Abstract
Mature seeds of the Cape Verde Islands (Cvi) ecotype of Arabidopsis thaliana (L.) Heynh. show a very marked dormancy. Dormant (D) seeds completely fail to germinate in conditions that are favourable for germination whereas non-dormant (ND) seeds germinate easily. Cvi seed dormancy is alleviated by after-ripening, stratification, and also by nitrate or fluridone treatment. Addition of gibberellins to D seeds does not suppress dormancy efficiently, suggesting that gibberellins are not directly involved in the breaking of dormancy. Dormancy expression of Cvi seeds is strongly dependent on temperature: D seeds do not germinate at warm temperatures (20–27°C) but do so easily at a low temperature (13°C) or when a fluridone treatment is given to D seeds sown at high temperature. To investigate the role of abscisic acid (ABA) in dormancy release and maintenance, we measured the ABA content in both ND and D seeds imbibed using various dormancy-breaking conditions. It was found that dry D seeds contained higher amounts of ABA than dry ND after-ripened seeds. During early imbibition in standard conditions, there was a decrease in ABA content in both seeds, the rate of which was slower in D seeds. Three days after sowing, the ABA content in D seeds increased specifically and then remained at a high level. When imbibed with fluridone, nitrate or stratified, the ABA content of D seeds decreased and reached a level very near to that of ND seeds. In contrast, gibberellic acid (GA3) treatment caused a transient increase in ABA content. When D seeds were sown at low optimal temperature their ABA content also decreased to the level observed in ND seeds. The present study indicates that Cvi D and ND seeds can be easily distinguished by their ability to synthesize ABA following imbibition. Treatments used here to break dormancy reduced the ABA level in imbibed D seeds to the level observed in ND seeds, with the exception of GA3 treatment, which was active in promoting germination only when ABA synthesis was inhibited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the later stages of seed maturation, morphological changes are relatively limited, but physiological and biochemical changes, amongst them the establishment of dormancy, are marked and crucial for the survival of plants in the seed stage (Koornneef and Karssen 1994). Seed dormancy has been defined as the temporary failure of a viable seed to germinate under favourable conditions of germination (Simpson 1990). In the natural environment, dormancy is usually broken by after-ripening and/or stratification, which occur, respectively, during mild/hot temperature conservation of dry seeds, and during low-temperature treatment of imbibed seeds (Bewley and Black 1994). Dormancy can be re-induced (secondary dormancy) when non-dormant seeds encounter conditions unfavourable for germination (Karssen 1982). The cycling of dormancy leading to seedling emergence in specific periods of the year is thought to reflect the seed’s responsiveness to environmental factors (Hilhorst 1995). For instance, in Arabidopsis thaliana accessions of the winter annual type, seeds are matured and dispersed in the spring, are dormant during summer, and germinate in autumn, allowing the plant to persist in a summer-arid habitat (Baskin and Baskin 1972).
The hormone-balance theory explains seed dormancy by the more or less simultaneous operation of hormones promoting (gibberellins: GAs) and inhibiting (abscisic acid: ABA) germination (Wareing and Saunders 1971). The study of numerous mutant plants impaired in GAs and/or ABA synthesis or sensitivity (Hilhorst and Karssen 1992; Hilhorst 1995), supports the hormone theory, demonstrating definitively the essential roles of ABA and GAs in seed dormancy and germination. More recently, the study of the germination characteristics of ethylene-insensitive and brassinosteroid-deficient mutants has underlined a role for these two hormones in the control of dormancy, probably by interacting with the transduction pathways of ABA and GAs (Beaudoin et al. 2000; Steber and McCourt 2001). These results did not fundamentally modify the scheme of dormancy control by the antagonistic effects of ABA and GAs on germination.
ABA synthesis and sensitivity to ABA are clearly involved in the onset of dormancy during seed maturation (Koornneef et al. 1982, 1984; Karssen et al. 1983; Walker-Simmons 1987; Le Page-Degivry et al. 1990). However, controversy has existed for a long time concerning the antagonistic role of ABA and GAs in dormancy breaking. For instance, decreasing levels of ABA and increasing levels of GAs have rarely been correlated with the efficiency of dormancy-breakage treatments (Wareing and Saunders 1971). Results obtained with Arabidopsis thaliana have shown that after-ripening and stratification alleviate the dormancy of GA-deficient mutants by increasing their sensitivity to GAs (Karssen and Laçka 1986). This has led to the suggestion that GAs are probably not directly active in the breaking of primary dormancy. In species like sunflower and barley, where after-ripening does not reduce the ABA content of seeds, it has been shown that it does suppress the capacity for ABA synthesis in imbibed embryos (Bianco et al. 1994; Wang et al. 1995). These latter results indicate an essential role for ABA synthesis in dormancy control in imbibed seeds. Furthermore, Grappin et al. (2000) demonstrated that dormant seeds of Nicotiana plumbaginifolia produced ABA during the first hours of imbibition and that this increase in ABA level was repressed by a gibberellic acid (GA3) treatment, which alleviated dormancy. In the same way, Toyomasu et al. (1994) reported that in dark-imbibed lettuce seeds, red light or GA3 treatments both promoted germination and substantially lowered endogenous ABA levels. Recently, it was shown that in barley the dormant embryos synthesized ABA during imbibition whereas in non-dormant embryos the ABA level decreased by degradation to phaseic acid (Jacobsen et al. 2002). It appears, therefore, that the expression of dormancy is strongly dependent on ABA levels in imbibed seeds. After-ripening can counteract dormancy by making the seeds able to catabolize ABA when imbibed, eventually through GA production, which can stimulate the germination and the catabolism of ABA.
Arabidopsis thaliana (L.) Heynh. (Cruciferae), the green model for geneticists and molecular biologists, is native to Europe and now widely distributed in the world. Both summer and winter annual varieties occur, although ecophysiologists have mainly studied winter-annual varieties (Baskin and Baskin 1972). Genetic variations for germination and dormancy are available among natural accessions (Ratcliffe 1976). The commonly used accessions such as Landsberg erecta or Columbia show a low level of dormancy. In comparison, the seeds of the Cape Verde Islands (Cvi) accession show a strong dormancy and have been used in a quantitative trait locus (QTL) mapping analysis to identify seed dormancy loci (Alonso-Blanco et al. 2003). Therefore Cvi could be considered an excellent model for studying the characteristics of dormancy expression in Arabidopsis seeds. In Arabidopsis also the scheme of dormancy control by the antagonistic effect of ABA and GAs on germination has been proposed (Debeaujon and Koornneef 2000). We examine here the effect of conditions defined as efficient in breaking the dormancy of various Arabidopsis accessions: dry storage, stratification, low temperature (Baskin and Baskin 1972; Debeaujon and Koornneef 2000), GAs, nitrates (Derkx and Karssen 1993, Alonso-Blanco et al. 2003) and norflurazon or fluridone (Debeaujon and Koornneef 2000; Jullien et al. 2000), on dormancy expression and the related variations of ABA content in imbibed Cvi seeds. Our results will contribute to a better understanding of the expression of dormancy during seed imbibition, a phenomenon largely unclear at present.
Materials and methods
Plant material
The seeds used in these experiments were derived from the Arabidopsis thaliana (L.) Heynh. ecotype “Cape Verde Islands” (Cvi), and were supplied by Dr. M. Koornneef (Wageningen). Seeds were sown in small containers filled with wet compost. The containers were incubated for 48 h at 4°C in the dark to release seed dormancy and then maintained in a culture room at 19/20°C with a 16-h photoperiod of artificial light (Osram L 58/31830 luminux plus Wanton Wan White, 45 µmol photons m−2 s−1) and 70% relative humidity (RH). Plants were watered every day for 2 months with tap water. After the full maturation of seeds, the plants were no longer watered and were left to dry for 3 weeks, and then mature seeds were harvested at the same time from dehydrated siliques. The collected seeds were dormant (D) and were systematically sterilized for 13 min in an aqueous solution of 10% chlorine (commercial bayrochlore tablets) in 95% ethanol, washed with absolute ethanol and dried for 48 h under a laminar flow hood. This sterilization is without evident effect on seed dormancy (not shown). The sterilized seeds were either used immediately or stored dry in plastic “Copro” boxes at 7°C, 40% RH, in a storage cabinet, to maintain the dormant state. In these conditions, dormancy was maintained for around 1 year and was always checked before using D seeds. Non-dormant (ND) seeds were obtained by keeping D seeds at room temperature at approx. 21/24°C (35–40% RH) for about 7–12 months (after-ripening).
Germination assays
Seeds used in each experiment originated from the same seed lot. The results of each experiment were confirmed in general five times. Germination was expressed as the cumulative percentage of germinated seeds. Each value was the mean ± SE of measurements of 100 seeds. Seeds were sown in lots of 25, in four replicates, in 5-cm Petri dishes containing 7 ml of a basal medium consisting of distilled water buffered with Mes (3 mM, pH 5.7) and solidified with agar (7 g l−1 Noble agar; Difco). In some experiments, additions were made to the basal medium: the growth regulators GA3, GA4, or GA7 (100 μM; Sigma); the herbicide 1-methyl-3-phenyl-5-(3-trifluoromethyl-(phenyl))-4-(1H)-pyridinone (fluridone, 10 μM; Duchefa), an inhibitor of carotenoid synthesis; and nitrates as 7 mM KNO3. These chemicals were prepared as follows: GA3 was dissolved in water; nitrates were dissolved in the basal medium directly prior to agar solidification; and fluridone, GA4 and GA7 were dissolved in a small volume of DMSO and then diluted in water. The DMSO dose used did not have any effect on seed germination. A concentration of 100 μM GA3 was found to be optimal in stimulating germination at the pH used here. Petri dishes were sealed with Parafilm (American National Can, Neenah, WI) to minimise moisture loss. Seeds were germinated in a controlled culture room under a 16-h photoperiod (Philips TRM HOW/33 RS tubes, 170 μmol photons m−2 s−1) at 25°C (light period)/20°C (dark period) and a constant 70% RH (standard conditions). The long-day (LD) photoperiod gave the best expression of dormancy. The stratified seeds were sown on the basal medium at 4°C in darkness for 1, 2, 3, 4 or 5 days and then Petri dishes were transferred to the standard conditions of germination. The percentage of germinated seeds was scored under a lens. Radicle protrusion was taken as the criterion for germination.
Temperature assays
The temperature assays were conducted on a two-way thermogradient plate (SNES, France). This plate allows 25 conditions of different alternate and constant temperatures between a cool corner adjusted to 13°C and a warmer one (27°C). Here germination occurs under a regime of 8 h light (Osram L 36 W/31–380, 120 µmol photons m−2 s−1) and16 h dark (short day: SD). Also in SD conditions, dormancy was strongly expressed at temperatures above 20°C (Fig. 3a,b). The seeds were sown as described previously and 4 replicates of 50 seeds per condition were incubated over all 25 thermal regimes of the thermogradient plate. Germination was recorded daily for each condition till 28 days after sowing.
Abscisic acid (ABA) measurements
For the quantification of ABA, 300 mg of each seed sample was sown in 14-cm Petri dishes on nylon filters covering the basal medium supplemented or not with 100 µM GA3, 10 µM fluridone or 7 mM NO3 −. After each period of imbibition, seed samples were transferred to microfuge tubes, frozen in liquid nitrogen and stored at −80°C. Before extraction of ABA from dry or imbibed seeds, the samples were ground to a powder in liquid nitrogen. Hormone extraction, purification, quantification by enzyme-linked immunosorbent assay (ELISA) and identification of immunoreactive molecules, have been previously described (Juillard et al. 1994; Kraepiel et al. 1994). We used a monoclonal anti-ABA antibody (LPDP 229; Jussieu, Paris, France) labelled with peroxidase-conjugated goat antibody to mouse immuno-globulins (Sigma). The hormone content was determined five times for each sample.
Results
Breaking the dormancy of Arabidopsis thaliana Cvi seeds
Effect of after-ripening
The germination kinetics of freshly harvested seeds and of after-ripened seeds were compared in order to characterize seed dormancy (Fig. 1). Freshly harvested Cvi mature seeds were strongly dormant, with a maximum germination (G max) of only 5%, 28 days after being sown on basal medium. This level of germination varied in accordance with the seed lots (0–10%; data not shown). In the course of after-ripening, dormancy was progressively alleviated; 5–7 months of dry storage were necessary to release dormancy. In this case, seeds germinated rapidly: the time for 50% germination (t 50) was 48 h with G max reaching more than 90%.
Effect of after-ripening on the germination of seeds of Arabidopsis thaliana line Cvi. Mature D seeds were freshly harvested (control: ●) and after-ripened for 5 weeks (◊), 14 weeks (▲), 5 months (■) and 7 months (○) at room temperature (≈20°C). Standard conditions of germination: 25°C (16 h light)/20°C (8 h dark)
Effect of stratification
Dormancy was rapidly suppressed by cold treatments of imbibed D seeds increasing from 1 to 4 days (Fig. 2). Four days of cold treatment broke dormancy completely, as after their transfer to standard conditions seeds germinated quickly (t 50≈1.5 days) and completely (G max≈95%).
Effect of temperature on the germination of dormant seeds
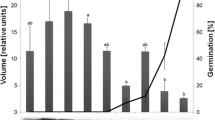
To investigate the effects of temperature, D seeds were sown on a thermogradient plate. Figure 3a shows that at the highest alternate or constant temperatures tested (23.5–27°C) D seeds do not germinate or germinate poorly. On the other hand, at the lowest alternate or constant temperatures tested (16.5–13°C), D seeds germinated efficiently with a G max of 92% at a constant 13°C. Under the latter conditions, seeds germinated quite rapidly (t 50≈5 days; Fig. 3b) but clearly more slowly than after-ripened (ND) seeds (t 50≈2 days) sown in standard conditions (Fig. 1). When alternate temperatures were used, the length of the cold period was critical in stimulating germination, e.g. the alternate 27°C (16 h night)/13°C (8 h light) cycle allowed only 52% germination whereas the inverse alternate cycle 13°C/27°C produced an 84% germination rate. The inhibitory effect of high temperature (e.g. 27°C) on the germination of D seeds was largely reversed by fluridone treatment (Fig. 3b); however, even when G max reached 95%, the t 50 (15 days) remained very high in comparison with the t 50 values for D seeds germinated at 13°C (5 days) or ND seeds sown in standard conditions (2 days). The same fluridone treatment produced no effects when D seeds were incubated at 13°C (Fig. 3b). ND (after-ripened) seeds were little affected by temperature; they germinated efficiently in all the conditions presented in Fig. 3a (not shown).
Effect of temperature on the germination of A. thaliana Cvi dormant seeds. a The experiment was conducted on a thermogradient plate with a short day photoperiod (see Materials and methods). In each rectangle are indicated the temperatures tested (bold characters: the first value is the temperature of the night period and the second that of the light period, e.g. 27°/13°) and the G max at 24 days after sowing (%). b Germination of D seeds imbibed on basal medium at 13°C (○) and 27°C (□) or with addition of 10 μM fluridone at 13°C (●) and 27°C (■)
Effect of chemicals (hormones, fluridone, nitrate) on dormancy expression
Mature D seeds of the Cvi ecotype were sown on basal medium supplemented with some reputed dormancy-breaking compounds, and germination was conducted under standard conditions (Fig. 4). The gibberellins tested here, GA3, GA4 and GA7 (100 μM) did not break dormancy efficiently: they needed a lot of time to improve germination, as shown by t 50 values of around 28 days and G max values reaching only 65% at 32 days after sowing. D seeds appeared to be more efficiently stimulated by 7 mM NO3 − since t 50 was reduced to 11 days and G max reached 91% in these conditions (a KCl control had no stimulatory effect, not shown). Germination of D seeds showed a marked stimulation by 10 μM fluridone, with a t 50 reduced to 8 days and a G max reaching 96%. Lastly, in comparison with fluridone alone, fluridone plus GA3 was the most efficient treatment for breaking dormancy, with t 50 reduced to 6.5 days. However, the germination kinetics observed under the latter conditions were clearly slower than those of ND seeds (compare with Fig. 1). The GAs, NO3 − and fluridone concentrations used here were optimal for breaking dormancy (data not shown).
Effect of delayed addition of fluridone on dormancy breaking
Transfer of imbibed D seeds from the basal medium to a fluridone-containing medium with increasing time periods after sowing was always efficient in inducing germination, which started 2–4 days after the transfer, then developed with the same kinetics as the control directly sown on fluridone (Fig. 5). This result suggests that a continuous long-lasting synthesis of ABA is the main reason for the germination defect of dormant seed. This fact, together with the previous results, could indicate that treatments efficient in breaking dormancy reduce the level of endogenous ABA in imbibed D seeds.
Effect of delayed fluridone treatments on the germination of A. thaliana Cvi dormant seeds. D seeds initially imbibed on basal medium were transferred to medium containing 10 µM fluridone at the indicated times: 3 days (○), 6 days (□), 14 days (◊) and 16 days (◆), and germination recorded. Controls: basal medium (●), and constantly on 10 µM fluridone (▲)
Changes in ABA contents during seed imbibition in relation to the dormancy status
Endogenous ABA levels in D, ND and stratified seeds
The amounts of endogenous ABA in both D and after-ripened (ND) dry seeds (Fig. 6a) were statistically different: 419 and 360 pmol g−1 DW, respectively. During the imbibition of ND seeds, in the first 6 h, the ABA level first increased and then decreased rapidly to 150 pmol g−1 DW 1 day after the start of imbibition. The ABA level was stabilized thereafter at a 3-fold lower value than in dry seeds (around 125 pmol g−1 DW), just before germination began around day 3 (Fig. 6a). During the imbibition of D seeds, which germinate poorly (Fig. 1), the ABA content decreased rapidly for the first 12 h and then slowly for the next 2 days to half the ABA level of dry seeds (about 220 pmol g−1 DW). The level of ABA in imbibed D seeds then increased again to reach a steady state around 400 pmol g−1 DW, which was maintained for at least 20 days after sowing (Fig. 6a).
Change in endogenous ABA levels in A. thaliana Cvi non-dormant and dormant seeds during the time course of imbibition under different conditions of dormancy breaking. a D seeds (●) and ND seeds (○) were imbibed on water in standard conditions. D seeds were stratified 4 days at 4°C then transferred to standard conditions (□). b D seeds were imbibed on 100 µM GA3 (▲), 10 µM fluridone (■), 100 µM GA3 + 10 µM fluridone (◆), or 7 mM NO3 − (◊). c Effect of temperatures on the endogenous ABA level in A. thaliana dormant seeds during the time course of imbibition. D seeds were imbibed on basal medium at 13°C (□) and 27°C (○). Data are the means ± SE of five independent measurements; missing error bars are smaller than the symbols
During the stratification treatment of D seeds (4 days at 4°C, in the dark, on basal medium) the ABA level first decreased rapidly and then stabilized at day 3, as in D seeds sown in standard conditions (Fig. 6a). However, after day 4 the ABA level decreased again in stratified seeds to about 140 pmol g−1 DW. Twelve hours after the transfer of stratified seeds to standard germination conditions we found a new and very marked decrease in the ABA content to 70 pmol g−1 DW, occurring just before the seeds began to germinate.
Effect of dormancy-breaking compounds on endogenous ABA levels
Dormant seeds were sown on media containing NO3 −, GA3, fluridone and fluridone plus GA3. In every case, the ABA levels decreased markedly on the first day following imbibition but the decrease was more pronounced in the presence of NO3 − and fluridone + GA3, where the ABA levels fell to around 200 pmol g−1 DW (Fig. 6b), with values statistically different from those for the other conditions. During the next 3 days, the ABA levels either stabilized (NO3 −, fluridone + GA3) around 150 pmol g−1 DW or continued to decline markedly (fluridone) to 80 pmol g−1 DW, enabling seed germination to occur (Fig. 4). With GA3 treatment, the ABA content of seeds increased again after 24 h to 450 pmol g−1 DW 6 days after sowing and seeds did not germinate; later, the ABA level decreased again to 200 pmol g−1 DW around 20 days after sowing (Fig. 6b) when germination began (Fig. 4).
Effect of temperature of sowing on endogenous ABA levels
Dormant seeds were sown at 13°C and 27°C, creating conditions that were permissive and non-permissive for germination, respectively (Fig. 3). The initial ABA level in the D seed lot used here was around 600 pmol g−1 (Fig. 6c). This level decreased very rapidly in the first day after sowing when D seeds were incubated at 13°C, before reaching a plateau around 250 pmol g−1 at day 3, when germination began. At 27°C the ABA level decreased at a markedly slower rate before stabilizing at a higher level, around 480 pmol g−1 (Fig. 6c). At this temperature, the D seeds did not germinate.
Discussion
Release from dormancy
The seeds of Arabidopsis thaliana ecotype Cvi are highly dormant when harvested in the culture conditions used here. Their dormancy can be alleviated by dry storage at room temperature: 20°C and 40% relative humidity (Fig. 1), conditions that are generally efficient for dormancy breaking by after-ripening (Laïbach 1956; Bewley and Black 1994). The time required for after-ripening depends on the species, e.g. few weeks for barley or as long as 60 months for Rumex crispus (Bewley and Black 1994). Dormancy of Arabidopsis Cvi seeds was alleviated after 5 months of dry storage (the same result was found by Alonso-Blanco et al. 2003), which is a rather short period compared to Rumex. However, 5 months is a long period for Arabidopsis because, for example, the poorly dormant Ler accession needs less than 2 months of after-ripening to break seed dormancy (Alonso-Blanco et al. 2003). How after-ripening promotes the capacity to germinate is still unclear. Possible mechanisms would involve temperature-dependant modifications in the properties of cellular membranes and membrane proteins or the accessibility of various “receptors” to their ligands: phytochrome, NO3 −, GAs, etc (Hooley et al. 1991; Hilhorst and Cohn 2000).
Dormancy of A. thaliana seeds can efficiently be relieved by chilling (Cone and Spruit 1983). In Cvi seeds, 4 days of cold treatment in darkness given to imbibed D seeds (Fig. 2) were equivalent to 7 months of dry storage. In previous studies on the germination of D seeds of common weeds, it was found that the stimulatory effect of chilling was not expressed unless at least one or more of the stimulating factors (light or alternating temperatures or nitrate) were applied after chilling (Vincent and Roberts 1979). Our results are in agreement with these findings because Cvi D seeds submitted to 4 days of dark stratification never germinated during this treatment but did so when transferred to light in standard conditions.
In A. thaliana Cvi seeds, the expression of dormancy is very dependent on the temperature at sowing (Fig. 3a). Dormant seeds germinated poorly, especially at constant temperatures above 23°C, but more efficiently at constant low temperatures of 16 or 13 °C. Alternating cold and hot temperatures can allow the germination of D seeds, especially when a low temperature was given during the 16 h night period. After-ripened ND seeds became largely insensitive to temperature as they germinated very efficiently in all the thermic conditions used in Fig. 3. In this respect, the Cvi accession exhibited the same behaviour as the A. thaliana ecotype tested by Baskin and Baskin (1972). A. thaliana Cvi seeds consequently show the same properties as in many other species where D seeds can only germinate in a narrow temperature range which becomes progressively larger as dormancy is alleviated (Bewley and Black 1994). Fluridone remarkably stimulated the germination of D seeds sown at 27°C (Fig. 3b), indicating a very probable role of ABA synthesis in the incapacity of D seeds to germinate at high temperature, as was shown for lettuce seeds, which were unable to germinate in the dark at high temperature (Yoshioka et al. 1998).
GAs are frequently very efficient in breaking the dormancy of imbibed seeds, but many exceptions are described in the literature (Bewley and Black 1994). A. thaliana can be classified among the species where GAs are poorly efficient (Karssen and Laçka 1986), which is confirmed in the present study with the Cvi accession (Fig. 4). Three of the GAs generally active in stimulating germination, GA3, GA4 and GA7 (and even GA4+7, not shown) allowed only a late stimulation of the germination of D seeds, which moreover germinated slowly.
A positive effect of nitrate on dormancy breaking of seeds has been shown in many species: Avena fatua (Adkins et al. 1984a), Sisymbrium officinale (Derkx and Karssen 1993), Arabidopsis thaliana (Hilhorst and Karssen 1988) and others (Bewley and Black 1994). Sowing Cvi D seeds on potassium nitrate largely suppressed dormancy (Fig. 4). According to Derkx and Karssen (1993), the combined action of light and nitrate could be reverted by tetcyclasis, an inhibitor of GA biosynthesis, possibly indicating that the combination of the two factors stimulated GA biosynthesis. However, in the present study nitrate was found to be clearly more efficient than exogenous GAs in breaking dormancy, indicating that its action was probably not directly mediated by these hormones.
Fluridone is very efficient in breaking dormancy in Cvi seeds (Fig. 4) whatever the time of application after sowing (Fig. 5). The effect of fluridone in alleviating various types of seed dormancy has begun to be seriously documented (primary dormancy in N. plumbaginifolia: Jullien and Bouinot 1997, Grappin et al. 2000; photodormancy and thermodormancy in lettuce: Yoshioka et al. 1998). Moreover, fluridone and GA3 given together (Fig. 4) showed an additive effect on the alleviation of dormancy, which has only been demonstrated previously with N. plumbaginifolia (Jullien and Bouinot1997; Grappin et al. 2000).
In conclusion, in A. thaliana Cvi the compounds or treatment that stimulate the germination of dormant seeds are, in increasing order of efficiency: GAs << NO3 − < fluridone < GA3 + fluridone < 13°C sowing < 4 days chilling. However, considering the t 50 values, even the best of these conditions cannot mimic completely the efficiency of after-ripening in alleviating seed dormancy.
Changes in ABA contents during the imbibition of D and ND seeds
In contrast to N. plumbaginifolia (Grappin et al. 2000) where the ABA content of D seeds was found to be strongly reduced during after ripening, the ABA levels in after-ripened Cvi seeds were only slightly reduced (Fig. 6a). The ABA content in ND after-ripened seeds increased transiently during the first 6 h of imbibition then decreased rapidly during the following 24 h and thereafter reached a steady state of around 120 pmol g−1 DW just before germination began (Fig. 6a). In D seeds, the ABA level also decreased in the first hours following sowing, but to a lesser extent than in ND seeds. The steady state was reached earlier (12 h after sowing) at around 250 pmol g−1 DW and the ABA level then tended to increase again 3–6 days after sowing to a higher level, around 400 pmol g−1 DW, which was maintained for a long time, and seeds did not germinate. This ABA level is efficient in inhibiting germination as the application of fluridone (6, 14 or 16 days after sowing, Fig. 5) allowed a rapid germination of the seed population. The ratio of endogenous ABA level between imbibed D seeds and imbibed non-germinated ND seeds was therefore approximately 3:1 (400/120). Such a ratio in ABA content between imbibed D and ND seeds has been found in Lactuca sativa in the past (Braun and Kahn 1975) and more recently (Yoshioka et al. 1998) in barley (Wang et al. 1995; Jacobsen et al. 2002) and in N. plumbaginifolia (Grappin et al. 2000).
In small seeds the whole process of imbibition is generally completed in a short time: about 30 min in celery (Simon 1984). Consequently, the variations in ABA content observed in D and ND seeds in the 24 h following sowing are developed during phases I and II of imbibition (Bewley and Black 1994). These two phases are characterized by a very active resumption of metabolism, terminating in radicle emergence in ND seeds (Obroucheva and Antipova 1997). One explanation for the rapid decrease in the ABA content during the first 24 h after sowing could be diffusion of the hormone into the germination medium. However, we never detected any traces of ABA in this medium (data not shown). The same observations were done (Toyomasu et al. 1994) for lettuce seeds where the ABA content was found to be decreasing after illumination. If ABA cannot diffuse outside of the seed, the most probable explanation is a catabolic degradation, which would occur in the first day following imbibition. The predominant pathway by which ABA is catabolized in plants is through oxidative degradation to 8′-hydroxy-ABA with subsequent conversion to phaseic acid then reduction to dihydrophaseic acid (Walton and Li 1995). This pathway operates in the seeds of various species, for instance Fraxinus americana (Sondheimer et al. 1974), Fagus sylvatica (Le Page-Degivry et al. 1997) and barley (Jacobsen et al. 2002), where it has been shown to be more intense in ND seeds versus D seeds. The same mechanism could operate in Cvi seeds.
Changes in ABA contents during the dormancy-breaking treatments
Hole et al. (1989; maize) and Le Page-Degivry and Garello (1992; Helianthus annuus) demonstrated that application of the inhibitor of ABA synthesis, fluridone, caused a strong reduction of ABA levels and prevented the development of embryo dormancy in developing seeds. Le Page-Degivry et al. (1990), Bianco et al. (1994), Jullien and Bouinot (1997), Yoshioka et al. (1998), Grappin et al. (2000) demonstrated that fluridone application during imbibition was very efficient in dormancy breaking. In the corresponding species, when D seeds were imbibed on fluridone they germinated rapidly and their ABA level was reduced to the low level observed in ND seeds just before germination occurred. The same results were observed in Cvi D seeds but notably the evolution of the ABA content during the first 3 days of imbibition was identical between the control D seeds and the fluridone treated D seeds, then the ABA content increased only in D seeds (Fig. 6a,b). This means that Cvi D seeds did not produce ABA while their ABA content decreased (from sowing to day 3). Day 3 is clearly a central point because from this time D seeds begin to produce ABA, as demonstrated by the sudden increase in the difference in ABA content between control D seeds unable to germinate and fluridone-treated D seeds which will germinate correctly (Fig. 6a,b). As D seeds did not produce ABA until day 3, it is clear that the difference in ABA content between D and ND seeds during this period should be related to a more intense catabolism of ABA in ND seeds.
The addition of GA3 to D seeds (not differing much in effects compared with GA4, the main effective endogenous GA in Arabidopsis, and GA7, Fig. 4) did not alleviate dormancy efficiently and the ABA level became rapidly higher than in control D seeds (Fig. 6b), suggesting an antagonistic effect of GA3 on ABA degradation or a stimulatory effect on ABA synthesis, or both. These results can be compared with those of Wang et al. (1998) and Grappin et al. (2000) with barley and N. plumbaginifolia, respectively, where a GA3 treatment was reported to be very efficient in breaking dormancy and also resulted in a rapid decrease in the ABA content of imbibed D seeds. In fact, our results show that GA3 acted later (15 days after sowing) in dormancy breaking which is correlated with a late decline of ABA levels in treated D seeds (Fig. 6b). Why GA3 is ineffective in dormancy breaking and reducing the ABA level in Cvi D seeds imbibed for 1–6 days, but becomes efficient 10 days later is a matter of debate.
Chilling did not appear to exert a specific effect on the ABA level in D seeds in comparison with the control during the first 3 days following sowing (Fig. 6a). The ABA content then slightly decreased during the following days. However, when chilled seeds were returned to standard germination conditions we then observed a new and strong decrease in the ABA level to 70 pmol g−1 DW, just before chilled seeds germinated. These results were in accordance with previous observations of Le Page-Degivry et al. (1997), which established that the main effect of chilling on Fagus sylvatica seeds was a strong reduction in their ABA content following their transfer from chilling conditions to standard conditions for germination.
D seeds sown on nitrate presented an accelerated catabolism of ABA during the 2 days following sowing, in comparison with control D seeds. In addition, nitrate treatment prevented the de-novo synthesis of ABA operating after day 3 in control D seeds. To our knowledge there is no information in the literature about any interaction of nitrate with ABA metabolism, with the exception of the work of Wang et al. (1998) on barley, who found nitrate to belong to class I of dormancy-breaking compounds that caused a decrease in the ABA content of imbibed D seeds. The dormancy-breaking effects of nitrogenous compounds on seeds have been well known for a long time (Adkins et al. 1984a). In Avena fatua, nitrate probably induced the germination of dormant caryopses by increasing the oxygen uptake, because the dose–response curves of germination stimulation by nitrates and of oxygen uptake were similar (Adkins et al. 1984b). The catabolism of ABA by the (+)-abscisic acid 8′-hydroxylase requires both NADPH and molecular oxygen and was inhibited at O2 concentrations less than 10% (v/v) (Krochko et al. 1998). Consequently, we can hypothesize that the dormancy-breaking effect of nitrate in general and that observed in Cvi seeds in particular results from a stimulatory effect of nitrate on the oxidative catabolism of ABA. Variations in the activity of the (+)-abscisic acid 8′-hydroxylase in relation, for instance, to the O2 availability could explain the variation observed in the rate of ABA decay in various conditions. Oxygen availability in imbibed seed is strongly dependant on low temperatures, which improve the dissolved oxygen concentration in water (Bewley and Black 1994; Corbineau and Côme 1995). This could explain why the ABA content decreased faster at 13°C, the optimal temperature for D seed germination, than at the non-optimal 27°C temperature (Fig. 6c). The same variations in ABA contents were obtained in Lactuca sativa seeds placed in an analogous temperature situation (Yoshioka et al. 1998). Moreover, in tomato, it was shown that male sterility could be reverted by low-temperature treatments, which decreased the ABA content in the stamen (Shing and Sawhney 1998). In addition, it was shown that the sensitivity of dormant wheat embryos to ABA inhibition of germination decreased at low temperature (Walker-Simmons 1988), which could be interpreted as an indication of ABA decay in cold-incubated embryos. Equally, the ABA decay observed when chilled seeds were transferred to standard conditions (Fig. 6a) could have been induced by the high availability of oxygen produced in imbibed D seeds during the cold treatment. Taken together, all these results could indicate a central role for oxygen availability in A. thaliana Cvi seed dormancy expression through the control of ABA catabolism during imbibition.
According to Le Page-Degivry et al. (1996), ABA is the primary hormone involved in any step during dormancy maintenance and release, and GAs are present at sufficient levels to promote germination as soon as ABA synthesis is inhibited. This is in agreement with our results since in GA3-treated D seeds, which did not germinate rapidly, there is an accumulation of ABA that can be counteracted by fluridone treatment (Fig. 6b), allowing seed to germinate efficiently (Fig. 4). In this way, in Cvi D seeds, GA3 is active in promoting germination only when ABA synthesis is inhibited. Fundamentally, in A. thaliana Cvi, as in N. plumbaginifolia PbH1D (Grappin et al. 2000), dormancy expression in seeds is mediated by ABA synthesis, which is rather transitory in N. plumbaginifolia where dormant seeds germinated 5 days later than ND seeds but is sustained for days in A. thaliana, where the intensity of dormancy is notably more pronounced. In the two species, all the treatments found to be efficient in dormancy breaking reduced the post-imbibition ABA synthesis specific to D seeds in a manner largely proportional to their efficiency. A very intriguing point is the fact that de-novo synthesis of ABA in the Cvi D seeds begins as late as 3 days after sowing. Before this time, D and ND seeds can only be differentiated by the rate of decrease in ABA content, highest in ND seeds, which seems to be only related to ABA catabolism. Some treatments suppressing dormancy (fluridone + GA3, NO3 −, 13°C sowing), clearly hastened the ABA decay characteristic of the first day of imbibition of D seeds. Other treatments (chilling and fluridone) did not hasten the initial decrease in ABA content. In after-ripened seeds the two mechanisms seemed to be linked, resulting in the faster decrease in ABA content being associated with the earliest germination. In this way, after-ripening appeared to be the most efficient treatment in reducing ABA production in imbibed seeds.
Abbreviations
- ABA:
-
Abscisic acid
- Cvi:
-
Cape Verde Islands
- D:
-
Dormant
- GA:
-
Gibberellin
- GA3 :
-
Gibberellic acid
- ND:
-
Non dormant
References
Adkins SW, Simpson GM, Naylor JM (1984a) The physiological basis of seed dormancy in Avena fatua. III. Action of nitrogenous compounds. Physiol Plant 60:227–233
Adkins SW, Simpson GM, Naylor JM (1984b) The physiological basis of seed dormancy in Avena fatua. IV. Alternative respiration and nitrogenous compounds. Physiol Plant 60:234–238
Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M (2003) Analysis of natural allelic variation at dormancy loci of Arabidopsis thaliana. Genetics 164:711–729
Baskin JM, Baskin CC (1972) Ecological life and physiological ecology of seed germination of Arabidopsis thaliana. Can J Bot 50:353–360
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12:1103–1115
Bewley DJ, Black M (1994) Seeds: physiology of development and germination. Plenum Press, New York
Bianco J, Garello G, Le Page-Degivry MT (1994) Release of dormancy in sunflower embryos by dry storage: involvement of gibberellins and abscisic acid. Seed Sci Res 4:57–62
Braun JW, Khan AA (1975) Endogenous abscisic acid levels in germinating and nongerminating lettuce seed. Plant Physiol 56:731–733
Cone JW, Spruit CJP (1983) Imbibition conditions and seed dormancy of Arabidopsis thaliana. Physiol Plant 59:416–420
Corbineau F, Côme D (1995) Control of seed germination and dormancy by the gaseous environment. In: Kigel J, Galili G (eds) Seed development and germination, Dekker, New York, pp 397–424
Debeaujon I, Koornneef M (2000) Gibberellin requirements for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122:415–424
Derkx MPM, Karssen CM (1993) Changing sensitivity to light and nitrate but not to gibberellins regulates seasonal dormancy patterns in Sisymbrium officinale seeds. Plant Cell Environ 16:469–479
Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M (2000) Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210:279–285
Hilhorst HWM (1995) A critical update on seed dormancy. I. Primary dormancy. Seed Sci Res 5:61–73
Hilhorst HWM, Cohn M (2000) Are cellular membranes involved in the control of seed dormancy? In: Crabbé J, Viemont JD (eds) Dormancy in plants: from whole plant behaviour to cellular control. 2nd international symposium on plant dormancy, Angers. CAB International, Wallingford, pp 275–290
Hilhorst HWM, Karssen CM (1988) Dual effect of light on the gibberellin- and nitrate-stimulated seed germination of Sisymbrium officinale and Arabidopsis thaliana. Plant Physiol 86:591–597
Hilhorst HWM, Karssen CM (1992) Seed dormancy and germination: the role of abscisic acid and gibberellins and the importance of hormone mutants. Plant Growth Regul 11:225–238
Hole DJ, Smith JD, Cobb BG (1989) Regulation of embryo dormancy by manipulation of abscisic acid in kernels and associated cob tissue of Zea mays L cultured in vitro. Plant Physiol 91:101–105
Hooley R, Beale MH, Smith SJ (1991) Gibberellin perception at the plasma membrane of Avena fatua aleurone protoplasts. Planta 183:274–280
Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002) Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115:428–441
Juillard J, Maldiney R, Sotta B, Miginiac E, Kherhoas L, Einhorn J (1994) HPLC–ELISA and MS as complementary techniques to study plant hormone metabolites. Analysis 22:483–489
Jullien M, Bouinot D (1997) Seed dormancy and responses of seeds to phytohormones in Nicotiana plumbaginifolia. In: Ellis RH, Black M, Murdoch AJ, Hong TD (eds) Basic and applied aspects of seed biology. Kluwer, Dordrecht, pp 203–214
Jullien M, Bouinot D, Ali-Rachedi S, Sotta B, Grappin P (2000) Abscisic acid control of seed dormancy expression in Nicotiana plumbaginifolia and Arabidopsis thaliana. In: Crabbé J, Viemont JD (eds) Dormancy in plants: from whole plant behaviour to cellular control. 2nd international symposium on plant dormancy, Angers. CAB International, Wallingford, pp 195–210
Karssen CM (1982) Seasonal patterns of dormancy in weed seeds. In: Kahn AA (ed) The physiology and biochemistry of seed development, dormancy and germination. Elsevier, Amsterdam, pp 243–270
Karssen CM, Laçka E (1986) A revision of the hormone balance theory of seed dormancy: studies on gibberellin and/or abscisic acid-deficient mutants of Arabidopsis thaliana. In: Bopp M (ed) Plant growth substances. Springer, Berlin Heidelberg New York, pp 315–323
Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M (1983) Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157:158–165
Koornneef M, Karssen CM (1994) Seed dormancy and germination. In: Meyerowitz EM, Somerville CR (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 313–334
Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection on induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 61:385–393
Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61:377–383
Kraepiel Y, Rousselin P, Sotta B, Kerhoas L, Einhorn J, Caboche M, Miginiac E (1994) Analysis of phytochrome- and ABA-deficient mutants suggests that ABA degradation is controlled by light in Nicotiana plumbaginifolia. Plant J 6:665–672
Krochko JE, Abrams GD, Lewen MK, Abrams RS, Cutler AJ (1998) (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol 118:849–860
Laïbach F (1956) Über die Brechung der Samenruhe bei Arabidopsis thaliana (L.) Heynh. Naturwissenschaften 7:164
Le Page-Degivry MT, Garello G (1992) In situ abscisic acid synthesis: a requirement for induction of embryo dormancy in Helianthus annuus. Plant Physiol 98:1386–1390
Le Page-Degivry MT, Barthe P, Garello G (1990) Involvement of endogenous abscisic acid in onset and release of Helianthus annuus embryo dormancy. Plant Physiol 92:1164–1168
Le Page-Degivry MT, Bianco J, Barthe P, Garello G (1996) Change in hormone sensitivity in relation to onset and breaking of sunflower embryo dormancy. In: Lang GA (ed) Plant dormancy: physiology, biochemistry and molecular biology. CAB International, Wallingford, pp 221–231
Le Page-Degivry MT, Garello G, Barthe P (1997) Changes in abscisic acid biosynthesis and catabolism during dormancy breaking in Fagus sylvatica embryo. J Plant Growth Regul 16:57–61
Obroucheva NV, Antipova OV (1997) Physiology of the initiation of seed germination. Russ J Plant Physiol 44:250–264
Ratcliffe D (1976) Germination characteristics and their inter- and intra-population variability in Arabidopsis. Arabidopsis Inf Serv 132:34–45
Shing S, Sawhney VK (1998) Abscisic acid in a male sterile tomato mutant and its regulation by low temperature. J Exp Bot 49:199–203
Simon EW (1984) Early events in germination. In: Murray DR (ed) Seed physiology, vol 2. Germination and reserve mobilization. Academic Press, New York
Simpson GM (1990) Seed dormancy in grasses. Cambridge University Press, Cambridge
Sondheimer E, Galson EC, Tinelli E, Walton DC (1974) The metabolism of hormones during seed germination and dormancy IV. The metabolism of (S)-2-14C-abscisic acid in ash seed. Plant Physiol 54:803–808
Steber CM, McCourt P (2001) A role for brassinosteroids in germination in Arabidopsis. Plant Physiol 125:763–769
Toyomasu T, Yamane H, Murofushi N, Inoue Y (1994) Effects of exogenously applied gibberellin and red light on the endogenous levels of abscisic acid in photoblastic lettuce seeds. Plant Cell Physiol 35:127–129
Vincent EM, Roberts EH (1979) The influence of chilling, light and nitrate on the germination of dormant seeds of common weed species. Seed Sci Technol 7:3–14
Walker-Simmons M (1987) ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol 84:61–66
Walker-Simmons M (1988) Enhancement of ABA responsiveness in wheat embryos by high temperature. Plant Cell Environ 11:769–775
Walton DC, Li Y (1995) Abscisic acid biosynthesis and metabolism. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, Dordrecht, pp 140–157
Wang M, Heimovaara-Dijkstra S, Van Duijn B (1995) Modulation of germination of embryos isolated from dormant and non-dormant barley grains by manipulation of endogenous abscisic acid. Planta 195:586–592
Wang M, Van der Meulen RM, Visser K, Van Schalk HK, Van Duijn B, De Boer AH (1998) Effects of dormancy-breaking chemicals on ABA levels in barley grain embryos. Seed Sci Res 8:129–137
Wareing PF, Saunders PF (1971) Hormones and dormancy. Annu Rev Plant Physiol 22:261–288
Yoshioka T, Endo T, Satoh S (1998) Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol 39:307–312
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali-Rachedi, S., Bouinot, D., Wagner, MH. et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana . Planta 219, 479–488 (2004). https://doi.org/10.1007/s00425-004-1251-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1251-4