting for the clustering of plants according to taxonomy and plot.
We also compared similarities of the interaction scenarios (i.e., identities and frequencies of plant-pollination interactions) between invaded and uninvaded visitation matrices by constructing one similarity matrix estimated with Kulczynski’s distance coefficient [K = 1/2(W/A + W/B); where W is the sum of the minimum visits of the various species, this minimum being defined as the visits at the site where the species is the rarest and, A and B are the sums of the visits of all species at each site, respectively]. The index was calculated between all pairs of plots (Legendre and Legendre 1998; Vázquez and Simberloff 2003). This index was semi-metric, meaning that it accounted for differences in contribution to the coefficient between abundant species and rare species. We used the resulting pair-wise similarity matrix as input for non-metric multidimensional scaling (NMDS), an ordination technique used to provide a visual representation of the pattern of proximities among plots (Legendre and Legendre 1998; Vázquez and Simberloff 2003). To test differences in the array of interactions between plots we performed a Wilcoxon test for each pair of plots where all paired plant–insect interactions in the two plots, including empty cells, were used as input.
To explore in depth the similarity of plant–pollinator interactions, we calculated the number of common plant–pollinator interactions and the number of plant–pollinator interactions that occurred in only one of the paired plots (Ie). In invaded plots we could distinguish between exclusive pollinator–invasive species interactions (Ii) and exclusive pollinator–native species interactions (In). Therefore, in invaded plots: Ie = Ii + In.
We further analyzed the structure of the matrix with the index of matrix nestedness (N) and the importance of each plant species in terms of interaction strength. Matrix nestedness is an estimation of the network organization (Bascompte et al. 2003) and is calculated from temperature (T) as N = (100−T)/100. Values range from 0 (random structure) to 1 (maximum nestedness). To calculate T, we organized the matrix in rows of pollinators and columns of plants, ordering the entries from those presenting the most to the least interactions. This maximally packed matrix was used to calculate the isocline of perfect nestedness for each matrix. The absence of a pair-wise interaction below the isocline or the presence of a pair-wise interaction above the isocline was then recorded as unexpected. Therefore, T is the measure of physical disorder calculated from the normalized measure of global distance from unexpected records to the isocline. To assess the significance of N for each matrix, we compared the observed value of N with a benchmark provided by null model 2 in Bascompte et al. (2003) in which each cell in the interaction matrix has the following probability (Pri + Pci)/2, where Pri is the fraction of interactions of row i and Pci is the fraction of interactions of the column i equivalent. We generated 1,000 random matrices with this null model. Analyses were conducted with the ANINHADO software (Guimaraes and Guimaraes 2006). T values for plants represented how far from the nested pattern each plant species was, and idiosyncratic T values were used to assess changes in the community. Differences in N between invaded and uninvaded plots for each invader species were compared with a paired t-test.
The strength of each plant species in the matrix is a measure of the dependence of pollinators on each particular plant species (Bascompte et al. 2006). Strength is defined as the sum of pollinator dependencies on a particular plant species. Dependence of a pollinator on a plant species is the fraction of all visits by a particular pollinator species to a particular plant (Da,P = Va-p/Va; where Da,P is the dependence of pollinator a on plant species p, Va-p is the number of visits of pollinator a to plant p and Va is the total number of visits of pollinator a to all plant species in the community). We tested for differences in the strength of plant species between invaded and uninvaded plots with a paired t-test. Furthermore, to test whether the two invasive plant species had a higher strength than native species, within each site we compared the invasive species strength with a normal distribution fitted to the strength of the native species by means of a Z-statistic.
Results
Pollinators on invader versus native plant species
We observed a total of 23 and 17 insect taxa on Carpobrotus and Opuntia, respectively. Both species were visited by several species of Coleoptera (e.g., Oedemera lurida and Oxythyrea funesta on Carpobrotus and Myrabilis quadripunctata on Opuntia) and Hymenoptera (e.g., Bombus terrestris, Anthidium sticticum to Carpobrotus and Xylocopa violacea, Apis mellifera to Opuntia). The native plants were also visited by some Diptera and Lepidoptera. Almost no pollinators were exclusive to Carpobrotus, which were visited by 43.4% of the observed insect taxa. Opuntia was visited by 30.9% of the observed insect taxa. X. violacea (i.e., carpenter bees) exclusively visited Opuntia plants and were a frequent pollinator (19.5% of the total visits to Opuntia).
Both invader species received on average more visits than any native species (Z-tests: P < 0.001 in all sites). Carpobrotus received 27.8 ± 7.2% (mean ± SE) of the total visits observed. On average, native species in invaded plots received 7.61 ± 1.23% of the visits. Opuntia received 44.1 ± 11.2% of the observed visits, whereas on average, native species received 6.16 ± 2.33%.
Pollinator structure in invaded versus uninvaded communities
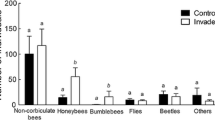
In total we found 53 and 55 different pollinator taxa in invaded and uninvaded Carpobrotus and Opuntia sites, respectively. There were differences in the composition of invaded and uninvaded plots measured with S; S = 0.52 ± 0.02 for Carpobrotus sites and S = 0.54 ± 0.04 for Opuntia sites. However, the presence of an invasive species did not affect pollinator species richness (invaded vs. uninvaded: 29.33 ± 3.71 vs. 20.33 ± 1.21, t = 2, P = 0.18 for Carpobrotus sites; 23.67 ± 1.20 vs. 21.00 ± 2.00, t = 1.02, P = 0.29 for Opuntia sites). We only found significantly more Coleoptera visits (t = −5.46, P = 0.03) in Carpobrotus-invaded plots compared to uninvaded plots. This difference was principally due to the frequency of visits to the invasive species and to Asteracea and Cistacea. There was no detectable difference in the insect orders observed in Opuntia-invaded and uninvaded sites, probably because of the large variability among plots (Fig. 1; paired t-tests: P > 0.10).
The frequency of visits to the invasive and native species as a whole did differ. There were marginally more visits in invaded than in uninvaded Carpobrotus plots (159.33 ± 22.26 vs. 83.33 ± 6.76, t = 2.97, P = 0.10). In the invaded plots, visitation rates increased significantly in more than 70% of the native species (GLMM test: fixed effects—presence of the invader, F 1,29 = 6.23, P = 0.02; species within families, F 14, 1 = 18.01, P = 0.18; random effects—plot, Z = 0.93, P = 0.35; family, Z = 1.21, P = 0.23) In contrast, the overall number of visits to Opuntia plots did not differ between invaded and uninvaded plots (95.33 ± 10.14 vs. 70.67 ± 4.10, t = 2.51, P = 0.13). However, visitation rates decreased in more than 60% of the native species in invaded plots (fixed effects—presence of the invader, F 1,27 = 4.91, P = 0.04; species within families, F 14, 1 = 14.99, P = 0.20; random effects—plot, Z = 1.41, P = 0.16; family, Z = 1.25, P = 0.21).
Plant–pollinator network in invaded vs. uninvaded communities
The greater number of visits in Carpobrotus-invaded vs. uninvaded plots resulted in a marginal increase in the number of plant–pollinator interactions (67.67 ± 6.34 vs. 39.33 ± 16.37, t-test = 3.47, P = 0.07). Nevertheless, paired Carpobrotus-invaded and uninvaded plots shared 57.66 ± 8.01% species interactions. Invaded plots had marginally more exclusive interactions than uninvaded plots (t-test = 3.46, P = 0.07). Most of the exclusive interactions, almost 70% in invaded plots, involved native plants. In fact, pollinators as a whole interacted with more plant species in Carpobrotus-invaded plots than in uninvaded plots (invaded, 2.33 ± 0.09 interactions/pollinator species; uninvaded, 1.94 ± 0.07 interactions/pollinator species; t-test = 4.64, P = 0.04). Opuntia-invaded and uninvaded plots shared even fewer common interactions (31.43 ± 3.56%). There were no significant differences in the total number of exclusive interactions between invaded and uninvaded plots (t-test = 0.24, P = 0.4). However, almost 50% of the exclusive interactions in invaded plots were due to interactions with Opuntia (Fig. 2).
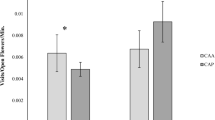
The two-dimensional representation of the NMDS presents a stress of 0.001 for Carpobrotus and 0.05 for Opuntia, showing a high correspondence with the input data (similarities of the interaction identities). Carpobrotus-invaded plots were distinctly separated from uninvaded plots (Fig. 3) and tended to cluster together along dimension 2. In two out of three sites, interaction identities differed significantly between paired native versus non-native plots (Wilcoxon test: site BAT, Z = 5.08, P < 0.0001; site MED, Z = 3.86, P < 0.001). In contrast, although Opuntia-invaded plots also showed a consistent segregation from uninvaded plots along dimension 2 of the MDS, these differences were not significant in the identity of interactions (Wilcoxon test: P > 0.1 in all sites).
Representation of similarities of the plant–pollinator identity interactions in a C. aff. acinaciformis- and b O. stricta-uninvaded (close circles) and invaded (open circles) plots, generated in two dimensions by multidimensional scaling analysis. See Table 1 for study site names and characteristics
The structure of the plant–pollinator network was significantly nested (P < 0.05) in half of the plots when compared with the benchmark of 1,000 random matrices for each plot with the null model 2 (Table 2). All Opuntia-invaded plots were significantly more nested than expected by random outcome, but none of the uninvaded plots were (Table 2). Nestedness did not differ between invaded and uninvaded plots for Carpobrotus (uninvaded vs. invaded: 0.82 ± 0.002 vs. 0.77 ± 0.001, t = 1.34, P = 0.3). However, Opuntia-invaded plots were marginally more nested than uninvaded plots (0.83 ± 0.0007 vs. 0.73 ± 0.0002, t = −3.49, P = 0.07).
The strength of each native plant species was not significantly different between invaded and uninvaded plots (t-test: P > 0.05 in all plots). The invasive plants had high strength values in all plots and on average these were significantly higher than the strength value of native plant species (Table 2).
Discussion
Our work, along with that of Lopezaraiza-Mikel et al. (2007), is unique in empirically testing how an invasive plant species can affect plant–pollinator networks. While Lopezaraiza-Mikel et al. (2007) looked at the effect of a zygomorphic invasive plant, we investigated two actinomorphic species with different floral phenologies. The two invaders we studied had larger flowers than any of the co-occurring native species and attracted significantly more pollinator species than any native. However, the consequences for plant–pollinator networks differed between species. Carpobrotus-invaded plots received a larger number of visits, mainly from Coleoptera, resulting in an increasing number of plant–pollinator interactions. Although Opuntia-invaded plots received a similar number of visits as uninvaded plots, most visits were to the invasive species, thus reducing the visits to native plants. We examined the consequences of the presence of the two invaders from the perspective of the native plants, the pollinators, and the plant–pollinator network as a whole.
Implications for the native plants
In general, pollinator limitation does not appear to be a major barrier for the spread of introduced plants (Richardson et al. 2000). Many invasive plants are pollination generalists, with attractive nectar and pollen-rich flowers that constitute an easy resource (Campbell 1989). In our study, Carpobrotus received more visits than any native species; still, the average number of interactions (3–4 pollinator species/plant species) was within the range reported by Olesen et al. (2002) for oceanic islands. In invaded plots, the general trend was for an increased visitation rate to most native plant species. Only a few specialized species with zygomorphic flowers, such as Labiatae (Lavandula stoechas) and Papilonaceae (Lathirus clymenum) received fewer visits in invaded than in uninvaded plots. These results seem to contradict the “floral market” hypothesis (Chittka and Schürkens 2001), which suggests that animals choose between products (plant species) on the basis of their quality (nectar and pollen). According to this hypothesis showy, resource-rich invasive plants with higher reward levels might receive higher visitation rates, thus decreasing visitation rates to the natives. Instead, Carpobrotus appears to have a facilitative effect on the visitation to natives. Similar observations were made by Fleishman et al. (2005), who found that the higher the total amount of resources available in the community, the more pollinators are attracted to all plants. Whether this enhanced pollinator visitation translates to a positive effect on the native species seed set is not known. In these circumstances the pollen transport web might be dominated by the invader as found in Lopezaraiza-Mikel et al. (2007), and invasive pollen could be deposited on native stigmas interfering with fecundity. Preliminary results show that Carpobrotus pollen is indeed transported on pollinator’s bodies, but few pollen grains reach native stigmas suggesting that the ultimate impact on natives is low (I. Bartomeus et al., unpublished data).
We expected Carpobrotus to have a higher impact on competition for pollinators than Opuntia because there were more species with flowering phenologies overlapping with Carpobrotus than with Opuntia. However, we found the contrary: when Opuntia overlaps with late spring flowering native species, we found that it tended to monopolize the available pollinators, depriving native plant species of their service. This result suggests that the “floral market” hypothesis best fits situations where resources are scarce for pollinators. Overall, assuming that visitation rates are good surrogates for pollination efficiency (Vázquez et al. 2005; Sahli and Conner 2006), these two invasive plants could have contrasting effects on native plant reproduction: enhancement by Carpobrotus, but reduction by Opuntia.
Implications for the pollinator community
Although pollinator diversity in Mediterranean communities is not well known (Petanidou and Ellis 1993), this region is a well-established hotspot of bee diversity and speciation (Michener 2000). Despite current concern about the homogenization of pollinator diversity (Kearns et al. 1998; Steffan-Dewenter et al. 2005), the two invasive plants we studied did not reduce the diversity of pollinators. This may be a transient pattern, because our study was conducted in the early stages of invasion, and so native plant diversity and composition are only slightly affected. Given that pollinators tend to show a high degree of floral constancy (Kevan and Baker 1983), in sites at more advanced stages of invasion, in which native plant diversity has been reduced (Lockwood and McKinney 2001; Vilà et al. 2006), pollinator diversity is more likely to be affected (Steffan-Dewenter and Tscharntke 2001). Additionally, we do not know the effects that the invaders might have on pollinator fitness and behavior, such as altering nesting sites (Graves and Shapiro 2003). To date, evidence for the impact of invasive plants on native pollinator population size and dynamics remains speculative (Traveset and Richardson 2006).
In Carpobrotus-invaded plots there were more visits by Coleoptera than in uninvaded plots. Coleoptera is a very generalist group of pollinators and little is known about their importance in Mediterranean plant pollination. Honey bees, which are maintained for honey production in several places in the Cap de Creus area, were not especially abundant neither in the Carpobrotus-invaded plots nor on Carpobrotus flowers (only 1.4 ± 0.1% of total visits). They were nonetheless present, visiting principally Labiatae flowers such as Lavandula stoechas and Rosmarinus officinalis. In Opuntia-invaded plots the presence of honey bees was high, especially on Opuntia flowers (11.1 ± 3.7% of total visits). In our study super-generalist pollinators are not the main pollinators supporting invasive plants (Olesen et al. 2002).
In Opuntia-invaded networks, Xilocopa violacea [usually considered a generalist pollinator (Westrich 1990)] visited Opuntia flowers exclusively. The absence of X. violacea from uninvaded networks confirms that insects can easily learn to choose and exploit new resources (Dukas and Bernays 2001; Weiss and Papaj 2003) and that, depending on the scale of observation, interactions that appear to be specialized can be interpreted as facultative or local specializations. Unraveling the pollination ecology of invasive plants in introduced and native ranges is a future challenge. A study on this has shown that although the identity of pollinators visiting the invader might differ between introduced and native habitats, flower visitation rates might not be significantly different at home than abroad (Stout et al. 2006).
Implications for the plant–pollinator network
Invasion can also affect the identity of plant-pollination interactions. In Carpobrotus paired sites, the similarity of interaction scenarios reflected on the MDS showed few differences within uninvaded plots, especially between those with a similar floral composition, and a large separation with their paired invaded plots. In contrast, for Opuntia differences between invaded and uninvaded sites were not significant.
Generalization among plants and pollinators is a common pattern in mutualistic interactions, with strict specialists (i.e., one-to-one interaction) being the exception rather than the norm (Waser et al. 1996; Vázquez and Aizen 2004). In addition, asymmetric specialization predominates as reflected by the nestedness of the plant–pollinator networks (Bascompte et al. 2003). Our results indicate that although the nested plant–pollinator structure is very stable (Bascompte et al. 2003; Jordano et al. 2003) it can be modified with introduced species that have not coevolved in the system. Nestedness increased in Opuntia plots, probably because while no important links disappeared, invasive plants interacted with many pollinators, attaining a central role in the plant–pollinator network. Furthermore, the high strength value and the central role invasive species played in the plant–pollinator networks suggest that invasion could have long-term consequences, and the dependence of pollinators on the invader may not be reversible. Manipulation experiments are needed to test this prediction.
Conclusion
Both C. aff. acinaciformis and O. stricta are insect-pollinated invasive plant species that in our study behaved as super generalists, occupying a central role in the community and reducing the protagonism of pollinator interactions with native species. However, they had contrasting effects on the relationships between plant and pollinators. Because these invasive species attract a wide diversity and abundance of pollinators, the spatial and temporal scale at which this attraction operates can result in either positive or negative effects on the visitation to native plants. In our study, visitation rates to native plants were enhanced in plots that had been invaded by Carpobrotus and reduced in plots invaded by Opuntia. Carpobrotus attracted pollinators which increased visitation to natives, whereas Opuntia diverted pollinators away from the native species in the community. This altered the structure of the plant–pollinator networks and resulted in an increase in nestedness in Opuntia-invaded plots.
Invasive plants are considered to be the third major cause of pollinator diversity loss through changes in native flora (Kearns et al. 1998). However, few studies have tested this hypothesis except in agroecosystems (but see Steffan-Dewenter and Tscharntke 2001). We envision that the outcomes of biological invasions on plant–pollinator interactions will be context specific, and will depend on the invader identity and abundance, the overlap in phenology of the invader and the native plants in a local community, the flower similarity between the invader and native species as well as the indirect effects of the invader on native plant composition and diversity. For example, Vázquez et al. (2007), found that a large proportion of the variation in the observed plant–pollinator structure was explained by differences in species abundance. Consequently, the impact of an invader will depend not only on qualitative traits but also on their dominance in the community. More experimental studies on different invasion scenarios are needed to disentangle the mechanisms involved in the impact of invaders on plant–pollinator networks.
References
Aigner PA (2004) Ecological and genetic effects on demographic processes: pollination, clonality and seed production in Dithyrea maritima. Biol Conserv 116:27–34
Bascompte J, Jordano P, Melián CJ, Olesen J (2003) The nested assembly of plant–animal mutualistic networks. Proc Natl Acad Sci USA 100:9383–9387
Bascompte J, Jordano P, Olesen J (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312:431–433
Bascompte J, Jordano P (2007) Plant–animal mutualistic networks: the archictecture of biodiversity. Annu Rev Ecol Evol Syst 38:567–593
Bjerknes AL, Totland O, Hegland SJ, Nielsen A (2007) Do alien plant invasions really affect pollination success in native plant species? Biol Conserv 138:1–12
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (Purple loosestrife) and a native congener. Ecology 83:2328–2336
Campbell DR (1989) Inflorescence size: test of the male function hypothesis. Am J Bot 76:730–738
Chittka L, Schürkens S (2001) Successful invasion of a floral market. Nature 411:653–653
Devoto M, Medan D, Montaldo NH (2005) Patterns of interaction between plants and pollinators along an environmental gradient. Oikos 109:461–472
Dukas R, Bernays E A (2001) Learning improves growth rate in grasshoppers. Proc Natl Acad Sci USA 97:2637–2640
Fleishman E, Mac Nally R, Murphy D D (2005) Relationships among non-native plants, diversity of plants and butterflies, and adequacy of spatial sampling. Biol J Linn Soc 85:157–166
Graves SD, Shapiro AM (2003) Exotics as host plants of the California butterfly fauna. Biol Conserv 110:413–433
Guimaraes PR Jr, Guimaraes P (2006) Improving the analyses of nestedness for large sets of matrices. Environ Model Softw 21:1512–1513
Jordano P (1987) Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Am Nat 129:657–677
Jordano P, Bascompte J, Olesen J (2003) Invariant properties in coevolutionary networks of plant–animal interactions. Ecol Lett 6:69–81
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112
Kevan PG, Baker HG (1983) Insects as flower visitors and pollinators. Annu Rev Entomol 28:407–453
Kunin WE (1997) Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. J Ecol 85:225–234
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Griguils K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond 270:775–781
Lockwood JL, McKinney ML (2001) Biotic homogenization. New York Press, New York
Lodge DM (1993) Biological invasions—lessons for ecology. Trends Ecol Evol 8:133–137
Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J (2007) The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecol Lett 10:539–550
Memmott J, Wasser NM (2002) Integration of alien plants into a native flower-pollinator visitation web. Proc R Soc Lond 269:2395–2399
Michener CD (2000) Bees of the world. Johns Hopkins University Press, Baltimore
Olesen J, Eskildsen LI, Venkatasami S (2002) Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic spear generalists. Diver Distr 8:181–192
Olesen J, Jordano P (2002) Geographic patterns in plant-pollinator mutualistic networks. Ecology 83:2416–2424
Ollerton J, Cranmer L (2002) Latitudinal trends in plant-pollinator interactions: are tropical plants more specialised? Oikos 98:340–345
Petanidou T, Ellis WN (1993) Pollinating fauna of a phryganic ecosystem: composition and diversity. Biodiv Lett 1:9–22
Petanidou T, Lamborn E (2005) A land for flowers and bees: studying pollination ecology in Mediterranean communities. Plant Biosyst 139:279–294
Richardson DM, Allsopp N, D’Antonio CM (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Richardson DM, Pyšek P (2000) Naturalization and invasion of alien plants: concepts and definitions. Diver Distr 6:93–107
Sahli HF, Conner JK (2006) Characterizing ecological generalization in plant-pollination systems. Oecologia 148:365–372
Sanz-Elorza M, Dana ED, Sobrino D (2006) Atlas de las plantas alóctonas invasoras de España. Dirección General para la Biodiversidad, Madrid
SAS (2001) SAS (data analysis software system), version 9. SAS institute, Cary
Shmida A, Dafni A (1989) Blooming strategies, flower size and advertising in the “lily-group” geophytes in Israel. Herbertia 45:111–123
StatSoft (2001) STATISTICA (data analysis software system), version 6. Statsoft, Tulsa
Steffan-Dewenter I, Potts SG, Packer L (2005) Pollinator diversity and crop pollination services are at risk. Trends Ecol Evol 20:651–652
Steffan-Dewenter I, Tscharntke T (2001) Succession of bee communities on fallows. Ecography 24:83–93
Stout JC, Parnell JAN, Arroyo J, Crowe TP (2006) Pollination ecology and seed production of Rhododendron ponticum in native and exotic habitats. Biodivers Conserv 15:755–777
Suehs CM, Affre L, Médail F (2004) Invasion dynamics of two alien Carpobrotus taxa on a Mediterranean island. Heredity 92:550–556
Traveset A, Moragues E (2005) Effect of Carpobrotus spp. on the pollination success of native plant species of the Balearic Islands. Biol Conserv 122:611–619
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Vázquez DP, Aizen MA (2004) Asymetric specialization: a pervasive feature of plant-pollinator interactions. Ecology 85:1251–1257
Vázquez DP, Morris WF, Jordano P (2005) Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol Lett 8:1088–1094
Vázquez DP, Simberloff D (2003) Changes in interaction biodiversity induced by an introduced ungulate. Ecol Lett 6:1077–1083
Vázquez DP, Melián CJ, Wiliams NM, Blüthgen N, Krasnov BR, Poulin R (2007) Species abundance and asymmetric interaction strength in ecological networks. Oikos 116:1120–1127
Vilà M, D’Antonio CM (1998) Hybrid vigor for clonal growth in Carpobrotus (aizoaceae) in costal California. Ecol Appl 8:1196–1205
Vilà M, et al. (2006) Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33:853–861
Waser NM, Chittka L, Price MV, Williams NM, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060
Weiss MR, Papaj DR (2003) Colour learning in two behavioural contexts: how much can a butterfly keep in mind? Anim Behav 65:425–434
Westrich P (1990) Die Wildbienen Baden-Württembergs. Ulmer, Stuttgart
Acknowledgements
We thank J. Bosch for insect identification supervision, L. Marco for the field assistance; and J. Bascompte, P. Jordano, A. Valido, K. Gross and three anonymous reviewers for comments on a previous version of the manuscript. Partial research support was provided by the Integrated European Project Assessing Large Scale Risks to Biodiversity with Tested Methods (ALARM: http://www.alarmproject.net), contract 506675 and the Ministerio de Ciencia y Tecnología projects Efecto de las Especies Invasoras en las Redes de Polinización (INVASRED) and Determinantes Biológicos del Riesgo de Invasiones Vegetales (RINVE). This work complies with the current laws of Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katherine Gross.
Rights and permissions
About this article
Cite this article
Bartomeus, I., Vilà, M. & Santamaría, L. Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 155, 761–770 (2008). https://doi.org/10.1007/s00442-007-0946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0946-1