Abstract
Background & aims
Elevated atmospheric CO2 (eCO2) can affect soil-plant systems via stimulating plant growth, rhizosphere activity and the decomposition of added (crop residues) or existing (priming) soil organic carbon (C). Increases in C inputs via root exudation, rhizodeposition and root turnover are likely to alter the decomposition of crop residues but will ultimately depend on the N content of the residues and the soil.
Methods
Two soil column experiments were conducted under ambient CO2 (aCO2, 390 ppm) and eCO2 (700 ppm) in a glasshouse using dual-labelled (13C/15N) residues of wheat (Triticum aestivum cv. Yitpi) and field pea (Pisum sativum L. cv. PBA Twilight). The effects of eCO2 and soil N status on wheat rhizosphere activity and residue decomposition and also N recovery from crop residues with different N status (C/N ratio 19.4–115.4) by different plant treatments (wheat, wheat + 25 mg N kg−1 and field pea).
Results
Total belowground CO2 efflux was enhanced under eCO2 despite no increases in root biomass. Plants decreased residue decomposition, indicating a negative rhizosphere effect. For wheat, eCO2 reduced the negative rhizosphere effect, resulting in greater rates of decomposition and recovery of N from field pea residues, but only when N fertiliser was added. For field pea, eCO2 enhanced the negative rhizosphere effect resulting in lower decomposition rates and N recovery from field pea residue.
Conclusions
The effect of eCO2 on N utilisation varied with the type of residue, enhancing N utilisation of wheat but repressing that of field pea residues, which in turn could alter the amount of N supplied to subsequent crops. Furthermore, reduced decomposition of residues under eCO2 may slow the formation of new soil C and have implications for long-term soil fertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impact of rising atmospheric CO2 concentrations on soil fertility is of critical importance for the future productivity and sustainability of agricultural systems. High concentrations of CO2 in the atmosphere, which have increased by over 42 % since the industrial revolution (IPCC 2001), are known to stimulate photosynthesis and enhance net primary productivity of a wide range of plant species (Ainsworth and Long 2005; de Graaff et al. 2006). Comparable increases in the growth of annual crops such as field pea and wheat have been observed in dryland agricultural systems (Jin et al. 2012; Lam et al. 2012a; Butterly et al. 2015). Elevated CO2 concentrations can increase rhizodeposition of root exudates, cellular material from living roots and fine-root turnover, leading to greater concentrations of labile C in the soil (Carrillo et al. 2011). For non-legumes, eCO2 often reduces the N concentrations in plant tissue (Cotrufo et al. 1998; Jensen and Christensen 2004), even when N fertiliser is applied (Butterly et al. 2015). Changes in the amounts and quality (C/N ratio) of organic matter inputs under eCO2 could alter soil C and N cycling.
In addition to higher C/N ratio, crop residues derived from plants grown under eCO2 often contain greater proportion of structural compounds (Pritchard et al. 1999). These changes in litter chemistry under eCO2 are expected to slow their decomposition. However, experimental evidence utilising agricultural plants is inconclusive with most reporting reduced decomposition and N release from residues produced under eCO2 or no effect of CO2 (Torbert et al. 2000; van Vuuren et al. 2000; Norby et al. 2001; Marhan et al. 2008; de Graaff et al. 2011). Furthermore, neither eCO2 nor N fertilisation altered the decomposition of white clover and ryegrass pasture materials (de Graaff et al. 2004). It is likely that these contrasting results in the literature are due to differences in ecosystem N status. Reductions in N concentration of crop residues could have little effect on decomposition if the C/N ratio remains relatively low (<35). Additionally, effects of C/N ratio may be negated in fertile soils if N mineralization can supply microbes with sufficient N for residue decomposition. For cropping systems dominated by cereals, residues remaining after harvest have C/N ratios (>60) much greater than used in many previous studies. The importance of changes in litter chemistry under eCO2 and subsequent impacts on C and N cycling in agricultural systems is unclear. Although slowly, higher C/N ratios of plants under eCO2 can alter that of the soil organic matter (SOM) (Yang et al. 2011). However, Carrillo et al. (2014) suggest that many incubation studies may not accurately predict eCO2 effects because of the absence of active plant roots.
Plant roots and their associated soil microbes in the rhizosphere are known to be fundamental for SOM cycling. Decomposition of SOM may be enhanced (by up to 380 %) or inhibited (by 50 %) in rhizospheres compared with non-planted soils (Cheng et al. 2014). The relative difference between species appears to be related to the volume of roots and rhizosphere (Paterson et al. 2008) and the quantity and quality of rhizodeposits (Zhu and Cheng 2012; Zhu et al. 2014). The direction and magnitude of priming effects are primarily driven by the availability of soil nutrients, especially N and P (Dijkstra et al. 2013). Positive priming effects occur via microbial mining of SOM for N when supplied with high amounts of labile C substrate (Fontaine et al. 2004; Craine et al. 2007). In contrast, negative priming effects are thought to result from increased competition for N and P in infertile soils or the preferential utilisation of labile rhizodeposits in fertile soils, which ultimately reduce the decomposition of existing SOM (Cheng 1999; Dijkstra et al. 2013).
The effects of eCO2 on rhizosphere priming has gained recent attention. Rhizosphere priming effects, which are mediated by soil microbes, are expected to be intensified under eCO2 due to greater C flow and increased competition for N between plants and microbes (Billings et al. 2010). Besides roots per se, C inputs are dominated by root exudates (Shahzad et al. 2015) and their quantity is commonly proportional to root mass (Jones et al. 2009). Trees are known to enhance root exudation under eCO2 (Phillips et al. 2011), but evidence of specific changes in exudation (per unit of root) in agricultural crop species is lacking (Billes et al. 1993; Martens et al. 2009). Greater rhizodeposits under eCO2 increase microbial biomass, predominantly bacteria, and the subsequent immobilisation of nutrients (Jin et al. 2014). Increased competition for N and changes in N availability under eCO2 are likely to alter plant C allocation, stoichiometric constraints to microbial growth and rhizosphere chemistry (Cheng et al. 2014). Furthermore, eCO2-induced changes in root growth and greater water-use efficiency from reduced evapotranspiration could alter the volume, distribution and activity of plant rhizospheres (Allard et al. 2006; Paterson et al. 2008).
Nevertheless, few studies have directly investigated eCO2-induced rhizosphere effects on residue decomposition (Paterson et al. 2008), a critical pathway for the formation of new soil C and N. Little net change in SOM content under eCO2 in agricultural cropping systems (Martens et al. 2009) and pastures (van Groenigen et al. 2003) indicates that additional C inputs, including residues, are offset by increased decomposition (Hopkins et al. 2014; van Groenigen et al. 2014). Similarly, reduced SOM content under eCO2 could be due to enhanced SOM decomposition via rhizosphere priming (Finzi et al. 2015). Greater microbial activity in wheat rhizosphere under free-air CO2 enrichment (FACE) did not enhance residue decomposition, possibly due to the high background soil N content (Lam et al. 2014). Consistent with other studies, N status of the soil-plant system appears critical.
The present study investigated the rhizosphere effect of two key agricultural crop species on the decomposition of crop residues. In particular, the study aimed to quantify the influence of three important soil N components on residue decomposition under eCO2, namely residue C/N ratio, soil N status and legume versus non-legume rhizospheres. We hypothesised that eCO2-induced rhizosphere effects would (a) enhance residue decomposition due to greater availability of labile substrate and subsequent increases in microbial activity and capacity, (b) be greater for field pea (legume) than wheat (non-legume) due to additional N deposition, and (c) be quantitatively smaller for systems with a lower N status (i.e. for residues with high C/N ratio and when fertiliser N was not applied).
Materials and methods
CO2 glasshouse facility
Two experiments were conducted in the CO2-regulated glasshouse facility at Horsham (36°43′S 142°10′E). The facility consisted of four adjoining glasshouse rooms at either ambient CO2 (aCO2, 390 ppm) or eCO2 (700 ppm) (two rooms for each CO2 level). Ambient CO2 was maintained by continual introduction of outside air via a non-recirculating air-conditioner in each room. Elevated CO2 was achieved by injecting pure CO2 into the air-conditioner airstreams from cylinders fitted with solenoid values and controlled using infra-red gas analysers (Guardian SP97301, Edinburgh Instruments).
Soil and crop residues
Surface soil (0–10 cm) of a Calcisol (WRB 2014) or Calcarosol (Isbell 1996) was collected from Warracknabeal, VIC, Australia (36°14′S 142°31′E) on 6th July 2012. The field was under an annual cropping rotation with lentil in 2010, wheat in 2011 and barley in 2012. The soil was air-dried, passed through a 4-mm sieve and thoroughly mixed. Initial physiochemical properties of the soil were: total C 18 mg g−1, total N 1.7 mg g−1, pH 6.6 (1:5 in 0.01 M CaCl2), clay 41 %.
Dual 13C/15N labelled residues of wheat (Triticum aestivum cv. Yitpi) and field pea (Pisum sativum L. cv. PBA Twilight) were generated under free-air CO2 enrichment (FACE) conditions in 2011 as outlined in Butterly et al. (2015). Briefly, wheat and field pea were grown under aCO2 (390 ppm) or eCO2 (550 ppm) with either 40 (low) or 100 (high) mg N kg−1 [Ca(15NO3)2, 20 % atom excess] and pulse-labelled with 13CO2 7 times throughout the growing season. Aboveground biomass was collected at physiological maturity, grain removed, and the remaining residues were ground (<2 mm). The initial 13C and 15N abundances of residue are presented in Table S1.
Soil column experiments
Experiment 1 (Exp 1) aimed to quantify the effects of eCO2 and soil N status on wheat rhizosphere activity and residue decomposition. PVC columns (7.5 cm ID × 20 cm long) cut lengthways and re-joined with tape and silicon sealant were filled with 600 g dry soil (Bulk density 1.0 g cm−3). The soil was supplied with basal nutrients (mg kg−1: KH2PO4, 180; K2SO4, 120; CaCl2.2H2O, 180; MgSO4.7H2O, 50; MnSO4.H2O, 6; ZnSO4.7H2O, 8; CuSO4.5H2O, 6; CoCl2.6H2O, 0.4; FeEDTA, 1.3; Na2MoO4.2H2O, 0.4) and amended with either wheat or field pea residues (0.5 % w/w, equivalent to 7.6 t ha−1 on surface area basis). The C/N ratio of the wheat and field pea were 59.7 and 20.7, respectively. Two sets of columns, one containing 25 mg N kg−1 [Ca(NO3 −)2] in the topsoil (0–7.5 cm) and one containing no added N, were included. To facilitate the construction of columns, soils were dried at 25 °C following nutrient addition, mixed with residues and assembled in an air-dried state. The top of each column was sealed with a plastic lid fitted with a central tube (3 cm ID × 6 cm long, inserted into the soil ~1 cm) to allow plants to be grown while maintaining an airtight soil headspace. Columns were wet to 80 % of field capacity (θg = 0.388 g g−1), allowed to equilibrate for 5 h, and three pre-germinated wheat seeds were sown at 1-cm depth in each on the 26th July 2012. After 1 week, plants were thinned to 2 seedlings per column. Planted columns were arranged with 4 replicates (2 in each CO2-regulated room). Overall this experiment consisted of a nested factorial design with 2 CO2 concentrations (main-plots) × 2 residues (sub-plots) × 2 N levels (sub-plots) with 4 replicates (32 columns). Non-planted controls were also included.
Experiment 2 (Exp 2) aimed to examine the relative importance of plant treatments (wheat, wheat + 25 mg N kg−1 and field pea) and residue N status (C/N ratio 19.4–115.4) on N recovery from crop residues. PVC columns were constructed as previously outlined, except that 25-cm long columns containing 790 g dry soil were used. In this experiment, four residues (0.5 % w/w, equivalent to 10 t ha−1 surface area basis) and three plant treatments were investigated. The residues were wheat previously grown under low N (C/N = 115) and high N (C/N = 31) and field pea previously grown under low N (C/N = 21) and high N (C/N = 19) (Soil and crop residues section). The three plant treatments were wheat, wheat + N and field pea. The wheat + N treatment received 25 mg N kg−1 [Ca(NO3 −)2] in the topsoil (0–10 cm). Columns were planted on the 1st August 2012 into moist (80 % field capacity) soil as previously described. Field pea was inoculated using commercial Group E peat inoculum (Rhizobium leguminosarum). Wheat and field pea were thinned to 2 plants after 7 and 10 days, respectively. For each CO2 treatment, columns were randomly arranged and rotated at least once per week between the two CO2-regulated rooms. Overall the experiment consisted of a randomised block design with 2 CO2 concentrations × 4 residues × 3 plant treatments with 3 replicates. Plant-free controls were also included.
Plants were grown under natural light conditions with glasshouse air-conditioners set to 25 °C with no diurnal change. Mean minimum and maximum temperatures over the experimental period were 19.4 and 26.2 °C, respectively. Columns were watered to 80 % field capacity. All wheat-plant treatments received a single addition of 494 μg N kg−1 week−1 in the last 3 weeks for Exp 1 (total 0.89 mg N column−1) and in the last 4 weeks for Exp 2 (total 1.56 mg N column−1) added as dilute Ca(NO3)2. solution prior to normal watering.
Total belowground CO2 efflux and residue decomposition
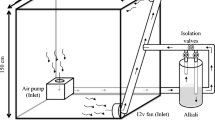
An alkali trapping approach was used to quantify total belowground CO2 efflux in all treatments in Exp 1 and the residue treatments with the most contrasting C/N (low-N-wheat and high-N-field pea) in Exp 2, plus relevant controls. Two vials containing 14 ml NaOH solution (28 ml total; 1 M for Exp 1 and 1.5 M for Exp 2) were placed within the sealed headspace of each column. Solutions were exchanged periodically via two holes in the lids that were sealed using Blu-tack®. Two vials were used to ensure sufficient surface area of the traps (24.8 % of soil surface). The remaining columns of Exp 2 without alkali traps (and lids) were covered with a 2-cm layer of white polyethylene beads to minimise evaporation.
Cumulative CO2 release (μg CO2-C g−1 soil) was estimated according to Zibilske (1994) with the following modifications. Briefly, 5 ml of each trap and 1.72 M BaCl2 (1:1) were titrated with 0.25 N HCl (Exp 1) or 0.5 N HCl (Exp 2) and phenolphthalein indicator (1 % w/v in ethanol) using a digital burette (Brand Titrette, Germany). Precipitates of each trap were formed for δ13C analysis using Isotope Ratio Mass Spectrometry (IRMS). Specifically, 2 ml of each trap was neutralised with 0.5 M HCl and combined with 2 ml 1 M SrCl2 and then dried in an oven at 60 °C for 3 days. The proportion of CO2 derived from residue (CO2 RES) was estimated using an isotopic approach according to the following equation;
where δ13C residue-amended soil and δ13C soil are the δ13C of the precipitates formed from residue- and non-amended soil columns, respectively, and δ13C residue is the δ13C value of the added residue. The amount of CO2 derived from residue was calculated by multiplying CO2 RES by the cumulative CO2 released at each sampling time.
Plant and soil sampling
Columns from Exp 1 and 2 were destructively sampled after 8 weeks (26th September) and 9 weeks (3rd October), respectively. Shoots were cut off at the soil surface, soil columns split and roots were carefully extracted. Shoot and root material was washed with reverse osmosis (RO) water and dried at 70 °C for 3 days. The soil was thoroughly mixed, stored at 5 °C overnight, and C and N in the microbial biomass and soil was determined the following day using moist soil. The remaining soil was air-dried at 25 °C for subsequent analyses. Dried plant samples were ground (<2 mm) using a centrifugal mill to reduce sample volume, and sub-samples of both ground plant material and whole soil were then finely ground using a Retsch MM400 mixer mill.
Soil and plant analyses
Soil pHCaCl2 was determined using a pH meter (Thermo Orion 720A+, Beverly, MA, USA) following extraction of 5 g air-dried soil with 0.01 M CaCl2 (1:5) by shaking end-over-end for 1 h and centrifugation at 492 g for 10 min. Soil texture was characterised by determining the particle-size distribution using a Laser Particle Size Analyser (Malvern Mastersizer 2000, Worcestershire, UK) following dispersion of soil (~10 g) with 10 ml of 0.164 M Na6P5O18 in 800 ml of RO water. Total C (TC) and N (TN) as well as the 13C (δ 13C Pee Dee Belemnite, PDB) and 15N (%15N) content of soil and plant samples was determined using IRMS (Hydra 20–22, SerCon, Crewe, UK). The proportion of N derived from residue (pNDFR) was estimated directly according to the following equation;
where %15N plant + residue is the atom% 15N of plants growing in residue-amended soil, %15N soil is the natural 15N abundance of the soil (0.368811 atom% 15N) and %15N residue is the atom% 15N value of the added residue. The amount of N derived from residue (NDFR) was calculated by multiplying pNDFR by the total N uptake.
Microbial biomass C (MBC) and N (MBN) were quantified using 24-h fumigation-extraction according to Vance et al. (1987) but with the following modifications. Soil (20 g DW) was extracted with 80 ml of 0.5 M K2SO4 by shaking end-over-end for 1 h. Extracts were passed through a Whatman #42 filter and stored at −20 °C until analysis. Organic C concentrations in fumigated and non-fumigated extracts were determined using wet-oxidation (Vance et al. 1987) as outlined in Heanes (1984). Briefly, 5 ml of extract, 5 ml of 1 N K2Cr2O7 and 10 ml of 98 % H2SO4 were mixed and heated at 130 °C for 30 min, allowed to cool, made up to 50 ml with RO water and the C concentration was determined spectrophotometrically at 600 nm. Each sample was analysed in duplicate. Sucrose solutions with known concentrations were included as standards. The C contained within digested non-fumigated samples was denoted extractable organic C (EOC). Microbial biomass C (MBC) was estimated as the difference between fumigated and non-fumigated samples using a k EC of 0.37 (Sparling and Zhu 1993; Joergensen 1996).
Total N contained within fumigated and non-fumigated extracts was determined using the wet-oxidation method of Cabrera and Beare (1993). Specifically, 2.5 ml of extract and digestion mix (50 g K2S2O8 and 30 g H3BO4 in 100 ml of 3.75 M NaOH adjusted to 1 l with H2O) (1:1) were autoclaved (121 °C, 104 kPa) for 30 min and stored at 4 °C until analysis. Solutions with known concentrations of urea were included as controls. The N (NH4 + + NO3 −) concentration of extracts was determined using a flow injection analyser (Lachat QuickChem 8500 Series II, USA). The N contained within digested non-fumigated samples was denoted extractable organic N (EON). Microbial biomass N (MBN) was estimated as the difference between fumigated and non-fumigated samples using a k EN of 0.54 (Brookes et al. 1985).
Statistical analyses
For Exp 1, a three-way analysis of variance (ANOVA) was used to test the effects of CO2, residue and N level on soil and plant properties of planted columns. A one-way residual maximum likelihood (REML) analysis was used to test the effects of CO2, residue and N level on soil chemical properties between planted and non-planted columns. For Exp 2, a three-way ANOVA was used to test the effects of CO2, plant treatment and residue on soil and plant properties of planted columns. A one-way REML analysis was used to test the effects of CO2, plant treatment and residue on soil properties between planted and non-planted columns. Differences between means were tested using least significance difference (LSD) test at P = 0.05.
Results
Plant biomass, N content and 15N abundance
In Exp 1, the biomass of wheat plants was significantly affected by residue type and N level but not CO2 concentration (Table 1). Specifically, wheat biomass was ~3 times greater when grown in soil containing field pea than wheat residue. Wheat shoot and root biomass were 78 and 73 % greater at the higher N level, respectively. N level significantly affected the N concentration within wheat shoot (P = 0.012) and root (P = 0.045). However, added N reduced the N concentration (by 17 %) of wheat grown under aCO2 in soil containing wheat residue (Table 1).
In Exp 2, plant shoot biomass increased in the order of wheat, wheat + N then field pea (Table 2). Furthermore, shoot biomass greatly differed between low-N-wheat and high-N-wheat residues (56 times) and less between high-N-wheat and both low-N-field pea and high-N-field pea residues (14 times) (Table 2). Elevated CO2 altered the N concentration of shoots (P < 0.001) but not roots (P = 0.107). For shoots, N concentration was 5.1–18.5 % lower under eCO2, except for field pea with high-N-wheat residue, where N concentration increased (by 5.2 %). However, the relative magnitude of the CO2 treatment effect on N concentration was much lower than that of residue and plant treatments. The effects of plant and residue treatments on N concentrations in roots were the same as shoots, but the N concentration was lower in roots than shoots for the wheat and wheat + N treatments.
Total N uptake, N derived from residue and % residue N recovered
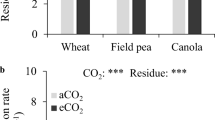
In Exp 1, eCO2 tended to increase total N uptake from field pea residue (P = 0.062) (Fig. 1a). Total N uptake increased with residue N concentration (field pea > wheat) and with N addition. Notably, total N uptake increased by 6 mg N column−1 when N was added with wheat residue, and by 13.5 mg N column−1 when N was added with field pea residue. The addition of N fertiliser decreased the amount of N derived from wheat residue for the aCO2 treatment but not the eCO2 treatment (Fig. 1b). In contrast, adding fertiliser N reduced the amount of N derived from field pea residue by ~6 % for both CO2 treatments. The %N recovered in the plant from residues, which accounts for the differences in N content between residues and the control, was significantly (P = 0.028) greater under eCO2 (Fig. 1c). The recovery of N from field pea residue was greater than from wheat residue, and increased further when fertiliser N was added, although only main effects of residue (P < 0.001) and N level (P < 0.001) were significant.
Total N uptake (a), % N derived from residue (b) and % residue N recovered (c) by wheat grown under ambient CO2 (aCO2, 390 ppm) and elevated CO2 (eCO2, 700 ppm) in soil containing wheat or field pea residues and either no added N (No N) or 25 mg N kg−1 soil (+N). Standard errors of the mean of 4 replicates. Bars indicate least significant difference (LSD) (P = 0.05) (Exp 1)
In Exp 2, eCO2 increased total N uptake (P = 0.002) with the magnitude of the increase being greatest for the field pea, and least for the wheat plant treatment (Fig. 2a). A reduction in total N uptake by wheat was observed for the high-N-field pea residue treatment. Adding fertiliser N (9.9 mg N column−1) increased total N uptake by 5.9–15.7 mg N column−1 under eCO2, and by 4.8–8.3 mg N column−1 under aCO2. For field pea, %NDFR decreased when fertiliser N was added and also with increased N content in the residues (Fig. 2b). Interestingly, eCO2 had a positive effect on %NDFR in the wheat and wheat + N plant treatments but a negative effect in the field pea plant treatment. Also, %NDFR was greater for the high-N than low-N field pea residue, despite these having similar C/N ratios. Adding fertiliser N increased the amount of N recovered from residues by wheat and facilitated the positive effect of CO2 concentration (Fig. 2c). Without fertiliser N, there was generally no eCO2 response, except that eCO2 decreased N recovery from high-N-field pea residue was reduced under eCO2. For field pea, eCO2 reduced the N recovered from high-N-wheat and high-N-field pea residues.
Total N uptake (a), % N derived from residue (b) and % residue N recovered (c) by three plant treatments (wheat, left; wheat + 25 mg N kg−1, middle and field pea, right) under ambient CO2 (aCO2, 390 ppm) and elevated CO2 (eCO2, 700 ppm) in soil containing residues of wheat (WT) or field pea (FP) previously grown with low N (HN) or high N (HN). Standard errors of the mean of 3 replicates. Bars indicate least significant difference (LSD) (P = 0.05) (Exp 2)
Total belowground CO2 efflux and CO2-C partitioning
In Exp 1, total belowground CO2 efflux did not differ between non-planted columns, except in the wheat + N treatment where total CO2 efflux was greater than the other treatments (Fig. 3a). Furthermore, total CO2 efflux was greater for field pea than wheat residue, and N fertiliser increased total CO2 compared to when wheat residue only was applied. For columns planted with wheat, total CO2 efflux was 22 and 52 % greater than non-planted columns for soils containing wheat and field pea residues, respectively. Importantly, eCO2 significantly (P = 0.004) increased total CO2 efflux by 8.6 % for wheat grown in soil containing field pea residue. CO2 derived from wheat residue was greater under eCO2 when no fertiliser N was added but lower than aCO2 and plant-free columns in the + N treatment (Fig. 3b). Between 31 and 35 % of wheat residue C and 31 % of the field pea residue C was recovered in the alkali traps over the 8-week experiment.
Total belowground CO2-C (a), CO2-C derived from residue (b) and % residue C recovered (c) in alkali traps of soil columns planted with wheat grown under ambient CO2 (aCO2, 390 ppm) and elevated CO2 (eCO2, 700 ppm) in soil containing wheat and field pea residues and either no added N (No N) or 25 mg N kg−1 soil (+N) and non-planted (NP) controls. Standard errors of the mean of 4 replicates. Bars indicate least significant difference (LSD) (P = 0.05) (Exp 1)
In Exp 2, there was a trend for total belowground CO2 efflux from non-planted columns to be greater for low-N-wheat than high-N-field pea residues (P = 0.066) (Fig. 4a). Elevated CO2 increased (P = 0.001) total CO2 efflux by up to 12 %, with the largest effects for wheat growing in soil containing high-N-field pea residue and for field pea growing with low-N-wheat residue. There was no effect of plant treatment on total CO2 produced (P > 0.05). The amount of CO2 derived from low-N-wheat residue was the same for wheat and wheat + N plant treatments, but this was lower than the non-planted control for the field pea plant treatment (Fig. 4b). Similarly, all plant treatments reduced the CO2 derived from high-N-field pea residue compared with the non-planted controls. Over the 9-week experiment, approximately 42 % of the residue-C were recovered in the alkali traps of non-planted controls and the recovery of residues was lower with higher residue and plant N status (Fig. 4c).
Total belowground CO2-C (a), CO2-C derived from residue (b) and % residue C recovered (c) in alkali traps of three plant treatments (wheat, left; wheat + 25 mg N kg−1, middle and field pea, right) under ambient CO2 (aCO2, 390 ppm) and elevated CO2 (eCO2, 700 ppm) in soil containing wheat (WT) or field pea (FP) residues grown with low N (LN) or high N (HN) and non-planted (NP) controls. Standard errors of the mean of 3 replicates. Bars indicate least significant difference (LSD) (P = 0.05) (Exp 2)
Soil C and N pools
For Exp 1, total C was reduced compared with the non-amended soil (18 g C kg−1) with the greatest reductions occurring for field pea residue with no N (−27 %) and wheat residue with + N (−20 %) (Table 3). Total N was also reduced by up to 33 % compared with the original soil. The abundance of 13C in soil (δ 13C PDB) was significantly reduced under eCO2 representing loss of 13C from residues. However, the 13C abundance of the non-planted controls was the same or lower than under eCO2 treatments, indicating similar loss of residue 13C without plants compared to the aCO2 treatment. MBC was lower under eCO2 and this reduction was greater for the + N treatment. MBN was reduced under aCO2 for the field pea + N treatment, increasing the MBC-to-MBN ratio. The MBC-to-MBN ratio was also lower under eCO2 for wheat + N treatment. Overall, EOC was lower for wheat than field pea amended treatments. EON was reduced compared with non-planted controls but was not affected by the treatments.
In Exp 2, no effect of CO2 on total C, total N or C/N ratio was observed. In contrast to Exp 1, total C and total N did not differ from the non-amended soil, except that total C was 34 % higher for the wheat + N plants with the low-N-wheat residues (Table 4). Total N ranged from 17.7 to 22.9 g N kg−1 soil and C/N ratio from 10.5 to 12 (data not shown). The abundance of 13C in soil was lower under eCO2 for wheat plants with low-N-wheat residues and lower under aCO2 for the field pea plants with low-N-wheat residues. Elevated CO2 greatly decreased MBC (up to 178 mg kg−1 soil) for low-N-field pea and high-N-wheat residues; however CO2 had a much smaller effect for high-N-field pea and low-N-wheat residues. In contrast, MBN was lower in all planted treatments compared with non-planted controls and consequently MBC-to-MBN ratio was increased. The field pea plant treatment and N rich residues (high-N-field pea, low-N-field pea and high-N-wheat) had much greater reductions in MBN than the low-N-wheat residue treatment. Furthermore, EON was reduced compared with non-planted controls, with the magnitude of the reduction in the order of low-N-wheat, high-N-wheat, low-N-field pea and high-N-field pea residue. For the field pea plant treatment, EON was ~30 % greater with low-N-field pea and ~200 % for high-N-field pea residues but did not differ between other treatments. Generally, EOC was similar across all treatments including non-planted controls, except for high-N-field pea residue where aCO2 reduced EOC in the wheat-planted treatment and eCO2 increased EOC in the field pea-planted treatment. Wheat plants with low-N-wheat residues had lower EOC than the non-planted controls for both CO2 levels.
Discussion
Residue decomposition
Enhanced residue decomposition in the presence of plants (a positive rhizosphere effect) was anticipated. In particular, higher amounts of labile C substrates in the form of rhizodeposits were expected to stimulate microbial activity and induce greater mineralisation of residues for N, consistent with theories of rhizosphere priming effects on soil organic matter decomposition (Fontaine et al. 2004; Craine et al. 2007). However, we observed little or no change in residue decomposition in the presence of plant compared with residues alone. Therefore, the 16–52 % increase in CO2 efflux between planted and non-planted treatments likely occurred via plant-derived substrate, although distinction between plant and soil C sources cannot be made in this study. Around 32–34 % and 42 % of residues were decomposed in Exp 1 and 2, respectively. The greater decomposition in the later experiment probably reflected the larger column size and longer duration of the study. Reduced residue decomposition that was observed between planted and non-planted treatments could have been due to a negative rhizosphere effect via increased competition for N and P (Cheng 1999; Dijkstra et al. 2013). However, since a negative rhizosphere effect also occurred under field pea, preferential mineralization of labile rhizodeposits by microbes could have reduced the decomposition of other more recalcitrant sources such as crop residues (Cheng 1999; Dijkstra et al. 2013).
Elevated CO2 had both positive and negative effects on residue decomposition, compared with aCO2. However, changes in residue decomposition under eCO2 were not proportional to total belowground CO2 efflux. Enhanced turnover of rhizodeposits under eCO2 may not increase rhizosphere effects since these labile compounds are primarily degraded by intracellular enzymes and more recalcitrant components like crop residues require an array of extracellular enzymes (Kuzyakov 2010). Instead, the three main observations appeared to be related with N availability. Firstly, eCO2 decreased wheat decomposition under wheat with added N in Exp 1 but not Exp 2. In this case, microbial competition for N with plants under eCO2 most likely occurred, which was overcome in Exp 2 by additional N from both N fertiliser and high-N-field pea residues. This competition for N under eCO2 can also have a negative impact on wheat growth (Lam et al. 2013b) and hence rhizodeposition. Secondly, the reduction in field pea decomposition under wheat was less under eCO2 than aCO2 and adding additional N had no effect (Exp 2). In this case, the N-mining hypothesis could explain enhanced decomposition under aCO2 triggered by low N availability. Elevated CO2 enables microbes to access more recalcitrant SOM pools, including residues (Carney et al. 2007; de Graaff et al. 2009). Although the high-N-field pea residue had a low C/N ratio, residue derived N was greater under eCO2 and was reduced by fertiliser N (discussed later). Thirdly, decomposition of the high-N-field pea residue was significantly reduced by eCO2 under field pea. In this case, preferential mineralization of labile substrates was likely to have occurred given the high N content of the residue and the likely N deposition by the legume. The current study showed a much greater influence of residue N content and plant species than fertiliser N on enhancing residue decomposition. However, the effect of eCO2 on field pea and wheat decomposition was minimal in a cropping soil with high soil N content (Lam et al. 2014). Importantly, the relative importance of different mechanisms on residue decomposition was supported by differences in the N balance within each plant-soil system. Future studies should examine the links between eCO2, the quantity and quality of root exudates and changes in rhizosphere microbial community composition and C and N functional capacity.
Total belowground CO2 efflux
The relative differences in CO2 efflux between planted and non-planted soils amended with residues (10–48 %) were smaller than expected. High amounts of CO2 were released by microbes in the non-planted treatments. The additional CO2 released in planted columns was mainly from root-derived CO2 such as root exudates and root respiration, as residue-derived CO2 efflux was reduced or not affected by the presence of plants. However, the design of this study cannot discriminate between microbial decomposition of soil organic matter and root exudates and root respiration. Total belowground CO2 efflux was greatest for wheat grown with field pea residue. However, considering wheat plants had 3–4 times more root mass than field pea (Exp 2), total belowground CO2 efflux per unit root length was proportionally smaller under wheat than field pea, consistent with other studies (Wang et al. 2016). de Graaff et al. (2006) also observed that microbial biomass was a poor indicator of total belowground CO2 efflux. Since only a small component of the microbial biomass is active (<2 %) (Blagodatskaya and Kuzyakov 2013), increases in the proportion of active organisms and the rate of microbial turnover could have been more important than overall biomass (Blagodatskaya et al. 2010). However, we expected that total CO2 efflux would be proportional to root and/or shoot biomass, which mediate photosynthetic capacity and rhizosphere volume (van Veen et al. 1991; Rogers et al. 1994). This was not the case in this study.
Total belowground CO2 efflux under eCO2 was not related to root mass. However, eCO2 did not increase plant biomass as expected (Ainsworth and Long 2005; de Graaff et al. 2006; Madhu and Hatfield 2013) even for the same species and soil types (Jin et al. 2012; Lam et al. 2012a; Butterly et al. 2015). Despite the lack of change in root biomass, root respiration was generally less affected or constrained by eCO2 (Kou et al. 2007). Therefore, greater C flow from root exudation and rhizodeposition under eCO2 due to enhanced photosynthetic activity was the likely source of additional CO2-C in our study, although microbial decomposition of root exudates and root respiration could not be separated. Higher rhizodeposition of wheat under FACE (Martens et al. 2009) and greater efflux of labile C substrate from the plant and subsequent mineralisation by soil microbes (Reinsch et al. 2013) support this theory. However, direct evidence of higher specific exudation (per unit of root) for crop species under eCO2 is limited (Cheng et al. 1993). Enhanced C release of wheat under eCO2 has been associated with both greater root biomass (Billes et al. 1993) and increases in specific root activity (Cheng and Johnson 1998). Furthermore, 13CO2-pulse-labelling of wheat and field pea and greater belowground 13C abundance were associated with increased root biomass (Jin et al. 2014; Butterly et al. 2015).
Increased competition for N between microbes and plant roots can be an important bottleneck which limits rhizodeposit mineralisation under eCO2 (Paterson et al. 1996). Increased total belowground CO2 efflux under eCO2 occurred for wheat in field pea-amended soil but there was no effect of N fertiliser (Exp 1 and 2). Hence, greater N status of the residues enhanced the CO2 effect for wheat (non-legume). In contrast, Martin-Olmedo et al. (2002) showed greater difference in CO2 efflux under barley between aCO2 and eCO2 at low N than high N supply via stimulation of root biomass. It is likely that wheat plants were more competitive for fertiliser N in our study and rhizosphere microbes were only stimulated in the presence of N-rich residues. For field pea, eCO2 increased total belowground CO2 efflux only in soil amended with low-N-wheat residue (Exp 2), and the relative effect of eCO2 on total belowground CO2 efflux decreased with increasing C/N ratio. Rhizosphere effects are known to depend on soil nutrient status, particularly N and P (Dijkstra et al. 2013). Cheng and Johnson (1998) showed that rhizosphere effects were positive with added N but negative without fertiliser N, highlighting that non-legumes require N to be above a critical level in order to have a functioning rhizosphere. Our study highlights that the C/N ratio of residues has opposite effects on total belowground CO2 efflux under cereals and legumes.
N uptake and recovery from residues
Greater N uptake of wheat (de Graaff et al. 2009; Lam et al. 2012b; Butterly et al. 2015) and field pea (Jin et al. 2012; Lam et al. 2013b; Butterly et al. 2016a) under eCO2 is commonly observed, primarily via increased plant biomass. In the current study, eCO2 only increased total N uptake for field pea (Exp 2). For legumes, N2 fixation provides an important source of additional N under eCO2 (Butterly et al. 2016a). Greater total N uptake under eCO2 primarily occurs via enhanced productivity, despite small decreases in N concentration of cereals (Jensen and Christensen 2004; Madhu and Hatfield 2013). However, reduced N concentration under eCO2 with no change in biomass can reduce total N uptake such as that for wheat growing in high-N-field pea amended soil. CO2 concentration had a comparatively smaller effect on total N uptake than C/N ratio and soil N status, consistent with previous reports (Martin-Olmedo et al. 2002; Lam et al. 2013b; Butterly et al. 2015).
Overall, plants obtained a small component of their N derived from residues. As expected the greatest levels of NDFR occurred under wheat (max 39 %), were lower when fertiliser N was added (max 33 %) and were the least under field pea (max 15 %). Although temporal changes in N availability were not quantified, plants are expected to preferentially utilise other N sources before residue N. Microbial mineralisation of residues and the availability of residue N is likely to occur only once other N sources were exhausted. Interestingly, the N content of field pea residues was a poor indicator of their contribution to plant N nutrition. Specifically, the high-N-field pea residue contributed a significantly greater amount of N to plants than low-N-field pea for all plant treatments, despite similar N concentrations (C/N of 20.5 for low-N-field pea and 19.4 for high-N-field pea). Furthermore, N concentrations in plant tissues were greater for soils amended with the residue of the same plant species. The differences in decomposition and N release from field pea residues could be due to the types of N present within the residues or differences in the decomposability (i.e. structural C, protein content, soluble N concentration) (Pritchard et al. 1999).
The effect of eCO2 on NDFR and the residue N recovery depended on the plant type (cereal v legume). Consistent with overall residue decomposition, eCO2 enhanced NDFR and residue N recovery for wheat but decreased these parameters for field-pea-planted treatments. Generally, the effects of eCO2 on the NDFR were not significant given their small contribution to overall N fertility. However, the 15N approach revealed clear effects of CO2 concentration on residue N recovery. Residue recovered in wheat plant were greater at eCO2 than aCO2 when fertiliser N was added, highlighting that residue N alone was insufficient to promote a positive rhizosphere effect under eCO2. The negative effect of eCO2 on N recovery from residues in the field-pea-planted treatment was likely preferential utilisation of other N sources, particularly N2 fixation, as discussed previously. Nevertheless, enhanced decomposition of SOM (priming) can be an important mechanism for increased N supply to wheat under eCO2 (de Graaff et al. 2009) and the results presented here indicate greater N-use efficiency of wheat under eCO2. This is consistent with a 21 % increase in N recovery by wheat from barley residues under eCO2 (Lam et al. 2013a). For field pea, reduced decomposition and utilisation of N from residues during a legume crop could mean a greater carryover of N to subsequent cropping phases. However, Lam et al. (2013a) showed that the contribution of field pea residue to the proceeding wheat crop could be significantly reduced (~8.6 %) under eCO2 if the C/N ratio of legume residue was increased (C/N from 44 to 52). Therefore, rhizosphere effects on residue decomposition and replenishment of soil N and C pools under eCO2 are likely to alter the C and N balance in cropping systems (Butterly et al. 2016b).
Conclusion
Understanding eCO2-induced rhizosphere effects on residue decomposition is critical for predicting changes in soil fertility of future agricultural productions systems. This study showed that residue decomposition was generally reduced in the presence of plant roots, due to enhanced competition for N between plants and microbes. Elevated CO2 both increased and decreased this negative rhizosphere effect. These changes were largely controlled by plant treatment, residue C/N ratio, less so by fertiliser N, and were not related to root mass nor microbial biomass. Importantly, eCO2 lessened the negative rhizosphere effect of aCO2-grown wheat but exacerbated that of field pea. However, temporal changes in the contribution of rhizosphere effects are likely to occur within and between growing seasons. Although residues only contributed a small component of overall plant N uptake, wheat utilised a greater amount of N from residues under eCO2, and this stimulation of rhizosphere N-recovery only occurred when N fertiliser was added. Hence, residues with high N (low C/N ratio) alone did not induce a positive rhizosphere effect. Our results indicate that reduced decomposition of residues under eCO2-grown field pea could potentially increase the N available to subsequent crops. Consistent with our previous study, the C content in the rhizosphere soil of wheat appeared to decrease under eCO2. A reduction in soil C could indicate that reduced residue decomposition in the presence of plants and the subsequent replenishment of soil C are interrupted under eCO2. These mechanisms need to be investigated over an extended period of growth as rhizosphere effects are likely to amplify during later stages of growth.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–371
Allard V, Robin C, Newton PCD, Lieffering M, Soussana JF (2006) Short and long-term effects of elevated CO2 on Lolium perenne rhizodeposition and its consequences on soil organic matter turnover and plant N yield. Soil Biol Biochem 38:1178–1187
Billes G, Rouhier H, Bottner P (1993) Modifications of the carbon and nitrogen allocations in the plant (Triticum aestivum L.) soil system in response to increased atmospheric CO2 concentration. Plant Soil 157:215–225
Billings SA, Lichter J, Ziegler SE, Hungate BA, Richter DD (2010) A call to investigate drivers of soil organic matter retention vs. mineralization in a high CO2 world. Soil Biol Biochem 42:665–668
Blagodatskaya E, Kuzyakov Y (2013) Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67:192–211
Blagodatskaya E, Blagodatsky S, Dorodnikov M, Kuzyakov Y (2010) Elevated atmospheric CO2 increases microbial growth rates in soil: results of three CO2 enrichment experiments. Glob Chang Biol 16:836–848
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Butterly CR, Armstrong R, Chen D, Tang C (2015) Carbon and nitrogen partitioning of wheat and field pea grown with two nitrogen levels under elevated CO2. Plant Soil 391:367–382
Butterly CR, Armstrong RD, Chen D, Tang C (2016a) Free-air CO2 enrichment (FACE) reduces the inhibitory effect of soil nitrate on N2 fixation of Pisum sativum L. Ann Bot 117:177–185
Butterly CR, Phillips LA, Wiltshire JL, Franks AE, Armstrong RD, Chen D, Mele PM, Tang C (2016b) Long-term effects of elevated CO2 on carbon and nitrogen functional capacity of microbial communities in three contrasting soils. Soil Biol Biochem 97:157–167
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc Natl Acad Sci 104:4990–4995
Carrillo Y, Pendall E, Dijkstra FA, Morgan JA, Newcomb JM (2011) Response of soil organic matter pools to elevated CO2 and warming in a semi-arid grassland. Plant Soil 347:339–350
Carrillo Y, Dijkstra F, Pendall E, LeCain D, Tucker C (2014) Plant rhizosphere influence on microbial C metabolism: the role of elevated CO2, N availability and root stoichiometry. Biogeochemistry 117:229–240
Cheng WX (1999) Rhizosphere feedbacks in elevated CO2. Tree Physiol 19:313–320
Cheng WX, Johnson DW (1998) Elevated CO2, rhizosphere processes, and soil organic matter decomposition. Plant Soil 202:167–174
Cheng WX, Coleman DC, Carroll CR, Hoffman CA (1993) In-situ measurement of root respiration and soluble C concentrations in the rhizosphere. Soil Biol Biochem 25:1189–1196
Cheng WX, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44
Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob Chang Biol 4:43–54
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
de Graaff MA, Six J, Harris D, Blum H, van Kessel C (2004) Decomposition of soil and plant carbon from pasture systems after 9 years of exposure to elevated CO2: impact on C cycling and modeling. Glob Chang Biol 10:1922–1935
de Graaff M-A, van Groenigen K-J, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077–2091
de Graaff MA, Van Kessel C, Six J (2009) Rhizodeposition-induced decomposition increases N availability to wild and cultivated wheat genotypes under elevated CO2. Soil Biol Biochem 41:1094–1103
de Graaff M-A, Schadt CW, Rula K, Six J, Schweitzer JA, Classen AT (2011) Elevated CO2 and plant species diversity interact to slow root decomposition. Soil Biol Biochem 43:2347–2354
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21:2082–2094
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Heanes DL (1984) Determination of total organic‐C in soils by an improved chromic acid digestion and spectrophotometric procedure. Commun Soil Sci Plant Anal 15:1191–1213
Hopkins FM, Filley TR, Gleixner G, Lange M, Top SM, Trumbore SE (2014) Increased belowground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biol Biochem 76:57–69
IPCC (2001) Climate change 2001: impacts, adaptation and vulnerability. Cambridge University Press, New York
Isbell RF (1996) The Australian soil classification. CSIRO Publishing, Melbourne
Jensen B, Christensen BT (2004) Interactions between elevated CO2 and added N: effects on water use, biomass, and soil 15N uptake in wheat. Acta Agric Scand Sect B Soil Plant Sci 54:175–184
Jin J, Tang CX, Armstrong R, Sale P (2012) Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 358:86–99
Jin J, Tang C, Robertson A, Franks AE, Armstrong R, Sale P (2014) Increased microbial activity contributes to phosphorus immobilization in the rhizosphere of wheat under elevated CO2. Soil Biol Biochem 75:292–299
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Kou T, Zhu J, Xie Z, Hasegawa T, Heiduk K (2007) Effect of elevated atmospheric CO2 concentration on soil and root respiration in winter wheat by using a respiration partitioning chamber. Plant Soil 299:237–249
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Lam SK, Chen D, Norton R, Armstrong R (2012a) Does phosphorus stimulate the effect of elevated [CO2] on growth and symbiotic nitrogen fixation of grain and pasture legumes? Crop Pasture Sci 63:53–62
Lam SK, Chen DL, Norton R, Armstrong R (2012b) Nitrogen demand and the recovery of 15N-labelled fertilizer in wheat grown under elevated carbon dioxide in southern Australia. Nutr Cycl Agroecosyst 92:133–144
Lam SK, Chen D, Norton R, Armstrong R (2013a) The effect of elevated atmospheric carbon dioxide concentration on the contribution of residual legume and fertilizer nitrogen to a subsequent wheat crop. Plant Soil 364:81–91
Lam SK, Chen DL, Norton R, Armstrong R (2013b) Crop residue incorporation negates the positive effect of elevated atmospheric carbon dioxide concentration on wheat productivity and fertilizer nitrogen recovery. Plant Soil 366:551–561
Lam SK, Norton R, Armstrong R, Chen D (2014) Increased microbial activity under elevated [CO2] does not enhance residue decomposition in a semi-arid cropping system in Australia. Soil Biol Biochem 72:97–99
Madhu M, Hatfield JL (2013) Dynamics of plant root growth under increased atmospheric carbon dioxide. Agron J 105:657–669
Marhan S, Derain D, Erbs M, Kuzyakov Y, Fangmeier A, Kandeler E (2008) Soil organic matter mineralization and residue decomposition of spring wheat grown under elevated CO2 atmosphere. Agric Ecosyst Environ 123:63–68
Martens R, Heiduk K, Pacholski A, Weigel HJ (2009) Repeated 14CO2 pulse-labelling reveals an additional net gain of soil carbon during growth of spring wheat under free air carbon dioxide enrichment (FACE). Soil Biol Biochem 41:2422–2429
Martin-Olmedo P, Rees RM, Grace J (2002) The influence of plants grown under elevated CO2 and N fertilization on soil nitrogen dynamics. Glob Chang Biol 8:643–657
Norby RJ, Cotrufo MF, Ineson P, O’Neill EG, Canadell JG (2001) Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127:153–165
Paterson E, Rattray EAS, Killham K (1996) Effect of elevated atmospheric CO2 concentration on C-partitioning and rhizosphere C-flow for three plant species. Soil Biol Biochem 28:195–201
Paterson E, Thornton B, Midwood AJ, Osborne SM, Sim A, Millard P (2008) Atmospheric CO2 enrichment and nutrient additions to planted soil increase mineralisation of soil organic matter, but do not alter microbial utilisation of plant- and soil C-sources. Soil Biol Biochem 40:2434–2440
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Glob Chang Biol 5:807–837
Reinsch S, Ambus P, Thornton B, Paterson E (2013) Impact of future climatic conditions on the potential for soil organic matter priming. Soil Biol Biochem 65:133–140
Rogers HH, Runion GB, Krupa SV (1994) Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ Pollut 83:155–189
Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S (2015) Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol Biochem 80:146–155
Sparling G, Zhu C (1993) Evaluation and calibration of biochemical methods to measure microbial biomass C and N in soils from Western Australia. Soil Biol Biochem 25:1793–1801
Torbert HA, Prior SA, Rogers HH, Wood CW (2000) Review of elevated atmospheric CO2 effects on agro-ecosystems: residue decomposition processes and soil C storage. Plant Soil 224:59–73
van Groenigen K-J, Six J, Harris D, Blum H, van Kessel C (2003) Soil 13C–15N dynamics in an N2-fixing clover system under long-term exposure to elevated atmospheric CO2. Glob Chang Biol 9:1751–1762
van Groenigen KJ, Qi X, Osenberg CW, Luo YQ, Hungate BA (2014) Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 344:508–509
van Veen JA, Liljeroth E, Lekkerkerk LJA, Vandegeijn SC (1991) Carbon fluxes in plant-soil systems at elevated atmospheric CO2 levels. Ecol Appl 1:175–181
van Vuuren MMI, Robinson D, Scrimgeour CM, Raven JA, Fitter AH (2000) Decomposition of 13C-labelled wheat root systems following growth at different CO2 concentrations. Soil Biol Biochem 32:403–413
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang X, Tang C, Severi J, Butterly CR, Baldock JA (2016) Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol. doi:10.1111/nph.13966
WRB IWG (2014) World reference base for soil resources. International soil classification system for naming soils and creating legends for soil maps. FAO, Rome
Yang Y, Luo Y, Lu M, Schaedel C, Han W (2011) Terrestrial C:N stoichiometry in response to elevated CO2 and N addition: a synthesis of two meta-analyses. Plant Soil 343:393–400
Zhu B, Cheng W (2012) Nodulated soybean enhances rhizosphere priming effects on soil organic matter decomposition more than non-nodulated soybean. Soil Biol Biochem 51:56–65
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng W (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192
Zibilske LM (1994) Carbon mineralization. In: Methods of soil analysis, Part 2. Microbial and biochemical properties. Soil Science Society of America, Madison, pp 835–863
Acknowledgments
This research was supported by an Australian Research Council Linkage Project (LP100200757), and was conducted at the CO2 glasshouse facility of the Department of Economic Development, Jobs, Transport and Resources (DEDJTR), Victoria at Horsham. We thank Dr Shu Kee Lam for his useful comments on the manuscript, Kaien Ra for her excellent technical support, Leanne Lisle for performing the IRMS analyses and Nicholas Sitlington Hansen who worked on this project as part of his Honours project.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Zucong Cai.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Butterly, C.R., Wang, X., Armstrong, R.D. et al. Elevated CO2 induced rhizosphere effects on the decomposition and N recovery from crop residues. Plant Soil 408, 55–71 (2016). https://doi.org/10.1007/s11104-016-2911-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2911-4