Abstract
Key message
Copper deficiency and excess differentially affect iron homeostasis in rice and overexpression of the Arabidopsis high-affinity copper transporter COPT1 slightly increases endogenous iron concentration in rice grains.
Abstract
Higher plants have developed sophisticated mechanisms to efficiently acquire and use micronutrients such as copper and iron. However, the molecular mechanisms underlying the interaction between both metals remain poorly understood. In the present work, we study the effects produced on iron homeostasis by a wide range of copper concentrations in the growth media and by altered copper transport in Oryza sativa plants. Gene expression profiles in rice seedlings grown under copper excess show an altered expression of genes involved in iron homeostasis compared to standard control conditions. Thus, ferritin OsFER2 and ferredoxin OsFd1 mRNAs are down-regulated whereas the transcriptional iron regulator OsIRO2 and the nicotianamine synthase OsNAS2 mRNAs rise under copper excess. As expected, the expression of OsCOPT1, which encodes a high-affinity copper transport protein, as well as other copper-deficiency markers are down-regulated by copper. Furthermore, we show that Arabidopsis COPT1 overexpression (C1 OE) in rice causes root shortening in high copper conditions and under iron deficiency. C1 OE rice plants modify the expression of the putative iron-sensing factors OsHRZ1 and OsHRZ2 and enhance the expression of OsIRO2 under copper excess, which suggests a role of copper transport in iron signaling. Importantly, the C1 OE rice plants grown on soil contain higher endogenous iron concentration than wild-type plants in both brown and white grains. Collectively, these results highlight the effects of rice copper status on iron homeostasis, which should be considered to obtain crops with optimized nutrient concentrations in edible parts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous interactions between copper (Cu) and iron (Fe) homeostasis have been described in Saccharomyces cerevisiae (Puig and Thiele 2002), humans (Gulec and Collins 2014) and plants (Puig et al. 2007). Cu and Fe are essential transition metals for living organisms. Both elements have an electronic configuration that allows them to form coordination complexes with organic molecules and to act as redox cofactors in many proteins, being key components in respiratory and photosynthetic electronic transport chains (Puig and Peñarrubia 2009; Yruela 2013). For instance, Fe serves as a cofactor for several proteins in chloroplasts, including ferredoxin (Fd), which is involved in the electron transport chain (Briat 1996). Cu and Fe display similar physical and chemical properties, and changes in their bioavailability during atmosphere evolution have driven metalloprotein substitution (Crichton and Pierre 2001). Fe uptake and mobilization depend on Cu in yeast and humans (Gulec and Collins 2014), although this connection remains needs in depth investigation in plants (Bernal et al. 2012; Waters et al. 2012; Perea-García et al. 2013; Waters and Armbrust 2013). Under metal deficiency conditions, induction of reactive oxygen species (ROS) could also affect Cu–Fe interactions (Ravet and Pilon 2013). Mechanisms of excess metal detoxification include the synthesis of metallothioneins (MTs), which contain cysteine-rich domains with the capacity to interact with metals (Leszczyszyn et al. 2013).
Cu enters the plant through members of the CTR family of high-affinity Cu transport proteins, denoted as COPT in plants. The CTR/COPT protein family is conserved in eukaryotes and functions in Cu+ uptake (Puig 2014). These proteins contain three transmembrane segments with the amino-terminus in the extracytosolic space and the carboxyl-terminus facing the cytosol. Most CTR-type proteins contain methionine-rich domains at their amino-terminus that form Cu-binding centers. Arabidopsis contains six COPT genes, annotated as COPT1-6 (Sancenón et al. 2003; Puig 2014; Peñarrubia et al. 2015), whereas Oryza sativa expresses seven COPT family members listed as OsCOPT1-7 (Yuan et al. 2010, 2011). Transgenic Arabidopsis lines that overexpress the AtCOPT1 transport protein (COPT1 OEor C1 OE) are Cu sensitive (Andrés-Colás et al. 2010), whereas Arabidopsis copt2 mutants, which lack of COPT2 expression, exhibit resistance to Fe deficiency (Perea-García et al. 2013). Specifically, the copt2 lines exhibit reduced chlorosis and produce a larger number of seeds under simultaneous Cu and Fe deficiencies, which supports the interaction between both metals (Perea-García et al. 2013).
Fe deficiency, which leads to anemia, is a widespread health problem that affects more than 2 billion people worldwide (White and Broadley 2009; Stevens et al. 2013). Multiple interventions have been encouraged to reduce micronutrient malnutrition, and genetic engineering-based biofortification is one of the most effective. Since rice (Oryza sativa L.) is one of the most widely consumed crops worldwide, biofortification strategies for increased Fe transport and delivery to rice grains have been prioritized as a suitable approach to provide Fe to anemia-susceptible populations (Sperotto et al. 2012; Bhullar and Gruissem 2013).
Nutrient uptake and distribution in plants depend on many processes, which makes difficult the use of conventional breeding techniques to improve crop nutritional quality. Unlike other grass species, rice utilizes strategy II and partially strategy I to transport Fe (Ishimaru et al. 2006). Strategy I consists in proton excretion, ferric-chelate reduction, and Fe uptake through IRON REGULATED TRANSPORTER (IRT1). A lack of inducible ferric-chelate reductase activity at the root surface has been described in rice (Ishimaru et al. 2006). In Strategy II, Fe(III)-phytosiderophore chelates of the mugineic acid family (MAs) are synthesized from S-adenosylmethionine (SAM) through nicotianamine (NA), an intermediate produced, among others, by NA synthase (NAS) and NA aminotransferase (NAAT) enzymes. Although originally identified as Fe(III)-phytosiderophore transporters, some of the members of the yellow-stripe (YS1/YSL) family transport structurally similar compounds, such as Fe(II)–NA. In this sense, the rice OsYSL2 member of this family transports Fe(II)- and Mn(II)-NA, but not Fe(III)–deoxymugineic acid (Koike et al. 2004). OsYSL2 expression also leads to higher Fe concentration in seeds (Ishimaru et al. 2010). A successful strategy has consisted in increasing Fe transport to the aerial part through NA synthesis, which acts as an Fe chelator in the phloem by overexpressing NAS genes (Lee et al. 2009; Johnson et al. 2011). Plastidic Fe storage protein ferritin, which aimed to prevent cell damage caused by the free metal, was specifically expressed in the endosperm leading to increased Fe concentration in rice grains (Goto et al. 1999; Oliva et al. 2014).
The distinct networks that regulate Fe sensing, uptake and intracellular distribution in rice are being uncovered (Kobayashi and Nishizawa 2012). Two candidates of putative Fe-sensing factors have been described in rice: iron deficiency-responsive element-binding factor 1 (OsIDEF1) and hemerythrin motif-containing really interesting new gene- and zinc-finger (OsHRZ) proteins (Kobayashi and Nishizawa 2014). OsIDEF1, whose transcript levels do not change in response to Fe availability, is a positive regulator of iron-related transcription factor 2 (OsIRO2), a member of the basic helix-loop-helix (bHLH) transcription factor family induced under Fe deficiency (Ogo et al. 2006). Rice lines overexpressing OsIRO2 exhibit improved tolerance to Fe deficiency, which indicates that OsIRO2 plays a key role in Fe uptake and transport in both germination and seed development (Ogo et al. 2011). OsHRZ1 and OsHRZ2 are E3 ubiquitin ligases that negatively regulate Fe accumulation under Fe sufficiency. However, the nature of the detected Fe signal, which may involve other metals, oxygen or redox status, remains elusive (Kobayashi et al. 2013; Kobayashi and Nishizawa 2014).
The present work aims to explore the interaction between the homeostatic networks of Cu and Fe in a relevant crop like rice. In order to explore new Fe and Cu homeostatic connections, we analyze the expression of Fe-related genes when adding or not Cu to the media and in transgenic rice plants that overexpress AtCOPT1.
Materials and methods
Plant growth conditions and treatments
Rice (O. sativa L. cv. Nipponbare) seeds were surface-sterilized and stratified for 2 days at 4 °C, and then germinated on 1/2 MS (Sigma) plates with 1% sucrose (Murashige and Skoog 1962) considered as the standard commercial control condition. Variable CuSO4 concentrations were added whenever indicated. For severe Cu-deficient conditions, 1/2 MS was supplemented with 100 µM bathocuproinedisulfonic acid disodium (BCS). In order to test the effect of Cu and Fe deficiency on plants, the components of 1/2 MS were prepared separately according to the following conditions: macronutrients (10 mM NH4NO3, 9.4 mM KNO3, 0.37 mM MgSO4, 0.62 mM KH2PO4, and 1.13 mM CaCl2), micronutrients (50 μM H3BO3, 36.6 μM MnSO4, 15 μM ZnSO4, 0.57 μM NaMoO4, and 0.05 μM CoCl2), 50 μM Fe-EDTA, 0.25 mM KI, 1 μM CuSO4, 0.05% MES, 1% sucrose, and 0.8% phytoagar, pH 5.7. The plates prepared as specified were considered as Fe- and Cu-sufficient medium (+Cu +Fe). The rest of the media were prepared as follows: Cu-deficient (with no added Cu) and Fe-sufficient medium (−Cu +Fe), Cu-sufficient and Fe-deficient (with no added Fe) medium (+Cu −Fe), and Cu and Fe-deficient (with no added Cu and Fe) medium (−Cu −Fe). Seedlings were grown under neutral day photoperiod (12 h light, 23 °C/12 h darkness, 16 °C) (65 mmol m−2cool-white fluorescent light) in a Sanyo Growth Cabinet MLR-350 T for 8 days.
For hydroponic cultures, rice grains were germinated in Petri dishes filled with deionized water at 30 °C in darkness. Then plantlets were cultivated in hydroponic boxes that contained standard Hoagland solution (0.1×) pH 5.8, as described by Hermans et al. (2005). After 2 weeks of adaptation, the −Cu −Fe treatment (corresponding to Hoagland medium without Cu and Fe) applied, for 2 more weeks. For these experiments, plants were grown under long day conditions (16 h light, 23 °C/8 h darkness, 16 °C; 65 mmol m− 2 cool-white fluorescent light) for 1 month.
The obtained 5 month-old plants, unless other age is stated, were grown in the greenhouse in 22 × 20 cm pots that contained a 3:1 mixture of substrate (SEED PRO5050 Projar Professional) and perlite (pH 6–6.5). Plants were grown under long day conditions (16 h light, 23 °C/ 8 h dark, 27 °C). Relative humidity was maintained between 65–83% until the emergence of 6–7 leaves in panicles. Root and shoot lengths from 8 day-old seedlings were measured by the Image J (http://rsb.info.nih.gov/ij) software.
Chlorophyll quantification
Chlorophylls from the excised rice aerials parts were extracted with cold acetone 90% (v/v) and gentle agitation at 4 °C overnight. Subsequently, chlorophyll concentration was spectrophotometrically determined as described in Garcia-Molina et al. (2011), and the resulting values were normalized to fresh weight (FW).
Rice transformation
The expression cassette CaMV35S::AtCOPT1-HA::NOS (Andrés-Colás et al. 2010) was excised from the pBI121 plasmid by HindIII and SacI digestion and was cloned into a pCAMBIA1305.1 binary vector (Cambia, http://www.cambia.org/). The resulting plasmid was transformed into the Agrobacterium tumefaciens C58 strain. Rice (O. sativa L. cv. Nipponbare) plants were transformed according to Hiei et al. (1994).
Gene expression by semi-quantitative and real-time quantitative PCR
Total RNA was extracted from 8 day-old seedling shoots grown under the indicated conditions by the RNAeasy mini plant kit (Qiagen) following the manufacturer’s instructions. RNA was quantified by UV spectrophotometry and its integrity was visually assessed on ethidium bromide-stained agarose gels. Total RNA (1.5 µg) was first converted into cDNA by reverse transcription using SuperScript II reverse transcriptase (Invitrogen) and anchored oligo(dT)15 (Roche). Semi-quantitative PCRs were performed with specific primers (Supplemental Table S1). Real-time quantitative PCRs were carried out with SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen) and a specific primer (Supplemental Table S1) in a CFX96 touch real-time PCR detection system (Bio-Rad) with one cycle of 95 °C for 2 min, and 40 cycles that consisted in 95 °C for 30 s and 60 °C for 30 s. Values were normalized to the ACTIN1 mRNA levels, and the wild-type (WT) used under the control conditions was taken as a reference.
Gene expression analysis by long oligonucleotide microarrays
Genome-wide gene expression studies were carried out by rice 45K whole-genome oligonucleotide DNA microarrays, provided by the University of Arizona (http://www.ag.arizona.edu/microarray). The RNA from four independent biological replicates of 8 day-old seedling shoots grown under each condition (1/2 MS and 1/2 MS + 75 µM CuSO4) was amplified by the MessageAmp aRNA kit (Ambion). The obtained amplified RNA (aRNA) was labeled by using the CyDye Post-Labeling Reactive Dye Pack, which generates fluorescent Cy3- and Cy5-labeled probes by a post-labeling (amino allyl) method (GE Healthcare). Each biological replicate was labeled with Cy3 and Cy5 to produce four pairs of replicate dye-swaps, which were used for microarray hybridization. Both amplified RNA and labeled aRNA were quantified in a NanoDrop ND-1000 spectrophotometer (Thermo scientific). Manual hybridization was performed according to the following protocol: microarrays were re-hydrated by exposing them to water vapor for 10 s (four times). Next microarrays were cross-linked by ultraviolet (UV) irradiation of 180 mJ in a Stratalinker 1800 UV (Stratagene). Subsequently, microarrays were washed with 1% sodium dodecyl sulfate (SDS; w/v) for 5 min and ten times with H2O milliQ (Millipore), washed with 100% ethanol five times and then dried by centrifugation at 200 g. After this pre-treatment, microarrays were hybridized overnight at 55 °C with hybridization solution [48 pmol of each labeled sample; deionized formamide 50% (v/v)] 3× saline-sodium citrate (SSC); Denhardt’s solution [5× and SDS 0.1% (w/v)]. The hybridization solution was denaturalized for 5 min at 65 °C and applied between the microarray and a coverslip LifterSlipTM (Erie Scientific). After hybridization, microarrays were washed with SSC and SDS at decreasing concentrations and dried by centrifugation at 1000 rpm. The hybridized microarrays were scanned using a Gene Pix Autoloader 4200AL (Axon/Molecular Devices) at 532 nm (Cy3) and 635 nm (Cy5). The expression values were obtained with the GenePix Pro 6.0 microarray-analysis software (Axon Molecular Devices). Quality control, normalization and determination of the differentially expressed genes were conducted in R using the Limma package (Smyth 2005), as previously described in Andrés et al. (2009). To determine the false-positive ratio (FDR), the P-values were adjusted by a multiple test according to the Benjamini and Hochberg (1995) method. Genes that displayed a differential expression with fold-change (FC) minimum of 2 for both conditions, represented as log2(FC) ≥ |1| (−1 > log-fold change [1/2 MS + 75 µM/ 1/2 MS] > 1) with a P-value of 0.05 (FDR = 0.5%) were selected. The microarray datasets have been deposited in the public NCBI database (GSE8910).
Western blot
Crude extracts from the WT and transgenic lines grown on 75 µM CuSO4 were obtained by crushing the material in a mortar with N2 liquid and adding 3× SDS buffer [Tris–HCl 250 mM, pH 6.8; glycerol 20% (v/v); SDS 4% (w/v); β-mercaptoethanol 10% (v/v) and bromophenol blue 0.025% (w/v)] in a 1:2 ratio (w/v). Samples were boiled for 30 min at 90 °C and centrifuged at 12,000 rpm at 4 °C for 30 min to remove cell debris. Twenty microlitre of the protein extract were analyzed by SDS-PAGE, transferred to a nitrocellulose membrane and blotted with an antibody against human influenza Hemaglutinin epitope (HA; Roche). Ponceau staining was used as a loading control. Antigen/antibody-peroxidase detection was carried out with the ECL system (Amersham) following the manufacturer’s recommendations and exposing the membrane in an ImageQuant 4000 instrument (GE Healthcare).
Metal accumulation and hormone measurements
For the biochemical analyses, fresh 8 day-old O. sativa seedling shoots grown under the indicated conditions were washed once with 20 µM EDTA and three times with MilliQ water. For the ABA, IAA and JA determinations, plant material was lyophilized and then analyzed by UHPLC (ultra-high-pressure liquid chromatography) (Q-Exactive, ThermoFisher Scientific) at the IBMCP (Valencia, Spain). Lyophilized samples and seedlings were digested with 65% (v/v) HNO3 at 80–90 °C. Digested samples were diluted with millipore water (Purelab Ultra), and Cu and Fe concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS) at the Servei Central d’Instrumentació Científica (Universitat Jaume I) and Servei Central de Suport a la Investigació Experimental (SCSIE; Universitat de València).
Perl’s staining
Seeds from the WT and transgenic plants were germinated in ½MS and embedded with equal volumes of 4% (v/v) HCl and 4% (w/v) K-ferrocyanide (Perl’s stain solution) for 15 min and incubated for 30 min at room temperature (Stacey et al. 2007).
Statistical analysis
A statistical analysis of the relative expression studies was performed by comparing the relative expression of the genes based on the pair-wise fixed reallocation randomization test (P < 0.05; (Pfaffl 2001)). For the remaining parameters, this was carried out by a two-way ANOVA with the means compared by the Duncan test (P < 0.05), or by a Kruskal–Wallis test when the data distribution was non parametric, using the InfoStat software, version 2010 (http://www.infostat.com.ar) (Di Rienzo et al. 2011).
Results
Characterization of copper nutritional responses in Oryza sativa
To evaluate how rice Cu status changes in accordance with the Cu present in the medium, 8 day-old seedlings were grown on 1/2 MS commercial plates supplemented with Cu at concentrations between 0 and 100 μM (Fig. 1a). As expected, we observed a positive correlation between the endogenous Cu concentration found in shoots from 8 day-old seedlings and the increasing of Cu added to the medium. According to the Cu sufficiency range established for higher plants (5–20 μg/g DW; Marschner 2012), the shoots of rice seedlings grown in medium with no Cu supplementation (0 µM CuSO4) contained Cu levels at the lower limit of sufficiency (5.1 μg/g DW), whereas Cu excess was found in plants grown on the media supplemented with concentrations of 10 μM CuSO4 (28.6 μg/g DW) and higher (Fig. 1a).
Characterization of O. sativa plants grown in media with different Cu concentrations. a Endogenous Cu concentration of the 8 day-old seedling shoots from the WT plants grown on a Cu scale that ranged from control conditions (0 μM CuSO4) with slight Cu deficiency to severe excess (100 µM CuSO4). Cu was determined by atomic absorption spectrophotometry. b Root length of the WT plants grown under the same conditions indicated in (a). c IAA concentration of WT seedlings shoots grown under severe Cu deficiency (BCS 100 µM) or Cu sufficiency (10 µM CuSO4). Values are the mean ± SD of n = 3 replicates. The samples with a cocommon letter re not significantly different (p-value > 0.05) Kruskal–Wallis test
The physiological toxic effect of Cu in rice seedlings was demonstrated as root growth inhibition. Cu led to a significant reduction in root length, with a 50% reduction in the medium supplemented with 50 µM CuSO4 (Fig. 1b). A similar trend has been previously observed in Arabidopsis (Andrés-Colás et al. 2010). Cu supply did not impact on the phytohormones abscisic acid (ABA) and jasmonate (JA) content in shoots (Fig. S1). However, auxins (IAA) concentrations in shoots analyzed in a Cu deficiency medium, obtained by adding a Cu chelator (Cu deficiency: 0 μM CuSO4 + 100 μM BCS) or in Cu excess (10 μM CuSO4) revealed significant differences, with decreased levels under Cu excess (Fig. 1c).
Since plasma membrane high-affinity Cu transport proteins are well-recognized as Cu deficiency markers in Arabidopsis (Yamasaki et al. 2009; Bernal et al. 2012; Andrés-Colás et al. 2013), the OsCOPT1 (LOC_Os01g56420) transcript level was determined by quantitative RT-PCR in the rice seedlings grown under the aforementioned wide range of Cu conditions. The OsCOPT1 transcript levels were high at 0 μM CuSO4 and dropped dramatically when the medium was supplemented with Cu (Fig. 2). Conversely, the transcript levels of metallotionein OsMT1c (LOC_Os12g38010), which is considered a Cu-excess marker in Arabidopsis (Andrés-Colás et al. 2013), raised in the seedlings cultivated on the media that contained more than 10 μM CuSO4 (Fig. 2). This trend agreed with the endogenous Cu concentration variation (Fig. 1a) and further supported that 10 μM CuSO4 can be considered a Cu excess medium. However, for 0 μM CuSO4, plants underwent slight Cu deficiency at the molecular level by expressing the high-affinity Cu transport protein OsCOPT1.
Expression of genes involved in metal homeostasis at different Cu contents. a Relative expression of the genes induced under low Cu. Expression of OsCOPT1, OsFER2 and OsFd1 was determined by qRT-PCR from the 8 day-old WT seedling shoots grown on a Cu scale that ranged from control conditions (0 µM CuSO4) with slight Cu deficiency to metal excess (75 µM CuSO4). b Relative expression of the genes induced under Cu excess. Expression of OsMT1c, OsNAS2 and OsIRO2 under the same conditions indicated in (a). The Actin gene was used as a loading control. The mRNA level is expressed in relative units (ru). The values are relative to the expression level at 0 µM CuSO4 in (a) and also to the expression level at 75 µM CuSO4 in (b). Values are the mean ± SD of n = 3 replicates. Samples with a common letter are not significantly different (p-value > 0.05)
Thus, we established Cu nutritional ranges for rice seedlings under our experimental conditions based on metal concentration, physiologic parameters and molecular responses, which reflect the endogenous cellular metal status.
Transcriptomic profile unveils an influence of copper on iron homeostasis
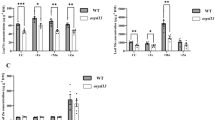
To gain further insight into the molecular responses affected by the Cu nutritional status, 8 day-old O. sativa seedling shoots were used to perform a global gene expression analysis by DNA microarrays. Two different Cu concentration conditions were compared, 1/2 MS standard commercial control (no Cu added; 0 μM CuSO4) and Cu excess (75 μM CuSO4), as the latter has been shown to affect root length and metal concentration, and practically eliminates OsCOPT1 expression (Figs. 1, 2). Table 1 contains the list of the differentially expressed genes with fold-change (FC) minimum of 2 for the Cu excess condition, represented as log2(FC) ≥ |1|. OsCOPT1 and metallothionein OsMT1c, which are respectively down- and up-regulated under Cu excess, validated our experimental settings (Fig. 2). Interestingly, the expression of several genes involved in Fe homeostasis appeared to be significantly altered. Ferritin (OsFER2) (LOC_Os12g01530) and ferredoxin (OsFd1) (LOC_Os08g01380) were down-regulated, whereas nicotianamine synthase (OsNAS2) (LOC_Os03g19420) and the OsIRO2 (LOC_Os01g72370) transcription factor were induced under Cu excess (Table 1).
To validate the transcriptomic profile and further assess Cu responsiveness, quantitative RT-PCR analyses were conducted in the samples from the seedlings grown within a range of Cu concentrations (0–75 μM CuSO4). The transcript levels of OsFER2 and OsFd1 lowered as the Cu concentration increased in the growth medium (Fig. 2). On the other hand, the expression levels of OsIRO2 and its target OsNAS2 were up-regulated as the Cu concentration increased (Fig. 2). Other OsIRO2-targets, such as OsNAS1 and OsNAAT1 (Ogo et al. 2007), as well as metallothionein OsMT1f were also induced under Cu excess (Table 1). All of them were confirmed by quantitative RT-PCR (Fig. S2). Since the expression of OsCOPT5 and OsCOPT7 is induced by Cu deficiency (Yuan et al. 2010), they were also included as controls in the analysis. OsCOPT7 was indeed up-regulated under the standard control condition that represents a slight Cu deficiency, as previously stated (Figs. 1a, 2a), whereas OsCOPT5 was not regulated under our experimental conditions (Fig. S2). Other Fe-related genes such as those encoding Fe superoxide dismutase, OsFSD1.1 and OsFSD1.2, and the Fe-sulfur domain-contain protein 1 OsCDGSH (displaying a Log2(FC) value of −0.615) were down-regulated under Cu excess (Fig. S3). Taken together, both global expression analysis and quantitative RT-PCR expression patterns indicate that rice Cu status influences Fe homeostasis by altering the expression of several Fe-related genes.
Ectopic expression of AtCOPT1 impacts iron homeostasis
Our global gene expression studies in rice seedling shoots suggested that Cu had an impact on Fe homeostasis (Table 1). To further investigate the cross-talk between Cu and Fe we generated two independent transgenic O. sativa lines that ectopically overexpressed the Arabidopsis AtCOPT1 transport protein fused to the carboxy-terminal end to the Human Influenza Hemaglutinin epitope (HA) under the control of the cauliflower mosaic virus 35 S (CaMV35S) promoter and the nopaline synthase (NOS) terminator. These CaMV35S::AtCOPT1-HA::NOS transgenic plants were named AtCOPT1 OE -1 (C1 OE -1) and AtCOPT1 OE -2 (C1 OE -2). To verify that the obtained transgenic lines expressed the CaMV35S::AtCOPT1-HA::NOS construct, both kanamycin-resistant plants were analyzed by different approaches. First, the cDNA obtained from 8 day-old WT, C1 OE -1 and C1 OE -2 the 8 day-old seedlings was analyzed by semiquantitative RT-PCR (Fig. 3a). The AtCOPT1 gene was detected only in the transgenic lines. Addition of the HA epitope allowed to detect AtCOPT1 protein by Western blotting (Fig. 3b). Although a similar amount of total protein was processed in the three samples (lower panel in Fig. 3b), the HA epitope was detected only in the C1 OE seedlings (upper panel in Fig. 3b). These results corroborate that the two transgenic lines (C1 OE -1 and C1 OE -2) express the AtCOPT1 gene, which is translated into protein.
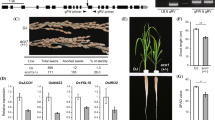
AtCOPT1 expression in O. sativa transgenic plants. a AtCOPT1 gene expression was determined by sqRT-PCR of the 8 day-old seedlings from the WT and C1 OE -1 and C1 OE -2 plants. The 18 S gene was used as a loading control. b A Western-blot analysis was used to detect the HA epitope of the seedlings from the WT and C1 OE -1 and C1 OE -2 plants grown under the same conditions indicated in (a). The anti-HA primary antibody labelled with peroxidase was used for Human Influenza Hemaglutinin epitope (HA) epitope detection. Ponceau staining was used as a loading control
To characterize the consequences of AtCOPT1 overexpression in rice, C1 OE -1 and C1 OE -2 seedlings were grown for 8 days in media with increasing Cu concentrations, and then shoot and root lengths were measured (Fig. 4). In both WT and transgenic plants, shoot and root lengths decreased when 50 µM or higher Cu concentrations were added to the medium (Fig. 4). Importantly, shoot and root shortening was more pronounced in the transgenic lines. Specifically, C1 OE -1 transgenic line displayed a significant decrease in shoot length as compared to WT seedlings, whereas the C1 OE -2 did not display a significant difference probably due to its higher variability (Fig. 4a). Regarding root length, 27 and 19% root growth reductions took place in the WT seedlings at 50 and 75 µM Cu SO4, whereas root growth inhibition was significantly more prominent (29–52%) in both C1 OE -1 and C1 OE -2 lines (Fig. 4b). These results indicate that C1 OE rice plants are sensitive to Cu.
Length of shoots and roots of O. sativa WT and C1 OE plants. a The relative shoot length of the 8 day-old seedlings from the WT, C1 OE -1 and C1 OE -2 lines grown on a Cu scale that ranged from control conditions (0 µM CuSO4) to Cu excess (75 µM CuSO4). b The relative root length was measured under the same conditions indicated in (a). The ratio was calculated and normalized against each genotype sufficiency condition (1 µM CuSO4). Values are the mean ± SD of n = 3 replicates. Samples with a cocommon letter re not significantly different (p-value > 0.05)
To check whether the transgenic plants altered Cu and Fe accumulation, the endogenous levels of both metals were determined in roots and shoots by ICP-MS (Fig. 5). At the Cu conditions assayed (0 and 75 µM added CuSO4), no significant differences in shoot and root endogenous Cu levels were observed for the transgenic plants as compared to WT. With regard to Fe content, under standard control conditions (no added CuSO4), the C1 OE -1 and C1 OE -2 roots accumulated 60–36% less Fe than the WT lines respectively (Fig. 5b). These data indicate that the expression of AtCOPT1 in rice significantly decreases root Fe accumulation.
Metal concentration of O. sativa C1 OE plants grown in media with a different Cu status. a Cu concentration of the shoots and roots of the 8-day-old seedlings from the WT, C1 OE -1 and C1 OE -2 lines grown under control conditions (0 µM CuSO4) or excess (75 µM CuSO4) of Cu. b Fe concentration of the shoots and roots of the seedlings from WT, C1 OE -1 and C1 OE -2 grown under the same conditions indicated in (a). Values are the mean ± SD of n = 3 replicates. Samples with a common letter are not significantly different (p-value > 0.05)
The expression of the Cu and Fe homeostasis genes was analyzed by quantitative RT-PCR in the C1 OE transgenic plants. We selected OsCOPT1 and OsMT1c as representative genes that were down- and up-regulated respectively by Cu excess (Fig. 2). The endogenous OsCOPT1 expression was lower in the two transgenic C1 OE lines at 0 μM CuSO4 (Fig. 6). This result suggests that, despite no global changes in plant Cu levels were observed by ICP-MS (Fig. 5), the transgenic plants might sense increased nucleo-cytosolic Cu concentration. Under 75 μM CuSO4, OsMT1c expression was higher in the two transgenic C1 OE lines than in WT seedlings (Fig. 6), which also indicates that C1 OE plants are sensing higher Cu levels. Altogether, these data indicate that the C1 OE lines display gene expression changes compatible with the perception of increased intracellular Cu levels and that the expression of specific marker genes could be more sensitive than total content of metals to assess nucleo-cytosolic Cu status.
Relative expression of OsCOPT1, OsCOPT7, OsMT1c, OsFd1, OsFER2, OsHRZ1, OsHRZ2 and OsIRO2 genes in the WT and AtCOPT1 overexpressing rice plants. Relative gene expression analyzed by qRT-PCR of the WT and C1 OE 8-day-old seedling shoots grown under control conditions (0 μM CuSO4) and excess (75 μM CuSO4). The Actin gene was used as a loading control. The mRNA level is expressed in relative units (ru). Expression values are relative to the WT seedlings grown under Cu deficiency conditions. Values are the mean ± SD of n = 3 replicates. Samples with a common letter are not significantly different (p-value > 0.05)
Then we analyzed the expression of Fe-related genes. Whereas OsFER2 expression remained mainly unchanged, OsHRZ1, OsHRZ2, OsIRO2 and OsFd1 expression was higher in the transgenic lines at least in 75 μM CuSO4 (Fig. 6). Given the postulated negative role of OsHRZ1 and OsHRZ2 in Fe acquisition (Kobayashi et al. 2013), these results point to altered Fe homeostasis when Cu uptake is increased in C1 OE lines. However, the expression of the putative Fe sensors OsHRZ1 and OsHRZ2 in WT plants was similar under different levels of Cu supply, with a decrease for OsHRZ2 at 75 μM CuSO4 (Fig. S3).
In Arabidopsis, substitution of Cu-Zn superoxide dismutase (SOD) for its Fe counterpart is a specific feature of the Cu deficiency response (Abdel-Ghany et al. 2005; Waters et al. 2012). Given that SOD substitution would implicate an increased Fe demand under Cu deficiency, we checked whether it also took place in Oryza sativa (Fig. S4). The expression of the different genes that encoded Cu-dependent SODs (OsCSD1.1, OsCSD1.2 and OsCSD2) remained higher under control than under Cu excess conditions, instead of being repressed by low Cu as described for Arabidopsis homologs (Abdel-Ghany and Pilon 2008; Waters et al. 2012). The expression of Fe-dependent SODs (OsFSD1.1 and OsFSD1.2) was also higher in Cu excess, which is indicative of increased superoxide radical production under the slight Cu deficiency shown by control conditions. However, no significant differences in the expression of SOD genes were detected between the WT and C1 OE transgenic lines (Fig. S4), which suggests that SOD substitution is not implicated in the observed phenotypes.
Phenotype of transgenic AtCOPT1 overexpressing lines with different copper and iron concentrations
To further investigate the potential interaction between Cu and Fe, we analyzed several parameters of the WT and C1 OE seedlings grown in the presence of different Cu and Fe concentrations (Fig. 7). The WT and both C1 OE lines displayed a similar root length when grown in plates under the control medium (+Cu +Fe). However, the C1 OE transgenic lines were more sensitive to single Cu (−Cu +Fe; 35–42% root length reduction), and especially to Fe (+Cu −Fe; 75–69% reduction) and the double (−Cu −Fe; 71–76% reduction) deficiencies (Fig. 7). Hydroponic cultures of the WT and C1 OE -1 lines were also performed under both the control (+Cu +Fe) and deficiency (−Cu −Fe) conditions. The adult C1 OE -1 plants also showed differences with the controls, as indicated by the 30% reduction of chlorophyll levels when grown on hydroponic cultures under both metal sufficiency and deficiency (Fig. S5).
Characterization of the O. sativa C1 OE plants grown in media with different Cu and Fe contents. a Representative photographs of the WT, C1 OE -1 and C1 OE -2 plants grown with different media: Cu and Fe sufficiency (+ Cu + Fe), Cu deficiency (−Cu + Fe), Fe deficiency (+ Cu −Fe), Cu and Fe deficiency (−Cu −Fe). b Root length of the 8 day-old seedlings of the WT, C1 OE -1 and C1 O -2 plants grown under the same conditions as in (a). Represented values are the mean ± SD of n = 3 replicates. Samples with a common letter are not significantly different (p-value > 0.05)
In order to check how the WT and C1 OE rice lines performed under regular greenhouse conditions, different agronomic parameters were analyzed (Fig. 8). Plant heights remained mostly unaltered, except for a slight decrease (about 15%) in the C1 OE -2 line (Fig. 8a, b). Metal concentrations were determined both in young and old leaves from 4- and 5-months old plants. Whereas metals remained mostly unchanged in old leaves from 5 month-old plants, Cu and Fe concentrations increased in young leaves from C1 OE rice lines (Fig. S6). Chlorophyll concentration and panicle production per plant were not affected in the C1 OE lines under these growing conditions (Fig. 8b, c). However, the number of grains per panicle was slightly reduced in the C1 OE lines (Fig. 8c). The localization of Fe detected by the Perl’s staining method (Stacey et al. 2007) indicated that Fe was present in both the WT and C1 OE lines (Fig. S7). However, Cu and Fe concentration in the unpolished rice grain from both C1 OE lines was higher than in the controls (Fig. 9a). Whereas Cu concentration slightly decreased in the polished grains from the C1 OE lines compared to the controls, Fe concentration was still higher than in the WT (Fig. 9b). Altogether, ectopic expression of AtCOPT1 in rice leads to altered Fe homeostasis with increased Fe concentration in sink organs, such as young leaves and unpolished rice grains.
Characterization of the O. sativa C1 OE plants grown under greenhouse conditions. a Representative photographs of the 5 month-old from the WT, C1 OE -1 and C1 OE -2 plants and panicles. b Plant height and leaf chlorophyll content per gram of fresh weight of the rice WT and C1 OE plants. c Panicles per plant and grain per panicle of the WT and C1 OE plants. Represented values are the mean ± SD of n = 3 replicates. Samples with a common letter are not significantly different (p-value > 0.05)
Metal seed concentration in the O. sativa C1 OE plants grown under greenhouse conditions. a Cu and Fe concentrations of the brown rice grain of the WT and C1 OE plants. b Cu and Fe concentrations of the white rice grain of the WT and C1 OE plants. Represented values are the mean ± SD of n = 3 replicates. Samples with a common letter are not significantly different (p-value > 0.05)
Discussion
In this work, we evaluated the physiological and molecular effects caused by Cu in O. sativa plants when adding Cu to the media and also when plants overexpressed the high-affinity Cu transport protein COPT1 from Arabidopsis thaliana. Rather than metal quantification, the expression of Cu homeostasis molecular markers seems a more reliable indicator of the symplastic metal status. Total metal determinations in plant cells are unable to discriminate the metal partitioning between intracellular and apoplastic compartments since metals are tightly bound to the cell walls (Ye et al. 2015). In A. thaliana, the expression of plasma membrane COPT transport genes (AtCOPT1, AtCOPT2 and AtCOPT6) has been shown to serve as markers of Cu deficiency conditions (Sancenón et al. 2003; Andrés-Colás et al. 2010; Garcia-Molina et al. 2013). The fact that the OsCOPT1 expression was finely regulated according to the Cu concentration in the medium suggests that this transporter is also an excellent marker of the endogenous cellular Cu status in shoots from rice seedlings (Fig. 2). Thus, when considering the OsCOPT1 expression pattern, three different Cu level conditions were established in the media in O. sativa. Cu deficiency was attained in the media with no supplemented Cu (0 μM CuSO4), where the OsCOPT1 transport protein was markedly induced. Slight Cu deficiency conditions are also corroborated by increased expression of OsCOPT7, OsATX1, OsFSD1.1 and OsFSD1.2 (Figs. S2 and S3). Cu sufficiency conditions corresponded to the presence of 1 μM and 10 μM CuSO4 in the media, where OsCOPT1 displayed a basal expression. Cu excess in rice was observed at 50 μM CuSO4 and at higher Cu concentrations (75 μM CuSO4), when the OsCOPT1 expression was barely detected (Fig. 2). For Cu excess, the data from rice differed from those obtained in Arabidopsis since 10 μM CuSO4 resulted in metal excess concentration in Arabidopsis (Andrés-Colás et al. 2013).
To conduct a global gene expression study 75 μM CuSO4 was selected as Cu excess and compared to the standard control condition (0 μM CuSO4). OsMT1c induction confirmed that the presence of 50 μM and 75 μM CuSO4 in the media represented metal excess (Fig. 2). Another MT gene OsMT1f was also induced under Cu excess (Table 1 and Fig. S2), which is indicative of them being good candidates for molecular markers of Cu excess in rice. The induction of type 1 metallothioneins under Cu excess is a well-known protecting process that affects ROS scavenging or signaling (Hassinen et al. 2011). The expression of genes related to NA-metal complexes, such as OsNAS1, OsNAS2 and OsNAAT1 were also increased (Fig. 2, S2), and could be used as molecular markers of Cu excess in rice.
To examine the effect of altered Cu incorporation, we overexpressed AtCOPT1 in the O. sativa plants. AtCOPT1 shares 51% sequence identity and 66% sequence similarity with OsCOPT1 (Yuan et al. 2010). Moreover, AtCOPT1 is able to complement the phenotype of S. cerevisiae strains deficient in Cu transport (Puig and Thiele 2002; Sancenón et al. 2003). We confirmed that the C1 OE rice lines generated expressed the heterologous gene and produced the encoded protein (Fig. 3). At the phenotypic level, and under Cu excess conditions, the shortening of root length was more marked in the C1 OE plants than in the WT (Fig. 4). These results are consistent with those described in Arabidopsis (Andrés-Colás et al. 2010), and agree with previous studies in which Cu excess was found to cause a reduction in the weight of rice plants (Mostofa et al. 2014). This could be due to the incorporation of Cu into plants as Cu(I), which is harmful as it produces ROS (Rodrigo-Moreno et al. 2013). The reduced expression of the endogenous OsCOPT1 gene in the transgenic lines under standard control conditions indicated that the C1 OE lines perceived more endogenous Cu levels as a result of AtCOPT1 activity (Fig. 6). Likewise, the highest expression of the metallothionein OsMT1c under Cu excess (Fig. 6) further indicated increased Cu perception in the C1 OE lines.
There is evidence for the relationship between Cu and Fe in other organisms, such as yeast or algae, where Cu is necessary for Fe incorporation and distribution (Puig et al. 2007; Gulec and Collins 2014). In Arabidopsis, Cu and Fe deficiencies induce the expression of high-affinity Cu transport protein COPT2, and that copt2 mutants are more tolerant than WT plants to double Cu and Fe deficiencies (Colangelo and Guerinot 2004; Perea-García et al. 2013). In agreement with these data, AtCOPT1 overexpression in rice produced increased sensitivity to double Cu and Fe deficiencies (Fig. 7 and S5). Severe Cu deficiency leads to increased auxin synthesis in rice (Fig. 1c), as described for Fe deficiency in Arabidopsis, which results in the enhanced expression of Fe-deficiency genes (Chen et al. 2010). Although the role of IAA in the cross-talk between Cu and Fe homeostasis merits further characterization (Peñarrubia et al. 2015), our results envisage that plants subjected to multiple nutritional deficiencies also face an auxin dilemma.
The putative Fe and oxygen/redox state sensors OsHRZ1 and OsHRZ2 have been shown to act as a negative regulators of Fe homeostasis (Kobayashi et al. 2013). OsHRZ1 and OsHRZ2 expression could be affected by the effects of Cu(I)-uptake since increased in C1 OE plants (Fig. 6), but not under Cu excess (Fig. S3).
Cu-Fe crosstalk under Cu excess might be at least partially explained by the increased expression of the transcriptional regulator OsIRO2, which could justify the expression of the Fe strategy II uptake targets related to DMA biosynthesis, such as OsNAS1, OsNAS2 and OsNAAT1 (Table 1; Fig. 2 and S2) (Ogo et al. 2007). OsIRO2 expression is subjected to the control of postulated Fe sensor OsIDEF1. By using the recombinant OsIDEF1 protein in in vitro experiments, Kobayashi et al. (2012) demonstrated that OsIDEF1 binds Fe(II) and other divalent metals, including Cu(II), through its histidine-asparagine repeat and proline-rich regions. Based on its ability to bind metals in proportion to their cellular abundance, it is tempting to speculate that Cu(II) could exert the described influence by competing with Fe(II) in OsIDEF1 metal-binding domains. In agreement with this, OsIDEF1 has been proposed to sense the nutritional metal balance by detecting the ratio of Fe to other metals rather than absolute Fe concentration (Kobayashi et al. 2012). Thus, Cu excess could affect at least some of the Fe deficiency responses, such as those leading to the increased expression of OsIRO2 and its downstream targets.
Cu and Fe concentrations in the different plant organs are probably the result of multiple processes in which both metals face diverse and complex interactions in their way from source toward sink organs including seeds. NA functions in a phloem pathway for Fe translocation to sink organs (Tsukamoto et al. 2009). Since Cu(II)-NA complexes are very stable (von Wirén et al. 1999), increased expression of OsNAS1, OsNAS2 and OsNAAT1 under Cu excess (Fig. 2 and S2) could reflect not only the increased Cu content, but could also affect Fe mobilization and accumulation in sink organs. As observed here, Fe concentration increased in young leaves as the plants got older (Fig. S6). Based on the lower Fe concentration in COPT-overexpressing roots under Cu scarce media (Fig. 5b), it is tempting to speculate that a putative Fe deficiency signaling could result in increased Fe mobilization to the sink organs. Interestingly, the Fe concentration of polished and unpolished rice grains from C1 OE plants increased 60 and 30% respectively (Fig. 9). Otherwise, the C1 OE plants were similar to the WT in agronomic performance terms when grown under regular greenhouse conditions (Fig. 8). However, the approximate 8 μg of Fe/g DW obtained by this approach (Fig. 9) is lower than the concentrations obtained by other strategies which range from 10 to 19 μg of Fe/g DW (Johnson et al. 2011; Oliva et al. 2014; Boonyaves et al. 2016; Trijatmiko et al. 2016). Taken together, these results indicate that both Cu deficiency and Cu excess may interfere with Fe sensing and mobilization in rice. The understanding of these interactions could be useful to design biotechnological approaches to improve the nutritional Fe concentration of edible crop parts.
References
Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283:15932–15945. doi:10.1074/jbc.M801406200
Abdel-Ghany SE, Burkhead JL, Gogolin KA, Andrés-Colás N, Bodecker JR, Puig S, Peñarrubia L, Pilon M (2005) AtCCS is a functional homolog of the yeast copper chaperone Ccs1/Lys7. FEBS Lett 579:2307–2312. doi:10.1016/j.febslet.2005.03.025
Andrés F, Galbraith DW, Talón M, Domingo C (2009) Analysis of PHOTOPERIOD SENSITIVITY5 sheds light on the role of phytochromes in photoperiodic flowering in rice. Plant Physiol 151:681–690. doi:10.1104/pp.109.139097
Andrés-Colás N, Perea-García A, Puig S, Peñarrubia L (2010) Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiol 153:170–184. doi:10.1104/pp.110.153676
Andrés-Colás N, Perea-García A, Mayo de Andrés S, Garcia-Molina A, Dorcey E, Rodríguez-Navarro S, Pérez-Amador MA, Puig S, Peñarrubia L, Mayo de Andres S, García-Molina A, Dorcey E, Rodriguez-Navarro S, Perez-Amador MA, Puig S, Peñarrubia L (2013) Comparison of global responses to mild deficiency and excess copper levels in Arabidopsis seedlings. Metallomics 5:1234–1246. doi:10.1039/c3mt00025g
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300. doi:10.2307/2346101
Bernal M, Casero D, Singh V, Wilson GT, Grande A, Yang H, Dodani SC, Pellegrini M, Huijser P, Connolly EL, Merchant SS, Krämer U (2012) Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24:738–761. doi:10.1105/tpc.111.090431
Bhullar NK, Gruissem W (2013) Nutritional enhancement of rice for human health: The contribution of biotechnology. Biotechnol Adv 31:50–57. doi:10.1016/j.biotechadv.2012.02.001
Boonyaves K, Gruissem W, Bhullar NK (2016) NOD promoter-controlled AtIRT1 expression functions synergistically with NAS and FERRITIN genes to increase iron in rice grains. Plant Mol Biol 90:207–215. doi:10.1007/s11103-015-0404-0
Briat JF (1996) Roles of ferritin in plants. J Plant Nutr 19:1331–1342. doi:10.1080/01904169609365202
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819. doi:10.1104/pp.110.161109
Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16:3400–3412. doi:10.1105/tpc.104.024315
Crichton RR, Pierre JL (2001) Old iron, young copper: from mars to venus. Biometals 14:99–112. doi:10.1023/A:1016710810701
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2011) InfoStat.
Garcia-Molina A, Andrés-Colás N, Perea-García A, Del Valle-Tascõn S, Peñarrubia L, Puig S (2011) The intracellular Arabidopsis COPT5 transport protein is required for photosynthetic electron transport under severe copper deficiency. Plant J 65:848–860. doi:10.1111/j.1365-313X.2010.04472.x
Garcia-Molina A, Andrés-Colás N, Perea-García A, Neumann U, Dodani SC, Huijser P, Peñarrubia L, Puig S (2013) The Arabidopsis COPT6 transport protein functions in copper distribution under copper-deficient conditions. Plant Cell Physiol 54:1378–1390. doi:10.1093/pcp/pct088
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286. doi:10.1038/7029
Gulec S, Collins JF (2014) Molecular mediators governing iron-copper interactions. Annu Rev Nutr 34:95–116. doi:10.1146/annurev-nutr-071812-161215
Hassinen VH, Tervahauta AI, Schat H, Kärenlampi SO (2011) Plant metallothioneins—metal chelators with ROS scavenging activity? Plant Biol 13:225–232. doi:10.1111/j.1438-8677.2010.00398.x
Hermans C, Bourgis F, Faucher M, Strasser RJ, Delrot S, Verbruggen N (2005) Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 220:541–549. doi:10.1007/s00425-004-1376-5
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282. doi:10.1046/j.1365-313X.1994.6020271.x
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45:335–346. doi:10.1111/j.1365-313X.2005.02624.x
Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK (2010) Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J 62:379–390. doi:10.1111/j.1365-313X.2010.04158.x
Johnson AAT, Kyriacou B, Callahan DL, Carruthers L, Stangoulis J, Lombi E, Tester M (2011) Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS One 6:e24476. doi:10.1371/journal.pone.0024476
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152. doi:10.1146/annurev-arplant-042811-105522
Kobayashi T, Nishizawa NK (2014) Iron sensors and signals in response to iron deficiency. Plant Sci 224:36–43. doi:10.1016/j.plantsci.2014.04.002
Kobayashi T, Itai RN, Aung MS, Senoura T, Nakanishi H, Nishizawa NK (2012) The rice transcription factor IDEF1 directly binds to iron and other divalent metals for sensing cellular iron status. Plant J 69:81–91. doi:10.1111/j.1365-313X.2011.04772.x
Kobayashi T, Nagasaka S, Senoura T, Itai RN, Nakanishi H, Nishizawa NK (2013) Iron-binding Haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat Commun 4:2792. doi:10.1038/ncomms3792
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39:415–424. doi:10.1111/j.1365-313X.2004.02146.x
Lee SSJ, Jeon US, Lee SSJ, Kim Y-K, Persson DP, Husted S, Schjørring JK, Kakei Y, Masuda H, Nishizawa NK, An G (2009) Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Natl Acad Sci 106:22014–22019. doi:10.1073/pnas.0910950106
Leszczyszyn OI, Imam HT, Blindauer C a (2013) Diversity and distribution of plant metallothioneins: a review of structure, properties and functions. Metallomics 5:1146–1169. doi:10.1039/c3mt00072a
Marschner P (2012) Marschner’s Mineral Nutrition of Higher Plants, 3rd edn. Elsevier, Amsterdam
Mostofa MG, Seraj Z, Fujita M (2014) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251:1373–1386. doi:10.1007/s00709-014-0639-7
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:493–497
Ogo Y, Itai RN, Nakanishi H, Inoue H, Kobayashi T, Suzuki M, Takahashi M, Mori S, Nishizawa NK (2006) Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J Exp Bot 57:2867–2878. doi:10.1093/jxb/erl054
Ogo Y, Nakanishi Itai R, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK (2007) The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J 51:366–377. doi:10.1111/j.1365-313X.2007.03149.x
Ogo Y, Itai RN, Kobayashi T, Aung MS, Nakanishi H, Nishizawa NK (2011) OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Mol Biol 75:593–605. doi:10.1007/s11103-011-9752-6
Oliva N, Chadha-Mohanty P, Poletti S, Abrigo E, Atienza G, Torrizo L, Garcia R, Dueñas C, Poncio MA, Balindong J, Manzanilla M, Montecillo F, Zaidem M, Barry G, Hervé P, Shou H, Slamet-Loedin IH (2014) Large-scale production and evaluation of marker-free indica rice IR64 expressing phytoferritin genes. Mol Breed 33:23–37. doi:10.1007/s11032-013-9931-z
Peñarrubia L, Romero P, Carrió-Seguí A, Andrés-Bordería A, Moreno J, Sanz A (2015) Temporal aspects of copper homeostasis and its crosstalk with hormones. Front Plant Sci 6:1–18. doi:10.3389/fpls.2015.00255
Perea-García A, Garcia-Molina A, Andrés-Colás N, Vera-Sirera F, Pérez-Amador MA, Puig S, Peñarrubia L (2013) Arabidopsis copper transport protein COPT2 participates in the crosstalk between iron deficiency responses and low phosphate signaling. Plant Physiol 162:180–194. doi:10.1104/pp.112.212407
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45. doi:10.1093/nar/29.9.e45
Puig S (2014) Function and regulation of the plant COPT family of high-affinity copper transport proteins. Adv Bot. doi:10.1155/2014/476917
Puig S, Peñarrubia L (2009) Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol 12:299–306. doi:10.1016/j.pbi.2009.04.008
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–180. doi:10.1016/S1367-5931(02)00298-3
Puig S, Andrés-Colás N, García-Molina A, Peñarrubia L (2007) Copper and iron homeostasis in Arabidopsis: Responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ 30:271–290. doi:10.1111/j.1365-3040.2007.01642.x
Ravet K, Pilon M (2013) Copper and iron homeostasis in plants: the challenges of oxidative stress. Antioxid Redox Signal 19:919–932. doi:10.1089/ars.2012.5084
Rodrigo-Moreno A, Andrés-Colás N, Poschenrieder C, Gunsé B, Peñarrubia L, Shabala S (2013) Calcium- and potassium-permeable plasma membrane transporters are activated by copper in Arabidopsis root tips: linking copper transport with cytosolic hydroxyl radical production. Plant Cell Environ 36:844–855. doi:10.1111/pce.12020
Sancenón V, Puig S, Mira H, Thiele DJ, Peñarrubia L (2003) Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol 51:577–587. doi:10.1023/a:1022345507112
Smyth GK (2005) Limma: linear models for microarray data. Springer, New York
Sperotto RA, Ricachenevsky FK, Waldow V de A, Fett JP (2012) Iron biofortification in rice: It’s a long way to the top. Plant Sci 190:24–39. doi:10.1016/j.plantsci.2012.03.004
Stacey MG, Patel A, McClain WE, Mathieu M, Remley M, Rogers EE, Gassmann W, Blevins DG, Stacey G (2007) The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiol 146:589–601. doi:10.1104/pp.107.108183
Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M (2013) Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative. Lancet Glob Heal 1:e16–e25. doi:10.1016/S2214-109X(13)70001-9
Trijatmiko KR, Dueñas C, Tsakirpaloglou N, Torrizo L, Arines FM, Adeva C, Balindong J, Oliva N, Sapasap MV, Borrero J, Rey J, Francisco P, Nelson A, Nakanishi H, Lombi E, Tako E, Glahn RP, Stangoulis J, Chadha-Mohanty P, Johnson AAT, Tohme J, Barry G, Slamet-Loedin IH (2016) Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci Rep 6:19792. doi:10.1038/srep19792
Tsukamoto T, Nakanishi H, Uchida H, Watanabe S, Matsuhashi S, Mori S, Nishizawa NK (2009) 52Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol 50:48–57. doi:10.1093/pcp/pcn192
von Wirén N, Klair S, Bansal S, Briat J-F, Khodr H, Shioiri T, Leigh RA, Hider RC (1999) Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol 119:1107–1114. doi:10.1104/pp.119.3.1107
Waters BM, Armbrust LC (2013) Optimal copper supply is required for normal plant iron deficiency responses. Plant Signal Behav 8:e26611. doi:10.4161/psb.26611
Waters BM, McInturf SA, Stein RJ (2012) Rosette iron deficiency transcript and microRNA profiling reveals links between copper and iron homeostasis in Arabidopsis thaliana. J Exp Bot 63:5903–5918. doi:10.1093/jxb/ers239
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84. doi:10.1111/j.1469-8137.2008.02738.x
Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T (2009) SQUAMOSA Promoter binding Protein–like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21:347–361. doi:10.1105/tpc.108.060137
Ye Y, Yuan J, Chang X, Yang M, Zhang L, Lu K, Lian X (2015) The phosphate transporter gene OsPht1;4 is involved in phosphate homeostasis in rice. PLoS One 10:e0126186. doi:10.1371/journal.pone.0126186
Yruela I (2013) Transition metals in plant photosynthesis. Metallomics 5:1090–1109. doi:10.1039/c3mt00086a
Yuan M, Chu Z, Li X, Xu C, Wang S (2010) The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22:3164–3176. doi:10.1105/tpc.110.078022
Yuan M, Li X, Xiao J, Wang S (2011) Molecular and functional analyses of COPT/Ctr-type copper transporter-like gene family in rice. BMC Plant Biol 11:69–80. doi:10.1186/1471-2229-11-69
Acknowledgements
This work has been supported by grants BIO2011-24848 and BIO2014-56298-P from the Spanish Ministry of Economy and Competitiveness, and by FEDER funds from the European Union. We acknowledge Àngela Carrió-Seguí (Universitat de València) and Kiranmayee Pamidimukkala for their technical help with this manuscript. We also acknowledge the Servei Central d’Instrumentació Científica (Universitat Jaume I) and SCSIE (Universitat de València) for the ICP-MS and atomic absorption spectrophotometry determinations and greenhouse facilities.
Author information
Authors and Affiliations
Contributions
SP and LP conceived the idea and wrote the manuscript. AA-B, FA and CD perform the rice microarray and transformation experiments. AA-B, AG-M and AP-G perform the physiological and molecular experiments in rice plants.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2017_622_MOESM1_ESM.pdf
Fig. S1 Hormone concentration in O. sativa plants. (a) ABA concentration of the 8 day-old seedlings of the WT plants grown under severe deficiency (BCS 100 µM) or sufficiency (10 µM CuSO4) of Cu. (b) JA concentration of the 8 day-old seedlings of the WT plants grown under same conditions as in (a). Represented values are the mean±SD of n=3 replicates. Samples with a common letter are not significantly different (p-value >0.05).
Fig. S2 Gene expression in the WT plants with different Cu status. (a) Relative gene expression of OsCOPT5, OsCOPT7, OsATX1 of the WT 8-day-old seedlings grown on control conditions (0 µM CuSO4) and excess (75 µM CuSO4). (b) Relative gene expression of OsNAS1, NAAT1, OsMt1f of the WT 8-day-old seedlings shoots grown in same conditions as in (a). Expression values are relative to the values of seedlings grown under Cu deficiency conditions. Represented values are the mean±SD of n=3 replicates. Samples with a common letter are not significantly different (p-value>0.05).
Fig. S3 Gene expression in the WT plants with different Cu status. (a) Relative gene expression of OsFSD1.1, OsFSD1.2, OsCDGSH of the WT 8-day-old seedlings shoots grown on a Cu scale from control conditions (0 µM CuSO4) to excess (75 µM CuSO4). (b) Relative gene expression of OsHRZ1 and OsHRZ2 of the WT 8-day-old seedlings grown in same conditions as in (a). Expression values are relative to the values of seedlings grown under Cu deficiency conditions. Represented values are the mean±SD of n=3 replicates. Samples with a common letter are not significantly different (p-value>0.05).
Fig. S4 The SOD gene expression of the WT and AtCOPT1 overexpressing rice plants. Relative expression of genes OsCSD1.1, OsCSD1.2, OsCSD2 (encoding Cu, Zn SODs), OsFSD1.1 and OsFSD1.2 (encoding Fe SODs) of the WT and C1OE 8-day-old seedling shoots grown under control conditions (0 μM CuSO4) and excess (75 μM CuSO4). Expression values are relative to the WT seedlings grown under Cu deficiency. Represented values are the mean±SD of n=3 replicates.
Fig. S5 Characterization of the WT and AtCOPT1 overexpressing rice plants grown in hydroponic cultures. (a) Representative photographs of 1 month-old plants. The WT and C1OE-1 plants were grown in different hydroponic cultures under different Cu and Fe conditions: Cu and Fe sufficiency (+Cu+Fe) and double Cu and Fe deficiency (-Cu-Fe). (b) Leaf chlorophyll content. Values of chlorophyll per gram of fresh weight of the WT and C1OE-1 1 month-old plants grown under the same conditions as in (a). Represented values are the mean±SD of n=3 replicates. Samples with a common letter are not significantly different (p-value >0.05).
Fig. S6 Metal concentration of the WT and AtCOPT1 overexpressing rice plants grown in soil. (a) Cu concentration in young and old leaves of the WT and C1OE from 4 and 5 month‐old plants grown in soil (b) Fe concentration of the WT and C1OE plants grown under the same conditions as in (a). Represented values are the mean±SD of n=3 replicates. Samples with a common letter are not significantly different (p‐value> 0.05).
Fig. S7 Perl’s staining of the brown rice grain of the WT and C1OE plants. Seeds were germinated for 3 days in ½MS and photographed after Perl’s stained.
Rights and permissions
About this article
Cite this article
Andrés-Bordería, A., Andrés, F., Garcia-Molina, A. et al. Copper and ectopic expression of the Arabidopsis transport protein COPT1 alter iron homeostasis in rice (Oryza sativa L.). Plant Mol Biol 95, 17–32 (2017). https://doi.org/10.1007/s11103-017-0622-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0622-8