Abstract
Over-expression of group A bZIP transcription factor genes in plants improves abiotic stress tolerance but usually reduces yields. Thus, there have been several efforts to overcome yield penalty in transgenic plants. In this study, we characterized that expression of the hot pepper (Capsicum annuum) gene CaBZ1, which encodes a group S bZIP transcription factor, was induced by salt and osmotic stress as well as abscisic acid (ABA). Transgenic potato (Solanum tuberosum) plants over-expressing CaBZ1 exhibited reduced rates of water loss and faster stomatal closure than non transgenic potato plants under drought and ABA treatment conditions. CaBZ1 over-expression in transgenic potato increased the expression of ABA- and stress-related genes (such as CYP707A1, CBF and NAC-like genes) and improved drought stress tolerance. Interestingly, over-expression of CaBZ1 in potato did not produce undesirable growth phenotypes in major agricultural traits such as plant height, leaf size and tuber formation under normal growth conditions. The transgenic potato plants also had higher tuber yields than non transgenic potato plants under drought stress conditions. Thus, CaBZ1 may be useful for improving drought tolerance in tuber crops. This might be the first report of the production of transgenic potato with improved tuber yields under drought conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global warming and population growth are threatening the supply of food world-wide (Hussain et al. 2011). Thus, abiotic stress tolerance has recently been a major focus of plant science research. Plants encounter a wide range of abiotic stresses such as drought, high salinity and low or high temperature during a typical life cycle (Bohnert et al. 2006). Abiotic stresses lead to biochemical, physiological and morphological changes that adversely affect plant growth, development and productivity (Moore et al. 2009; Reynolds and Tuberosa 2008).
Changes in genome-wide gene expression patterns help plants adapt to adverse environments. Genetic, molecular and biochemical analyses have revealed numerous transcription factor (TF) genes that respond to abiotic stresses, including bZIP, WRKY, AP2/EREBPs and bHLHs (Golldack et al. 2011; Oh et al. 2009; Wang et al. 2003). These genes encode TFs that bind to specific cis-elements present in the promoters of many stress-responsive genes and function as important regulators of plant tolerance to abiotic stresses (Chinnusamy et al. 2007; Hussain et al. 2011). Many stress-inducible TFs (containing individual DNA binding domains) form a complicated signaling network that functions in response to abiotic stress (Shinozaki and Yamaguchi-Shinozaki 2007).
Basic leucine zipper (bZIP) TFs comprise of a large family of regulatory proteins in plants (Correa et al. 2008). The bZIP domain consists of a 40–80 amino acid region defined by two motifs: a DNA-binding basic domain consisting of approximately 16 amino acid residues and a leucine zipper dimerization domain (Jakoby et al. 2002). Numerous bZIP TFs have been identified in many plant species including Arabidopsis thaliana and rice (Oryza sativa), which contain 75 and 89 genes encoding bZIP TFs, respectively (Jakoby et al. 2002; Nijhawan et al. 2008). Recent studies have shown that bZIP TFs play multiple roles as regulators of diverse biological processes such as abiotic stress responses, pathogen defense, seed maturation and flower development (Izawa et al. 1994; Muszynski et al. 2006; Thurow et al. 2005). Group A bZIP genes, including ABFs/AREBs such as AREB1/ABF2, AREB2/ABF4 and ABF3, are induced by dehydration, salinity and ABA treatment and play major roles in regulating ABA-dependent gene expression (Yoshida et al. 2010). Thus, over-expression of group A bZIPs enhances abiotic stress tolerance in several plant species. Constitutive expression of AREB1 in Arabidopsis produces ABA hypersensitivity and enhances drought tolerance (Fujita et al. 2005). Transgenic Arabidopsis plants over-expressing ABF3 and ABF4 also exhibit reduced transpiration and increased drought tolerance via the up-regulation of several ABA/stress-responsive regulatory genes (Kang et al. 2002).
Hot pepper (Capsicum annuum) is an important vegetable crop (Lee et al. 2004). However, transformation of hot pepper is quite difficult and inefficient. Therefore, transformation of hot pepper is not currently performed in most laboratories, and the functions of hot pepper genes are instead studied by ectopic expression in other plant species. Among plants of solanaceae to which hot pepper, tomato, potato and etc. are belong, potato (Solanum tuberosum) is the world fourth major food crop next to rice, wheat and corn in terms of production and area cultivated (Camire et al. 2009). Potato is relatively sensitive to abiotic stresses such as drought and salinity, and potato tuber initiation, bulking and tuber growth stage are especially vulnerable to drought stress.
In previous studies we reported several TFs which is induced by several abiotic stresses in hot pepper (Hwang et al. 2005). We transformed those genes into potato to identify whether over-expression of those genes can improve abiotic stress tolerance of crops. Among them, CaBZ1 showed drought tolerance in this study. Several studies have been aimed at overcoming potato yield loss under abiotic stress conditions through molecular biological approaches, which introduced stress inducible transcription factors into the potato plant (Evers et al. 2010; Stiller et al. 2008). However, even though it can improve the stress tolerance of potato, transgenic potato showed stunted growth or tuber yield reduction (Pino et al. 2007). In this study, over-expression of CaBZ1 didn’t give any negative effects for plant growth and tuber yield interestingly. Thus this study will give valuable information to improve the tuber crop productivities using molecular breeding technology.
Materials and methods
Plant growth conditions and stress treatments
Hot pepper seeds (C. annuum cv. Chung-Ryong Cho) were obtained from the Red Pepper Research Institute (Young Yang, Korea). To examine the expression level of CaBZ1 under various abiotic stress conditions, hot pepper plants were grown in soil for 3 weeks in a growth chamber maintained at 25 °C and 50 % relative humidity under long day conditions (16 h light/8 h dark cycle). The seedlings were treated with abiotic stresses, including cold (seedlings were transferred to a growth chamber at 4 °C), drought (150 mM Mannitol), and salt (150 mM NaCl) stress, followed by sampling at the designated time points. For ABA treatment, 0.1 mM ABA was sprayed onto the leaves of hot pepper plants.
Potato (S. tuberosum cv. ‘Sumi’) was used for Agrobacterium-mediated transformation. Plants were grown in soil in the greenhouse or on MS (Murashige and Skoog) agar (per liter: 4.4 g MS salt, 30 g sucrose, 0.5 g 2-(N-morpholino)ethanesulfonic acid (MES), 8 g plant agar, pH 5.8) in a growth chamber maintained between 21 and 23 °C and 60 % relative humidity under long day conditions.
RNA gel blot analysis and quantitative RT-PCR
For RNA blotting, total RNA was extracted from both hot pepper seedling and T0 transgenic potato plants using Trizol Regent (MRC, USA). Total RNA (15 μg per lane) was separated on 1.2 % formaldehyde agarose gels, and transferred to nylon membranes (Amersham, UK) by capillary blotting followed by UV-cross-linked. Pre-hybridization, hybridization, and washing conditions were based on standard protocols (Sambrook et al. 2001). The membranes were then exposed to BAS cassette (Fuji Film, Japan) for 1 day.
For qRT-PCR analysis, DNase-treated total RNA (1 μg) was transcribed with SuperScript III reverse transcriptase (Invitrogen, USA). PCR was performed in an optical 96-well plate with a MyiQ real-time PCR system (Bio-Rad, USA) using SYBR Premix Ex Taq™ (Takara, Japan). Amplification parameters were as follows: one cycle of 95 °C for 10 min and 65 °C for 5 min, followed by 40 cycles of 95 °C for 5 s, 58 °C for 25 s and 72 °C for 30 s. A final step was carried out at 65–95 °C (1 °C/sec) for melting curve analysis. Data analysis was based on the relative quantitative method and ΔΔCT value was used to determine the relative fold change in expression. All data were normalized to the expression level of the housekeeping β-tubulin gene. The qRT-PCR reactions were performed at least three times on independent biological replicates.
Subcellular localization analysis
For subcellular localization analysis, the coding region of CaBZ1 (except for the terminator codon) was amplified by PCR using specific primer set: 5′-TCTAGAATGGCTTCTTCAAGTGGTAC-3′ (underlining indicates XbaI site) and 5′-TTGGATCCTGTACTGCAAGACATGTG-3′ (underlining indicates BamHI site). The amplified fragment was fused to the N-terminus of vector p326GFP under the control of CaMV35S promoter to generate 35S-CaWRKY1:GFP (Niwa et al. 1999). A fusion construct containing a nuclear localization signal and red fluorescent protein gene (NLS-RFP) was used as a positive control. The plasmids were introduced into potato protoplasts that had been prepared from leaf and stem tissue by PEG-mediated transformation (Jin et al. 2001; Moon et al. 2014). Expression of the fusion constructs was monitored and images were captured with an Axioplan fluorescence microscope (Carl Zeiss, Jena, Germany).
Generation of transgenic plants and phenotype observation
To generate CaBZ1 overexpression plants, the sequence-confirmed coding region of CaBZ1 cDNA was cloned into pB7WG2D (Karimi et al. 2002) under the control of the CaMV35S promoter. The construct harboring the CaBZ1 gene was transferred into Agrobacterium tumefaciens LBA4404 by electroporation using a MicroPulser Electroporator (BioRad, USA). The Agrobacterium was transformed into wild type potato (‘Sumi’ cultivar) plants using the method of Lee et al. (2007). CaBZ1 overexpression potato plants were also created in which, the coding region of CaBZ1 was cloned into pPZP-3′PinII-Bar under the control of the CaMV35S promoter.
T0 transgenic potato lines were selected and confirmed by l-phosphinothricin (PPT) selection and RNA gel blot or qRT-PCR analysis. Among the transgenic potato lines, transgenic potato plants overexpressing CaBZ1 were chose for further experiments. To survey the phenotypes under drought conditions, 3-week-old T0 transgenic and non transgenic potato plants grown on MS medium and hydroponic solution were transferred to soil (150 g of soil per pot). The plants were grown for an additional week in the greenhouse to increase their adaptation ability to environmental changes in the soil. Before drought treatment, individual plants grown in pots were watered equally for 2 day. Water was then withheld for 10 day. Subsequently, watering was resumed, and the survival rate was determined 7 day later. To minimize positional effects, pot positions were changed daily during the drought treatment. Photographs were taken on the tenth day after withdrawal of watering and on the fifth day after re-watering. Drought tests were performed in three independent experiments (8 plants per experiment).
Determination of chlorophyll contents
To measure chlorophyll content, leaves from transgenic and non transgenic potato plants used in the phenotype observation experiment were collected on the seventh day after resuming watering. The leaf tissue (100 mg) was ground in 1 mL 80 % acetone with a Micro Smash MS-100R (Tomy, Japan). The homogenate was shaken and centrifuged (13,000 rpm for 5 min). Then, 500 μL supernatant was combined with 500 μL 80 % acetone. For the chlorophyll content assays, three plants were used per lines, and the experiments were twice. The chlorophyll contents were measured as described by Arnon (Arnon 1949).
Measurement of leaf water loss
To measure water loss under drought stress conditions, six leaves (two detached leaves per plants) at similar developmental stages (5-weeks-old) from T0 transgenic and non transgenic potato progenies cultivated in the greenhouse were detached and floated in sterilized water for 16 h at 4 °C. The detached leaves were the placed into weighing dishes and incubated in the growth chamber at 22 °C with 60 % relative humidity under light conditions. The fresh weights of the leaves were measured at the designated time points. Water loss was calculated as the percentage of initial fresh weight at each time point. Each experiment was repeated at least twice.
Stomatal aperture assay
For guard cell stomatal aperture observations, detached leaves from 3-week-old T0 transgenic and non transgenic potato plants grown in MS medium were floated in sterilized water and exposed to light conditions for 3 h. For experiments we used three independent plants for wild type and transgenic plant and two leaves per plants. We observed the >50 stomata, repeated experiments twice. Subsequently, 3 μM ABA was added to the fresh sterilized water to assay for stomatal closure. After treatment for 1 h treatment, the abaxial epidermal layers of the leaves were examined and images were captured through a bright-field Axioskop 2 microscope (Carl Zeiss, Germany). Stomatal aperture (the length and width of the stomata) was measured from the photographs using the Interactive Measurement software package AxioVison Rel. 4.8 (Carl Zeiss, Germany).
Survey of agronomic characteristics
To survey agronomic traits such as plant height, leaf shape, tuber formation and tuber yield, 3-week-old T0 transgenic and non transgenic potato plants grown on MS medium were transferred hydroponic solution and cultured for 1 week. Six independent potato plants per each line were then planted in soil (900 g soil per pot) and cultivated in the greenhouse. After 8 weeks of cultivation, various growth characteristics were examined. In addition to compare potato tuber yields between transgenic and non transgenic potato plants, the plants were grown for an additional 2 weeks under identical greenhouse conditions.
Results
Sequence and expression analysis of CaBZ1
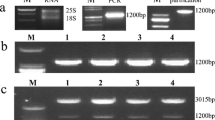
The pepper bZIP TF gene, CaBZ1, was isolated from a hot pepper cDNA library produced from the aerial portions (leaves and stem) treated with cold stress (Hwang et al. 2005). We performed RNA gel bolt analysis to clarify the expression pattern of CaBZ1 under different abiotic stress conditions. To examine the effect of ABA, we sprayed 3-week-old pepper plants with 100 μM ABA and analyzed the expression of this gene at 0–48 h after treatment. The expression of CaBZ1 was rapid and strongly induced at 3 h after treatment (Fig. 1c). We also investigated the responses of CaBZ1 to osmotic stress and salt treatment. CaBZ1 was induced at 12–24 h mannitol treatment. CaBZ1 was also induced by salt stress, with the highest mRNA level detected at 24–48 h treatment (Fig. 1c). Cold treatment slightly increased CaBZ1 expression at 1 h, and the increase remained at 24 h after treatment (Fig. 1c). These results indicate that CaBZ1 was induced by abiotic stresses and that it may encode a stress-responsive bZIP TF in pepper plants.
Phylogenetic analysis and expression patterns of CaBZ1 under different stress treatments. a Phylogenetic analysis of CaBZ1. The phylogenetic tree was constructed based on the deduced amino acid sequence of CaBZ1 and other bZIP-like domain protein sequences using the DNASTAR program (DNASTAR, Inc., Madison, WI, USA). b Multiple sequence alignment of CaBZ1 and homologous proteins. Accession numbers are as follows: CaBZ1, AY789639; NtbZIP, AAK92213; GmbZIP, NP_001238344; AtbZIP11, NP_195185; AtbZIP02, NM_127373; ZmbZIP, ADX60208; OsbZIP14, NP_001045798. c CaBZ1 is up-regulated in response to stress treatments. Total RNA was isolated from 3-week-old pepper plants treated with ABA (100 μM), mannitol (150 mM) and NaCl (150 mM). A gel pre-stained with ethidium bromide (lower) was used to confirm equal loading in all wells. d Transient expression of CaBZ1-GFP fusion protein using potato protoplasts. Potato protoplasts co-transformed with CaBZ1-GFP and NLS-RFP were observed by fluorescence microscopy 48 h after transformation. Bar represents 10 μm
To determine whether CaBZ1 is a functional TF, we examine the subcellular localization of CaBZ1 protein. A construct harboring CaBZ1-GFP driven by the CaMV35S promoter, was introduced into potato protoplast cells together with a vector expressing NLS-RFP, a nuclear marker, revealing that. CaBZ1-GFP fusion protein co-localized with NLS-RFP in the nucleus (Fig. 1d). To analyze the phylogenetic relationship between CaBZ1 and bZIP protein from other plant species, a phylogenetic tree was constructed based on the amino acid sequences of these proteins. Phylogenetic tree analysis indicated that CaBZ1 is grouped with, AtbZIP02 and AtbZIP11, which are class S group Arabidopsis bZIP proteins (Fig. 1a). CaBZ1 has a basic region and a leucine zipper for DNA-binding (Fig. 1b).
Performance of the CaBZ1-overexpressing transgenic plants under drought stress
To assess the effect of CaBZ1 overexpression on abiotic stress tolerance in plants, we generated transgenic potato plants overexpressing the coding sequence of CaBZ1 under the control of the CaMV35S promoter. The expression level of CaBZ1 was investigated by RNA gel blot analysis, and three independent transgenic potato plants were selected for further analysis (Fig. 2a). Firstly we analyzed the phenotypes for salt and cold in greenhouse. We couldn’t find out any clear tolerant phenotype for them (data are not shown). However, we could observe the drought tolerant phenotype in CaBZ1 overexpressing potato. For drought stress treatment, one-month-old transgenic and non transgenic potato plants were deprived of water for 10 day, after which watering was resumed in the greenhouse. Ten days after watering was stopped, we observed severely wilted leaves in non transgenic potato plants, whereas leaf wilting and rolling were substantially delayed in transgenic potato plants overexpressing CaBZ1 (Fig. 2b). Five days after re-watering, the transgenic potato plants exhibited stronger growth recovery; the plants survived and eventually developed green leaves. However, non transgenic potato plants did not go on to develop green leaves (Fig. 2b). We monitored the survival rates of transgenic and non transgenic potato plants. More than 50 % of CaBZ1-overexpressing plants survived and grew normally after drought stress treatment. However, only approximately 20 % of the non transgenic potato plants survived (Fig. 2c). We also measured the chlorophyll content after drought stress. Specifically, we measured the chlorophyll contents in leaves from transgenic and non transgenic potato plants used in the phenotype observation experiment on the seventh day after re-watering was resumed. All three independent transgenic potato lines had higher chlorophyll contents (by more than 50 %) than wild type potato plants (Fig. 2d).
Effect of CaBZ1 over-expression on drought tolerance and, on growth and tuber yields under non-stress treatment condition. a Expression analysis of CaBZ1 in three independent T0 transgenic potato plants by RNA gel blot analysis. Each lane was loaded with total RNA (15 μg per lane) isolated from 3-week-old potato plants. b Drought tests were performed on 1 month-old T0 transgenic potato plants. Watering was stopped for 10 day and resumed for 5 day. c Survival rates of non transgenic potato and transgenic potato plants overexpressing CaBZ1 under drought stress conditions. d Chlorophyll contents of non transgenic potato and CaBZ1-overexpressing potato plants under drought stress conditions. Chlorophyll contents (mg per fresh weight) measurements were performed on leaves of non transgenic potato and T0 transgenic potato plants. e and f Whole plants, tuber formation and plant height of non transgenic potato and T0 transgenic potato plants were evaluated in 10-week-old plants grown in a greenhouse under non-stress treatment condition. g Potato tuber yields of non transgenic potato and T0 transgenic potato plants shown each line that produced tubers under non-stress treatment condition. Error bars indicate the standard errors. Asterisks indicate a significant difference (one-way ANOVA with Turkey’s test, *p value <0.05 and **p value <0.01)
Agronomic performance of transgenic potato plants overexpressing CaBZ1
To evaluate whether ectopic expression of CaBZ1 affect the growth and development of potato, we cultivated the three CaBZ1-overexpressing transgenic lines under normal greenhouse conditions. After 8 weeks of cultivation, we examined growth characteristics, such as plant height, foliar tissue structure and tuber formation. As shown in Fig. 2e, the expression of CaBZ1 in potato did not substantially alter plant height, foliar tissue structure or tuber formation. For example, CaBZ1-overexpressing transgenic potato lines #23 and #24 exhibited reduced plant heights (by approximately 3 ~ 8 %) compared to wild type potato plants, but the plant height of line #19 was similar to that of non transgenic potato plants (Fig. 2f).
To compare potato tuber yields, wild type and three transgenic potato lines were cultivated for more than 2 weeks. The tuber weights of the three transgenic potato lines were higher (by approximately 10 ~ 25 %) than those of non transgenic potato plants (Fig. 2g). These data indicate that ectopic expression of CaBZ1 in potato does not noticeably alter the growth and development of potato plants.
Overexpression of CaBZ1 reduced water loss and increased ABA-induced stomatal closure in transgenic potato plants
To investigate the physiological mechanism underlying the regulation of water loss by CaBZ1 under drought stress conditions, we performed a water loss assay with detached leaves of transgenic and non transgenic potato plants. Water loss rates were calculated by measuring the fresh weights of detached leaves 10 times over the course of 5 h. As shown in Fig. 3a, detached leaves of CaBZ1-overexpressing transgenic potato plants lost water more slowly than those of non transgenic potato plants under drought stress condition. After 5 h drought stress treatment, the fresh weights of non transgenic potato leaves decreased by approximately 40 %, but the leaves of the three CaBZ1-overexpressing transgenic potato lines lost only approximately 34, 31 and 30 % fresh weight, respectively (Fig. 3a).
Water loss assay and ABA-induced stomatal closure of transgenic potato plants overexpressing CaBZ1. a Relative water loss rate in detached leaves from non transgenic potato and T0 transgenic potato plants overexpressing CaBZ1 under drought stress conditions. Water loss is presented as the percentage of weight loss versus the initial fresh weight. b Stomatal observation of non transgenic potato and transgenic potato plants treated with 3 μM ABA and without ABA treatment. Bar = 20 μm. (c) Analysis of stomatal aperture of non transgenic potato and transgenic potato plants treated with 3 μM ABA for 1 h. ABA-induced stomatal closure was analyzed by measuring stomatal aperture. More than 50 stomata from non transgenic potato and transgenic potato plants were examined and error bars represent the standard errors. The graph is a representative result of two independent experiments which showed similar results. Asterisks indicate a significant difference (one-way ANOVA with Turkey’s test, **p value <0.01)
In plants, water loss is regulated by guard cells, which cause stomates to open and close; ABA can induce stomatal closure. To further determine whether CaBZ1 is involved in regulating stomatal movement in potato, we compared the ABA-dependent stomatal phenotypes of the non transgenic potato and CaBZ1-overexpressing transgenic potato line #23. To induce full stomatal opening, we incubated leaves in the light for 3 h, after which we measured the widths of guard cells. The stomata of non transgenic potato plants were 12.7 ± 1.66 μm wide, compared with 13.2 ± 1.88 μm wide in transgenic potato line #23. However, in the presence of 3 μM ABA, there was a significant decrease in the size of stomatal pores (ratio of width to length) in transgenic potato line #23 compared with wild type (Fig. 3b, c). As shown in Fig. 3, after 1 h ABA treatment, most stomata in transgenic potato line #23 exhibited more ABA-sensitive stomatal closure (0.28 ± 0.05 μm) than those in non transgenic potato plant (0.4 ± 0.06 μm).
Collectively, these results suggest that expressing CaBZ1 in potato affects drought tolerance via ABA-sensitive stomatal closure, leading to reduced water loss under drought conditions. This tolerance phenotype of CaBZ1-overexpressing transgenic potato plants is consistent the slower water loss from leaves compared with non transgenic potato plants.
CaBZ1 regulates the transcription levels of drought stress-related genes
To investigate whether the drought tolerance of CaBZ1-overexpressing plants is associated with changes in the expression levels of stress-responsive genes, we conducted microarray analysis of transgenic potato plants using potato chip (135 K) (GGBio, Korea). A number of genes were up-regulated twofold or more in transgenic plants compared with non transgenic potato plants (Supplemental Table 1). Based on the microarray data, we chose five putative stress-related genes (Supplemental Table 2) and analyzed their expression by qRT-PCR. Among them, the expression of the genes encoding NAC-domain protein, NAC TF, and AP2 domain CBF protein, C-repeat binding factor, was elevated more than twofold in all three independent CaBZ1-overexpressing plants compared with the non transgenic potato plant (Fig. 4). In addition, a major ABA 8′-hydroxylase gene, CYP707A1, was up-regulated 2.5-fold, 4.4-fold, and 3.8-fold, respectively, in the three independent transgenic lines compared with non transgenic potato plants (Fig. 4).
Up-regulation of stress-responsive genes in transgenic potato plants overexpressing CaBZ1. a Expression levels of CYP707A1, CBF and NAC in potato plants genes increased in response to ABA (100 μM) and mannitol (150 mM) treatment. b Expression levels of stress-inducible genes increased in T0 transgenic potato plants overexpressing CaBZ1. Error bars indicate the standard errors. The experiments were performed three times with at least three independent repetitions. Asterisks indicate a significant difference (one-way ANOVA with Turkey’s test, *p value <0.05 and **p value <0.01)
Transgenic potato overexpressing CaBZ1 exhibit improved tuber yields under drought stress conditions
After confirming the improved drought tolerance in early stage leaves of transgenic potato plants, we measured their tuber yields under drought stress conditions. For this experiment, we generated additional transgenic potato plants using a different vector harboring CaBZ1 under the control of the CaMV35S promoter (Supplemental Materials and Methods). The expression levels of were examined by qRT-PCR, and five independent transgenic potato plants were chosen for further analysis (Supplemental Fig. 1b). Seven-week-old transgenic and non transgenic potato plants were deprived of water for 12 day and watering was subsequently resumed in the greenhouse. As described above, all transgenic potato lines showed strong drought stress tolerance compared with non transgenic potato plants (Supplemental Fig. 1a). We determined the survival rates of transgenic potato and non transgenic potato plants after 7 day re-watering. More than 80 % of the transgenic potato plants survived after the period of drought stress; however, only approximately 20 % of non transgenic potato plants survived (Supplemental Fig. 1c).
To compare potato tuber yields, potato plants re-watered after drought treatment and cultivated for two more weeks under normal greenhouse conditions. Potato tubers from transgenic and non transgenic potato plants were harvested and their tuber yields were compared (Fig. 5a). Although the tuber sizes were diverse for each plant, the number of tubers in the transgenic lines averaged 6–7 after drought stress treatment whereas that of non transgenic potato plants averaged only 4 (Fig. 5b). In addition, the total tuber weight of non transgenic potato plants was approximately 23.47 g, but that of the transgenic potato lines was more than 70 g (Fig. 5c). Although transgenic potato line #3 exhibited slightly reduced total tuber yields under normal conditions, these results indicate that the ectopic expression of CaBZ1 in potato confers increased stress tolerance and tuber yields under drought stress conditions.
Transgenic potato overexpressing CaBZ1 exhibits improved tuber yields under drought stress conditions. a Representative potato tubers of non transgenic potato and T0 transgenic potato plants are shown for each line that produced tubers under normal and drought stress conditions. b Number of tuber in non transgenic potato and T0 transgenic potato plants after drought treatment. c Potato tuber yields of non transgenic potato and T0 transgenic potato plants under normal and drought stress condition. Error bars indicate the standard errors. Asterisks indicate a significant difference (one-way ANOVA with Turkey’s test, *p value <0.05 and **p value <0.01)
Discussion
In this study, we examined the gene expression pattern and cloned a novel bZIP gene from hot pepper. Amino acid alignment showed that CaBZ1, which harbors a basic region and leucine zipper region for DNA-binding, is similar to other plant bZIP proteins and phylogenetic analysis classified it with group S bZIP proteins compared with Arabidopsis (Fig. 1). Lee et al. 2006 showed that several bZIP genes (AtbZIP11 and AtbZIP53), which belong to the same phylogenetic group S as CaBZ1, are induced only by salt stress treatment, whereas AtbZIP2 is down-regulated under the same conditions (Lee et al. 2006). Here, we determined that CaBZ1, unlike the bZIP genes of Arabidopsis group S, is strongly induced by multiple stress stimuli, such as ABA, cold, mannitol and salinity (Fig. 1).
TFs can up- or down-regulate the expression of a group of genes containing specific cis-elements in their promoters (Fujita et al. 2006; Hussain et al. 2011). Therefore, downstream genes of TFs can contribute to stress tolerance in plants synergistically, producing stronger phenotypes than those produced by a single down-stream functional gene. In this study, over-expression of CaBZ1 in potato altered the expression levels of a group of stress-inducible genes and TF genes, such as NAC and CBF (Fig. 4). Transgenic rice plants constitutively expressing AtCBF3 are more tolerant to drought and high salinity stress conditions that non-transgenic control plants (Oh et al. 2005). Ectopic expression of AtNAC2 in groundnut (Arachis hypogaea L.) also increases tolerance to drought and salinity, and it improves yields as well (He et al. 2005). Taken together, our results suggest that ectopic expression of the CaBZ1 TF gene from hot pepper regulates the expression of several important TFs and functional genes to improve stress tolerance in potato.
To date, many studies have focused on increasing plant tolerance to environmental stress conditions through expressing stress-inducible TFs, including bZIP TFs (Golldack et al. 2011; Kim 2006, 2014). However, overexpressing stress-responsive TF genes driven by constitutively expressed promoters might produce undesirable growth characteristics such as smaller leaves, stunted plants, delayed flowering and yield penalty (Hsieh et al. 2002; Kasuga et al. 2004; Pino et al. 2007). Therefore, although many TFs related to abiotic stress tolerance have been reported in several different plants, few TF genes have been used to develop commercial transgenic plants. For example, in potato, constitutive expression of three Arabidopsis CBF genes causes severe growth retardation and reduced tuber production. Among these TFs, the expression of AtCBF2 abolishes tuber yield (except in one line), while the expression of AtCBF3 completely abolishes tuber production in all transgenic lines (Pino et al. 2007). Furthermore, Kasuga et al. (2004) developed transgenic tobacco over-expressing DREB1A, which improved drought and low temperature stress tolerance. However, these transgenic plants exhibited a severe dwarf phenotype under normal growth conditions (Kasuga et al. 2004). In several different plants such as soybean, tomato and Brassica junsea, over-expression of DREBs repressed the growth of plants (Cong et al. 2008; Li et al. 2012; Suo et al. 2012). Microarray analysis of SIDREB over-expressing tomato showed the alteration of GA metabolism related gene expression (Li et al. 2012). In AtDREB1 over-expressing soybean endogenous gibberellins levels were decreased (Suo et al. 2012). Thus the alteration of GA metabolism might be a cause of dwarfism commonly observed in stress inducible TF over-expressing transgenic plants. The yield penalty of transgenic plants is one of the most important bottlenecks in the application of transgenic crops. However, despite the constitutive over-expression of CaBZ1, the transgenic potato plants did not exhibit altered plant growth and development phenotypes (such as altered foliar tissue shape, plant height, tuber formation and yield) under normal conditions (Fig. 2). We also couldn’t find out the significant alteration of GA metabolism related genes in microarray data and qPCR analysis (Supplemental Fig. 3). In addition, the transgenic potato plants exhibited strong drought tolerance and higher tuber number and yields than that wild type potato plants under drought stress conditions (Fig. 5).
Some reports demonstrate that the use of stress-inducible promoters can minimize the negative effects of the exogenous gene on the plant growth (Kasuga et al. 1999, 2004; Pino et al. 2007). In Arabidopsis and tobacco, expressing DREB1A under the control of the stress-inducible rd29A promoter resulted in much less growth inhibition than expressing this gene under the control of the constitutive CaMV35S promoter (Kasuga et al. 1999, 2004). In addition, Pino et al. (2007) found that in potato, expression of Arabidopsis CBF1 and CBF3 under the control of a cold-inducible promoter alleviated the stunting phenotype and restored tuber yield to roughly wild type potato levels (Pino et al. 2007). Therefore, using a stress-inducible promoter might be a useful strategy for minimizing the effects of a TF gene on plant growth and productivity. However, the development of an abiotic stress-tolerant gene without yield penalty and increasing productivity still represents the best choice for such applications because it alleviates the necessity of overcoming negative effects. CaBZ1 exhibits this valuable characteristic. Our current study represents the first report of improving the drought tolerance of a tuber crop without reducing tuber yields, using transgenic technology.
References
Arnon DL (1949) A copper enzyme is isolated chloroplast pholyphenol oxidase in Beta vulgaries. Plant Physiol 24:1–15
Bohnert HJ, Gong QQ, Li PH, Ma SS (2006) Unraveling abiotic stress tolerance mechanisms: getting genomics going. Curr Opin Plant Biol 9:180–188
Camire ME, Kubow S, Donnelly DJ (2009) Potatoes and human health. Crit Rev Food Sci Nutr 49:823–840
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Cong L, Zheng HC, Zhang YX, Chai TY (2008) Arabidopsis DREB1A confers high salinity tolerance and regulates the expression of GA dioxygenases in Tobacco. Plant Sci 174:156–164
Correa LG, Riano-Pachon DM, Schrago CG, dos Santos RV, Mueller-Roeber B, Vincentz M (2008) The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PLoS One 3:e2944
Evers D, Lefevre I, Legay S, Lamoureux D, Hausman JF, Rosales RO, Marca LR, Hoffmann L, Bonierbale M, Schafleitner R (2010) Identification of drought-responsive compounds in potato through a combined transcriptomic and targeted metabolite approach. J Exp Bot 61:2327–2343
Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17:3470–3488
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Golldack D, Luking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY (2005) AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J 44:903–916
Hsieh TH, Lee JT, Charng YY, Chan MT (2002) Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol 130:618–626
Hussain SS, Kayani MA, Amjad M (2011) Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol Prog 2:297–306
Hwang EW, Kim KY, Park SC, Jeong MJ, Byun MO, Kwon HB (2005) Expression profiles of hot pepper (Capsicum annuum) genes under cold stress conditions. J Biosci 30:657–667
Izawa T, Foster R, Nakajima M, Shimamoto K, Chua NH (1994) The rice bZIP transcriptional activator RITA-1 is highly expressed during seed development. Plant Cell 6:1277–1287
Jakoby M, Weisshaar B, Droge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7:106–111
Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13:1511–1526
Kang JY, Choi HI, Im MY, Kim SY (2002) Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14:343–357
Karimi M, Inze D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45:346–350
Kim SY (2006) The role of ABF family bZIP class transcription factors in stress response. Physiol Plant 126:519–527
Kim TH (2014) Mechanism of ABA signal transduction: agricultural highlights for improving drought tolerance. J Plant Biol 57:1–8
Lee YH, Kim HS, Kim JY, Jung M, Park YS, Lee JS, Choi SH, Her NH, Lee JH, Hyung NI, Lee CH, Yang SG, Harn CH (2004) A new selection method for pepper transformation: callus-mediated shoot formation. Plant Cell Rep 23:50–58
Lee SS, Yang SH, Bererich T, Miyazaki A, Kusano T (2006) Characterization of AtbZIP2, AtbZIP11 and AtbZIP53 from the group S basic region-leucine zipper family in Arabidopsis thaliana. Plant Biotechnol 23:249–258
Lee HE, Shin D, Park SR, Han SE, Jeong MJ, Kwon TR, Lee SK, Park SC, Yi BY, Kwon HB, Byun MO (2007) Ethylene responsive element binding protein 1 (StEREBP1) from Solanum tuberosum increases tolerance to abiotic stress in transgenic potato plants. Biochem Biophys Res Commun 353:863–868
Li JH, Sima W, Ouyang B, Wang TT, Ziaf K, Luo ZD, Liu LF, Li HX, Chen ML, Huang YQ, Feng YQ, Hao YH, Ye ZB (2012) Tomato SlDREB gene restricts leaf expansion and internode elongation by downregulating key genes for gibberellin biosynthesis. J Exp Bot 63:6407–6420
Moon SJ, Han SY, Kim DY, Kim BG, Yoon IS, Shin D, Kwon HB, Byun MO (2014) Ectopic expression of CaWRKY1, a pepper transcription factor, enhances drought tolerance in transgenic potato plants. J Plant Biol 57:198–207
Moore JP, Le NT, Brandt WF, Driouich A, Farrant JM (2009) Towards a systems-based understanding of plant desiccation tolerance. Trends Plant Sci 14:110–117
Muszynski MG, Dam T, Li B, Shirbroun DM, Hou Z, Bruggemann E, Archibald R, Ananiev EV, Danilevskaya ON (2006) Delayed flowering1 encodes a basic leucine zipper protein that mediates floral inductive signals at the shoot apex in maize. Plant Physiol 142:1523–1536
Nijhawan A, Jain M, Tyagi AK, Khurana JP (2008) Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol 146:333–350
Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J 18:455–463
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK (2009) Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol 150:1368–1379
Pino MT, Skinner JS, Park EJ, Jeknic Z, Hayes PM, Thomashow MF, Chen TH (2007) Use of a stress inducible promoter to drive ectopic AtCBF expression improves potato freezing tolerance while minimizing negative effects on tuber yield. Plant Biotechnol J 5:591–604
Reynolds M, Tuberosa R (2008) Translational research impacting on crop productivity in drought-prone environments. Curr Opin Plant Biol 11:171–179
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Stiller I, Dulai S, Kondrak M, Tarnai R, Szabo L, Toldi O, Banfalvi Z (2008) Effects of drought on water content and photosynthetic parameters in potato plants expressing the trehalose-6-phosphate synthase gene of Saccharomyces cerevisiae. Planta 227:299–308
Suo H, Ma Q, Ye K, Yang C, Tang Y, Hao J, Zhang ZJ, Chen M, Feng Y, Nian H (2012) Overexpression of AtDREB1A causes a severe dwarf phenotype by decreasing endogenous gibberellin levels in soybean [Glycine max (L.) Merr]. PLoS One 7:e45568
Thurow C, Schiermeyer A, Krawczyk S, Butterbrodt T, Nickolov K, Gatz C (2005) Tobacco bZIP transcription factor TGA2. 2 and related factor TGA2. 1 have distinct roles in plant defense responses and plant development. Plant J 44:100–113
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61:672–685
Acknowledgments
This work was supported by the Research Program for Agricultural Science and Technology Development (Project No. PJ010885) of the National Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moon, SJ., Han, SY., Kim, DY. et al. Ectopic expression of a hot pepper bZIP-like transcription factor in potato enhances drought tolerance without decreasing tuber yield. Plant Mol Biol 89, 421–431 (2015). https://doi.org/10.1007/s11103-015-0378-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0378-y