Abstract
Seed dormancy facilitates to endure environmental disadvantages by confining embryonic growth until the seeds encounter favorable environmental conditions for germination. Abscisic acid (ABA) and gibberellic acid (GA) play a pivotal role in the determination of the seed dormancy state. ABA establishes seed dormancy, while GA triggers seed germination. Here, we demonstrate that MYB96 contributes to the fine-tuning of seed dormancy regulation through the coordination of ABA and GA metabolism. The MYB96-deficient myb96-1 seeds germinated earlier than wild-type seeds, whereas delayed germination was observed in the activation-tagging myb96-1D seeds. The differences in germination rate disappeared after stratification or after-ripening. The MYB96 transcription factor positively regulates ABA biosynthesis genes 9-CIS-EPOXYCAROTENOID DIOXYGENASE 2 (NCED2), NCED5, NCED6, and NCED9, and also affects GA biosynthetic genes GA3ox1 and GA20ox1. Notably, MYB96 directly binds to the promoters of NCED2 and NCED6, primarily modulating ABA biosynthesis, which subsequently influences GA metabolism. In agreement with this, hyperdormancy of myb96-1D seeds was recovered by an ABA biosynthesis inhibitor fluridone, while hypodormancy of myb96-1 seeds was suppressed by a GA biosynthesis inhibitor paclobutrazol (PAC). Taken together, the metabolic balance of ABA and GA underlies MYB96 control of primary seed dormancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seeds are products of plant sexual reproduction and facilitate the dispersal of offspring to new favorable locations. Dormancy enables seeds to tolerate unfavorable environmental conditions and limit embryonic growth until they encounter optimal growth conditions (Finch-Savage and Leubner-Metzger 2006; Bentsink and Koornneef 2008; Graeber et al. 2012). Primary seed dormancy is established during the seed maturation stages and peaks in freshly harvested seeds (Holdsworth et al. 2008; Graeber et al. 2012). This quiescent state can be broken by stratification (cold imbibition) or after-ripening (dry storage) (Baskin and Baskin 2004), and the subsequent germination process is started to initiate a plant life cycle. The developmental transition from seed dormancy to germination is elaborately regulated in order to minimize physiological damage and enhance reproductive success (Willis et al. 2014).
ABA is a central phytohormone that regulates the induction and maintenance of seed dormancy (Grappin et al. 2000; Gubler et al. 2005; Kermode 2005; Finkelstein et al. 2008). While endogenous contents of ABA are low during early embryogenesis, the ABA levels increase gradually as seeds mature (Finkelstein et al. 2002; Gutierrez et al. 2007). During the late stages of seed maturation, high levels of ABA ensure inhibition of pre-harvest sprouting or viviparity (Nambara and Marion-Poll 2003; Gubler et al. 2005; Finch-Savage and Leubner-Metzger 2006; Holdsworth et al. 2008). ABA-dependent accumulation of seed storage compounds, such as carbohydrates, oils, and LATE EMBRYOGENESIS ABUNDANT (LEA) proteins, also occurs during the late stages of seed maturation to facilitate desiccation tolerance and to establish primary seed dormancy (Finkelstein and Somerville 1990; Meinke et al. 1994; Baud et al. 2008).
A number of molecular components regulating ABA-dependent seed dormancy have been identified. One group includes proteins associated with ABA metabolism. The NCED3, NCED5, NCED6, and NCED9 proteins and additional ABA-biosynthetic enzymes, such as ABA1, ABA2, and ABSCISIC ALDEHYDE OXIDASE 3 (AAO3), contribute to the induction and maintenance of seed dormancy (Koornneef et al. 1982; Seo and Koshiba 2002; González-Guzmán et al. 2004; Lefebvre et al. 2006; Lin et al. 2007). Consistently, ABA-deficient mutant seeds show reduced dormancy (Lefebvre et al. 2006; Frey et al. 2012), whereas ABA overproduction leads to deep seed dormancy (Martínez-Andújar et al. 2011). Meanwhile, ABA breakdown is also important for the removal of seed dormancy. ABA catabolism is catalyzed by members of the CYP707A family (CYP707A1-CYP707A4) that produce an inactive form of ABA, 8′-hydroxy ABA (Kushiro et al. 2004; Okamoto et al. 2006). The genetic mutants that have defects in CYP707A1, CYP707A2, and CYP707A3 accumulate high levels of ABA and exhibit hyperdormancy in seeds (Kushiro et al. 2004).
The other group includes proteins regulating ABA signal transduction, such as protein kinases, phosphatases, and transcription factors. Core ABA perception components, including PYRABACTIN RESISTANCE 1/PYR-LIKE PROTEINs/REGULATORY COMPONENTS OF ABA RECEPTORs (PYR1/PYLs/RCARs), group A protein phosphatase 2Cs (PP2Cs), and SNF1-related protein kinase 2s (SnRK2s) (Fujii et al. 2009; Cutler et al. 2010; Hubbard et al. 2010), are involved in the regulation of seed dormancy. The loss-of-function mutants of ABA-binding PYR1/PYLs/RCARs are hyposensitive to ABA during seed germination (Gonzalez-Guzman et al. 2012; Kim et al. 2012). Two PP2C proteins, ABA INSENSITIVE 1 (ABI1) and ABI2, negatively regulate ABA signaling, and accordingly, the dominant-negative abi1-1 and abi2-1 mutant seeds exhibit hypodormancy (Koornneef et al. 1984; Finkelstein 1994). Furthermore, mutations in genes encoding SnRK2.2, SnRK2.3, and SnRK2.6, which phosphorylate ABA-response element (ABRE)-binding factors/(ABRE)-binding proteins (ABFs/AREBs) and thereby activate ABA signaling, result in shallow seed dormancy (Fujii et al. 2007, 2009; Nakashima et al. 2009). In support of this, an ABA signaling transducer ABI3 transcription factor, together with FUSCA3 (FUS3), LEAFY COTYLEDON 1 (LEC1), and LEC2, controls seed maturation and dormancy (Parcy et al. 1997; Nambara et al. 2000; Raz et al. 2001; To et al. 2006). ABI4 and ABI5 are also involved in seed dormancy establishment (Xi et al. 2010; Shu et al. 2013; Vaistij et al. 2013), demonstrating the unequivocal role of ABA in seed dormancy.
GA is another key hormone that promotes the transition from seed dormancy to germination by antagonizing the action of ABA (Koornneef et al. 2002; Peng and Harberd 2002; Ogawa et al. 2003; Yamauchi et al. 2004). In accordance with this, GA-deficient ga1-3 seeds exhibit hyperdormancy (Koornneef and van der Veen 1980), whereas mutations in the GA2-OXIDASE (GA2ox) gene, which inactivates bioactive GA, reduce seed dormancy (Varbanova et al. 2007; Yamauchi et al. 2007).
GA signaling components are also implicated in the control of seed dormancy and germination. The GA receptor proteins, GIBBERELLIN-INSENSITIVE DWARF1s (GID1s), which ubiquitinate and degrade DELLA proteins along with an F-box protein SLEEPY1 (SLY1) in the presence of GA, stimulate GA signal transduction (McGinnis et al. 2003; Sun and Gubler 2004; Ueguchi-Tanaka et al. 2007; Schwechheimer 2008). Consistently, the GID1- or SLY1-deficient mutants show reduced germination rate (Griffiths et al. 2006; Iuchi et al. 2007). In addition, five DELLA proteins, GA INSENSITIVE (GAI), REPRESSOR OF GA1-3 (RGA), RGA-LIKE 1 (RGL1), RGL2, and RGL3, and an additional negative regulator of GA signaling SPINDLY (SPY), inhibit seed germination by maintaining the dormancy state (Lee et al. 2002; Peng and Harberd 2002; Cao et al. 2005; Penfield et al. 2006; Piskurewicz and Lopez-Molina 2009).
Accumulating evidence supports that ABA and GA signaling is coordinated to fine-tune the transition from seed dormancy to germination (Gubler et al. 2008; Toh et al. 2008; Footitt et al. 2011). Although several regulatory components have been suggested (Yano et al. 2009; Shu et al. 2013), the molecular mechanisms behind the crosstalk between ABA and GA signaling in seed dormancy remain yet to be discovered.
The MYB96 transcription factor regulates a wide array of ABA responses, such as drought tolerance, salicylic acid biosynthesis, lateral root development, and cuticular wax accumulation (Seo et al. 2009, 2011; Seo and Park 2010). In this work, we report that MYB96 also regulates seed dormancy primarily through the regulation of ABA metabolism, which further influences GA metabolism. The MYB96-deficient mutant showed reduced seed dormancy, whereas delayed germination was observed in myb96-1D seeds. The MYB96 transcription factor positively regulates ABA biosynthesis and also affects GA biosynthesis. In agreement with this, hyperdormancy of myb96-1D seeds was recovered by an ABA biosynthesis inhibitor, the fluridone, and hypodormancy of myb96-1 seeds was suppressed by a GA biosynthesis inhibitor, PAC. Taken together, this study demonstrates that MYB96 regulation of GA and ABA metabolism contributes to establishing primary seed dormancy and provides insight into how developing seeds achieve metabolic balance of ABA and GA.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana (Columbia-0 ecotype) was used for all experiments described, unless specified otherwise. Plants were grown under long day conditions (LDs; 16-h light/8-h dark cycles) with cool white fluorescent light (100 μmol photons m−2 s−1) at 23 °C. The myb96-1D and myb96-1 mutants (GABI_120B05) were previously reported (Seo et al. 2009, 2011).
Quantitative real-time RT-PCR analysis
Total RNA was extracted using TRI agent (TAKARA Bio, Singa, Japan) according to the manufacturer’s recommendations. Reverse transcription (RT) was performed using Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Dr. Protein, Seoul, South Korea) with oligo dT(18) to synthesize first-strand cDNA from 2 μg of total RNA. Total RNA samples were pretreated with an RNAse-free DNAse. The synthesized cDNAs were diluted to 100 μL with TE buffer, and 1 μL of diluted cDNA was used for PCR amplification.
Quantitative RT-PCR reactions were performed in 96-well blocks using the Step-One Plus Real-Time PCR System (Applied Biosystems). The PCR primers used are listed in Supplementary Table S1. The values for each set of primers were normalized relative to the EUKARYOTIC TRANSLATION INITIATION FACTOR 4A1 (eIF4A) gene (At3g13920). All RT-qPCR reactions were performed in biological triplicates using total RNA samples extracted from three independent replicate samples. The comparative CT method was used to evaluate relative quantities of each amplified product in the samples. The threshold cycle (CT) was automatically determined with default parameters. The specificity of RT-qPCR reactions was determined by melt curve analysis.
Seed germination assays
All genotypes were grown at 23 °C under LD conditions, and seeds were collected at the same time. Harvested seeds were dried at room temperature at least 1 week before the germination assays. For the seed germination assays, 40–50 seeds of each line were sterilized and plated on MS-medium (half-strength MS salts, 0.05 % MES, pH 5.7, and 0.7 % agar) with or without stratification treatment for 3 days at 4 °C. Plates were incubated in a culture room set at 23 °C with a 16-h light/8-h dark cycle. Germination was scored at the indicated time points by counting the frequency of radicle emergence from the seed coat and endosperm. For each germination assay, biological triplicates were performed.
To investigate the effects of ABA and GA biosynthetic inhibitors, fluridone (MB-F4369) and PAC (MB-P5699) were purchased from MB cell (Los Angeles, CA) and used at final concentrations of 10 μM and 15 μM, respectively, as previously described (Seo et al. 2006; Martínez-Andújar et al. 2011; Barua et al. 2012; Shu et al. 2013).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed as previously described (Yang et al. 2011). 35S:MYB96-MYC transgenic plants, anti-MYC antibodies (Millipore, Billerica, USA), and salmon sperm DNA/protein A agarose beads (Millipore, Billerica, USA) were used for ChIP. DNA was purified using phenol/chloroform/isoamyl alcohol and sodium acetate (pH 5.2). The level of precipitated DNA fragments was quantified by quantitative real-time PCR using specific primer sets (Supplementary Table S2). Values were normalized with the level of input DNA. The values in pBA002 control plants were set to 1 after normalization against eIF4a for quantitative PCR analysis.
Results
myb96-1 seeds exhibit hypodormancy
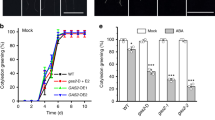
The MYB96 transcription factor mediates a variety of ABA responses (Seo et al. 2009, 2011; Seo and Park 2010). Since seed dormancy is one of the representative traits regulated by ABA, we wanted to know whether MYB96 is involved in establishing primary seed dormancy. To that end, we first compared the germination rate of myb96-1D, myb96-1, and wild-type seeds, which were subjected to dry storage for 1 week. Without cold stratification, higher germination rate was observed in myb96-1 seeds, whereas myb96-1D seeds exhibited delayed germination relative to wild-type seeds (Fig. 1a). At 2 days after sowing, the germination rate of myb96-1 seeds reached to 85 %, while 65 % of wild-type seeds germinated. The germination rate of myb96-1D seeds was only about 40 % at this time point (Fig. 1a).
Germination rate of myb96-1D, myb96-1, and wild-type seeds. Seeds were stored for 1 week (a, b), 2 weeks (c, d), or 6 months (e, f) after harvest and germinated on MS-medium with or without stratification treatment. The seed germination percentage of the indicated genotypes was quantified. Radicle emergence was used as a morphological marker for germination. At least 50 seeds per genotype were measured in each replicate. Biological triplicates were averaged. Bars indicate the SE of the mean
Cold stratification breaks seed dormancy and promotes seed germination (Wang et al. 2009; Arc et al. 2012). We examined whether cold imbibition reduces dormancy of myb96-1D seeds. Following cold stratification of 1-week-stored seeds for 3 days, the seeds germinated under long day (LD) conditions. The germination rates of myb96-1D and myb96-1 mutant seeds were comparable to those of wild-type seeds, and all genotypes were fully germinated 2 days after sowing (Fig. 1b). Two-week-stored seeds showed similar results. Without stratification, the myb96-1 seeds germinated earlier than wild-type seeds, but delayed germination was observed in myb96-1D seeds (Fig. 1c). The differences in germination rate were compromised by stratification treatment (Fig. 1d).
After-ripening is a developmental factor that reduces seed dormancy (Finch-Savage et al. 2007; Holdsworth et al. 2008). To examine the effect of after-ripening on seed dormancy, 6-month-stored fully ripened seeds of myb96-1D, myb96-1, and wild-type were used for scoring germination rates. Seed germination rates of myb96-1D and myb96-1 were indistinguishable from those of wild-type seeds, regardless of stratification treatment (Fig. 1e, f).
Vivipary is a result of reduced seed dormancy. Hence, we examined whether myb96-1 seeds show vivipary in developing long-green siliques (Martínez-Andújar et al. 2011). Wild-type, myb96-1D, and myb96-1 seeds in developing siliques were put on either MS-medium or soil and germinated under LD conditions. The myb96-1 seeds germinated more quickly than wild-type seeds (Fig. 2a, b), whereas myb96-1D seeds showed delayed germination rate (Fig. 2a, b). Ten days after sowing, the germination rate of myb96-1D seeds was approximately half that of wild-type seeds, but most of the myb96-1 seeds had already produced cotyledons (Fig. 2c), indicating that the MYB96 protein is a positive regulator of seed dormancy establishment.
Viviparous phenotype of myb96-1 seeds. Immature developing siliques at the long-green stage were collected and germinated on MS-medium (a) or soil (b) without stratification treatment. The siliques were germinated for 10 days under LD conditions. The seed germination percentage of the indicated genotypes was quantified (c). Biological triplicates were averaged. Bars indicate the SE of the mean
MYB96 positively regulates ABA biosynthesis
The role of MYB96 in seed dormancy is likely to be independent of DELAY OF GERMINATION 1 (DOG1) and ABI5 (Supplementary Fig. 1), master regulators of the dormancy acquisition (Lopez-Molina et al. 2001; Bentsink et al. 2006; Vaistij et al. 2013). We therefore supposed that MYB96 may be involved in the control of hormone homeostasis.
Seed dormancy is intimately associated with ABA contents in seeds. ABA promotes seed dormancy (Ali-Rachedi et al. 2004), but cold stratification or after-ripening reduces the ABA contents in mature seeds to stimulate germination (Corbineau et al. 2002; Gubler et al. 2008). To look into the molecular mechanism underlying MYB96 regulation of seed dormancy, we analyzed the expression of ABA biosynthetic genes, such as NCED2, NCED3, NCED5, NCED6, NCED9, ABA1, ABA2, and AAO3, and ABA catabolic genes CYP707A1, CYP707A2, and CYP707A3 in myb96-1D, myb96-1, and wild-type seedlings. Quantitative real-time RT-PCR (RT-qPCR) analysis revealed that most genes examined were not significantly altered in myb96-1D and myb96-1 mutants, except for NCED2, NCED5, NCED6, and NCED9. The NCED genes were substantially up-regulated in myb96-1D but slightly down-regulated in myb96-1 (Fig. 3), suggesting that MYB96 induces ABA biosynthesis to establish primary seed dormancy.
Expression of genes involved in ABA metabolism in myb96-1D and myb96-1 mutants. Ten-day-old seedlings grown under LD conditions were harvested for total RNA isolation. Transcript accumulation was analyzed by quantitative real-time RT-PCR (RT-qPCR). The eIF4a gene was used as an internal control. Biological triplicates were averaged. Bars indicate the SE of the mean. Statistically significant differences between the wild-type and mutants are indicated by asterisks (Student’s t test, *P < 0.05). The y-axis is presented on a logarithmic scale for better comparison of fold changes
GA biosynthetic genes are down-regulated in myb96-1D
GA is also an important hormone that governs the transition from seed dormancy to germination (Koornneef et al. 2002; Graeber et al. 2012). Given the intensive metabolic crosstalk between ABA and GA in seeds (Gubler et al. 2008; Toh et al. 2008; Yano et al. 2009; Shu et al. 2013), we speculated that GA metabolism may also be altered in myb96-1D and myb96-1 mutants. To estimate the role of MYB96 in the regulation of GA metabolism, we analyzed the expression of GA biosynthetic genes, including GA3, GA3ox1, GA20ox1, GA20ox2, GA20ox3, ENT-KAURENOIC ACID OXYDASE 1 (KAO1), and KAO2, and a GA catabolic gene GA2ox8 (Ogawa et al. 2003; Rieu et al. 2008). RT-qPCR analysis showed that the expression of examined GA metabolism genes was largely unaltered, except for GA3ox1 and GA20ox1. Their expression levels were significantly reduced in myb96-1D, but slightly elevated in myb96-1 (Fig. 4). These results support that MYB96 also influences GA biosynthetic activity in the control of seed dormancy.
Expression of genes involved in GA metabolism in myb96-1D and myb96-1 mutants. Ten-day-old seedlings grown under LD conditions were harvested for total RNA isolation. Transcript accumulation was analyzed by RT-qPCR. The eIF4a gene was used as an internal control. Biological triplicates were averaged. Bars indicate the SE of the mean. Statistically significant differences between the wild-type and mutants are indicated by asterisks (Student’s t test, *P < 0.05)
MYB96 balances accumulation of ABA and GA in mature seeds
To confirm the involvement of ABA and GA metabolism in MYB96 regulation of seed dormancy, we analyzed the germination rates of 1-week-stored myb96-1, myb96-1D, and wild-type seeds in the presence of either ABA biosynthesis inhibitor fluridone or GA biosynthesis inhibitor PAC. Without stratification, earlier germination was observed in myb96-1 seeds, whereas myb96-1D seeds showed delayed germination (Fig. 5a). In the presence of fluridone, hyperdormancy of myb96-1D seeds was recovered to a level comparable to wild-type seeds (Fig. 5b). In addition, hypodormancy of myb96-1 seeds was suppressed by PAC (Fig. 5c), indicating that a metabolic balance of GA and ABA underlies MYB96 control of seed dormancy.
Effects of ABA and GA biosynthesis inhibitors on seed dormancy of myb96-1 and myb96-1D. Seeds were stored for 1 week after harvest and germinated on MS-medium (a) or MS-medium supplemented with 10 μM fluridone (b) or 15 μM PAC (c). The seed germination percentage of the indicated genotypes was quantified using radicle emergence as a morphological marker for germination. At least 50 seeds per genotype were measured in each replicate. Biological triplicates were averaged. Bars indicate the SE of the mean
MYB96 binds to promoters of NCED2 and NCED6
MYB96 regulates ABA and GA metabolism to properly establish seed dormancy. Given the regulation hierarchy between ABA and GA in seed germination (Seo et al. 2006) and the biochemical nature of MYB96 as a transcriptional activator (Seo et al. 2009, 2011), it was most likely that MYB96 primarily regulates ABA biosynthesis. We therefore asked whether MYB96 binds directly to the consensus motifs on the promoters of the NCED genes. Sequence analysis revealed that their promoters contain at least three conserved sequence motifs that are analogous to the R2R3-type MYB-binding sequences (Fig. 6a–d). The presence of MYB-binding cis-elements led us to examine whether MYB96 is targeted to the NCED promoters.
Binding of MYB96 to NCED2 and NCED6 promoters. The putative MYB binding sites are indicated by arrowheads. Black lines indicate the regions for PCR amplification after chromatin immunoprecipitation (ChIP). Enrichment of putative MYB binding regions of NCED2 (a), NCED5 (b), NCED6 (c), and NCED9 (d) promoters was analysed by qPCR. The values in the pBA002 plants were set to one after normalization against eIF4a for qPCR analysis. Biological triplicates were averaged and statistically analysed by Student’s t test (*P < 0.05). Bars indicate the SE of the mean
To perform ChIP assays, we employed 35S:MYB96-MYC transgenic plants (Seo et al. 2011). Total protein extracts from control pBA002 and 35S:MYB96-MYC transgenic plants were immunoprecipitated with anti-MYC-antibody. DNA bound to MYB96 proteins was analyzed by qPCR assays. ChIP analysis showed that the B region of the NCED2 promoter and the H region of the NCED6 promoter were enriched by MYB96 (Fig. 6a, c). In contrast, genomic fragments on the promoters of NCED5 and NCED9 were not enriched (Fig. 6b, d), supporting the specific interaction of MYB96 with the NCED2 and NCED6 promoters. In addition, the control ChIP with resin alone did not enrich the B and H fragments (Supplementary Fig. 2). These results indicate that MYB96 specifically targets promoter sequences of the NCED2 and NCED6 genes to activate its expression.
Taken together, MYB96 regulates the seed dormancy state by coordinating ABA and GA metabolism. The transcription factor directly binds to the promoters of ABA biosynthetic genes, such as NCED2 and NCED6, and activates their expression. MYB96 activation of ABA biosynthesis probably leads to reduced GA accumulation by repressing the expression of GA3ox1 and GA20ox1, re-establishing GA and ABA homeostasis.
Discussion
Regulation of seed dormancy through coordinated ABA and GA metabolism
Seed dormancy and germination processes are intricately regulated in order to ensure plant productivity and reproduction. Deep seed dormancy delays germination and thereby decreases the time period for growth in the growing season, whereas shallow seed dormancy results in preharvest sprouting and/or viviparity. Hence, complex molecular mechanisms have been evolved to optimize the timing of germination.
ABA and GA are key hormones that regulate seed dormancy and germination (Grappin et al. 2000; Koornneef et al. 2002; Graeber et al. 2012). Consistent with their importance, ABA and GA levels are dynamically regulated and closely interconnected with multiple crosstalk in the transition from seed development to germination. One notable example is ABA suppression of GA biosynthesis in seeds (Seo et al. 2006). The aba2 seeds accumulate higher levels of GA (Seo et al. 2006).
Several molecular components have been recently identified, which are involved in the coordination of ABA and GA metabolism during the establishment of seed dormancy. The APETALA2 (AP2)/ETHYLENE-RESPONSIVE FACTOR (ERF) transcription factor ABI4 is a possible regulator in this process (Finkelstein et al. 1998; Shu et al. 2013). The germination rate of abi4-1 seeds is higher than that of wild-type seeds without stratification (Shu et al. 2013). The difference in germination rate between wild-type and abi4-1 mutant seeds is compromised after stratification or after-ripening (Shu et al. 2013), indicating the role of ABI4 in seed dormancy. Notably, ABI4 promotes ABA accumulation but decreases GA levels in mature seeds (Shu et al. 2013). GA biosynthetic and ABA catabolic genes are up-regulated, while GA catabolic and ABA biosynthetic genes are down-regulated in abi4 mutant seeds (Shu et al. 2013). This coordination is accomplished by direct binding of ABI4 to the CYP707A1 and CYP707A2 promoters.
The HONSU (HON) protein, a member of the group A PP2C family, also negatively regulates seed dormancy by modulating ABA and GA metabolism. In the presence of ABA, PYR1/PYLs/RCARs interact with HON and inactivate its activity. Subsequently, SnRK2s are liberated from the negative action of HON (Kim et al. 2013), activating ABA signaling. The hon-deficient mutant shows delayed germination, whereas transgenic plants overexpressing HON (HON-OX) exhibit shallow seed dormancy (Kim et al. 2013). Consistent with the fact that ABA signaling influences ABA metabolic genes through a negative feedback regulation (Nakashima et al. 2009), the HON-OX plants show increased expression of an ABA biosynthetic gene NCED6, whereas the expression of an ABA catabolic CYP707A2 gene is elevated in the hon mutant (Kim et al. 2013). In addition, expression of GA3ox1 and GA3ox2 is reduced in the hon mutant, but the mutant shows increased expression of the GA catabolic gene GA2ox2, indicating that HON coordinates ABA and GA metabolism in order to properly control seed dormancy. Altogether, the dormancy state of seeds is elaborately regulated by hormonal interactions and multiple molecular components that coordinate the hormonal metabolic activities.
MYB96 and seed dormancy
The MYB96 transcription factor is also involved in establishing primary seed dormancy. The germination rate of myb96-1D seeds was significantly lower than that of wild-type seeds, whereas myb96-1 seeds quickly germinated. Stratification or after-ripening compromised the differences in the germination rate of the genotypes. Thus, MYB96 is an important regulator of primary seed dormancy that is established during seed maturation on mother plants, rather than secondary seed dormancy that is induced in seeds with post-dispersal non-deep physiological dormancy.
MYB96 promotes ABA biosynthesis but suppresses GA accumulation. MYB96 positively regulates ABA biosynthetic genes NCED2, NCED5, NCED6, and NCED9, and also affects GA biosynthetic genes, such as GA3ox1 and GA20ox1. In support of this, strong seed dormancy of myb96-1D was suppressed by fluridone, whereas PAC recovered the early germination phenotype of myb96-1 seeds.
Given that MYB96 possesses a transcriptional activation domain (Seo et al. 2009, 2011) and that GA signaling is epistatic to ABA biosynthesis in the control of seed dormancy and germination (Seo et al. 2006; Liu et al. 2010; Shu et al. 2013), it is most likely that MYB96 primarily regulates ABA biosynthesis (Seo et al. 2009; Seo and Park 2010). Indeed, MYB96 directly binds to the promoters of NCED2 and NCED6. The MYB96-mediated changes in ABA levels may lead to altered GA metabolic activities to ensure a dormancy state. Collectively, metabolic coordination of ABA and GA underlies MYB96 regulation of seed dormancy. MYB96 forms a positive feedback loop with ABA biosynthesis and further bolsters ABA signaling to enhance adaptive fitness under unfavorable conditions.
References
Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, Jullien M (2004) Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219:479–488
Arc E, Chibani K, Grappin P, Jullien M, Godin B, Cueff G, Valot B, Balliau T, Job D, Rajjou L (2012) Cold stratification and exogenous nitrates entail similar functional proteome adjustments during Arabidopsis seed dormancy release. J Proteome Res 11:5418–5432
Barua D, Butler C, Tisdale TE, Donohue K (2012) Natural variation in germination responses of Arabidopsis to seasonal cues and their associated physiological mechanisms. Ann Bot 109:209–226
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14:1–16
Baud S, Dubreucq B, Miquel M, Rochat C, Lepiniec L (2008) Storage reserve accumulation in Arabidopsis: metabolic and developmental control of seed filling. Arabidopsis Book 6:e0113
Bentsink L, Koornneef M (2008) Seed dormancy and germination. Arabidopsis Book 6:e0119
Bentsink L, Jowett J, Hanhart CJ, Koornneef M (2006) Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA 103:17042–17047
Cao DN, Hussain A, Cheng H, Peng JR (2005) Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 223:105–113
Corbineau F, Bianco J, Garello G, Côme D (2002) Breakage of Pseudotsuga menziesii seed dormancy by cold treatment as related to changes in seed ABA sensitivity and ABA levels. Physiol Plant 114:313–319
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Finch-Savage WE, Cadman CS, Toorop PE, Lynn JR, Hilhorst HW (2007) Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J 51:60–78
Finkelstein RR (1994) Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5:765–771
Finkelstein R, Somerville C (1990) Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol 94:1172–1179
Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10:1043–1054
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl 1):S15–S45
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387–415
Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE (2011) Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc Natl Acad Sci USA 108:20236–20241
Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A (2012) Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J 70:501–512
Fujii HM, Verslues PE, Zhu JK (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth and gene expression in Arabidopsis. Plant Cell 19:485–494
Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462:660–664
Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, Rodriguez PL (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24:2483–2496
González-Guzmán M, Abia D, Salinas J, Serrano R, Rodríguez PL (2004) Two new alleles of the abscisic aldehyde oxidase 3 gene reveal its role in abscisic acid biosynthesis in seeds. Plant Physiol 135:325–333
Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJ (2012) Molecular mechanisms of seed dormancy. Plant Cell Environ 35:1769–1786
Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M (2000) Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210:279–285
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Gubler F, Millar AA, Jacobsen JV (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8:183–187
Gubler F, Hughes T, Waterhouse P, Jacobsen J (2008) Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiol 147:886–896
Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C (2007) Combined networks regulating seed maturation. Trends Plant Sci 12:294–300
Holdsworth MJ, Bentsink L, Soppe WJ (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179:33–54
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24:1695–1708
Iuchi S, Suzuki H, Kim YC, Iuchi A, Kuromori T, Ueguchi-Tanaka M, Asami T, Yamaguchi I, Matsuoka M, Kobayashi M, Nakajima M (2007) Multiple loss-of-function of Arabidopsis gibberellin receptor AtGID1s completely shuts down a gibberellin signal. Plant J 50:958–966
Kermode AR (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24:319–344
Kim H, Hwang H, Hong JW, Lee YN, Ahn IP, Yoon IS, Yoo SD, Lee S, Lee SC, Kim BG (2012) A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth. J Exp Bot 63:1013–1024
Kim W, Lee Y, Park J, Lee N, Choi G (2013) HONSU, a protein phosphatase 2C, regulates seed dormancy by inhibiting ABA signaling in Arabidopsis. Plant Cell Physiol 54:555–572
Koornneef M, van der Veen JH (1980) Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) heynh. Theor Appl Genet 58:257–263
Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in nongerminating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 61:385–393
Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61:377–383
Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5:533–536
Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23:1647–1656
Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16:646–658
Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion-Poll A (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45:309–319
Lin PC, Hwang SG, Endo A, Okamoto M, Koshiba T, Cheng WH (2007) Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol 143:745–758
Liu Y, Ye N, Liu R, Chen M, Zhang J (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61:2979–2990
Lopez-Molina L, Mongrand S, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98:4782–4787
Martínez-Andújar C, Ordiz MI, Huang Z, Nonogaki M, Beachy RN, Nonogaki H (2011) Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc Natl Acad Sci USA 108:17225–17229
McGinnis KM, Thomas SG, Soule FD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15:1120–1130
Meinke DW, Franzmann LH, Nickle TC, Yeung EC (1994) Leafy cotyledon mutants of Arabidopsis. Plant Cell 6:1049–1064
Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K (2009) Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol 50:1345–1363
Nambara E, Marion-Poll A (2003) ABA action and interactions in seeds. Trends Plant Sci 8:213–217
Nambara E, Hayama R, Tsuchiya Y, Nishimura M, Kawaide H, Kamiya Y, Naito S (2000) The role of ABI3 and FUS3 loci in Arabidopsis thaliana on phase transition from late embryo development to germination. Dev Biol 220:412–423
Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15:1591–1604
Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E (2006) CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol 141:97–107
Parcy F, Valon C, Kohara A, Miséra S, Giraudat J (1997) The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 9:1265–1277
Penfield S, Gilday AD, Halliday KJ, Graham IA (2006) DELLA-mediated cotyledon expansion breaks coat-imposed seed dormancy. Curr Biol 16:2366–2370
Peng J, Harberd NP (2002) The role of GA-mediated signalling in the control of seed germination. Curr Opin Plant Biol 5:376–381
Piskurewicz U, Lopez-Molina L (2009) The GA-signaling repressor RGL3 represses testa rupture in response to changes in GA and ABA levels. Plant Signal Behav 4:63–65
Raz V, Bergervoet JH, Koornneef M (2001) Sequential steps for developmental arrest in Arabidopsis seeds. Development 128:243–252
Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, Woolley L, Benlloch R, Nilsson O, Thomas SG, Hedden P, Phillips AL (2008) Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell 20:2420–2436
Schwechheimer C (2008) Understanding gibberellic acid signaling—are we there yet? Curr Opin Plant Biol 11:9–15
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7:41–48
Seo PJ, Park CM (2010) MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol 186:471–483
Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T, Kamiya Y, Yamaguchi S, Nambara E (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48:354–366
Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM (2009) The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol 151:275–289
Seo PJ, Lee SB, Suh MC, Park MJ, Go YS, Park CM (2011) The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23:1138–1152
Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S, Liu C, Feng Y, Cao X, Xie Q (2013) ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet 9:e1003577
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223
To A, Valon C, Savino G, Guilleminot J, Devic M, Giraudat J, Parcy F (2006) A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 18:1642–1651
Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N, Iuchi S, Kobayashi M, Yamaguchi S, Kamiya Y, Nambara E, Kawakami N (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146:1368–1385
Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19:2140–2155
Vaistij FE, Gan Y, Penfield S, Gilday AD, Dave A, He Z, Josse EM, Choi G, Halliday KJ, Graham IA (2013) Differential control of seed primary dormancy in Arabidopsis ecotypes by the transcription factor SPATULA. Proc Natl Acad Sci USA 110:10866–10871
Varbanova M, Yamaguchi S, Yang Y, McKelvey K, Hanada A, Borochov R, Yu F, Jikumaru Y, Ross J, Cortes D, Ma CJ, Noel JP, Mander L, Shulaev V, Kamiya Y, Rodermel S, Weiss D, Pichersky E (2007) Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell 19:32–45
Wang WQ, Song SQ, Li SH, Gan YY, Wu JH, Cheng HY (2009) Quantitative description of the effect of stratification on dormancy release of grape seeds in response to various temperatures and water contents. J Exp Bot 60:3397–3406
Willis CG, Baskin CC, Baskin JM, Auld JR, Venable DL, Cavender-Bares J, Donohue K, Rubio de Casas R, NESCent Germination Working Group (2014) The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol 203:300–309
Xi W, Liu C, Hou X, Yu H (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 22:1733–1748
Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16:367–378
Yamauchi Y, Takeda-Kamiya N, Hanada A, Ogawa M, Kuwahara A, Seo M, Kamiya Y, Yamaguchi S (2007) Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol 48:555–561
Yang SD, Seo PJ, Yoon HK, Park CM (2011) The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 23:2155–2168
Yano R, Kanno Y, Jikumaru Y, Nakabayashi K, Kamiya Y, Nambara E (2009) CHOTTO1, a putative double APETALA2 repeat transcription factor, is involved in abscisic acid-mediated repression of gibberellin biosynthesis during seed germination in Arabidopsis. Plant Physiol 151:641–654
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A1004831). H.G.L. and K.L. were supported by the BK21 PLUS program in the Department of Bioactive Material Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong Gil Lee and Kyounghee Lee have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, H.G., Lee, K. & Seo, P.J. The Arabidopsis MYB96 transcription factor plays a role in seed dormancy. Plant Mol Biol 87, 371–381 (2015). https://doi.org/10.1007/s11103-015-0283-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0283-4