Abstract
Objective
Full blood count (FBC) and serum inflammation-based scores reflect systemic inflammation and predict outcomes in cancer, but little is known in pituitary adenomas (PAs). We aimed to characterise FBC and inflammation-based scores in PA patients and investigate their usefulness in predicting challenging disease course.
Methods
We studied 424 PA patients first operated at our centre with available pre-operative biochemical data. Patients with infection, malignancies, autoimmune or haematological conditions were excluded. Inflammation-based scores studied: Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Lymphocyte-to-Monocyte Ratio (LMR), Systemic Immune-Inflammation Index (SII), Neutrophil-Platelet Score (NPS), Prognostic Nutrition Index (PNI), and Glasgow Prognostic Score (GPS).
Results
Cushing’s disease patients had more platelets, leucocytes, neutrophils and monocytes, and higher NLR, NPS and SII. Serum inflammation-based scores didn’t differ among non-Cushing PA subtypes. The glucocorticoid excess severity influenced leucocyte, eosinophil, basophil and platelet counts, and GPS in Cushing’s disease. Patients with functioning non-Cushing PAs with suprasellar extension, cavernous sinus invasion and hypopituitarism had GPS ≥ 1, while NPS ≥ 1 was associated with suprasellar extension. More invasive and difficult to treat corticotrophinomas were associated with fewer platelets pre-operatively (< 299.5 × 109/L predicting multimodal treatment). Non-functioning PA patients who suffered apoplexy had more leucocytes, neutrophils and monocytes, higher GPS ≥ 1 and fewer platelets; re-operated cases had fewer lymphocytes, higher NLR and PLR.
Conclusions
Serum inflammation-based scores may predict invasive/refractory PAs: GPS and PNI in non-functioning and functioning non-Cushing PAs; NPS in functioning non-Cushing PAs; NLR and PLR in non-functioning PAs. Platelets < 299.5 × 109/L predict multimodal treatment in Cushing’s disease. Further studies are needed to confirm these observations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas (PAs) account for 15% of all intracranial tumours, being the third most common intracranial neoplasm after meningiomas and gliomas, and the vast majority of PAs are benign and follow an indolent course, although they can be associated with significant morbidity due to mass effects on surrounding tissues and/or excessive or insufficient hormone secretion (caused by both the tumour mass effects and therapeutical approaches such as surgery and radiotherapy) [1]. A subset of PAs present a challenging disease course being refractory to the conventional treatments, and may recur/regrow after surgical or medical therapy requiring multiple or multimodal treatment. Although upfront discrimination of an aggressive PA is not straightforward, some markers of PA aggressiveness have been identified, including radiological invasiveness (into cavernous or sphenoid sinus), and histological markers such as Ki-67, mitotic count, p53 staining and certain histiotypes including Crooke’s adenoma, sparsely-granulated somatotrophinomas or null-cell PAs [2, 3]. However, to date it is still not possible to reliably predict the prognosis or recurrence in patients with PAs, and therefore new prognostic markers would definitely fulfil an important unmet clinical need.
Inflammation plays an important role in tumour biology, not only in the local tumour microenvironment [4], but also systemically [5, 6]. Responses to systemic inflammation include alterations in haematopoiesis and in secretion of acute-phase proteins, cytokines, growth factors and hormones [7, 8]. Full blood count (FBC), C-reactive protein (CRP), albumin and serum inflammation-based scores can reflect the systemic inflammatory status and predict outcomes in cancer patients [5]. Different pre-operative serum inflammation-based scores have been used and can easily be calculated from FBC data (Table 1): Neutrophil-to-Lymphocyte Ratio (NLR) [5, 9, 10], Platelet-to-Lymphocyte Ratio (PLR) [5, 11], Lymphocyte-to-Monocyte Ratio (LMR) [12, 13], Neutrophil-Platelet Score (NPS) [14] and Systemic Immune-Inflammation Index (SII) [15]. These scores not only reflect systemic inflammation, but also the patient’s anti-tumour response and immunosurveillance status. Increased neutrophil counts or neutrophilia in cancer occur due to the secretion of myeloid growth factors by tumour cells triggering neutrophil production, or due to cancer-related inflammation secondary to tissue destruction or hypercytokinaemia. On the other hand, lymphocytes are important anti-tumour cells, and their reduction may indicate immunosuppression or weakened anti-tumour response [5, 10, 16]. Both the Prognostic Nutrition Index (PNI) [17] and Glasgow Prognostic Score (GPS) [5, 7] take into account serum albumin levels (Table 1), and they reflect the nutritional and immunological status of the patient [5, 7, 16, 18].
In general, increased NLR, PLR, NPS, SII and GPS, as well as low LMR and low PNI are associated with poor clinical outcomes in cancer (Table 1) [5, 7, 9, 13]. Several lines of research support the role of pre-operative FBC and serum inflammation-based scores in predicting surgical outcomes and prognosis in oncological patients [5, 16], including in non-metastatic tumours, such as meningiomas [19] and gliomas [15, 18, 20]. Endocrine neoplasms have also been studied for serum inflammatory scores including thyroid cancer [21, 22], neuroendocrine tumours [23,24,25,26], and craniopharyngiomas [8, 27]. To our knowledge, there is only one study describing FBC and serum inflammation-based scores in PA patients, comparing these with data from patients with craniopharyngiomas and Rathke’s cleft cysts and healthy subjects; however, details on PA subtypes or their correlation with clinical features and outcomes were not assessed [8].
Identifying any of these pre-operative serum inflammation-based scores as predictors of invasiveness, challenging disease course and/or clinical outcomes in PA patients would provide added value to risk stratification and management algorithms for these patients. Hence, in this study, we aimed to characterise the FBC and serum inflammation-based scores in patients with PAs, as well as to investigate the applicability of such biochemical parameters in predicting invasive, challenging disease course or treatment refractory disease.
Materials and methods
Study population
Clinical records of 424 patients with PAs (68 prolactinomas, 72 acromegaly, 70 Cushing’s disease, 208 non-functioning PAs (NFPAs) and 6 thyrotrophinomas) who underwent first operation at the Center for Endocrine Tumors at Leiden University Medical Center were retrospectively analysed. Patients who had first pituitary operation at our centre between 2006 and 2019 and with pre-operative FBC, differential leucocyte count, CRP and albumin data were included in our study (Supplementary Table 1). For the clinical and treatment outcome analyses only patients with a minimum of 1 year post-operative follow-up were considered. Patients with infection, malignancies, autoimmune, haematological conditions, serious cardiac, hepatic or renal disease, and patients on supraphysiological doses of glucocorticoids or on immunosuppressives at time of the pre-operative blood test were excluded. Data were obtained after waiver of medical ethical review approved by our institutional ethical board (G19.011).
Clinical and outcome data and study definitions
Patients’ demographic, clinical, radiological, treatment and outcome data were retrieved from medical records. Based on their clinical and histological diagnoses, PA patients were grouped as follows: patients with acromegaly (GHomas), prolactinomas (PRLomas), clinically NFPAs, Cushing’s disease (ACTHomas) and thyrotrophinomas (TSHomas). “Functioning non-Cushing PAs” subgroup included patients with GHomas, PRLomas and TSHomas. Invasiveness was evaluated using the Knosp classification [28], with grades 3 and 4 considered as presence of cavernous sinus invasion [28]. Hypopituitarism was defined as the presence of at least 1 pituitary deficiency documented biochemically through basal pituitary function tests, and when necessary dynamic tests were performed as appropriate [29]. The number of treatments corresponded to the number of individual treatments received (each medication, surgery and radiotherapy) including the first operation at our centre. Multimodal treatment was defined as the employment of 2 or more distinct forms of treatment in patient’s management including the first operation at our centre. Multiple treatment was defined as the employment of 3 or more treatments received by the patient including the first operation at our centre. Re-operation subgroup involved patients who had at least 1 additional surgery following their first operation at our centre. Active disease at last follow-up was considered in case of persistent or recurrent progressive tumour remnants in both functioning PAs and NFPAs; small persistent tumour remnants after operation, stable over time and requiring no further intervention, were regarded as not active at last follow-up. For functioning PAs, biochemical remission at the last follow-up assessment was interpreted according to current guidelines: normalisation of IGF-I and nadir GH levels < 0.4 ng/L during oral glucose suppression test for acromegaly [30, 31], normalisation of prolactin (< 23.3 µg/L for women and < 15.2 µg/L for men) for prolactinoma patients [32, 33], and for Cushing’s disease a post-operative cortisol < 50 nmol/L, a cortisol < 138 nmol/L measured 3 months after surgery, a normal 24 h-urinary free cortisol (UFC) on two consecutive samples and/or a 1 mg dexamethasone suppression to cortisol < 50 nmol/L, as clinically appropriate [34, 35]. The mean follow-up duration of our study was calculated from the date of the first pituitary operation at our centre until the last clinical follow-up observation.
The occurrence of apoplexy as well as the presence of large, compressive and invading tumours at the pre-operative evaluation, e.g. macroadenoma, visual field defects, suprasellar extension and cavernous sinus invasion, were regarded as potentially indicative of challenging disease course (i.e. PAs with challenging or more eventful disease course). During follow-up, the presence of tumour remnant, tumour regrowth or persistent hormone excess requiring any additional treatment after first operation at our centre, were regarded as suggestive of poorer clinical outcome and/or more refractory pituitary disease (i.e. PAs more difficult to treat).
Pre-operative biochemical data collection and serum inflammation-based scores definitions
Blood samples from each patient with confirmed diagnosis of a PA were taken before the first pituitary surgery at our centre, as part of the pre-operative work-up, which included also a baseline pituitary hormones assessment performed in our center. Serum FBC, albumin and CRP were performed in certified health service laboratories in a standardised manner on automated counters. From these retrospectively available pre-operative biochemical data, the following scores were calculated (Table 1): NLR by dividing the absolute neutrophil count by the absolute lymphocyte count; PLR by dividing the absolute platelet count by the absolute lymphocyte count; LMR by dividing the absolute lymphocyte count by the absolute monocyte count [5]; SII by multiplying the absolute platelet count and NLR [15]; NPS giving a score of 0 if neutrophils ≤ 7.5 × 109/L and platelets ≤ 400 × 109/L, a score of 1 if neutrophils > 7.5 × 109/L or platelets > 400 × 109/L, or a score of 2 if neutrophils > 7.5 × 109/L and platelets > 400 × 109/L [14]; PNI by applying the formula albumin level (g/L) + (5 × total lymphocyte count) [17]; and GPS giving a score of 0 if CRP ≤ 10 mg/dL and albumin ≥ 35 g/L, a score of 1 if CRP > 10 mg/dL or albumin < 35 g/L, and a score of 2 if CRP > 10 mg/dL and albumin < 35 g/L [7].
Statistical analysis
Data are presented as mean and standard deviation for continuous variables, and as absolute number or percentages for categorical variables. Qualitative variables were analysed with the χ2 test to compare two or more groups. Quantitative or continuous variables were tested for Gaussian distribution with the Shapiro–Wilk test, and non-parametric and parametric data were further analysed with Mann–Whitney U and Student’s T-tests, respectively. Correlations between continuous variables (r) were determined by Pearson correlation coefficient for two variables with normal distribution or Spearman’s correlation coefficient for abnormally distributed variables. Logistic regression was performed to assess predictive performance of continuous variables on dichotomous outcomes, data shown as odds ratio (OR) with 95% confidence interval (95%CI). For variables that showed a significant predictive value (defined as P < 0.05), Receiver Operator Characteristics (ROC)-curves were prepared. The ROC analysis was used to evaluate a cut-off point for these predictive markers and to calculate the sensitivity and specificity of these cut-offs. An arbitrary optimal cut-off point was chosen with a high sensitivity and a specificity > 50%. To assess the effect of length of follow-up on outcomes, Cox-regression was performed, with data shown as hazard ratio (HR) with 95%CI. As length of follow-up and HRs differed between subgroups, the performance of the inflammation-based scores was only assess within these subgroups. Statistical analyses were carried out using the SPSS software version 20 (IBM, USA) and GraphPad version 6 (Prism, USA). The α for statistical significance was set at 0.05. Correction for multiple testing was applied using family-wise Benjamini–Hochberg procedure, with an accepted false discovery rate of 10%, which means we accept that 10% of reported significant associations are false-positive (data shown in Supplementary Table 2). Families of tests were based on performed statistical test and type of variables, e.g. all correlation analyses between inflammation-based scores and serum or urinary hormone levels. Because this is an exploratory study, all factors with a crude (i.e. before multiple testing correction) p-value < 0.05 are reported, so they may be explored in further cohorts. However, factors that lost significance after correction for multiple testing are marked with (ns), while those factors that remained significant are marked with an asterisk (*).
Results
General characterisation of pre-operative biochemical parameters and serum inflammation-based scores in patients with PAs
Pre-operative FBC data, albumin and CRP (when available) and the respective serum inflammation-based scores from our cohort of 424 patients with PAs are shown in Table 2, while patients’ demographic, clinical and outcome data are presented in Supplementary Table 1. Sixty out of 68 prolactinoma patients received dopamine agonists at any point before the pituitary surgery, 13 of whom were refractory to this medical therapy, while 40 were intolerant for medication. Two prolactinoma patients suffered from apoplexy, 2 had a cerebrospinal fluid leak, 7 optic chiasm compression, 3 preferred surgical management and 1 patient underwent a biopsy. The mean follow-up duration of the whole cohort of PA patients was 5.0 ± 3.6 years, and longer (6.5 ± 4.2 years) for the subgroup of Cushing’s disease (Supplementary Table 1). Hazard for recurrence and multimodal and multiple treatment were higher in prolactinoma, acromegaly and Cushing’s disease as compared to NFPAs. Additionally, Cushing’s disease patients had an increased hazard for additional surgery and radiotherapy (Supplementary Table 1).
In general, the different FBC parameters among PA patients were within the normal reference range, with only a few patients displaying thrombocytosis (0.6%), thrombocytopenia (3.0%) and leucopenia (2.1%). Leucocytosis was seen in 15.1% of cases (30 of 64 cases with leucocytosis had Cushing’s disease), and a CRP > 10 mg/dL was observed in 11.5% of the patients (Table 2).
Pre-operative FBC data and serum inflammation-based scores among the different PA subtypes are shown in Figs. 1 and 2 (and in more detail in Supplementary Table 3). Cushing’s disease patients had significantly higher leucocyte and neutrophil counts than other PA subtypes, and subsequently also higher NLR, SII and NPS (Figs. 1 and 2). Cushing’s disease patients had higher platelet and monocyte counts than acromegaly (284.21 ± 81.71 vs 237.22 ± 63.90; p = 0.001*, and 0.77 ± 0.31 vs 0.48 ± 0.24; p = 0.001*) and higher monocyte counts than NFPA (0.58 ± 0.18; p < 0.001*) patients (Fig. 1). Apart from Cushing’s disease, FBC parameters and serum inflammation-based scores did not differ among the other PA subtypes (Figs. 1 and 2).
Continuous pre-operative biochemical parameters and serum inflammation-based scores in patients with different subtypes of pituitary adenomas. Data are shown as mean ± standard deviation for the biochemical parameters that showed crude significant differences, which also remained significant after Benjamini–Hochberg correction. *, < 0.05, ** < 0.01, ***, < 0.001 (One-way ANOVA test with post-hoc Bonferroni multiple comparison test). ACTHoma Cushing’s disease, GHoma acromegaly, NFPA non-functioning pituitary adenoma, PRLoma prolactinoma, TSHoma thyrotrophinoma
Categorical serum inflammation-based scores in patients with different subtypes of pituitary adenomas. Data are shown as percentage of total pituitary adenomas within each subtype and per categorical biochemical variable. The crude statistical differences observed regarding the Neutrophil-Platelet Score remained significant after Benjamini–Hochberg. ***, < 0.001 (Chi-squared test with post-hoc multiple comparison tests). ACTHoma Cushing’s disease, GHoma acromegaly, NFPA non-functioning pituitary adenoma, PRLoma prolactinoma, TSHoma thyrotrophinoma
Serum inflammation-based scores and their correlation with the extent of pre-operative pituitary hormone excess in patients with functioning PAs
Within Cushing’s disease cohort, there was a negative correlation between 24 h-UFC levels and eosinophil (r = − 0.574; p < 0.001*) counts. There was a crude negative correlation between 24 h-UFC and platelet (r = -0.362; p = 0.006 (ns)) and basophil (r = − 0.425; p = 0.006 (ns)) counts (Fig. 3 and Supplementary Table 4). In addition, 24 h-UFC levels had a crude positive association with elevated CRP and GPS, and Cushing’s disease patients with GPS ≥ 1 also had a crude positive association with ACTH levels (p = 0.014 (ns)) (Fig. 4). In patients with an elevated CRP (> 10 mg/dL) random serum cortisol was higher than in patients with CRP < 5 mg/dL and than those with CRP comprised between 5 and 10 mg/dL (0.997 ± 0.367 vs 0.641 ± 0.195 µmol/L; p = 0.006*, and 0.997 ± 0.367 vs 0.681 ± 0.278 µmol/L; p = 0.050*, respectively). Finally, there was a crude positive correlation between leucocyte counts and random serum cortisol (r = 0.244; p = 0.047 (ns)) (Fig. 3).
Correlation between biochemical parameters and inflammation-based scores and serum pituitary hormone levels within the different hormone-secreting pituitary adenoma subtypes. Data are shown for correlations where a crude significant correlation was observed before correction; significant correlations after correction with Benjamini–Hochberg procedure are marked with an asterisk (*) while correlations that lost significance after correction are marked with (ns). P-values were determined by the Spearman’s correlation coefficient for variables without normal distribution and with Pearson correlation coefficient for correlations between two normally distributed variables. ACTHomas Cushing’s disease, GHomas acromegaly, IGF-1 insulin-like growth factor 1, ns non-significant after correction, UFC urinary free cortisol, ULN upper limit of the normal range
Categorical biochemical parameters and inflammation-based scores and degree of hormone excess within patients with Cushing’s disease. Data are shown for the correlations within the subgroup of Cushing’s disease where a crude significant association was observed prior to correction with Benjamini–Hochberg method; significant factors after correction are marked with an asterisk (*), and comparisons where significance was lost after correction are marked with (ns). ACTH adrenocorticotropic hormone, ns non-significant, UFC urinary free cortisol
Among acromegaly patients, a crude negative correlation between platelet count and IGF-1 xULN (r = -0.280; p = 0.033 (ns)) (Fig. 3) was observed, while in prolactinoma patients there was no correlation between serum prolactin and pre-operative FBC or serum inflammation-based scores (Supplementary Table 4). There were no associations between prolactin and GH or IGF-1 levels and elevated CRP, GPS or NPS among acromegaly or prolactinoma patients (data not shown).
Pre-operative serum inflammation-based scores and their relation with clinical features at presentation and follow-up and outcomes in patients with PAs
Functioning non-Cushing PAs (prolactinoma, acromegaly and thyrotrophinoma)
Functioning non-Cushing PA patients with pre-operative GPS ≥ 1 had a crude association with higher rates of pre-operative hypopituitarism (25.0% vs 3.8%; p = 0.048 (ns)) and suprasellar extension (25.0% vs 4.0%; p = 0.048 (ns)) than those with GPS = 0. Pre-operative NPS ≥ 1 had a crude association with higher rates of suprasellar extension (14.8% vs 2.9%; p = 0.028 (ns)), and lower PLR in patients with functioning non-Cushing macroadenomas (p = 0.039 (ns)) (Fig. 5-A and Supplementary Table 5).
Significant associations between biochemical parameters and serum inflammation-based scores data and clinical features and outcomes within functioning non-Cushing pituitary adenomas (a) and Cushing’s disease (b). For a certain clinical feature or outcome, the presence/absence are depicted in grey (for Yes) and in black (for No), respectively. In the bars is represented the number of cases with presence or absence of a certain feature or outcome versus the total number of cases with available data for categorical biochemical data, or simply the number of cases with available data for continuous biochemical data. Data are shown as percentage of cases with GPS ≥ 1 and NPS ≥ 1 regarding different clinical features. Continuous biochemical parameters/scores data are shown as mean ± standard deviation. Chi-squared and Mann Whitney U test were used as appropriate, and significant crude p-values (< 0.05) are shown. Significant factors after Benjamini–Hochberg correction are marked with an asterisk (*), and comparisons where significance was lost after correction are marked with (ns). GPS Glasgow Prognostic Score, NPS Neutrophil-Platelet Score, ns non-significant, PA pituitary adenoma, PLR Platelet-to-Lymphocyte Ratio, PNI Prognostic Nutrition Index
Functioning non-Cushing PA females had significantly lower platelet count and higher red cell and leucocyte counts than males (Supplementary Table 5). However, the distribution of males and females did not differ within the functioning non-Cushing PAs subgroup as well as within prolactinoma, acromegaly and thyrotrophinoma subgroups (Supplementary Table 1), therefore excluding a gender-related effect on the observed associations.
PNI seemed to be lower in functioning non-Cushing PA patients who required multiple treatments (35.85 ± 23.10 vs 55.42 ± 5.07; p = 0.048 (ns)) including post-operative radiotherapy (39.68 ± 20.58 vs 54.08 ± 9.71; p = 0.024 (ns)) (Fig. 5-A and Supplementary Table 6).
Cushing’s disease
Cushing’s disease patients with tumours invading the cavernous sinus had lower platelet counts (233.30 ± 46.19 vs 293.30 ± 83.58; p = 0.012 (ns)) (Fig. 5b and Supplementary Table 5).
Cushing’s disease patients who required multimodal treatment had a lower platelet count (242.14 ± 50.00 vs 304.03 ± 86.54; p = 0.001*). Pre-operative lower platelet count was also observed in Cushing’s disease patients who had multiple treatments (239.81 ± 49.94 vs 296.07 ± 84.51; p = 0.006 (ns)), including post-operative medical therapy (245.50 ± 54.85 vs 297.14 ± 83.77; p = 0.031 (ns)), radiotherapy (249.53 ± 41.75 vs 291.37 ± 87.70; p = 0.032 (ns)) (Fig. 5-B and Supplementary Table 6).
None of the studied serum inflammation-based scores correlated with clinico-pathological features or outcomes in patients with Cushing’s disease (Supplementary Tables 5 and 6).
NFPAs
NFPA patients who suffered apoplexy more often had a GPS ≥ 1 (40.0% vs 6.4%; p = 0.001*), and showed a crude association with more neutrophils (6.17 ± 3.23 vs 3.94 ± 1.70; p = 0.004 (ns)), monocytes (0.70 ± 0.19 vs 0.54 ± 0.16; p = 0.005 (ns)), leucocytes (8.65 ± 3.30 vs 7.22 ± 2.14; p = 0.013 (ns)) and elevated CRP (30.4% vs 8.5%; p = 0.008 (ns)) (Fig. 6 and Supplementary Table 5). NFPA patients with visual field defects at presentation showed a crude association with lower lymphocyte count (2.02 ± 0.80 vs 2.90 ± 0.39; p = 0.014 (ns)), higher NLR (2.61 ± 2.11 vs 1.20 ± 0.30; p = 0.024 (ns)) and lower LMR (3.84 ± 1.87 vs 5.45 ± 1.09; p = 0.031 (ns)) (Fig. 6 and Supplementary Table 5).
Significant associations between biochemical parameters and serum inflammation-based scores data and clinical features and clinical outcomes within non-functioning pituitary adenomas. For a certain clinical feature or clinical outcome, the presence/absence are depicted in grey (for Yes) and in black (for No), respectively. In the bars is represented the number of cases with presence or absence of a certain feature or outcome versus the total number of cases with available data for categorical biochemical data, or simply the number of cases with available data for continuous biochemical data. Continuous biochemical parameters/scores data are shown as mean ± standard deviation. Mann Whitney U tests were used as appropriate, and significant crude p-values (< 0.05) are shown. Significant factors after Benjamini–Hochberg correction are marked with an asterisk (*), and comparisons where significance was lost after correction are marked with (ns). NLR Neutrophil-to-Lymphocyte Ratio, ns non-significant, PLR Platelet-to-Lymphocyte Ratio, PNI Prognostic Nutrition Index, post-op post-operatively, VF visual fields
NFPA patients with a tumour remnant within 1-year after operation had fewer lymphocytes (1.83 ± 0.57 vs 2.58 ± 0.75; p < 0.001*), and a crude association with fewer leucocytes (6.90 ± 2.16 vs 7.68 ± 2.42; p = 0.010 (ns)) and higher PLR (131.07 ± 45.65 vs 109.33 ± 64.70; p = 0.021 (ns)). Patients who were reoperated had a crude association with fewer leucocytes (6.29 ± 2.27 vs 7.48 ± 2.42; p = 0.029 (ns)), lymphocytes (1.46 ± 0.36 vs 2.26 ± 0.74; p = 0.005 (ns)) and higher NLR (3.15 ± 0.96 vs 2.15 ± 1.34; p = 0.017 (ns)) than those requiring no further surgeries. NFPAs managed with multiple treatments had a crude association with lower lymphocyte count (1.56 ± 0.40 vs 2.21 ± 0.76; p = 0.047 (ns)) and higher NLR (3.12 ± 0.99 vs 2.20 ± 1.34; p = 0.049 (ns)), and NFPA patients with active disease at last follow-up with lower PNI (25.88 ± 22.32 vs 54.69 ± 6.10; p = 0.021 (ns)) (Fig. 6 and Supplementary Table 6).
Assessment of the diagnosis efficacy and usefulness of pre-operative biochemical parameters and inflammation-based scores in predicting challenging disease course
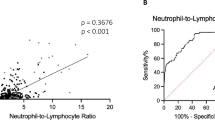
The value of pre-operative biochemical parameters and serum inflammation-based scores in predicting challenging disease course in patients with PAs were tested within each PA subgroup for those parameters with significant associations or trends with clinical features and outcomes. Platelet count showed the highest accuracy and was the best biochemical tool in predicting refractory disease in patients with Cushing’s disease. Univariate logistic regression showed an OR of 0.987 (95%CI: 0.978–0.997; p = 0.008*) for multimodal treatment, and an OR of 0.989 (95%CI: 0.980–0.998; p = 0.023*) for multiple treatment per 1 × 109 platelets. ROC-analysis showed an AUC of 0.758 (95%CI: 0.634–0.882) for multimodal treatment and an AUC of 0.735 (95%CI: 0.604–0.867) for multiple treatment, with an optimal platelet cut-off of 299.5 × 109/L for both. This cut-off of 299.5 × 109 corresponded with a 90.9% sensitivity (95%CI: 74.5–98.4%) and a 58.3% specificity (95%CI: 42.1–73.4%) in predicting multimodal treatment in Cushing’s disease (Fig. 7a), and with a 93.8% sensitivity and a 52.4% specificity in predicting multiple treatment in Cushing’s disease (Fig. 7b). No other specific cut-offs regarding other biochemical parameters were identified as useful or reliable in predicting invasive or refractory disease in the different PA subtypes (data not shown).
Discussion
FBC data and serum inflammation-based scores are widely used in cancer to predict outcomes and prognosis [5, 9, 10, 12, 13, 16], including in endocrine neoplasms [8, 21,22,23,24,25,26,27]; however, up until this study, there were no data in PAs. In this study, we characterised the FBC, CRP, albumin and several serum inflammation-based scores in patients with PAs, and we investigated the usefulness of such parameters in predicting challenging disease course or refractory disease aiming to provide advances in risk stratification and management of PA patients.
In our cohort of PA patients, FBC parameters were overall within normal range and the inflammation-based scores were rather unimpressive, with mean NLR and PLR being relatively low compared to those usually seen in highly malignant neoplasms [5, 12, 13], where NLR > 5 (> 4 in craniopharyngiomas [27]) and PLR > 300 are frequent and indicative of poor prognosis [5, 16]. On the other hand, the mean LMR in our PA cohort (3.82) was relatively high when compared to other neoplasms, where LMR < 2.18–2.71 indicate aggressive disease and poor outcomes [12, 36]. The proportion of PA patients with GPS ≥ 1 we observed (15.1%) was in general lower to what is often described in other cancers [7]. Chen et al. observed more leucocytes, lymphocytes and platelets as well as higher LMR and PNI in craniopharyngioma patients in comparison to PA patients with no differences regarding NLR or PLR individually, however the combinations NLR + PLR and dNLR + PLR were able to differentially diagnose papillary craniopharyngiomas, PAs and Rathke’s cleft cysts [8]. Overall, these data are not surprising considering that PAs are usually benign and lack metastatic properties, and despite the fact that pituitary tumour cells secrete factors such as cytokines and growth factors [4, 37] their release into the circulation and/or systemic repercussions may be less prominent than in other malignancies [5, 12, 13], or even than in craniopharyngiomas [8]. Nevertheless, the systemic inflammation appears to be higher in PA patients than healthy individuals, as suggested by the observations of higher NLR, lower PNI, more leucocytes, neutrophils, monocytes, and fewer platelets in PA patients in comparison to healthy controls [8].
PA secretome also includes hormones which are released in the circulation and may remarkably influence the haematopoiesis and circulating immune cells, as well as the degree of systemic inflammation (Fig. 8). This is well-known for Cushing’s disease, in which excessive glucocorticoid levels increase leucocyte and neutrophil counts [38, 39]. In fact, about 40–52% of Cushing’s disease patients present with leucocytosis (42.9% in our cohort), and in most cases (including those with normal baseline counts) the leucocyte and neutrophil counts decrease 20–30% after treatment, demonstrating the direct effect of hypercortisolism on these blood cells [38, 39]. We also observed higher platelet count in Cushing’s disease patients compared to other subtypes, particularly than acromegaly and NFPA patients. The effect of hypercortisolism on coagulation results in a hypercoagulability state in Cushing’s syndrome [40], as excessive glucocorticoid levels increase several plasma clotting factors and lead to defective fibrinolysis [41, 42]. The effects of cortisol on platelet count and function are less known, but higher numbers of platelets in patients with Cushing’s syndrome than in obese non-Cushingoid [43] and than in healthy controls [44] have been reported. Additionally, oxidative injury and platelet aggregation were enhanced in Cushing’s syndrome [45,46,47], processes that may further contribute to hypercoagulability, thromboembolic events, and cardiovascular disease recognised in this condition [41, 42]. When compared to other subtypes, our Cushing’s disease cohort had the highest absolute monocyte count and the lowest eosinophil count, consistent with previous reports [38, 48]. Cushing’s disease patients also had the lowest lymphocyte count (non-significant) and their serum inflammation-based scores differed from other subtypes, particularly those incorporating leucocyte and neutrophil counts (NLR, NPS and SII, all higher in Cushing’s disease), which is consistent with the Cushing’s disease-related inflammation and immunosuppression [49].
Overview of the interactions between pituitary tumour secreted factors and biological processes such as haematopoiesis and systemic inflammation, determining challenging disease course and clinical outcomes in patients with pituitary adenomas. Cytokines, chemokines, growth factors, hormones and other neuropeptides are secreted by a PA into the circulation, where they exert systemic effects such as modulation of haematopoiesis or systemic inflammation by altering the liver production of acute-phase proteins such as C-reactive protein or albumin. Relevant biochemical parameters and serum inflammation-based scores that showed a crude association (crude p-value < 0.05) with clinical features and outcomes within the subgroups of functioning non-Cushing PAs, Cushing’s disease and non-functioning PAs are shown in the colour boxes in the left side of the figure. In the right side is shown the clinical features at presentation and outcomes that were found significantly associated to biochemical full or serum inflammation-based scores, coloured as the respective PA subgroup where such significance was observed (blue corresponding to non-functioning PAs; purple corresponding to functioning non-Cushing PAs; and orange corresponding to Cushing’s disease). *denotes the parameters that remained significantly different after correction with Benjamini–Hochberg method (corrected p-value < 0.05). GPS Glasgow Prognostic Score, LMR lymphocyte-to-monocyte ratio, NLR neutrophil-to-lymphocyte ratio, NPS neutrophil-platelet score, PA pituitary adenoma, PLR platelet-to-lymphocyte ratio, PNI Prognostic Nutrition Index, post-op post-operatively, RT radiotherapy, SII Systemic Immune-Inflammation Index, tx treatment, VF visual fields
The extent of glucocorticoid excess appears to influence, at least in part, the degree of systemic inflammation in Cushing’s disease, as we noted a crude positive correlation between leucocytes and serum cortisol and ACTH levels; 24 h-UFC negatively correlated with eosinophil, platelet and basophil counts, and was higher in patients with elevated CRP and GPS. Other studies failed to find an association between pre-treatment leucocyte counts and UFC or other parameters of hypothalamic–pituitary–adrenal axis activity [38, 39]. However, Masri-Iraqi et al. reported a positive correlation between decrease in UFC and reduction in leucocyte counts following treatment for Cushing’s disease [39]. In another study, CRP did not differ between Cushing’s syndrome patients and healthy controls, however, interleukin-6 and soluble tumour necrosis factor-α receptor were more elevated in Cushing's syndrome [49]. To determine whether our findings are purely due to hormone excess and its extent, a comparison between patients with Cushing’s syndrome of pituitary versus adrenal origin could be performed in future studies.
Excessive levels of GH/IGF-1 or prolactin in acromegaly and prolactinoma patients do not seem to have similar effects as to those observed for glucocorticoid excess in Cushing’s disease, considering that FBC parameters and inflammation-based scores did not differ among other non-Cushing PA subtypes. Moreover, there were no correlations between GH, IGF-1 or prolactin and FBC parameters neither with serum inflammation-based scores among acromegaly or prolactinoma patients, except the negative crude correlation between IGF-1 and platelets in acromegaly, which was somewhat unexpected considering the thrombopoietic effects of GH [50]. Despite the fact that GH/IGF-1 or prolactin can influence haematopoiesis [51,52,53], these pituitary hormones may not be crucial for haematopoiesis, at least in comparison to other conventional immune-stimulating cytokines and myeloid factors [53,54,55]. Hence, our data suggest that PA-related hormone status may not be relevant in determining the haematopoiesis and systemic inflammation in patients with functioning non-Cushing PAs.
On the other hand, pituitary hormone deficiencies may not be significant in determining the haematopoiesis or the degree of systemic inflammation in PA patients, as we found no statistical associations between the presence of pre-operative hypopituitarism and the different FBC and serum inflammation-based scores, except within functioning non-Cushing PA patients in whom a GPS ≥ 1 had a crude association with higher rates of pre-operative hypopituitarism.
Our exploratory study suggests that some FBC parameters and serum inflammation-based scores may have a role in predicting invasive or refractory disease depending on the PA subtype (Fig. 8). GPS, NPS and PNI may be relevant for the subgroup of functioning non-Cushing PAs, but no value was noted for individual FBC elements. Consistently, Tam et al. reported no differences regarding leucocyte and platelet counts between prolactinoma patients and healthy controls, neither before or 6 months after cabergoline treatment among prolactinoma patients [56]. However, in our Cushing’s disease cohort, the pre-operative platelet count emerged as the most relevant biochemical parameter in predicting refractory disease, with an optimal cut-off of 299.5 × 109/L below which multimodal treatment is more likely required. In our Cushing’s disease cohort, lower platelet counts were noted for patients with invasive and multi-treated tumours, however thrombocytopenia was in general uncommon (only 3%). In cancer, platelet counts are often decreased as a result of thrombopoiesis impairment, platelet consumption or platelet aggregation [57]. Low platelet counts, regarded as “sentinels” of the tumour disease state, have been associated with more aggressive disease and worse outcomes [58], including bleeding or thrombotic events [59], however, to our knowledge, this has not been shown in Cushing’s disease. We found no association between leucocytes or neutrophil counts, or related inflammation-based scores, and features suggestive of invasive or refractory Cushing’s disease. These data suggest that leucocytosis and neutrophilia are probably a direct consequence of hypercortisolism but do not necessarily imply deleterious systemic inflammation or poorer outcomes in Cushing’s disease, despite the fact that leucocytes and NLR may be valuable in predicting outcomes in non-neoplastic cardiometabolic diseases [60, 61].
Regarding NFPAs, patients with more treatment refractory disease had fewer leucocytes and lymphocytes, higher NLR and PLR, and lower PNI pre-operatively. From a clinical perspective, the identification of such biochemical parameters in predicting challenging disease course may be relevant for decision-making and management of NFPA patients, and our observations here reported require confirmation in other cohorts. As this study included only NFPA patients who underwent surgery, our findings should be validated in a series involving NFPAs who had either surgery or only surveillance aiming to assess whether there is a value for leucocyte count (or any other parameter) in predicting which NFPAs will grow or require surgery, and thus contribute for the decision of advising early operation (or monitor instead) in NFPAs with no or borderline indication for surgery when first presented. More leucocytes, neutrophils, monocytes, as well as fewer lymphocytes, GPS ≥ 1 and higher NLR, and CRP levels were observed in NFPA patients who suffered apoplexy, which might be a consequence of the local inflammation, haemorrhage or infarction within the tumoural tissue, and/or a result of the patient being clinically unwell or critically unstable during an apoplexy episode [62], rather than depending on the PA characteristics or predicting clinical outcomes per se.
As this is the first study investigating the role of FBC and serum inflammation-based scores in patients with PAs, we explored several biochemical parameters and clinical/outcome variables. This inexorably resulted in a high amount of analyses, which makes our study very comprehensive and exploratory in nature, but also constitutes a limitation on its own. Therefore, to minimise the type I-errors associated with multiple testing, we applied Benjamini–Hochberg correction when reporting on significance (shown in Supplementary Table 2). As this is an exploratory study, we reported crude p-values up to 0.05 before correction worth being investigated in future studies. Another limitation of our study is that we have relatively small number of cases with available clinical and biochemical data for some subgroups, as some patients had missing clinical and/or biochemical data. In fact, this relatively small size of some subgroups limited the assessment of some serum inflammation-based scores we studied, and provides insufficient statistical power to detect significant differences after correction, particularly if we take into consideration that such biochemical parameters have substantial inter- and intra-individual variability. Hence, some of our negative findings may not reflect the lack of association but instead insufficient sample size for some comparative subanalyses performed. Thus, the positive and negative data from our exploratory study require further validation in larger series. Other limitations of our study include: i) single-centre study, mainly including a population of Dutch patients, hence limiting the generalizability of our findings to other populations or ethnicities; ii) retrospective study, iii) absence of healthy controls as comparator or other specific subgroups of (ultra-rare) cases, such as pituitary carcinomas; iv) our cohort does not reflect the usual prevalence distribution of the different PA subtypes, particularly the hyperfunctioning ones, due to its surgical nature; v) we cannot exclude that some patients would have unknown/unreported concomitant diseases capable of influencing haematopoiesis or systemic inflammation; vi) the specific effects of the hormonal excess (or the pre-operative medical therapies) in each serum inflammation-based score per PA subtype were not comprehensively assessed in our study, as the serum inflammation-based scores calculation and their usefulness were considered before the first operation at our centre, and not by the time of the PA diagnosis or prior any therapeutical intervention. Worth noting that most studies regarding serum inflammation-based scores have been conducted in aggressive cancers, hence clinical outcomes such as overall survival, progression-free survival and mortality rates are often reported in those studies, making difficult to contrast our results with those reported in the literature as such clinical variables are not applicable to a cohort of patients with benign and often non-aggressive PAs.
Conclusions
FBC and serum inflammation-based scores remarkably differ in Cushing’s disease comparing to other PA subtypes. The extent of pituitary hormone excess may influence, at least in part, the systemic inflammation in Cushing’s disease. Platelet count below 299.5 × 109/L predicts multimodal treatment in patients with Cushing’s disease. Some serum inflammation-based scores may have a role in predicting invasive, challenging disease course or treatment refractory disease, namely GPS and PNI in NFPAs and functioning non-Cushing PAs; NPS in functioning non-Cushing PAs; NLR or PLR in NFPAs. Further studies involving larger cohorts of patients are needed to confirm some of the observations from our exploratory study.
References
Marques P, Korbonits M (2017) Genetic aspects of pituitary adenomas. Endocrinol Metab Clin N Am 46(2):335–374. https://doi.org/10.1016/j.ecl.2017.01.004
Chatzellis E, Alexandraki KI, Androulakis II, Kaltsas G (2015) Aggressive pituitary tumors. Neuroendocrinology 101(2):87–104. https://doi.org/10.1159/000371806
Trouillas, J., Roy, P., Sturm, N., Dantony, E., Cortet-Rudelli, C., Viennet, G., Bonneville, J.F., Assaker, R., Auger, C., Brue, T., Cornelius, A., Dufour, H., Jouanneau, E., Francois, P., Galland, F., Mougel, F., Chapuis, F., Villeneuve, L., Maurage, C.A., Figarella-Branger, D., Raverot, G., members of, H., Barlier, A., Bernier, M., Bonnet, F., Borson-Chazot, F., Brassier, G., Caulet-Maugendre, S., Chabre, O., Chanson, P., Cottier, J.F., Delemer, B., Delgrange, E., Di Tommaso, L., Eimer, S., Gaillard, S., Jan, M., Girard, J.J., Lapras, V., Loiseau, H., Passagia, J.G., Patey, M., Penfornis, A., Poirier, J.Y., Perrin, G., Tabarin, A.: A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol 126(1), 123–135 (2013)
Marques P, Barry S, Carlsen E, Collier D, Ronaldson A, Awad S, Dorward N, Grieve J, Mendoza N, Muquit S, Grossman AB, Balkwill F, Korbonits M (2019) Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol Commun 7(1):172. https://doi.org/10.1186/s40478-019-0830-3
Bugada D, Allegri M, Lavand’homme P, De Kock M, Fanelli G (2014) Inflammation-based scores: a new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed Res Int 2014:142425. https://doi.org/10.1155/2014/142425
Balkwill FR, Mantovani A (2012) Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 22(1):33–40. https://doi.org/10.1016/j.semcancer.2011.12.005
McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12(3):223–226. https://doi.org/10.1097/MCO.0b013e32832a7902
Chen M, Zheng SH, Yang M, Chen ZH, Li ST (2018) The diagnostic value of preoperative inflammatory markers in craniopharyngioma: a multicenter cohort study. J Neurooncol 138(1):113–122. https://doi.org/10.1007/s11060-018-2776-x
Chang X, Zhang F, Liu T, Wang W, Guo H (2017) Neutrophil-to-lymphocyte ratio as an independent predictor for survival in patients with localized clear cell renal cell carcinoma after radiofrequency ablation: a propensity score matching analysis. Int Urol Nephrol 49(6):967–974. https://doi.org/10.1007/s11255-017-1554-6
Tang H, Lu W, Li B, Li C, Xu Y, Dong J (2017) Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget 8(22):36857–36868. https://doi.org/10.18632/oncotarget.16143
Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI (2010) Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 200(2):197–203. https://doi.org/10.1016/j.amjsurg.2009.08.041
Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ (2017) The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg 265(3):539–546. https://doi.org/10.1097/SLA.0000000000001743
Yang T, Zhu J, Zhao L, Mai K, Ye J, Huang S, Zhao Y (2017) Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J Surg Oncol 115(6):718–728. https://doi.org/10.1002/jso.24549
Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC (2015) The Neutrophil-Platelet Score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS ONE 10(11):e0142159. https://doi.org/10.1371/journal.pone.0142159
Liang R, Li J, Tang X, Liu Y (2019) The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg 184:105397. https://doi.org/10.1016/j.clineuro.2019.105397
Ahmad J, Grimes N, Farid S, Morris-Stiff G (2014) Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int 13(5):474–481
Wang PF, Meng Z, Song HW, Yao K, Duan ZJ, Yu CJ, Li SW, Yan CX (2018) Preoperative changes in hematological markers and predictors of glioma grade and survival. Front Pharmacol 9:886. https://doi.org/10.3389/fphar.2018.00886
Yang T, Mao P, Chen X, Niu X, Xu G, Bai X, Xie W (2019) Inflammatory biomarkers in prognostic analysis for patients with glioma and the establishment of a nomogram. Oncol Lett 17(2):2516–2522. https://doi.org/10.3892/ol.2018.9870
Lin M, Hu T, Yan L, Xiao D, Zhao H, Yan P (2019) Can systemic inflammatory markers be used to predict the pathological grade of meningioma before surgery? World Neurosurg 127:e677–e684. https://doi.org/10.1016/j.wneu.2019.03.241
Wang DP, Kang K, Lin Q, Hai J (2020) Prognostic significance of preoperative systemic cellular inflammatory markers in gliomas: a systematic review and meta-analysis. Clin Transl Sci 13(1):179–188. https://doi.org/10.1111/cts.12700
Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP (2013) Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol 107(5):493–497. https://doi.org/10.1002/jso.23270
Ozmen S, Timur O, Calik I, Altinkaynak K, Simsek E, Gozcu H, Arslan A, Carlioglu A (2017) Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) may be superior to C-reactive protein (CRP) for predicting the occurrence of differentiated thyroid cancer. Endocr Regul 51(3):131–136. https://doi.org/10.1515/enr-2017-0013
Luo G, Liu C, Cheng H, Jin K, Guo M, Lu Y, Long J, Xu J, Ni Q, Chen J, Yu X (2017) Neutrophil-lymphocyte ratio predicts survival in pancreatic neuroendocrine tumors. Oncol Lett 13(4):2454–2458. https://doi.org/10.3892/ol.2017.5716
McDermott SM, Saunders ND, Schneider EB, Strosberg D, Onesti J, Dillhoff M, Schmidt CR, Shirley LA (2018) Neutrophil lymphocyte ratio and transarterial chemoembolization in neuroendocrine tumor metastases. J Surg Res 232:369–375. https://doi.org/10.1016/j.jss.2018.06.058
Okui M, Yamamichi T, Asakawa A, Harada M, Saito M, Horio H (2017) Prognostic significance of neutrophil-lymphocyte ratios in large cell neuroendocrine carcinoma. Gen Thorac Cardiovasc Surg 65(11):633–639. https://doi.org/10.1007/s11748-017-0804-y
Salman T, Kazaz SN, Varol U, Oflazoglu U, Unek IT, Kucukzeybek Y, Alacacioglu A, Atag E, Semiz HS, Cengiz H, Oztop I, Tarhan MO (2016) Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for patients with neuroendocrine tumors: an Izmir Oncology Group Study. Chemotherapy 61(6):281–286. https://doi.org/10.1159/000445045
Zhang J, He M, Liu Z, Song Y, Wang Y, Liang R, Chen H, Xu J (2018) Impact of neutrophil-lymphocyte ratio on long-term outcome in patients with craniopharyngioma. Medicine (Baltimore) 97(37):e12375. https://doi.org/10.1097/MD.0000000000012375
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617 (discussion 617–618)
Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH (2016) Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101(11):3888–3921. https://doi.org/10.1210/jc.2016-2118
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA, Endocrine S (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A (2018) A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol 14(9):552–561. https://doi.org/10.1038/s41574-018-0058-5
Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JA, Giustina A (2006) Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 65(2):265–273. https://doi.org/10.1111/j.1365-2265.2006.02562.x
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA, Endocrine S (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(2):273–288. https://doi.org/10.1210/jc.2010-1692
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A, Endocrine S (2015) Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 100(8):2807–2831. https://doi.org/10.1210/jc.2015-1818
Pereira AM, van Aken MO, van Dulken H, Schutte PJ, Biermasz NR, Smit JW, Roelfsema F, Romijn JA (2003) Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing’s disease. J Clin Endocrinol Metab 88(12):5858–5864. https://doi.org/10.1210/jc.2003-030751
Wang X, Su S, Guo Y (2017) The clinical use of the platelet to lymphocyte ratio and lymphocyte to monocyte ratio as prognostic factors in renal cell carcinoma: a systematic review and meta-analysis. Oncotarget 8(48):84506–84514. https://doi.org/10.18632/oncotarget.21108
Tsagarakis S, Kontogeorgos G, Kovacs K (1998) The role of cytokines in the normal and neoplastic pituitary. Crit Rev Oncol Hematol 28(2):73–90
Ambrogio AG, De Martin M, Ascoli P, Cavagnini F, Pecori Giraldi F (2014) Gender-dependent changes in haematological parameters in patients with Cushing’s disease before and after remission. Eur J Endocrinol 170(3):393–400. https://doi.org/10.1530/EJE-13-0824
Masri-Iraqi H, Robenshtok E, Tzvetov G, Manistersky Y, Shimon I (2014) Elevated white blood cell counts in Cushing’s disease: association with hypercortisolism. Pituitary 17(5):436–440. https://doi.org/10.1007/s11102-013-0522-0
Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB (2015) Mechanisms in endocrinology: The spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol 173(3):R101-113. https://doi.org/10.1530/EJE-15-0308
Trementino L, Arnaldi G, Appolloni G, Daidone V, Scaroni C, Casonato A, Boscaro M (2010) Coagulopathy in Cushing’s syndrome. Neuroendocrinology 92(Suppl 1):55–59. https://doi.org/10.1159/000314349
Van Zaane B, Nur E, Squizzato A, Dekkers OM, Twickler MT, Fliers E, Gerdes VE, Buller HR, Brandjes DP (2009) Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab 94(8):2743–2750. https://doi.org/10.1210/jc.2009-0290
Sato T, Hiramatsu R, Iwaoka T, Fujii Y, Shimada T, Umeda T (1984) Changes of platelets, serum lactic dehydrogenase, gamma-glutamyltranspeptidase, choline esterase and creatine phosphokinase levels in patients with Cushing’s syndrome. Tohoku J Exp Med 142(2):195–200. https://doi.org/10.1620/tjem.142.195
Erem C, Nuhoglu I, Yilmaz M, Kocak M, Demirel A, Ucuncu O, Onder Ersoz H (2009) Blood coagulation and fibrinolysis in patients with Cushing’s syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activatable fibrinolysis inhibitor levels. J Endocrinol Invest 32(2):169–174. https://doi.org/10.1007/bf03345709
Fatti LM, Bottasso B, Invitti C, Coppola R, Cavagnini F, Mannucci PM (2000) Markers of activation of coagulation and fibrinolysis in patients with Cushing’s syndrome. J Endocrinol Invest 23(3):145–150. https://doi.org/10.1007/BF03343697
Karamouzis I, Berardelli R, D’Angelo V, Fussotto B, Zichi C, Giordano R, Settanni F, Maccario M, Ghigo E, Arvat E (2015) Enhanced oxidative stress and platelet activation in patients with Cushing’s syndrome. Clin Endocrinol (Oxf) 82(4):517–524. https://doi.org/10.1111/cen.12524
Patrassi GM, Sartori MT, Viero ML, Scarano L, Boscaro M, Girolami A (1992) The fibrinolytic potential in patients with Cushing’s disease: a clue to their hypercoagulable state. Blood Coagul Fibrinolysis 3(6):789–793. https://doi.org/10.1097/00001721-199212000-00013
Aranda G, Lopez C, Fernandez-Ruiz R, Esteban Y, Garcia-Eguren G, Mora M, Halperin I, Casals G, Ensenat J, Hanzu FA (2017) Circulatory Immune Cells in Cushing Syndrome: Bystanders or Active Contributors to Atherometabolic Injury? A Study of Adhesion and Activation of Cell Surface Markers. Int J Endocrinol 2017:2912763. https://doi.org/10.1155/2017/2912763
Aulinas A, Ramirez MJ, Barahona MJ, Valassi E, Resmini E, Mato E, Santos A, Crespo I, Bell O, Surralles J, Webb SM (2015) Dyslipidemia and chronic inflammation markers are correlated with telomere length shortening in Cushing’s syndrome. PLoS ONE 10(3):e0120185. https://doi.org/10.1371/journal.pone.0120185
Xu Y, Wang S, Shen M, Zhang Z, Chen S, Chen F, Chen M, Zeng D, Wang A, Zhao J, Cheng T, Su Y, Wang J (2014) hGH promotes megakaryocyte differentiation and exerts a complementary effect with c-Mpl ligands on thrombopoiesis. Blood 123(14):2250–2260. https://doi.org/10.1182/blood-2013-09-525402
Auernhammer CJ, Strasburger CJ (1995) Effects of growth hormone and insulin-like growth factor I on the immune system. Eur J Endocrinol 133(6):635–645. https://doi.org/10.1530/eje.0.1330635
Merchav S (1998) The haematopoietic effects of growth hormone and insulin-like growth factor-I. J Pediatr Endocrinol Metab 11(6):677–685. https://doi.org/10.1515/jpem.1998.11.6.677
Welniak LA, Tian ZG, Sun R, Keller JR, Richards S, Ruscetti FW, Murphy WJ (2000) Effects of growth hormone and prolactin on hematopoiesis. Leuk Lymphoma 38(5–6):435–445. https://doi.org/10.3109/10428190009059263
Welniak LA, Richards SM, Murphy WJ (2001) Effects of prolactin on hematopoiesis. Lupus 10(10):700–705. https://doi.org/10.1191/096120301717164930
Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K (1997) Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16(23):6926–6935. https://doi.org/10.1093/emboj/16.23.6926
Tam AA, Kaya C, Baser H, Ersoy R, Cakir B (2016) Mean platelet volume in patients with prolactinoma. Arch Endocrinol Metab 60(4):319–322. https://doi.org/10.1590/2359-3997000000054
Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV (2014) Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev 33(1):231–269. https://doi.org/10.1007/s10555-014-9498-0
Contursi A, Grande R, Dovizio M, Bruno A, Fullone R, Patrignani P (2018) Platelets in cancer development and diagnosis. Biochem Soc Trans 46(6):1517–1527. https://doi.org/10.1042/BST20180159
Girolami A, de Marinis GB, Bonamigo E, Treleani M, Vettore S (2013) Arterial and venous thromboses in patients with idiopathic (immunological) thrombocytopenia: a possible contributing role of cortisone-induced hypercoagulable state. Clin Appl Thromb Hemost 19(6):613–618. https://doi.org/10.1177/1076029612452114
Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, Banai S (2012) Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 225(2):456–460. https://doi.org/10.1016/j.atherosclerosis.2012.09.009
Gokhan S, Ozhasenekler A, Mansur Durgun H, Akil E, Ustundag M, Orak M (2013) Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur Rev Med Pharmacol Sci 17(5):653–657
Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P (2015) Pituitary Apoplexy. Endocr Rev 36(6):622–645. https://doi.org/10.1210/er.2015-1042
Funding
P.M. was supported by the Fellowship Program Grant ´3E´, the Exchange in Endocrinology Expertise, Board of Endocrinology of the UEMS and Novo Nordisk A/S and Novartis (2019).
Author information
Authors and Affiliations
Contributions
PM and FdV designed and performed the study, collected and analysed the data and wrote the manuscript; OMD helped with the statistical analysis and provided critical input to the design of the study; WRvF, MK and NRB provided critical input; AMP designed the study, provided critical input and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by our institutional ethical board (G19.011).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marques, P., de Vries, F., Dekkers, O.M. et al. Pre-operative serum inflammation-based scores in patients with pituitary adenomas. Pituitary 24, 334–350 (2021). https://doi.org/10.1007/s11102-020-01112-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01112-5