Abstract
Background and aims
Characterising the spatial distribution of tree fine roots (diameter ≤ 2 mm) is fundamental for a better understanding of belowground functioning when tree are grown with associated crops in agroforestry systems. Our aim was to compare fine root distributions and orientations in trees grown in an alley cropping agroforestry stand with those in a tree monoculture.
Methods
Fieldwork was conducted in two adjacent 17 year old hybrid walnut (Juglans regia × nigra L.) stands in southern France: the agroforestry stand was intercropped with durum wheat (Triticum turgidum L. subsp. durum) whereas the tree monoculture had a natural understorey. Root intercepts were mapped to a depth of 150 cm on trench walls in both stands, and to a depth of 400 cm in the agroforestry stand in order to characterise tree root distribution below the crop’s maximum rooting depth. Soil cubes were then extracted to assess three dimensional root orientation and to establish a predictive model of root length densities (RLD) derived from root intersection densities (RID).
Results
In the tree monoculture, root mapping demonstrated a very high tree RID in the top 50 cm and a slight decrease in RID with increasing soil depth. However, in the agroforestry stand, RID was significantly lower at 50 cm, tree roots colonized deeper soil layers and were more vertically oriented. In the agroforestry stand, RID and RLD were greater within the tree row than in the inter-row.
Conclusions
Fine roots of intercropped walnut trees grew significantly deeper, indicating a strong plasticity in root distribution. This plasticity reduced direct root competition from the crop, enabling trees to access deeper water tables not available to crop roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fine roots of trees, usually defined as those with a diameter ≤ 2 mm (Trumbore and Gaudinski 2003), play a fundamental role in the provision of multiple services in tree-based agroecosystems. The absorptive function of fine roots for water and nutrients (Hinsinger 2001; Newman and Hart 2006) is closely associated with aboveground tree performance, and is thus essential for wood and fruit production in mixed intercropping systems (i.e. trees grown in association with an annual crop). Fine roots are also the most active part of tree root systems with regard to carbon dynamics, mainly through production, respiration, exudation and decomposition (Norby et al. 1987; Desrochers et al. 2002; Marsden et al. 2008; Hobbie et al. 2010), and thus can play a major role in carbon sequestration (Kuzyakov and Domanski 2000; Rasse et al. 2005), especially in agroforestry systems (Nair et al. 2010; Haile et al. 2010).
To better evaluate the services of tree-based agroecosystems, characterising the spatial distribution of fine roots is vital (Moreno et al. 2005; Mulia and Dupraz 2006; Upson and Burgess 2013). In mixed trees and crop systems, the aboveground performance of trees is linked to the amount of competition experienced, especially with regard to root systems. This competition depends largely on the spatial distribution of roots which is modified if competition is high (Mulia and Dupraz 2006). Spatial root distribution and density have been studied considerably in monocultural tree stands using both observational and modelling approaches (Hoffmann and Usoltsev 2001; Zianis et al. 2005). By using simple statistical tools and establishing allometric equations, several studies found that root density increases significantly with greater tree size and decreases with distance to tree stem and increasing soil depth (see Hoffmann and Usoltsev 2001; Zianis et al. 2005). We hypothesize that root competition between trees and crops in mixed systems will lead to differences in horizontal and vertical root distributions.
In temperate agroforestry systems, crop species are usually cultivated between parallel tree rows in strips (Torquebiau 2000). This sort of system is described as an alley cropping agroforestry system, and has become increasingly popular in Europe as it has the capacity to optimize nutrient and water cycles and provide multiple ecosystem services (Quinkenstein et al. 2009). However, in alley cropping systems, tree root distributions may be constrained both vertically and horizontally, due to competition with crop roots (Casper and Jackson 1997; Fernández et al. 2008), that could reduce the availability of water and nutrients in the soil (Schroth 1995; van Noordwijk et al. 1996). Belowground competition of roots from different species have been described in intercropped agricultural fields with two or more herbaceous species (Ozier-Lafontaine et al. 1998; Li et al. 2001; Li et al. 2006; Gao et al. 2010 and Neykova et al. 2011), but has been seldom examined between trees and crops (but see Mulia and Dupraz 2006; Wang et al. 2014). This knowledge gap may hinder our understanding of ecological interactions between species and their consequences for providing ecosystem services, as well as developing sustainable management strategies in the context of climate change. By considering the fine root distribution of trees as a proxy of root competition, existing studies on alley cropping agroforestry systems have found the root interaction between trees and annual crops to be very complex both in time and space (Mulia and Dupraz 2006; Wang et al. 2014). In particular, trees intercropped with annual crops tend to have deeper root systems and greater root length densities (RLD) beneath the root systems of the neighbouring crop (Mulia and Dupraz 2006). We hypothesize that deeper roots will permit trees to obtain nutrients and water not available to crops. However, a better quantification of tree root distribution is needed to understand the complex interactions between trees and crops.
Traditionally, studies on tree root distribution related to crops are usually based on data obtained from soil coring. Considered as the most routine approach to detect root spatial distribution (van Noordwijk et al. 2000), root coring is not very laborious and can attain profound soil depths (van Noordwijk et al. 2000; Saint-André et al. 2005; Christina et al. 2011). However, coring is difficult to carry out when soils are extremely dry and stony such as is usually the case in Mediterranean climates. Another negative aspect of root coring is that it cannot be used determine the spatial variability of root patches in soil, since this method is discontinuous in the horizontal space (several cores and extrapolation techniques would be needed to do this). Root-profiling methods have therefore been developed to complement or replace coring techniques. Using root-profiling techniques, root maps can be created by manually counting roots intersecting the soil profile in a trench and the distribution of root patches on these maps can be characterised using geo-statistical methods (Laclau et al. 2013).
The study of fine root spatial distributions has been limited mainly to shallow soil depths, but the distribution of roots in deep soils, defined as those located at depths below 1.0 m (Maeght et al. 2013), has rarely been studied (but see Christina et al. 2011; Laclau et al. 2013). The lack of data concerning deep root spatial distributions can be explained by the difficulties associated with sampling in the field, especially when using root-profiling techniques (Maeght et al. 2013). When soil depth exceeds 1.0 m, excavation of a root profile becomes tedious and even dangerous, as soil walls are more prone to collapse. Using these and other methods, it has been shown that tree roots can extend to depths below 20 m (Haase et al. 1996; Hubble et al. 2010; Bleby et al. 2010), but these studies remain descriptive and lack a detailed characterisation of deep root distribution. Roots in deep soils perform important functions in particular with regard to mechanical anchorage, carbon sequestration, water uptake and transport (Stokes et al. 2009; Prieto et al. 2012; Maeght et al. 2013). Thus, it is important to characterise deep root distributions in contrasting ecosystems to understand the potential implications for ecosystem functioning and services.
Similarly to root density, root orientation is considered as an important trait related to the plant capacity to absorb water and nutrients (Nobel and Alm 1993; Ho et al. 2004). Root orientation is influenced by gravity, distribution of water (Cassab et al. 2013) and nutrients in the soil (Bonser et al. 1996). Compared to shallow roots, deep roots are more likely to uptake soil water supplied by water tables (Chen and Hu 2004) if not too deep. We therefore hypothesize that deep fine roots will be more vertically oriented than shallow roots due to the hydrotropism (Cassab et al. 2013). The spatial variability of preferential root orientation, or anisotropy, has been rarely studied, especially in field (Chopart et al. 2008; Maurice et al. 2010). Estimating root orientation also allows to determine RLD via root intersection density (RID, defined as the number of root tips counted on a given soil surface) which is an important trait defining the utilization of resources (Gregory 2006; Markesteijn and Poorter 2009). As the measurement of RLD is more time-consuming than that for RID (Chopart and Siband 1999), a series of studies has attempted to explore the relationship between RLD and RID by introducing the effect of root orientation (Chopart and Siband 1999; Chopart et al. 2008; Maurice et al. 2010). To our knowledge, no such relationship is as yet available for walnut, an economically valuable species for wood production, especially in agroforests. Establishing this relationship would allow a better characterisation and quantification of root biomass and when combined with data for root turnover and decomposition rates, can be used to quantify carbon sequestration.
Our aim was to characterise the spatial distribution of fine roots of hybrid walnut (Juglans regia × nigra L.) trees in a Mediterranean alley cropping agroforestry system mixed with durum wheat (Triticum turgidum L. subsp. durum) in southern France. We hypothesized that tree and crop roots are in competition and that this will be reflected in the distribution of tree roots. To do this, we used six root profiles excavated to a depth of 150 cm in an agroforestry (hybrid walnut trees × durum wheat) stand and in a tree monoculture (walnut trees × natural understorey). To study tree root spatial distribution beneath the maximum rooting depth of durum wheat, a 400 cm deep trench was also dug in the intercropped stand (four additional root profiles). To characterise the spatial variability of root distribution, we measured the RID and RLD, and calculated the orientation of roots along the soil profile. Our hypotheses were that (i) trees in the agroforestry stand would have lower RID and RLD near the soil surface compared to trees in the monocultural stand but have a greater root density deeper in the soil, (ii) RID and RLD would decrease with increasing distance from the trees and this effect would be greatest in the agroforestry stand, (iii) the orientation of roots would change with soil depth from isotropic to anisotropic, i.e. from a uniform root growth in all orientations to a preferential growth orientation. At the same time, we sought to highlight new methodologies for analysing root data by using (i) geo-statistical methods to better characterise and visualize root spatial heterogeneity and (ii) a segmented linear model to better describe deep root distribution (Qian and Cuffney 2012).
Materials and methods
Study site
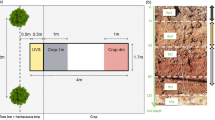
The study was conducted at the Restinclières experimental site, 15 km north of Montpellier, France (43°43′ N, 4°1′ E, 54 m a.s.l.). The climate is sub-humid Mediterranean with an average temperature of 14.5 °C and an average annual rainfall of 951 mm (years 1996–2003). Soils are silty deep alluvial fluvisols (IUSS Working Group WRB 2007), with 25 % clay and 60 % silt (Dupraz et al. 1999) with a slope < 1° within the site. The site is near the Lez river watershed and the depth from the soil surface to the water table usually oscillates between 5 m in winter and 7 m in the summer.
Root sampling was conducted in two adjacent types of hybrid walnut (Juglans regia × nigra L. cv. NG23) plantations. All the walnut trees at the site were planted in February 1995 in parallel tree rows with an east–west orientation (Fig. 1). The site comprised:
-
an alley cropping agroforestry stand (walnut trees at 13 × 4 m tree spacing) where walnut trees were usually intercropped with durum wheat (Triticum turgidum L. subsp. durum (Desf.) Husn.). However, rapeseed (Brassica napus L.) was also grown in 1998, 2001 and 2006, and pea (Pisum sativum L.) in 2010.
-
a tree monoculture with natural vegetation in the understorey (walnut trees at 7 × 4 m tree spacing). Understorey vegetation was dominated by Vicia lutea L. and composed mainly of herbaceous species, including Medicago sp., Avena sp. and Papaver sp.
In the agroforestry stand, the annual crop was fertilized with approximately 150 kg N ha−1 yr−1 , whereas the tree monoculture did not receive any fertilization. The soil in the inter-row was usually ploughed to 20 cm every year before the winter crop was sown. Durum wheat is sown in late October and harvested in late June. Exceptionally, the soil was tilled to 10–15 cm but not ploughed before durum wheat was sown in 2011. The last ploughing was performed in October 2010, i.e. 18 months before this study was performed. The soil has never been tilled along the tree rows in the intercropped stand or in the tree monoculture, and natural spontaneous vegetation was present in these areas.
Sampling locations
Two 200 (length, i.e. perpendicular to tree row) × 200 (width, i.e. parallel to tree row) × 160 cm (depth, i.e. vertical) trenches were dug in March 2012 in both the agroforestry (AF) stand and the tree monoculture (M) (Fig. 2). Tree root mapping and soil sampling were performed in April 2012. The trenches were located on the edge of the tree row, i.e. in the inter-row between two trees (Fig. 2). The distance of the nearest edge of the pits to a tree was 100 cm. The two trees surrounding the AF pit were 13.80 and 11.60 m tall, respectively, and had a diameter at breast height (DBH) of 29.0 and 25.2 cm, respectively. In the tree monoculture, the two surrounding trees were 14.00 and 13.30 m tall, respectively, and had a DBH of 31.5 and 26.4 cm, respectively. In order to study tree root spatial distribution beneath the maximum rooting depth of wheat (i.e. 150 cm), an additional trench was dug in the agroforestry stand (deep-AF). This pit was 500 (length) × 150 (width) × 400 cm (depth), and as the AF pit described previously, perpendicular to the tree row (Figs. 1 and 2). For safety reasons, before the deep-AF pit was dug, six iron posts were pushed 500 cm deep in the soil with a mechanical shovel corresponding to each corner of the future trench. Two additional posts were inserted in the middle of each of the longest trench walls. The pit was dug between the posts to a depth of 400 cm. A wooden framework was then built using the iron posts at different depths in order to secure access to the bottom of the pit and to leave spaces between wooden posts to perform measurements. This trench started on the tree row and ended in the middle of the intercropped row. The two trees surrounding the deep-AF pit were 13.80 and 11.70 m tall and had a DBH of 26.1 and 30.5 cm, respectively.
Tree root and soil cube sampling
In both the AF and M trenches (Fig. 2), fine roots (diameter ≤ 2 mm) of walnut were mapped using a grid with regular squares (10 × 10 cm) along three soil profiles of 150 (length) × 150 (width) × 150 cm (depth). Live fine roots were determined visually and their number per square was counted. It was easy to distinguish tree roots from the arable crop and the herbaceous understorey roots. Walnut roots are black whereas wheat and herbaceous roots were whitish/yellowish. A precision calliper was used to measure precisely root diameter when roots were considered to be close to 2 mm in diameter. We then calculated the mean root intersection density (\( \overline{RID} \), roots cm−2) for each square in the grid by dividing the number of roots counted in each square by the surface area (100 cm−2). Mean \( \overline{RID} \) profiles were calculated per depth interval (10 cm) along a width of 150 cm (the width of the grid).
In order to predict RLD from RID and to assess root anisotropy, we used a similar technique to that of Maurice et al. (2010). Two soil cubes (10 × 10 × 10 cm, Fig. 3) per profile were taken at depths of 10, 40, 70, 100 and 150 cm. Cubes of soil were extracted in the middle of each soil profile (Fig. 2). Cubes had one horizontal face (H) parallel to the soil surface and perpendicular to the profile; one lateral face (L) perpendicular to the soil surface and to the profile, and one transversal face (T) perpendicular to the soil surface and parallel to the profile (Fig. 3). The transversal face of these cubes corresponded to the plane of the soil profile walls where root impacts were mapped. This face is the most accessible for studying root spatial distribution but does not allow the three dimensional (3D) distribution of fine roots to be mapped. Therefore, two additional faces (H and L faces) were also sampled. Two cubes per depth and per profile were sampled close to each other, one in position A (lateral face on the right), one in position B (lateral face on the left, Fig. 3). Overall, a total of 30 cubes were sampled per pit (n = 2 positions of cube × 5 soil depths × 3 root profiles).
Sampling of cubes. a sampling device; b sampling positions. In (b), the grey faces, solid red lines and red letters at vertices represent the sampling device. The hollow faces dashed blue lines and blue letters in the middle of a plan represent the sampled soil cube; H horizontal, T transversal, L lateral
In the deep-AF trench, fine root impacts were mapped on all four lateral soil profiles (Fig. 2). Following the same protocol mentioned above, soil cubes were sampled at depths of 10, 40, 70, 100, 150, 200, 300 and 400 cm. Two replicates (cubes) were sampled in the middle of the tree row profile (see profile 1 in Fig. 2) and in the inter-row 100 cm from the tree row (see profile 4 in Fig. 2). Cubes were also sampled at 500 cm from the tree row (see profile 2 in Fig. 2). Overall, a total of 48 cubes were sampled in this trench (2 positions of cube × 8 soil depths × 3 root profiles). Cubes were taken to the laboratory and stored at 4 °C before measurements were performed, which were carried out within a few days after sampling.
Root counts on soil cubes
Live fine roots were determined visually and the number of fine root intercepts was counted on each of the three cube faces. The RIDs were then calculated for each cube face as the number of roots per cm−2 and were named RID H , RID L and RID T respectively for the H, L and T faces. The average number of fine root intercepts for each soil cube (\( \overline{RID} \), roots cm−2) was calculated as follows:
Root traits
After roots were counted, all roots from each cube were carefully extracted by gently washing the cube with tap water using a 0.2 mm mesh sieve. Coarse roots (>2 mm diameter) were removed from the analysis. Remaining roots were then rinsed, spread out onto a mesh tray and scanned at 400 dpi with a scanner (Epson Expression © 10000 XL, Japan). The resulting image was then processed using an image analysis software (WinRHIZO v. 2005b ©, Regent Instruments Inc., Québec, Canada) to determine the total fine root length (L, cm) and mean root diameter per diameter class, i.e. 0.0–0.5 mm, 0.5–1.0 mm, 1.0–1.5 mm, and 1.5–2.0 mm. We then calculated the proportion of root length in each diameter class, with regard to total root length for all diameter classes combined. Total RLD (cm cm−3) was then calculated as L/V, where L is the total root length in the cube and V is the volume of the soil cube (1000 cm3). Roots were then dried at 65 °C for 48 h and weighed to determine their dry mass (DM, g). We calculated the specific root length (SRL, m g−1) as the ratio between L/DM.
Root anisotropy
Root anisotropy (A) is considered one of the most important and commonly used metrics of root orientation (Lang and Melhuish 1970). Root distribution is fully isotropic when root growth is uniform in all orientations, whilst root distribution is fully anisotropic, when root growth is toward only one orientation. However, anisotropy is almost impossible to estimate in the field, as measuring the orientation of individual fine roots is extremely painstaking and time-consuming. Therefore, A was interpreted as the deviation degree from a random orientation of roots within a soil cube (Chopart and Siband 1999) and can be expressed as:
where, RID T, RID L and RID H are the root intersection densities (roots cm−2) on the T, L and H faces of a given soil cube, respectively. The denominator term in the equation allows for normalization of A (dimensionless) so that it ranges between 0 and 1. When A = 0, i.e. RID T = RID L = RID H , there is isotropy (i.e. root distribution is isotropic) and there is no specific orientation for fine root growth. When A = 1 (in a fully anisotropic status), this indicates that roots are counted only on one face but do not penetrate the other two faces of the cube (RID = 0 on these faces).
Data analysis
Separate generalized linear models (GLM) were used with either the proportion of root length in each diameter class, RLD, DM, or SRL as the dependent variables and the soil cube position (A or B), the stand (AF or M), the distance to the tree row (quantitative factor), and soil depth (quantitative factor) as factors and all interactions between factors. When a maximum soil depth of 150 cm was considered for analysis, soil profiles from the AF and deep-AF pits were considered as individual replicates. A Shapiro-Wilk test was performed before each GLM to guarantee that the investigated indicator followed a normal or quasi-normal distribution. These analyses were followed by a one-way analysis of variance (ANOVA) for each factor.
Root vertical profiles are usually described using logarithmic or exponential models (Jackson et al. 1996; Hartmann and Von Wilpert 2014). However, we used a hockey stick model (Qian 2009; Qian and Cuffney 2012) to compare rooting patterns between the different stands (AF and M). We applied this model to the mean RID between the profiles of the inter-row and of the tree row for each stand in order to determine if the vertical rooting pattern was distributed smoothly throughout the soil profile and compare rooting depths. Compared to these conventional models, the advantage of the hockey stick model is that the significant breakpoints in a series of data can be found. The hockey stick model can have several breakpoints and segments, but adding extra-breakpoints might not always be biologically meaningful, and would make the model less robust mathematically due to the high number of parameters. Therefore we decided to use this model with only one breakpoint:
where, z is soil depth (cm), α 1 , α 2 , δ and β are coefficients (see Fig. 4 for geometrical definitions, and supplementary material for the R code). If δ is statistically significant, i.e. there is a significant breakpoint along soil depth, and at a soil depth of z = δ, there is a root vertical distribution with RID = β. For the agroforestry stand, profiles from the AF and deep-AF pits were averaged for values to a depth of 150 cm and a distance of up to 150 cm from the tree row.
Geometric meanings of all the coefficients of the four-parameter hockey stick model, which comprises two linear segments. RID is the root intersection density (roots cm-2), z is soil depth (cm). β and δ are the breakpoint coordinates, α 1 , α 2 are the slope coefficients. Around the breakpoint, a quadratic curve was estimated between point A and B, which are sufficiently close to the breakpoint, so that the whole hockey stick model becomes smooth and derivable, and therefore can be fitted using non-linear regression approaches (Qian 2009)
The spatial distribution of roots was also analyzed using univariate geostatistical methods including kriging (Webster and Oliver 2007), that was performed using GS+ (Gamma Design Software 2004).
Ordinary least square regressions (OLS) between RLD and RID T or \( \overline{RID} \) were performed for the cubes of each pit (local model) and for the cubes of all pits at the same time (global model). Since A and B cubes were spatially correlated for each soil depth (taken next to each other but with opposing orientations), these cubes were not considered as individual replicates. Due to the chosen sampling method (Chopart and Siband 1999), the horizontal face of the B-type cube was 10 cm deeper than the horizontal face of the A-type cube (Fig. 3). Therefore, we considered both A- and B-type cubes at each sampling position (depth) as one sample using mean values between A- and B-type cubes as no significant differences were found between them (F = 0.31, P = 0.58). Since the intercepts of the OLS models were not significantly different from zero, we performed additional OLS forcing the intercept through the origin. This method is based on the assumption that if the mean number of root intercepts for a given cube equals zero there are no roots inside the cube. The slope of the regression line (α) as well as the 95 % confidence interval for the slope were calculated. Slopes were compared using an analysis of covariance (ANCOVA) (Andrade and Estévez-Pérez 2014). We performed a Fisher’s (F) test to determine which model should be chosen between a local model (containing a series of equations, one equation per level of factor) and a global model (containing one equation for all levels mixed).
With regard to anisotropy (A), a GLM and ANOVA were also applied in the same way as for the analysis of root traits. As A possesses no information on the preferential orientation of roots (Chopart and Siband 1999), when we detected anisotropy, we also analysed the proportion of root impacts per cube face (H, L, T) using the same methodology to determine the preferential orientation of roots at each depth.
All calculations were carried out with the R software, Version 2.15.3 (R Development Core Team 2013) at a significance level of <0.05.
Results
Root traits from soil cubes
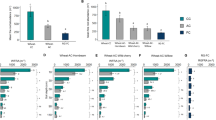
In both stands, fine roots from walnut trees were mainly constituted of roots ≤ 0.5 mm in diameter that represented almost 80 % of the total root length (Fig. 5). The only significant variable that impacted the proportion of root length in each diameter class in the upper 150 cm of soil was stand type. The finest roots (≤0.5 mm) represented 85 % of the total root length in the tree monoculture, but only 77 % in the agroforestry plot (F = 9.15, P = 0.003, df = 81). Within the additional trench dug in the agroforestry stand (deep-AF), there was a higher proportion of very fine roots (77 %) in the upper 150 cm of soil compared to the deeper soil (69 %) although the result was not significant (F = 3.61, P = 0.065, df = 41). There were no significant differences in the proportion of root length within each diameter class with regard to soil depth or distance to the tree.
The stand type, the distance to the tree, soil depth and interaction between distance to the tree and depth, had a significant effect on RLD and DM of fine roots from soil cubes (Table 1). The interaction between distance to the tree and stand type was not significant. RLD and DM were greater in the tree monoculture (0.26 ± 0.03 cm cm−3 and 0.17 ± 0.02 g, respectively) compared to the agroforestry stand (0.10 ± 0.01 cm cm−3 and 0.10 ± 0.02 g, respectively), and decreased with increasing distance from the tree row and depth.
The SRL of walnut roots to a depth of 150 cm did not differ significantly between the agroforestry stand (17.29 ± 1.84 m g−1) and the tree monoculture (17.19 ± 1.02 m g−1). SRL was not significantly different between soil cubes, depth or distance to the tree (Table 1).
Mapping tree fine root impacts
In the tree monoculture (Fig. 6a and Fig. S1a for raw data), most tree fine roots were concentrated in shallower depths compared to the agroforestry stand (Fig. 6b and Figure S1b for raw data), and appeared to be more homogeneously distributed along the horizontal plane. Using a hockey stick model, it was shown that the soil depth above which most root impacts were counted was > 150 cm (no breakpoint detected when a depth of 150 cm was considered) in the tree row in the agroforestry stand and 104 cm in the tree monoculture. In the inter-row, this depth was 122 cm in the agroforestry stand and 64 cm in the tree monoculture (Fig. 7a). Wheat roots were not mapped but we visually determined a maximum rooting depth of 150 cm.
a Walnut fine root intersection density (RID) profiles in the agroforestry stand and in the tree monoculture to a depth of 150 cm. For the agroforestry stand, profiles from the AF and deep-AF pits were combined for the values down to 150 cm, b Walnut fine root intersection density (RID) profiles in the agroforestry stand to a depth of 400 cm as a function of distance to the tree row
In the deep-AF pit, tree fine roots colonized the whole soil profile both vertically (400 cm deep) and horizontally (500 cm long) (Fig. 6c and Fig. S1c for raw data). In the inter-row, 2 m away from the tree, and to a depth of 75 cm, the RID of fine roots was still high (0.04 to 0.05 roots cm−2), and comparable with that under the tree row. Further away from the tree row, RID started to decrease in topsoil layers. Below 100 cm deep in the inter-row, no clear spatial pattern was observed, and fine roots appeared to be randomly distributed regardless of increasing soil depth and tree distance. In deeper layers, RID was generally lower but in the tree row it remained high (0.02 to 0.03 root cm−2) at depths < 150 cm. Fine roots tended to grow in clusters at depths greater than 150 cm (Fig. 6c). Consistent with results from the AF pit, an estimate with hockey stick models of the soil depth above which most root impacts occurred showed that, in the deep-AF pit, this depth was around 150 cm for the tree row (Fig. 7b) whereas it decreased to 79 and 104 cm in the inter-rows, respectively between 0 and 150 cm, and between 150 and 300 cm from the tree row. From 50 to 100 cm soil depth, the mean RID in the tree row was 0.028 roots cm−2, whereas it was 0.012 roots cm−2 in the inter-row at a distance of 150 cm from the tree row. Three meters away from the tree row, RID was low and constant along the whole profile so that the hockey stick model failed to detect a breakpoint.
Root anisotropy
GLM and ANOVA analysis revealed that the stand type and the distance to the tree row had a significant impact on root anisotropy where soil depth ≤ 150 cm (Table 2). Tree roots in the tree monoculture (A = 0.30) were significantly (P < 0.05) more isotropic than in the agroforestry stand (A = 0.45) (Fig. 8). Fine roots in the tree row were significantly more isotropic (A = 0.28) than in the inter-row (A = 0.46).
Variation of walnut fine root anisotropy according to sampling location (a, for all the pits) and soil depth (b, only for the 400 cm deep agroforestry pit). In a, “AF” and “M” represent agroforestry stand and tree monoculture, respectively. Black crosses on each boxplot represent the mean value of anisotropy (A)
In the deep-AF pit, GLM and ANOVA analysis revealed that depth had a significant impact on root anisotropy (Table 2). Shallow fine roots were more isotropic (A = 0.26 at a depth of 10 cm) than deep fine roots (A = 0.71 at an average depth of 400 cm) (Fig. 8). An analysis of the proportion of fine root counts on each cube face showed that the horizontal face of cubes had a higher proportion of root intercepts with increasing depth (F = 16.59, P < 0.001). About 24 % of root intercepts were counted on the horizontal face of cubes from the soil surface to a depth of 150 cm, but this proportion reached 62 % at a depth of 200 to 400 cm. Tree fine roots were preferentially vertically oriented with increasing depth.
Prediction of root length density (RLD)
The slopes of the OLS regressions between RLD and either RID T or \( \overline{RID} \), respectively, were not significantly different from each other (Table 3). The confidence interval was generally slightly narrower when \( \overline{RID} \) was used (Table 3, Fig. S2). The F test revealed that a local model (i.e. one model for each pit) was not more significant than a global model for all pits (F = 1.67, P = 0.185). Thus, we were able to link the mean number of root impacts and RLD for hybrid walnut trees as follows:
This equation was then used to predict RLD in both the agroforestry stand and the tree monoculture, in the tree row and in the inter-row (Fig. S3a, b).
Discussion
We showed that agroforestry practices promoted deeper rooting of walnut trees as root densities were much smaller near the soil surface in the agroforestry stand compared to the tree monoculture. Roots were also more heterogeneously distributed horizontally in the agroforestry stand, with larger root densities deeper in the soil in the tree row compared to the inter-row.
Plasticity of root distribution
The tree monoculture had a root distribution typical of that found in a forest stand, i.e. with a vertical heterogeneous root distribution and very high root densities in the top 0.5 m of soil (López et al. 2001; Yuan and Chen 2010; Hartmann and Von Wilpert 2014). In the agroforestry stand, root distribution was more vertically homogeneous and RLD was much smaller than in the tree monoculture, but roots occupied a higher volume of soil. These results confirm our first hypothesis that trees in the AF stand would have deeper root distributions induced by a greater belowground competition from the crop. This disparity between the two types of systems demonstrates the highly plastic behaviour of walnut trees in that their vertical root distribution was modified, likely in response to crop competition (Mulia and Dupraz 2006), even though soil type and environmental conditions were the same for both stands. This phenomenon has been commonly observed in other economically important tree species. For example, Dupraz and Liagre (2008) showed that poplars (Populus L.) possessed completely different rooting patterns when grown in a tree monoculture compared to an agroforestry stand, with significantly deeper rooting for the latter. Livesley et al. (2000) and Wang et al. (2014) found that Grevillea robusta L. grown with Zea mays L. and Jujube (Ziziphus jujuba L.) trees grown together with wheat (Triticum aestivum L.) in an arid climate, had a lower RLD than trees grown in a monospecific stand due to competition from the crops. However, the belowground interaction between trees and crops can be more complex and roots of both trees and crop can be overlapped in shallow soils despite strong competition (Moreno et al. 2005). Further support to our hypothesis comes from the fact that annual crop species often have high SRL near the soil surface and are thus able to absorb nutrients and shallow soil water faster than trees, which usually have lower SRL (Moreno et al. 2005). The SRL of walnut trees at our field site was lower than that of wheat, both at the surface (20 cm) and in deep soil layers (150 cm) (Prieto et al. 2014). Therefore, we suggest that walnut trees in the agroforestry stand had minimal competition from wheat plants through the production of deeper fine roots. Deep roots will enable trees to access water from the water table not available to root crops, and to benefit from nutrients leached beneath the crop root systems. On the contrary, in the tree monoculture, walnut trees laid down roots in shallow soil because the understorey herbaceous species were mostly leguminous (Prieto et al. 2014) and less competitive than winter wheat, mainly because of a lower root density. A parallel study estimated that root biomass of wheat was about 4.5 t DM ha-1 in 0–50 cm, whereas the root biomass of herbs was less than 1.5 t DM ha-1. Herbaceous herbs in the tree monoculture were brown/green in colour during the winter months and very short. As herbaceous roots are less active in the winter (Steinaker and Wilson 2008) compared to winter wheat roots, the surface soil may contain less roots in the tree monoculture compared to the agroforestry stand. Therefore, in the spring, tree root in the monoculture could rapidly occupy the neighbouring superficial soil poorly colonized by herbaceous species.
Another factor potentially affecting the vertical root distribution in the AF stand is soil tillage (Korwar and Radder 1994; Sinclair 1995). In our system, the soil in the inter-row of the agroforestry stand was regularly ploughed to a depth of 20 cm and coarse roots in the soil surface were frequently damaged, affecting tree fine root production in these layers. In this sense, tillage might also induce deeper rooting in trees. However, soil disturbances can also stimulate root growth through root pruning (Joslin and Wolfe 1999) and by releasing soil micronutrients (Balesdent et al. 2000). Whatever the case, this explanation would be valid only for the top 20 cm of soil, the maximum tillage depth. Below this depth, only root competition between durum wheat and walnut trees can explain the contrasting rooting patterns observed between the AF and M stands.
Temporal differences between the root growth of durum wheat and walnut trees may also influence the root distribution of walnut trees. Durum wheat is sown in late October at our field site, and is fully developed before walnut bud break, which occurs between late April and early May at the site (Mulia and Dupraz 2006). This period coincides with the peak fine root production for walnut trees (unpublished data). By this date, durum wheat, with a maximum rooting depth of 150 cm, will have already captured most of the nutrients and water contained in the topsoil (Burgess et al. 2004). We propose that temporal differences in growing periods between annual crops and tree species is therefore a key parameter for certain agroforestry systems to be successful (i.e. in Mediterranean ecosystems), and must be considered if new mixtures of crops and trees are to be successful (Schroth 1995; van Noordwijk et al. 1996; Burgess et al. 2004).
In the AF stand, the horizontal root distribution was heterogeneous and dependent on the distance from the tree, with higher root densities in the tree row or close to the tree row. The tree monoculture did not exhibit such a drastic decline in their root density, confirming our second hypothesis.
This unusual fine root distribution in the AF stand may promote carbon storage in the tree row and deep in the soil. Several studies have shown that carbon stocks in agroforestry systems were heterogeneously distributed, with more carbon in the tree row than in the inter row (Bambrick et al. 2010; Howlett et al. 2011; Lorenz and Lal 2014). A parallel study at this site confirmed that soil organic carbon (SOC) stocks were significantly higher in the AF stand (116.7 ± 1.5 Mg C ha-1) in the upper metre of soil compared to that in a control agricultural plot (110.4 ± 0.6 Mg C ha-1). SOC stocks were also significantly higher in the tree rows than in inter-rows (Cardinael et al. submitted). This additional SOC will not only be due to leaf litter from trees, but will also originate from fine root exudation and turnover (Haile et al. 2010).
Shallow roots and deep roots
We found smaller proportions of very fine roots in the soil 200 to 400 cm deep. These results are in accordance with Prieto et al. (2014) who found that fine roots deep in the soil were not only thicker than those near the surface, but presented traits associated to a more conservative strategy (i.e. lower root nitrogen and higher lignin concentrations). Prieto et al. (2014) attributed this result to thinner, more acquisitive roots in shallow soil layers being more efficient for absorbing nutrients, which usually accumulate near the soil surface (Jobbagy and Jackson 2000). Our third hypothesis was confirmed since we found that tree fine roots down to 150 cm showed no clear orientation patterns but that deeper roots (200–400 cm) were preferentially vertically orientated.
This result suggests that, once a certain depth threshold is achieved, deep roots are preferentially oriented to access more stable water resources (i.e. the water table). Groundwater is present in this agroforestry stand at a depth of 500–700 cm, depending on the season, and having access to groundwater will enable walnut trees to overcome the summer drought period (Rambal 1984; Bréda et al. 1995; Bréda et al. 2006). Deep roots may be able to reach and take up nitrate leached from fertilizers beneath the wheat crop rooting depth, which may also explain the deep rooting observed in the AF stand. This “tree root safety net” (Cadisch et al. 1997; Rowe et al. 1999) could also contribute to reducing groundwater nitrate levels (Bergeron et al. 2011; Tully et al. 2012) and therefore improve ecosystem services provided by agroforestry systems.
In spite of the disparity in the trait distribution and functioning between shallow and deep roots (Prieto et al. 2014), few studies have aimed at determining their distinct roles (Laclau et al. 2013; Maeght et al. 2013). Here, we show that a sharp breakpoint exists between two populations of roots within the soil profile: roots from upper soil layers, where root density declines sharply with increasing soil depth, and where roots have no determined spatial orientation, and roots in deeper soil layers (200–400 cm), where root density remains quite stable regardless of soil depth and roots are preferentially vertically oriented. Although we do not yet know the mechanisms behind this distribution, the breakpoints and identification of these thresholds in different ecosystems with deep-rooted species seem important to determine competition and foraging behaviours. These can be statistically estimated using the hockey stick model, which might be a promising tool to better define root distribution patterns than conventional linear, exponential or logarithmic functions.
Conclusion
Using deep soil profiles, we evidenced how tree fine root density can be both horizontally and vertically modified by the belowground competition from understory crops. Trees in the agroforestry stand rooted deeper in the soil than trees in the monocultural stand and had a higher root density in the tree rows compared to the cropped inter-rows. These results enrich our understanding of the functioning of agroforestry systems. The plasticity in tree root distribution seems to be an important feature to achieve efficient agroforestry systems. This may also have implications concerning carbon and nutrient cycling in these systems as exploration of deep soil layers by roots is favoured. Methodologically, we highlighted the interest of using the hockey stick model. This model has a strong potential for use in future studies when attempting to define shallow and deep rooting profiles and distribution.
References
Andrade JM, Estévez-Pérez MG (2014) Statistical comparison of the slopes of two regression lines: a tutorial. Anal Chim Acta 838:1–12
Balesdent J, Chenu C, Balabane M (2000) Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res 53:215–230
Bambrick AD, Whalen JK, Bradley RL, Cogliastro A, Gordon AM, Olivier A, Thevathasan NV (2010) Spatial heterogeneity of soil organic carbon in tree-based intercropping systems in Quebec and Ontario, Canada. Agrofor Syst 79:343–353
Bergeron M, Lacombe S, Bradley RL, Whalen J, Cogliastro A, Jutras MF, Arp P (2011) Reduced soil nutrient leaching following the establishment of tree-based intercropping systems in eastern Canada. Agrofor Syst 83:321–330
Bleby TM, McElrone AJ, Jackson RB (2010) Water uptake and hydraulic redistribution across large woody root systems to 20 m depth. Plant Cell Environ 33:2132–2148
Bonser AM, Lynch J, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132:281–288
Bréda N, Granier A, Barataud F, Moyne C (1995) Soil water dynamics in an oak stand I. Soil moisture, water potentials and water uptake by roots. Plant Soil 172:17–27
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644
Burgess PJ, Incoll LD, Corry DT, Beaton A, Hart BJ (2004) Poplar (Populus spp) growth and crop yields in a silvoarable experiment at three lowland sites in England. Agrofor Syst 63:157–169
Cadisch G, Rowe E, van Noordwijk M (1997) Nutrient harvesting - the tree-root safety net. Agrofor Forum 8:31–33
Cardinael R, Chevallier T, Barthès BG, Saby NPA, Parent T, Dupraz C, Bernoux M, Chenu C (submitted) Impact of agroforestry on stocks, forms and spatial distribution of soil organic carbon
Casper BB, Jackson RB (1997) Plant competition underground. Annu Rev Ecol Syst 28:545–570
Cassab GI, Eapen D, Campos ME (2013) Root hydrotropism: an update. Am J Bot 100:14–24
Chen X, Hu Q (2004) Groundwater influences on soil moisture and surface evaporation. J Hydrol 297:285–300
Chopart J-L, Siband P (1999) Development and validation of a model to describe root length density of maize from root counts on soil profiles. Plant Soil 214:61–74
Chopart J-L, Rodrigues SR, de Azevedo MC, de Conti MC (2008) Estimating sugarcane root length density through root mapping and orientation modelling. Plant Soil 313:101–112
Christina M, Laclau J-P, Gonçalves JLM, Jourdan C, Nouvellon Y, Bouillet J-P (2011) Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forests. Ecosphere 2:art27
Desrochers A, Landhäusser SM, Lieffers VJ (2002) Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol 22:725–732
R Development Core Team (2013) R: a language and environment for statistical computing
Dupraz C, Liagre F (2008) Agroforesterie: des arbres et des cultures. France Agr:413
Dupraz C, Fournier C, Balvay Y, Dauzat M, Pesteur S, Simorte V (1999) Influence de quatre années de culture intercalaire de blé et de colza sur la croissance de noyers hybrides en agroforesterie. Bois Forêts Des Agric:95–114
Fernández ME, Gyenge J, Licata J, Schlichter T, Bond BJ (2008) Belowground interactions for water between trees and grasses in a temperate semiarid agroforestry system. Agrofor Syst 74:185–197
Gamma Design Software (2004) Geostatistics for the environmental sciences
Gao Y, Duan A, Qiu X, Liu Z, Sun J, Zhang J, Wang H (2010) Distribution of roots and root length density in a maize/soybean strip intercropping system. Agric Water Manag 98:199–212
Gregory P (2006) Plant roots: growth, Activity and Interactions with soils. 318p
Haase P, Pugnaire FI, Fernandez EM, Puigdefabregas J, Clark SC, Incoll LD (1996) An investigation of rooting depth of the semiarid shrub Retama sphaerocarpa (L.) Boiss. by labelling of ground water with a chemical tracer. J Hydrol 177:23–31
Haile SG, Nair VD, Nair PKR (2010) Contribution of trees to carbon storage in soils of silvopastoral systems in Florida, USA. Glob Chang Biol 16:427–438
Hartmann P, Von Wilpert K (2014) Fine-root distributions of Central European forest soils and their interaction with site and soil properties. Can J For Res 44:71–81
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Ho MD, McCannon BC, Lynch JP (2004) Optimization modeling of plant root architecture for water and phosphorus acquisition. J Theor Biol 226:331–340
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513
Hoffmann CW, Usoltsev VA (2001) Modelling root biomass distribution in Pinus sylvestris forests of the Turgai depression of Kazakhstan. For Ecol Manage 149:103–114
Howlett DS, Moreno G, Mosquera Losada MR, Nair PKR, Nair VD (2011) Soil carbon storage as influenced by tree cover in the Dehesa cork oak silvopasture of central-western Spain. J Environ Monit 13:1897–1904
Hubble TCT, Docker BB, Rutherfurd ID (2010) The role of riparian trees in maintaining riverbank stability: a review of Australian experience and practice. Ecol Eng 36:292–304
IUSS Working Group WRB (2007) World reference base for soil resources 2006, first update 2007. World Soil Resources Reports No. 103. FAO, Rome
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Joslin JD, Wolfe MH (1999) Disturbances during minirhizotron installation can affect root observation data. Soil Soc Am J 63:218–221
Korwar G, Radder G (1994) Influence of root pruning and cutting interval of Leucaena hedgerows on performance of alley cropped rabi sorghum. Agrofor Syst 25:95–109
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. J Plant Nutr Soil Sci 163:421–431
Laclau J-P, da Silva EA, Rodrigues Lambais G, Bernoux M, le Maire G, Stape JL, Bouillet J-P, de Moraes Gonçalves JL, Jourdan C, Nouvellon Y (2013) Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Front Plant Sci 4:243
Lang ARG, Melhuish FM (1970) Lengths and diameters of plant roots in non-random populations by analysis of plane surfaces. Biometrics 26:421–431
Li L, Sun J, Zhang F, Li X, Yang S, Rengel Z (2001) Wheat/maize or wheat/soybean strip intercropping I. Yield advantage and interspecic interactions on nutrients. F Crop Res 71:123–137
Li L, Sun J, Zhang F, Guo T, Bao X, Smith FA, Smith SE (2006) Root distribution and interactions between intercropped species. Oecologia 147:280–290
Livesley SJ, Gregory PJ, Buresh RJ (2000) Competition in tree row agroforestry systems. 1. Distribution and dynamics of fine root length and biomass. Plant Soil 227:149–161
López B, Sabaté S, Gracia CA (2001) Vertical distribution of fine root density, length density, area index and mean diameter in a Quercus ilex forest. Tree Physiol 21:555–560
Lorenz K, Lal R (2014) Soil organic carbon sequestration in agroforestry systems. A review. Agron Sustain Dev 34:443–454
Maeght J-L, Rewald B, Pierret A (2013) How to study deep roots-and why it matters. Front Plant Sci 4:1–14
Markesteijn L, Poorter L (2009) Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J Ecol 97:311–325
Marsden C, Nouvellon Y, Epron D (2008) Relating coarse root respiration to root diameter in clonal Eucalyptus stands in the Republic of the Congo. Tree Physiol 28:1245–1254
Maurice J, Laclau J-P, Re DS, de Moraes Gonçalves JL, Nouvellon Y, Bouillet J-P, Stape JL, Ranger J, Behling M, Chopart J-L (2010) Fine root isotopy in Eucalyptus grandis plantations. Towards the prediction of root length densities from root counts on trench walls. Plant Soil 334:261–275
Moreno G, Obrador JJ, Cubera E, Dupraz C (2005) Fine root distribution in Dehesas of central-western Spain. Plant Soil 277:153–162
Mulia R, Dupraz C (2006) Unusual fine root distributions of two deciduous tree species in southern France: What consequences for modelling of tree root dynamics? Plant Soil 281:71–85
Nair PKR, Nair VD, Kumar BM, Showalter JM (2010) Carbon sequestration in agroforestry systems. Adv Agron:237–307
Newman GS, Hart SC (2006) Nutrient covariance between forest foliage and fine roots. For Ecol Manage 236:136–141
Neykova N, Obando J, Schneider R, Shisanya C, Thiele-Bruhn S, Thomas FM (2011) Vertical root distribution in single-crop and intercropping agricultural systems in Central Kenya. J Plant Nutr Soil Sci 174:742–749
Nobel PS, Alm DM (1993) Root orientation vs water uptake simulated for monocotyledonous and dicotyledonous desert succulents by a root-segment model. Funct Ecol 7:600–609
Norby RJ, O’Neill EG, Hood WG, Luxmoore RJ (1987) Carbon allocation, root exudation and mycorrhizal colonization of Pinus echinata seedlings grown under CO2 enrichment. Tree Physiol 3:203–210
Ozier-Lafontaine H, Lafolie F, Bruckler L, Tournebize R, Mollier A (1998) Modelling competition for water in intercrops: theory and comparison with field experiments. Plant Soil 204:183–201
Prieto I, Armas C, Pugnaire FI (2012) Water release through plant roots: new insights into its consequences at the plant and ecosystem level. New Phytol 193:830–841
Prieto I, Roumet C, Cardinael R, Dupraz C, Jourdan C, Kim JH, Maeght JL, Mao Z, Pierret A, Portillo N, Roupsard O, Thammahacksa C, Stokes A (2014) Root community traits along a land use gradient: evidence of a community-level economics spectrum. J Ecol
Qian SS (2009) Environmental and ecological statistics with R. 440p
Qian SS, Cuffney TF (2012) To threshold or not to threshold? That’s the question. Ecol Indic 15:1–9
Quinkenstein A, Wöllecke J, Böhm C, Grünewald H, Freese D, Schneider BU, Hüttl RF (2009) Ecological benefits of the alley cropping agroforestry system in sensitive regions of Europe. Environ Sci Pol 12:1112–1121
Rambal S (1984) Water balance and pattern of root water uptake by a Quercus coccifera L. evergreen scrub. Oecologia 62:18–25
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Rowe EC, Hairiah K, Giller KE, Van Noordwijk M, Cadish G (1999) Testing the safety-net role of hedgerow tree roots by 15N placement at different soil depths. Agrofor Syst 43:81–93
Saint-André L, M’Bou AT, Mabiala A, Mouvondy W, Jourdan C, Roupsard O, Deleporte P, Hamel O, Nouvellon Y (2005) Age-related equations for above- and below-ground biomass of a Eucalyptus hybrid in Congo. For Ecol Manage 205:199–214
Schroth G (1995) Tree root characteristics as criteria for species selection and systems design in agroforestry. Agrofor Syst 30:125–143
Sinclair FL (1995) Agroforestry: science, policy and practice. 287p
Steinaker DF, Wilson SD (2008) Phenology of fine roots and leaves in forest and grassland. J Ecol 96:1222–1229
Stokes A, Atger C, Bengough AG, Fourcaud T, Sidle RC (2009) Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 324:1–30
Torquebiau EF (2000) A renewed perspective on agroforestry concepts and classification. Life Sci 323:1009–1017
Trumbore SE, Gaudinski JB (2003) The secret lives of roots. Science 302:1344–1345
Tully KL, Lawrence D, Scanlon TM (2012) More trees less loss: nitrogen leaching losses decrease with increasing biomass in coffee agroforests. Agric Ecosyst Environ 161:137–144
Upson MA, Burgess PJ (2013) Soil organic carbon and root distribution in a temperate arable agroforestry system. Plant Soil 373:43–58
Van Noordwijk M, Lawson G, Soumaré A, Groot JJR, Hairiah K (1996) Root distribution of trees and crops: competition and/or complementary. Tree-crops interact. A physiol. Approach. CAB-International, Wallingford, pp 319–364
Van Noordwijk M, Brouwer G, Meijboom F, Oliveira MRG, Bengough AG (2000) Trench profile techniques and core break methods. In: Smit AL, Bengough AG, Engels C, Van Noordwijk M, Pellerin S, Van Geijn SC (eds) Root methods. Springer, Nerlin, pp 212–233
Wang BJ, Zhang W, Ahanbieke P, Gan YW, Xu WL, Li LH, Christie P, Li L (2014) Interspecific interactions alter root length density, root diameter and specific root length in jujube/wheat agroforestry systems. Agrofor Syst 88:835–850
Webster R, Oliver MA (2007) Geostatistics for environmental scientists:1–332
Yuan ZY, Chen H (2010) Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses. CRC Crit Rev Plant Sci 29:204–221
Zianis D, Muukkonen P, Mäkipää R, Mencuccini M (2005) Biomass and stem volume equations for tree species in Europe. 63p
Acknowledgments
This study was financed by the French ANR funded project ECOSFIX (Ecosystem Services of Roots - Hydraulic Redistribution, Carbon Sequestration and Soil Fixation, ANR-2010-STRA-003-01), and by the ADEME funded project AGRIPSOL. We thank the farmer Mr Breton, for his authorization to sample roots and open pits. We are very grateful to our French colleagues for their help with field and laboratory work and logistics, including Jean-François Bourdoncle (INRA), Lydie Dufour (INRA), Clément Enard (INRA), Alain Sellier (INRA), and the students (Jordan Chauliaguet, Hugo Fontenille, James Metayer, Floriane Schmith, Aurélien Schüller).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Angela Hodge.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
a) Raw data of walnut fine root intersection densities (RID) within the pit in the tree monoculture. DTR = Distance to the tree row. b) Raw data of walnut fine root intersection densities (RID) within the agroforestry pit. DTR = Distance to the tree row. c) Raw data of the walnut fine root intersection densities (RID) within the 400 cm deep agroforestry pit. DTR = Distance to the tree row. (ZIP 3170 KB)
Figure S2
Linear regressions between walnut fine root length density (RLD) and the mean fine root intersection density (RID) for cubes, for the different pits. Dotted lines: confidence interval of the regression line. (JPEG 3541 KB)
Figure S3
a) Estimated walnut fine root length density (RLD) profiles in the agroforestry and in the tree monoculture to a depth of 150 cm. For the agroforestry stand, profiles from the AF and deep-AF pits were combined for values to a depth of 150 cm. b) Estimated walnut fine root length density (RLD) profiles in the agroforestry stand to a depth of 400 cm as a function of distance to the tree row. (ZIP 3821 KB)
Rights and permissions
About this article
Cite this article
Cardinael, R., Mao, Z., Prieto, I. et al. Competition with winter crops induces deeper rooting of walnut trees in a Mediterranean alley cropping agroforestry system. Plant Soil 391, 219–235 (2015). https://doi.org/10.1007/s11104-015-2422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2422-8