Abstract
Peach fruit rapidly soften after harvest, a significant challenge for producers and marketers as it results in rotting fruit and significantly reduces shelf life. In this study, we identified two tandem genes, PpNAC1 and PpNAC5, within the sr (slow ripening) locus. Phylogenetic analysis showed that NAC1 and NAC5 are highly conserved in dicots and that PpNAC1 is the orthologous gene of Non-ripening (NOR) in tomato. PpNAC1 and PpNAC5 were highly expressed in peach fruit, with their transcript levels up-regulated at the onset of ripening. Yeast two-hybrid and bimolecular fluorescence complementation assays showed PpNAC1 interacting with PpNAC5 and this interaction occurs with the tomato and apple orthologues. Transient gene silencing experiments showed that PpNAC1 and PpNAC5 positively regulate peach fruit softening. Yeast one-hybrid and dual luciferase assays and LUC bioluminescence imaging proved that PpNAC1 and PpNAC5 directly bind to the PpPGF promoter and activate its transcription. Co-expression of PpNAC1 and PpNAC5 showed higher levels of PpPGF activation than expression of PpNAC1 or PpNAC5 alone. In summary, our findings demonstrate that the tandem transcription factors PpNAC1 and PpNAC5 synergistically activate the transcription of PpPGF to regulate fruit softening during peach fruit ripening.
Key message
PpNAC1 and PpNAC5 directly bind to the PpPGF promoters, synergistically activate its transcription to regulate peach fruit softening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peach [Prunus persica (L.) Batsch] is a fruit crop of economic importance and celebrated for its distinct flavor and nutrient content. However, peach fruits have a limited shelf life and are harvested before physiological ripening, resulting in inferior fruit quality (Eduardo et al. 2011). The ability to regulate fruit ripening could not only extend the shelf life but also allow strategic planning of the marketing season. As a climacteric fruit, peach ripening is influenced by various factors, with the plant hormone ethylene serving as a critical regulator. Ethylene exerts an effect on multiple physiological responses throughout peach ripening (Tatsuki et al. 2007, 2013; Monti et al. 2016). Furthermore, several transcription factors, including some in the NAC family, have been identified and functionally characterized as important regulators of fruit ripening (Lü et al. 2018; Gu et al. 2019; Wang et al. 2021; Dai et al. 2023; Cheng et al. 2023).
In tomato, the Non-ripening (NOR) gene, encoding a NAC transcription factor, serves as a pivotal regulator of fruit ripening. The SlNOR loss-of-function mutant exhibited disrupted pigment accumulation, abnormal fruit softening and an extended shelf life (Gao et al. 2020). Similarly, the silencing of NOR-like1 suppressed tomato fruit ripening and, NOR-like1 directly interacted with the promoters and positively regulated the expression of several genes involved in processes related to tomato ripening, including ethylene biosynthesis, color change, and cell wall metabolism (Gao et al. 2018). In the climacteric fruit melon, CmNAC–NOR directly induces the expression of CmACS5, CmNCED3, and CmZDS1 to promote fruit ripening (Wang et al. 2022a, b). In apple, MdNAC18.1 has been identified as an indicator of apple harvest date, and influences fruit firmness at harvest and after harvest (Migicovsky et al. 2021). Transgenic introduction of MdNAC18.1 into the tomato nor mutant complemented the ripening deficiency observed in the nor tomato (Migicovsky et al. 2021). Furthermore, the NAC transcription factor RIPENING INDUCING FACTOR (RIF), which is an orthologue of NOR, plays a crucial role in the regulation of ripening in strawberry, a non-climacteric fruit. Knockout mutations of FvRIF result in a complete blockade of fruit ripening (Martin-Pizarro et al. 2021; Li et al. 2023). Moreover, FvRIF modulates anthocyanin biosynthesis and fruit softening by directly regulating the expression of related core genes (Li et al. 2023). Collectively, these studies demonstrate that NOR and its orthologous genes play vital roles in both climacteric and non‐climacteric fruit ripening.
In peach, PpNAC1 (Prupe.4G187100), which shares homology with NOR, has been identified and shown to be a key participant in the ethylene synthesis positive feedback loop during peach fruit ripening. PpNAC1 exerts its influence by directly binding to the promoter regions of the ethylene synthesis genes ACS and ACO, triggering their expression (Tatsuki et al. 2007, 2013; Lü et al. 2018). Additionally, PpNAC1 also serves as an activator of biosynthesis of flavor-related volatile compounds and of anthocyanin metabolism during peach fruit ripening (Zhou et al. 2015; Cao et al. 2021; Jin et al. 2022). These findings collectively underscore the roles played by PpNAC1 during peach fruit ripening. However, fruit ripening involves dramatic and complex changes beyond the generation of volatiles and anthocyanin, and the precise contribution of PpNAC1 to other ripening-associated changes remains unclear. Furthermore, previous investigations have reported that PpNAC1 is located in the quantitative trait locus (QTL) qMD4.1, which controlls maturity date (Pirona et al. 2013). In conjunction with PpNAC1, another member of the NAC transcription factor family, PpNAC5 (Prupe.4G186800), is also located in the qMD4.1 locus and has been considered to be a candidate gene for maturity date (Nuñez-Lillo et al. 2015). Notably, a 9-bp insertion in the coding sequence of PpNAC5 has been consistently associated with the early-ripening phenotype (Pirona et al. 2013; Guo et al. 2020). However, the mechanisms by which PpNAC5 regulates fruit ripening remain unknown.
Fruit softening is one of the most important physiological changes during peach fruit ripening. The rapid softening of harvested peaches makes them susceptible to rotting and significantly shortens their shelf life, thereby limiting potential markets (Qian et al. 2021). It has been reported that the peach fruit texture and flesh adhesion to the pit are closely related traits controlled by the F-M locus in linkage group 4 (Dettori et al. 2001; Dirlewanger et al. 2006; Ogundiwin et al. 2009). In addition, two pectinases, namely Pp-endoPGM (PGM) and Pp-endoPGF (PGF), have been identified as key regulators of distinct fruit texture and flesh adhesion phenotypes across various peach accessions (Peace et al. 2005; Gu et al. 2016).
The slow ripening (SR) trait is a mutation that inhibits the normal fruit ripening process in peach. This trait is controlled by a single locus sr, which has been mapped on linkage group 4 (G4) andoverlapps with the qMD4.1 locus (Eduardo et al. 2015; Meneses et al. 2016; Nuñez-Lillo et al. 2015). A deletion of 26.6 kb in the sr locus correlates with the SR trait/maturity date, and the gene PpNAC5 was located in the deleted sequence (Eduardo et al. 2015; Nuñez-Lillo et al. 2015). In this study, we conducted an analysis of the 26.6-kb deleted sequence and found that it contains not only the PpNAC5 gene but also the entire promoter sequence of PpNAC1. The tandem arrangement of these two genes were was highly conserved in dicots. Furthermore, our findings demonstrate that both PpNAC1 and PpNAC5 play positive roles in fruit softening during ripening. Moreover, PpNAC1 physically interacted with PpNAC5 to form a protein heterodimer, resulting in greater PpPGF transcription.
Materials and methods
Plant materials
The peach cultivars used in this study were 7-year-old trees maintained at the Fruit Tree Germplasm Repository of Henan Agricultural University (Henan Province, China). Fruit samples from the early-ripening variety ‘FeiYu’ and the late-ripening peach variety ‘JinQiuHongMi’ were collected 15 days before (− 15) to 182 days after full bloom (DAFB). The two freestone melting peach cv. ‘YuMeiRen’ and ‘HuangShuiMi’ were collected at four development stages, including 30, 61, 74, and 87 DAFB for ‘YuMeiRen’ and 35, 54, 85, and 100 DAFB for ‘HuangShuiMi’. The fruits were immediately frozen in liquid nitrogen and stored at − 80 °C. Three biological replicates, each consisting of five fruits from different trees, were used for the experiments.
Nicotiana benthamiana seedlings were grown in pots under a 16/8 h photoperiod condition in a growth chamber at 25 °C for 4 weeks, and the leaves were subsequently used for further tests.
Methods
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using the FastPure® Universal Plant Total RNA Isolation Kit (Vazyme, Jiangsu, China), followed by the synthesis of first-strand cDNA using the HiScript® III RT SuperMix for qPCR(+ gDNA wiper) (Vazyme, Jiangsu, China) according to the manufacturer’s instructions. qRT-PCR reactions were conducted in a total reaction volume of 10 μL, containing 5 μL of SYBR Green I Master Mix, 0.5 μM of each primer, and 200 ng of template cDNA, using the SYBR® Green PCR kit (Takara, China). Amplification was carried out on an ABI Prism 7500 FAST Sequence Detection System (Applied Biosystems, USA). The peach PpTEF2 (Prupe.4G138900) and tobacco β-tubulin (U91564) genes were selected as reference genes (Tong et al. 2009; Schmidt and Delaney 2010). The primer sequences used for qRT-PCR are listed in Table S1. Three biological replicates were performed for each analysis.
Gene isolation and promoter cloning
The full-length Coding DNA Sequence (CDS) of each gene was isolated from the cDNA of peach cv. ‘HuangShuiMi’ using primers designed based on the reference sequences from the peach genome version 2.0 (https://phytozome-next.jgi.doe.gov/info/Ppersica_v2_1). Homologous genes used for phylogenetic analyses in other species were obtained from the NCBI database. Sequence alignments and phylogenic analyses were performed using MEGA7.
Yeast two-hybrid (Y2H) assay
The coding sequences of PpNAC1 (for amino acids 1–285), SlNAC1 and MdNAC5 were cloned into pGBKT7, while the coding sequences of PpNAC5, SlNAC5 and MdNAC1 were fused to pGADT7. The resulting AD and BD plasmids were co-transformed into the Y2H-gold yeast strain (TSC0502, Beijing Tsingke Biotech). P53 and 53-BD + AD were used as positive control and negative control, respectively. The interaction between the proteins was analyzed on SD/-Trp/-Leu (− TL) and SD/-Trp/-His/-Leu/-Ade (− THLA) media supplemented with X-α-gal medium (Clontech, Mountain View, CA, USA). Primers used for cloning in this study are listed in Table S1.
Bimolecular fluorescence complementation (BiFC) assay
The full-length CDSs of PpNAC1 and PpNAC5 were cloned into the pNC-Ecn and pNC-Enn vectors, respectively. These constructs were then introduced into A. tumefaciens GV3101 (pSoup). After incubation, the bacteria were collected and resuspended in infiltration buffer to an OD 600 = 0.8. The bacterial suspensions harboring pNC-Ecn-PpNAC1 and pNC-Enn-PpNAC5 were mixed at a ratio of 1:1 (v:v) and infiltrated into tobacco leaves. The green fluorescent protein (GFP) signals were visualized using a confocal microscope (Nikon, Japan).
Transient transformation in peach fruit
For silencing experiments, a 235-bp cDNA fragment of PpNAC1 and a 343-bp cDNA fragment of PpNAC5 were amplified and then individually inserted into the virus vector pTRV2 to generate the Virus-induced gene silencing (VIGS) constructs.
The recombinant plasmids, along with empty plasmid (pTRV1 and pTRV2) as control, were transformed into A. tumefaciens GV3101 and incubated at 28 ℃ until an OD600 of 0.6. The bacteria were then suspended in infiltration buffer (10 mM MES + 10 mM MgCl2 + 150 µM AS, pH5.6). For the VIGS experiment, the culture containing the recombinant plasmids pTRV1 and pTRV2 was mixed at a ratio of 1:1, and 500 µL of this culture was infiltrated into both sides of peach fruit growing on trees of ‘YuNongMiXiang’ at the end of the S3 developmental stage, following the protocol described by Wang et. al (2022). A total of 100 peach fruits were infiltrated, and the fruits were harvested to detect gene expression levels and measure fruit firmness 2 weeks after the injection.
Measurement of fruit firmness
Peel firmness and flesh firmness were measured using a TA-XTPlusC (Stable Micro Systems, UK). The texture analyzer was set with the following parameters: test depth of 5 mm, pre-test speed of 1 mm s−1, test speed of 1 mm s−1 and post-test speed of 10 mm s−1. A stainless-steel probe with a diameter of 5 mm was used for the measurements. Each side of the fruit was measured three times. Measurements were taken at 48-h intervals, and 10 fruits were tested for each treatment.
Yeast one-hybrid (Y1H) assay
The full-length CDSs of PpNAC1 and PpNAC5 were cloned into pB42AD vector as prey. Different lengths of the PpPGF promoter were cloned into pLacZi vector as bait using the SE Seamless Cloning and Assembly Kit (Beijing ZOMANBIO, China). The Y1H assay was performed following the protocol described in a previous study (Zhang et al. 2022). The primer sequences used for the Y1H assay are provided in Table S1.
Dual-luciferase (LUC) reporter assays
About 1.8-kb promoter regions of PpPGF were amplified and cloned into pGreen II 0800-LUC reporter vectors. The constructs of pSAK277-PpNAC1 and SAK277-PpNAC5 under the CaMV35S promoter were used as effectors. For the transient expression assay, the empty vector (EV) pSAK277, the effector and reporter vectors were separately transformed into A.tumefaciens GV3101 and incubated. After collection, the bacteria were suspended in solution (10 mM MgCl2 + 10 mM MES + 150 µM AS, pH5.6) to an OD600 of 0.8. The effector and the reporter were mixed at ratios of 4:1 or 2:2:1 and statically incubated for 3 h at room temperature. Then, the mixture was infiltrated into four-week-old tobacco leaves. The LUC and REN luciferase activities were measured using the Dual-Luciferase Reporter Assay Kit (Vazyme, China). The LUC signal intensity was also analyzed using the NightSHADE LB 985 in vivo imaging system (Berthold Technologies, Bad Wildbad, Germany). Three biological replicates with a mixture of 6 infiltrated leaves each replicates were performed for four different vector mixtures.
Statistical analysis
The data were presented as means and standard errors calculated from at least three biological replicates. Statistical analysis was conducted using Student’s t-test within SPSS version 21. Statistical significance is indicated with single or double asterisks at P < 0.05 and P < 0.01, respectively.
Results
The tandem genes PpNAC1 and PpNAC5 were located in the maturity date locus and were highly conserved in dicots
A 26.6-kb deletion in the sr locus was previously reported to co-localize with the SR and maturity date traits, and within this deleted region, PpNAC5 has been identified as a candidate gene for this trait (Eduardo et al. 2015; Nuñez-Lillo et al. 2015). In this study, the 26.6-kb deleted sequence was further analyzed and found to include not only the PpNAC5 gene but also the entire promoter region of PpNAC1 (Fig. 1a). This finding raises the possibility that both the PpNAC1 and PpNAC5 genes may be candidate genes for SR /maturity date.
Location of PpNAC1 and PpNAC5 in the maturity date locus. a The tandem genes PpNAC1 and PpNAC5 were located in the SR locus. All of PpNAC5 and promoter of PpNAC1 were located within a 26.6-kb sequence that is deleted in the SR genotype. b Phylogenetic analyses of PpNAC1 and PpNAC5 with their homologous genes in other dicotyledonous species
To further determine the functional roles of PpNAC1 and PpNAC5, an orthologous gene comparison was conducted across multiple species. The analysis showed that PpNAC1 is the ortholog of NOR and Nor-like in tomato and CmNAC–NOR in melon (Fig. 1b) (Gao et al. 2018; Wang et al. 2022a, b). The tandem arrangement of NAC1 and NAC5 in the genomes of various taxa revealed that monocotyledons solely possess NAC1, whereas dicotyledons carry both NAC1 and NAC5, but with variable lengths of their intergenic intervals (Table S2). Remarkably, the tandem configuration of NAC1 and NAC5 in dicots exhibits a notable evolutionary conservation (Table S2). These results suggest an integrated role of these tandemly arranged genes in modulating peach fruit ripening.
Transcript levels of PpNAC1 and PpNAC5 are increased at the onset of peach fruit ripening
To further investigate the roles of PpNAC1 and PpNAC5 in peach fruit development and ripening, the transcript levels of these two genes were assessed during fruit development in the early-ripening cultivar ‘Feiyu’ and late-ripening cultivar ‘JinQiuHongMi’. The transcriptional profiles of PpNAC1 and PpNAC5 were similar during fruit development in each cultivar, although the timing was shifted between the early- and late- ripening peach (Fig. 2a and b). At the preliminary stages of fruit development (S1 and S2), transcript abundance for both genes was relatively low. Significant increases in gene expression were observed concomitant with the initiation of ripening (S3), reaching peak expression levels prior to a subsequent decline during the ripening phase (S4). Additionally, tissue-specific expression analysis revealed higher expression levels of PpNAC1 and PpNAC5 in the fruit than in other tissues like stem, mature leaves and blooming flowers (Fig. S1). These results suggest an involvement of PpNAC1 and PpNAC5 in regulation of the ripening of peach fruits. Furthermore, a distinct peak in expression was observed at the S1 to S2 transition in both the early and late- ripening varieties. This expression peak indicates a potential role for these two genes not only in ripening stages but also in the initial phases of fruit development.
Transcript levels of PpNAC1 and PpNAC5 during peach fruit development and ripening. a Transcript levels of PpNAC1 and PpNAC5 in the early-ripening peach cultivar ‘FeiYu’. b Transcript levels of PpNAC1 and PpNAC5 in the late-ripening peach cultivar ‘JinQiuHongMi’. The fruit development stage was divided into four periods (S1, S2, S3 and S4; identified with black lines) according to the days after full bloom (DAFB). The minus days represent days before full bloom
PpNAC1 physically interacted with PpNAC5
NAC transcription factors are known to form homo- and heterodimers. The evolutionary conservation of the tandem NAC1/5 gene arrangement and their synchronized expression profiles in peach fruit suggest a potential for direct interaction between PpNAC1 and PpNAC5. Yeast two-hybrid (Y2H) assays were carried out to confirm this hypothesis. Since it has been reported that full-length PpNAC1 shows strong self-activation, a truncated PpNAC1, encoding the amino acids 1 to 285, was used according to the previous report (Zhou et al. 2015) for the protein interaction analysis. The results revealed an interaction between PpNAC1 and PpNAC5 (Fig. 3a). The interaction was further confirmed in planta via BiFC assays in tobacco leaves (Fig. 3b).
PpNAC1 physically interacted with PpNAC5. a Yeast two-hybrid analyses confirmed the interaction between PpNAC1 and PpNAC5. The pGBKT7-53 + pGADT7-RecT (P53) is a positive control, and pGBKT7-53 + pGADT7 (AD + 53-BD) is a negative control. b BiFC assay of the interaction between PpNAC1 and PpNAC5. ECN-PpNAC1 + ENN is used as a negative control. c Y2H analyses confirmed the interaction between PpNAC1 and PpNAC5 (PpNAC5 without the 9-base pair segment was identified with an asterisk). d Y2H analyses confirmed the interaction between the two tandem genes NAC1 and NAC5 in apple and in tomato
Variations of a 9-base pair insertion/deletion (INDEL) in the PpNAC5 coding sequence have been associated with the maturation date of peach fruits (Pirona et al. 2013; Guo et al. 2020). To delineate the impact of different PpNAC5 allelic variants on its interaction with PpNAC1, Y2H assays were employed. These assays demonstrated that the allele of PpNAC5 with the 9-base pair segment exhibited an enhanced interaction with PpNAC1 compared to the one with the deletion (PpNAC5*) (Fig. 3c).
The highly conserved tandem arrangement of NAC1 and NAC5 across different dicotyledonous species implies a functional conservation between the two genes. Additional Y2H assays confirmed the physical interaction between the two NAC proteins encoded within tomato and within apple (Fig. 3d). These results indicated this protein–protein interaction is likely preserved across various dicot plants. The implication of these findings is that PpNAC1 and PpNAC5 may operate synergistically in the regulatory network governing fruit ripening.
PpNAC1 and PpNAC5 regulate peach fruit softening
To further understand the contributions of PpNAC1 and PpNAC5 to fruit ripening, VIGS was utilized to suppress expression of these two genes in peach fruits. Peach fruit transiently injected with TRV2-PpNAC1 or TRV2-PpNAC5 were harvested 15 days after infiltration, along with control fruits injected with the empty vector TRV2 (Fig. 4a). Subsequent RT-qPCR analysis revealed a significant reduction in the transcript levels of PpNAC1 and PpNAC5 in the silenced fruits compared to the TRV2 controls (Fig. 4b). Post-infiltration observations revealed that both PpNAC1- and PpNAC5-silenced fruits demonstrated a significant increase in peel and flesh firmness after 7 days of storage when contrasted with fruits injected with TRV2 (Fig. 4c, d). In addition, the expression of PpPGF, one of the polygalacturonase (PG) genes, was markedly reduced in the silenced fruits compared to the TRV2 controls (Fig. 4e). We also investigated the transcript levels of PpPGF together with PpNAC1 and PpNAC5 in different developmental stages of two free stone cultivars and found that the expression of PpPGF was highly expressed at S4 stage, when peaches begin to soften, which is consistent with the high expression of PpNAC1 and PpNAC5 at S3 and S4 (Fig. S2). These results suggest that PpNAC1 and PpNAC5 are involved in promoting peach fruit softening through upregulation of PpPGF expression.
Silencing of PpNAC1 and PpNAC5 delayed peach fruit softening. a Appearance of peach fruits injected with TRV2, TRV2-PpNAC1 and TRV2-PpNAC5. The red circles indicate the injected sites. b Relative expression of PpNAC1 and PpNAC5 detected by RT-qPCR in the transiently silenced fruits. c, d Peel and flesh firmness of the silenced fruits at different days after harvest. Ten fruits were used for each treatment and two injected sides of each fruit were tested. e Relative expression of PpPGF in the transiently silenced fruits. Data are the means ± SD. The asterisks indicate statistically significant differences between the TRV2 and the silenced fruits areas, as determined by Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001)
PpNAC1 and PpNAC5 regulate fruit softening by directly binding to the PpPGF promoter and activating its transcription
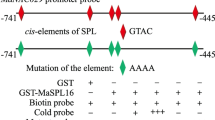
To validate the hypothesis that PpNAC1 and PpNAC5 modulate PpPGF expression, Y1H assays were conducted. About 1800-bp upstream of the PpPGF gene as well as four promoter fragments (P1–P4) were used for Y1H assays (Fig. 5a). The yeast cells co-transformed with the PpPGF promoter as bait and pGADT7 as prey exhibited blue colonies, indicating self-activation of the PpPGF promoter, while the dissected fragments showed no self-activation (Fig. 5b). The yeast cells co-transformed with PpNAC1 and PpPGF promoter fragment P2 or with PpNAC5 and fragment P4 indicated interactions (Fig. 5b). This suggested that PpNAC1 and PpNAC5 regulate PpPGF expression by interacting with separate regions within the PpPGF promoter.
PpNAC1 and PpNAC5 directly bind to the PpPGF promoter and activate its transcription. a Schematic diagrams of the full and four distinct PpPGF promoter fragments. b The growth and color of yeast cells co-transformed with prey (PpNAC1-pGADT7 or PpNAC5-pGADT7) and reporter constructs containing different fragments of the PpPGF promoter, on medium containing aureobasidin A and X-gal. c Schematic diagrams of reporter and effector vectors used for transformation of N. benthamiana leaves. d, e Dual-LUC activation assay and LUC bioluminescence imaging in tobacco leaves co-transformed with the reporter driven by pro-PpPGF and the two effectors, pSAK-PpNAC1 and pSAK-PpNAC5, alone or together. pGreenII 62-SK was used a negative control. Values are the means ± SD. The multiple comparisons were subjected to ANOVA using Duncan’s test, and statistically significant differences (P < 0.05) were indicated by diverse lowercase
LUC reporter assays and LUC bioluminescence imaging were also performed to further verify this regulation. The promoter sequence of PpPGF was cloned into the pGreenII 0800-LUC vector as reporter, and the CDSs of PpNAC1 and PpNAC5 under control of the 35S promoter were used as effectors (Fig. 5c). A significant enhancement of the LUC/REN ratio was recorded in the presence of pSAK-PpNAC1 or pSAK-PpNAC5 (Fig. 5d). Notably, simultaneous co-expression of both effectors (pSAK-PpNAC1 and pSAK-PpNAC5) with the PpPGF reporter vector resulted in an even greater increase in the LUC/REN ratio compared to the individual effectors or the co-expression of pSAK-PpNAC1 or pSAK-PpNAC5 with EV (Fig. 5d). These results were supported by visualization of the LUC fluorescence (Fig. 5e). Collectively, these findings demonstrate that PpNAC1 and PpNAC5 directly bind to the PpPGF promoter and activate its transcription, likely influencing peach fruit softening. Moreover, the evidence suggests a synergistic interaction between PpNAC1 and PpNAC5 in facilitating this transcriptional activation.
Discussion
The NAC (NAM, ATAF and CUC) proteins comprise a plant-specific transcription factor family, and several members have been identified as key regulators of fruit ripening (Olsen et al. 2005; Forlani et al. 2021; Liu et al. 2022). It has been reported that NAC proteins function through the formation of homodimers and/or heterodimers (Puranik et al. 2012). Zhou et al. (2015) demonstrated that one NAC transcription factor, BL, can interact with PpN.AC1 and that the heterodimer of BL and PpNAC1, rather than BL alone, activated the transcription of PpMYB10.1, leading to anthocyanin pigmentation. Here, we discovered that PpNAC1 physically interacts with PpNAC5, forming a protein heterodimer and that co-expression of two proteins positively regulates a softening-related gene to a greater extent than either gene alone. This is consistent with the recently published research that PpNAC1 and PpNAC5 can form heterodimers and both function as ripening enhancers (Zhang et al. 2023). The difference is that we found that the co-expression has a higher activation activity for PpPGF than the genes of PpNAC1 or PpNAC5 alone, while Zhang et al. (2023) suggested that the activation activity of following PpNAC1 and PpNAC5 infiltration was significantly higher than that of PpNAC5, but lower compared with the PpNAC1. These discrepancies may be caused by the different ripening-related genes that were directly activated. It has also been reported that PpNAC1 and DNA demethylase PpDML1 synergistically regulate peach fruit ripening by directly regulating expression of multiple genes required for peach ripening (Cao et al. 2023). Taken together, these findings suggest that PpNAC1 serves as a central regulator modulating various metabolic pathways during peach fruit ripening either independently or through the formation of homodimers and/or heterodimers with other proteins.
Phylogenetic analyses of PpNAC1 and PpNAC5, in conjunction with their respective homologous genes, reveals that these two tandemly arrayed genes are highly conserved in dicotyledonous species. Accordingly, the physical interactions between NAC1 and NAC5 were also verified in tomato and apple (Fig. 3d), which suggested a conserved biological role of NAC1 and NAC5. Extensive research has delineated the involvement of NAC1 homologs in fruit ripening. However, reports on NAC5 and its corresponding homologs remain scant. Notably, Wei et al. (2023) demonstrated that, in apple, phosphorylation of MdNAC72 (orthologous to PpNAC5) mediated by MdMAPK3 exerts influence over the softening of apple fruit during storage. Moreover, the overexpression of AdNAC72 in kiwifruit not only enhances expression of a methionine sulfoxide reductase (AdMsrB1), but also increases free Met and ACC content and ethylene production rates (Fu et al. 2021). In our study, both PpNAC1 and PpNAC5 have regulatory effects on the softening of peach fruit. Moreover, Zhang et al. have also demonstrated the regulatory roles of PpNAC1 and PpNAC5 in fruit enlargement and ripening by stable or transient overexpression in tomato and strawberry, respectively (Zhang et al. 2023). All these results suggest the NAC5 may participate in regulation of fruit ripening, thereby indicating the evolutionary conservation of the functional roles of PpNAC1 and PpNAC5.
Previous research has reported that PpNAC5 is a prime candidate gene linked to maturity date locus located on Chr. 4 (Pirona et al. 2013). A 9-bp insertion/deletion (INDEL) within the coding sequence of PpNAC5 has been correlated with phenotypic variation in maturity date, where early ripening individuals exhibit the 9-bp insertion and late ripening ones lack this segment. However, the explicit function and genetic variation of PpNAC5 in regulating developmental and ripening remained unclear. In the present study, phenotypic and molecular characterization of PpNAC5-knockdown transgenic peach fruits elucidated that PpNAC5 plays a key role in accelerating the ripening of peach fruits by regulating fruit softening. Additionally, our findings suggest that the presence of the 9-bp insertion within PpNAC5 could potentiate its interaction with PpNAC1, thereby potentially contributing to the manifestation of an earlier ripening phenotype.
In addition to PpNAC5, PpNAC1 is likewise posited as a key regulatory gene for peach maturity date, because of its significant contributions to fruit ripening. Here, our investigation supports the notion that both the PpNAC1 and PpNAC5 are viable candidate genes for the SR /maturity date traits. Peach fruit development and ripening encompass distinct growth phases: the first exponential growth phase (S1), the onset of pit hardening (S2), the second exponential growth phase (S3), and ripening (S4) (Tonutti et al. 1991). Notably, the span of the S2 phase varies according to the cultivar, being shorter in early ripening varieties and longer in late ripening ones (Bonghi et al. 2011). In this study, both PpNAC1 and PpNAC5 exhibited high expression levels during the S3 and S4 phases, which suggested their roles in peach ripening. Intriguingly, our data reveal expression peaks for PpNAC1 and PpNAC5 at the S1 to S2 transition in both early and late ripening varieties. This transitional juncture marks a deceleration in fruit growth and precedes the lignification of the endocarp, an event synonymous with the onset of pit hardening that persists until the end of the S2 stage (Dardick et al. 2010). The increasing expression of PpNAC1 and PpNAC5 at the onset of the S2 stage indicated their potential roles in influencing peach fruit development and consequently modulating the maturity date of the peach fruit. It has also been verified by the recently reported that PpNAC1 and PpNAC5 may participate in the regulation of fruit enlargement through activating cell elongation-related genes (Zhang et al. 2023).
References
Bonghi C, Trainotti L, Botton A, Tadiello A, Rasori A, Ziliotto F, Zaffalon V, Casadoro G, Ramina A (2011) A microarray approach to identify genes involved in seed-pericarp cross-talk and development in peach. BMC Plant Biol 11:107. https://doi.org/10.1186/1471-2229-11-107
Cao XM, Wei CY, Duan WY, Gao Y, Kuang JF, Liu MC, Chen KS, Klee H, Zhang B (2021) Transcriptional and epigenetic analysis reveals that NAC transcription factors regulate fruit flavor ester biosynthesis. Plant J 106:785–800. https://doi.org/10.1111/tpj.15200
Cao X, Li X, Su Y, Zhang C, Wei C, Chen K, Grierson D, Zhang B (2023) Transcription factor PpNAC1 and DNA demethylase PpDML1 synergistically regulate peach fruit ripening. Plant Physiol 22:kiad627. https://doi.org/10.1093/plphys/kiad627
Cheng X, Cui ZG, Jiang YB, Chen Y, Tan B, Cheng J, Zhang LL, Ye X, Wang XB, Zhang HP, Lian XD, Li JD, Li ZQ, Zheng XB, Feng JC (2023) PpeERF115 regulates peach fruit ripening by increasing polyamine turnover through up-regulation of genes involved in polyamine synthesis and catabolism. Postharvest Biol Technol 204:112432. https://doi.org/10.1016/j.postharvbio.2023.112432
Dai JY, Xu Z, Xu YT, Fang ZH, Shah K, Kang T, Wu HX, Zhang D, Xing LB, Ma JJ, Liu HK, Hu YN, Zhao CP (2023) A novel NAC transcription factor, PpNAP6, is involved in peach ripening by activating ethylene synthesis. Postharvest Biol Technol 201:112363. https://doi.org/10.1016/j.postharvbio.2023.112363
Dardick CD, Callahan AM, Chiozzotto R, Schaffer RJ, Piagnani MC, Scorza R (2010) Stone formation in peach fruit exhibits spatial coordination of the lignin and flavonoid pathways and similarity to Arabidopsis dehiscence. BMC Biol 8:13. https://doi.org/10.1186/1741-7007-8-13
Dettori MT, Quarta R, Verde I (2001) A peach linkage map integrating RFLPs, SSRs, RAPDs, and morphological markers. Genome 44:783–790. https://doi.org/10.1139/g01-065
Dirlewanger E, Cosson P, Boudehri K, Renaud C, Capdeville G, Tauzin Y, Laigret F, Moing A (2006) Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet Genomes 3:1–13. https://doi.org/10.1007/s11295-006-0053-1
Eduardo I, Pacheco I, Chietera G, Bassi D, Pozzi C, Vecchietti A, Rossini L (2011) QTL analysis of fruit quality traits in two peach intraspecific populations and importance of maturity date pleiotropic effect. Tree Genet Genomes 7(2):323–335. https://doi.org/10.1007/s11295-010-0334-6
Eduardo I, Picañol R, Rojas E, Batlle I, Howad W, Aranzana MJ, Arús P (2015) Mapping of a major gene for the slow ripening character in peach: co-location with the maturity date gene and development of a candidate gene-based diagnostic marker for its selection. Euphytica 205:627–636. https://doi.org/10.1007/s10681-015-1445-9
Forlani S, Mizzotti C, Masiero S (2021) The NAC side of the fruit: tuning of fruit development and maturation. BMC Plant Biol 21:238. https://doi.org/10.1186/s12870-021-03029-y
Fu BL, Wang WQ, Liu XF, Duan XW, Allan AC, Grierson D, Yin XR (2021) An ethylene-hypersensitive methionine sulfoxide reductase regulated by NAC transcription factors increases methionine pool size and ethylene production during kiwifruit ripening. New Phytol 232(1):237–251. https://doi.org/10.1111/nph.17560
Gao Y, Wei W, Zhao XD, Tan XL, Fan ZQ, Zhang YP, Jing Y, Meng LH, Zhu BZ, Zhu HL, Chen JY, Jiang CZ, Grierson D, Luo YB, Fu DQ (2018) A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic Res 5:75. https://doi.org/10.1038/s41438-018-0111-5
Gao Y, Wei W, Fan ZQ, Zhao XD, Zhang YP, Jing Y, Zhu BZ, Zhu HL, Shan W, Chen JY, Grierson D, Luo YB, Jemric T, Jiang CZ, Fu Q (2020) Re-evaluation of the nor mutation and the role of the NAC-NOR transcription factor in tomato fruit ripening. J Exp Bot 71:3560–3574. https://doi.org/10.1093/jxb/
Gu C, Guo ZH, Cheng HY, Zhou YH, Qi KJ, Wang GM, Zhang SL (2019) A HD-ZIP II HOMEBOX transcription factor, PpHB.G7, mediates ethylene biosynthesis during fruit ripening in peach. Plant Sci 278:12–19. https://doi.org/10.1016/j.plantsci.2018.10.008
Gu C, Wang L, Wang W, Zhou H, Ma BQ, Zheng HY, Fang T, Ogutu C, Vimolmangkang S, Han YP (2016) Copy number variation of a gene cluster encoding endopolygalacturonase mediates flesh texture and stone adhesion in peach. J Exp Bot 67(6):1993–2005. https://doi.org/10.1093/jxb/erw021
Guo J, Cao K, Deng C, Li Y, Zhu GR, Fang WC, Chen CW, Wang XW, Wu JL, Guan LP, Wu S, Guo WW, Yao JL, Fei ZJ, Wang LR (2020) An integrated peach genome structural variation map uncovers genes associated with fruit traits. Genome Biol 21:258. https://doi.org/10.1186/s13059-020-02169-y
Jin ZN, Wang JJ, Cao XM, Wei CY, Kuang JF, Chen KS, Zhang B (2022) Peach fruit PpNAC1 activates PpFAD3-1 transcription to provide ω-3 fatty acids for the synthesis of short-chain flavor volatiles. Hortic Res 9:85. https://doi.org/10.1093/hr/uhac085
Li XJ, Martín-Pizarro C, Zhou LL, Hou BZ, Wang YY, Shen YY, Li BB, Posé D, Qin GZ (2023) Deciphering the regulatory network of the NAC transcription factor FvRIF, a key regulator of strawberry (Fragaria vesca) fruit ripening. Plant Cell 35:4020–4045. https://doi.org/10.1093/plcell/koad210
Liu GS, Li HL, Grierson D, Fu DQ (2022) NAC Transcription factor family regulation of fruit ripening and quality: a review. Cells 11(3):525. https://doi.org/10.3390/cells11030525
Lü PT, Yu S, Zhu N, Chen YR, Zhou BY, Pan Y, Tzeng D, Fabi JP, Argyris J, Garcia-Mas J, Ye NH, Zhang JH, Grierson D, Xiang J, Fei ZJ, Giovannoni J, Zhong SL (2018) Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat Plants 4(10):784–791. https://doi.org/10.1038/s41477-018-0249-z
Martin-Pizarro C, Vallarino JG, Osorio S, Meco V, Urrutia M, Pillet J, Casanal A, Merchante C, Amaya I, Willmitzer L, Fernie AR, Giovannoni JJ, Botella MA, Valpuesta V, Pose D (2021) The NAC transcription factor FaRIF controls fruit ripening in strawberry. Plant Cell 33:1574–1593. https://doi.org/10.1093/plcell/
Meneses C, Ulloa-Zepeda L, Cifuentes-Esquivel A, Infante R, Cantin CM, Batlle I, Arús P, Eduardo I (2016) A codominant diagnostic marker for the slow ripening trait in peach. Mol Breeding 36:77. https://doi.org/10.1007/s11032-016-0506-7
Migicovsky Z, Yeats TH, Watts S, Song J, Forney CF, Burgher-MacLellan K, Somers DJ, Gong YH, Zhang ZQ, Vrebalov J, Velzen RV, Giovannoni JG, Rose JKC, Myles S (2021) Apple ripening is controlled by a NAC transcription factor. Front Genet 12:671300. https://doi.org/10.3389/fgene.2021.671300
Monti LL, Bustamante CA, Osorio S, Gabilondo J, Borsani J, Lauxmann MA, Maulión E, Valentini G, Budde CO, Fernie AR, Lara MV, Drincovich MF (2016) Metabolic profiling of a range of peach fruit varieties reveals high metabolic diversity and commonalities and differences during ripening. Food Chem 190:879–888. https://doi.org/10.1016/j.foodchem.2015.06.043
Nuñez-Lillo G, Cifuentes-Esquivel A, Troggio M, Micheletti D, Infante R, Campos-Vargas R, Orellana A, Blanco-Herrera F, Meneses C (2015) Identification of candidate genes associated with mealiness and maturity date in peach [Prunus persica (L.) Batsch] using QTL analysis and deep sequencing. Tree Genet Genomes 11:86. https://doi.org/10.1007/s11295-015-0911-9
Ogundiwin EA, Peace CP, Gradziel TM, Parfitt DE, Bliss FA, Crisosto CH (2009) A fruit quality gene map of Prunus. BMC Genomics 10:587. https://doi.org/10.1186/1471-2164-10-587
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10:79–87. https://doi.org/10.1016/j.tplants.2004.12.010
Peace C, Crisosto C, Gradziel T (2005) Endopolygalacturonase: a candidate gene for freestone and melting flesh in peach. Mol Breeding 16:21–31. https://doi.org/10.1007/s11032-005-0828-3
Pirona R, Eduardo I, Pacheco I, Linge CDS, Miculan M, Verde I, Tartarini S, Dondini L, Pea G, Bassi D, Rossini L (2013) Fine mapping and identification of a candidate gene for a major locus controlling maturity date in peach. BMC Plant Biol 13:166. https://doi.org/10.1186/1471-2229-13-166
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381. https://doi.org/10.1016/j.tplants.2012.02.004
Qian M, Xu Z, Zhang ZH, Li Q, Yan XY, Liu HK, Han MY, Li FR, Zheng JC, Zhang D, Zhao CP (2021) The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 254:22. https://doi.org/10.1007/s00425-021-03673-6
Schmidt GW, Delaney SK (2010) Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet 283:233–241. https://doi.org/10.1007/s00438-010-0511-1
Tatsuki M, Haji T, Yamaguchi M (2007) The peach 1-aminocyclopropane-1-carboxylic acid synthase isogene, Pp-ACS1, is required for fruit softening. Springer, Dordrecht, pp 227–228. https://doi.org/10.1007/978-1-4020-6014-4_48
Tatsuki M, Nakajima N, Fujii H, Shimada T, Nakano M, Hayashi K, Hayama H, Yoshioka H, Nakamura Y (2013) Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J Exp Bot 64(4):1049–1059. https://doi.org/10.1093/jxb/ers381
Tong ZG, Gao ZH, Wang F, Zhou J, Zhang Z (2009) Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol 10:71. https://doi.org/10.1186/1471-2199-10-71
Tonutti P, Casson P, Ramina A (1991) Ethylene biosynthesis during peach fruit development. J Am Soc Hortic Sci 116:274–279. https://doi.org/10.21273/jashs.116.2.274
Wang JF, Tian SW, Yu YT, Ren Y, Guo SG, Zhang J, Li MY, Zhang HY, Gong GY, Wang M, Xu Y (2022a) Natural variation in the NAC transcription factor NONRIPENING contributes to melon fruit ripening. J Integr Plant Biol 64(7):1448–1461. https://doi.org/10.1111/jipb.13278
Wang W, Liu SL, Cheng X, Cui ZG, Jiang YB, Zheng XB, Tan B, Cheng J, Ye X, Li JD, Li ZQ, Zhang LL, Wang XB, Zhang HP, Lian XD, Feng JC (2022b) Ethylene and polyamines form a negative feedback loop to regulate peach fruit ripening via the transcription factor PpeERF113 by regulating the expression of PpePAO1. Postharvest Biol Technol 190:111958. https://doi.org/10.1016/j.postharvbio.2022.111958
Wang XB, Pan L, Wang Y, Meng JR, Deng L, Niu L, Liu H, Ding YF, Yao JL, Nieuwenhuizen NJ, Ampomah-Dwamena C, Lu ZH, Cui GC, Wang ZQ, Zeng WF (2021) PpIAA1 and PpERF4 form a positive feedback loop to regulate peach fruit ripening by integrating auxin and ethylene signals. Plant Sci 313:111084. https://doi.org/10.1016/j.plantsci.2021.111084
Wei Y, Liu Z, Lv TX, Xu YX, Wei YJ, Liu WT, Liu L, Wang A, Li T (2023) Ethylene enhances MdMAPK3-mediated phosphorylation of MdNAC72 to promote apple fruit softening. Plant Cell 35(8):2887–2909. https://doi.org/10.1093/plcell/koad122
Zhang RX, Liu Y, Zhang X, Chen X, Sun J, Zhao Y, Zhang J, Yao JL, Liao L, Zhou H, Han Y (2023) Two adjacent NAC transcription factors regulate fruit maturity date and flavor in peach. New Phytol 241(2):632–649. https://doi.org/10.1111/nph.19372. (Epub. PMID: 37933224)
Zhang ST, Fu MM, Li ZQ, Li JW, Hai LF, Chen CY, Zheng XB, Tan B, Li JD, Cheng J, Wang W, Zhang LL, Ye X, Feng JC (2022) VvEIL2 and VvEIL4 regulate ethylene synthesis and carotenoid metabolism during senescence of grape rachis. Postharvest Biol Technol 187:111853. https://doi.org/10.1016/j.postharvbio.2022.111853
Zhou H, Kui LW, Wang H, Gu C, Dare AP, Espley RV, He H, Allan AC, Han Y (2015) Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J 82:105–121. https://doi.org/10.1111/tpj.12792
Funding
This work was supported by Innovative Research Group Project of the National Natural Science Foundation of China (32002014), the National Key Research and Development Program of China (2019YFD1000104), and the Special Fund for Henan Agriculture Research System (S2014-11-G02).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xiaofei Wang, Langlang Zhang and Kang Dong. The first draft of the manuscript was written by Langlang Zhang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Wang, X., Dong, K. et al. Tandem transcription factors PpNAC1 and PpNAC5 synergistically activate the transcription of the PpPGF to regulate peach softening during fruit ripening. Plant Mol Biol 114, 46 (2024). https://doi.org/10.1007/s11103-024-01429-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11103-024-01429-w