Abstract
N-nitrosamines, the potential hazardous pollutants, are classified as most mutagenic and probable carcinogenic compounds. One of the Potentially carcinogenic N-nitroso compounds is N-nitrosopiperidine (NPIP) which is produced by the oxidation or nitrosation of amine precursors. NPIP can be found in a variety of matrices including latex products, agricultural chemicals, cosmetic items, chlorinated water, alcoholic beverages, spices, and food products. Various physical (ionized radiation, and ultraviolet light), chemical (outdoor and indoor air pollution, second-hand smoke, asbestos, metals, and vinyl chloride), and biological (diet, physical activity, infection, mutagenic and carcinogenic compounds, nitrosamines) factors are identified as precursors associated with the formation of NPIP. In addition, various genetic factors (cell cycle genes, tissue organization genes, signal transduction genes, and DNA repair genes) are also involved in the development of NPIP-directed diseases. Under physiological conditions, NPIP is found to be stable but require cytochrome P450-directed hydroxylation at the carbon atoms adjacent to nitroso group to form α-hydroxy NPIP ester for their metabolic activation. Various acute, chronic, reproductive health hazards may produce after the reaction of α-acetoxy-N-nitrosopiperidine with 2′-deoxy guanosine which can last for months or years. Different types of cancers such as esophageal, hepatocellular, pulmonary, bronchial and alveologenic are induced in response of NPIP in different animal models at 33 or 66 mg/kg, 0.88 × 10–3 M, 0.2 mmol/kg of dosage. Tumours, such as tonofibrils, desmosomes, irregular nuclei, aggregated condensed chromatin with pars amorpha and fibrillar components, induced in lab animals show resemblance with their human counterparts with respect to their histological studies. Various studies have explored the role of food mutagen NPIP in generating caspase directed apoptosis. Apoptosis is well characterized by nucleus fragmentation, chromatin condensation, cell volume reduction, cytoplasmic shrinkage, and membrane blebbing. The safety and health organizations have taken various preventive measures to limit the exposure of NPIP carcinogenic compounds around residential areas and workplaces but this further requires population-based intervention and some policy implementation. Removal techniques like biological denitrifications, electrodialysis, ion-exchange chromatography, reverse osmosis, cellulose nanopaper membrane, etc., have also been applied to control the exposure of NPIP. Thus, NPIP has a role as an environmental pollutant, a mutagen, an apoptosis inducer, and a carcinogenic agent. Therefore, we have reviewed some basic features of NPIP and its contribution towards various types of cancers, along with some preventive measures and removal techniques of NPIP for the first time in this report.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrites and nitrates are extensively present in the environment, mainly, in natural water bodies and in vegetable food products (Ma et al. 2018). The role of drinking water in the intake of nitrate is quite low, i.e., less than 14%. However, excessive use of inorganic fertilizers increases the level of nitrate in water resources of various places across the globe. Notably, when the concentration of nitrate is less than 10 mg/L in drinking water, food is considered as the major nitrate source for the humans (Ward et al. 2018). In another condition, when the level of nitrate in drinking water exceeds 50 mg/L, water is generally considered as the main source of nitrate exposure to the human body. On the other hand, the process of malt drying in beer resulted in the formation of nitrosamines which are the products of reaction between the amines (present in barley) and the nitrogen dioxides (form during fuel combustion in the air) (Fan and Lin 2018). Nitrites/nitrates can also be utilized as food additives in animal originated food products. Nitrite is one of the important additives which are added to meat production for desirable texture and color, and for preventing lipid peroxidation and formation of toxins through Clostridium botulinum. It is mainly applied to stabilize processed cheese and meat (Ferysiuk and Wójciak 2020). Various investigations at different food institutions and research centres have shown that the contamination of N-nitroso compounds occurs with food products. Therefore, in some countries, there is a strict control over the utilization of nitrosating agents for curing meat or can be used along with some inhibitors such as ascorbic acid to restrict the generation of nitrosamine in the processing of various food products (Shakil et al. 2022).
Recent studies have reported the bactericidal nature of nitrite for skin, oral, and gastrointestinal infectious bacteria when mixed and ingested with gastric acid (Ma et al. 2018). There is a concern with respect to nitrite as it may react with amino acids and amines and may result in the production of N-nitrosamines. Around 50–70% of such compounds are rapidly absorbed and about 3% of them are excreted as ammonia and urea from urine. These compounds can also escape and survive the passage through stomach and get enter into the circulatory system (Masuda et al. 2000). A large number of reactive nitrogen species (RNS) are produced in tissues and blood or under the acidic gastric conditions. Such species may be responsible for the formation of nitrosamine of toxicological nature when these are available in the stomach (Karwowska and Kononiuk 2020). The N-nitrosamines have been classified in the group B2 of carcinogenic compounds by United States Environmental Protection Agency (US EPA). Some N-nitrosamine, such as, N-nitrosodiphenylamine (NDPhA), N-nitrosodi-n-butylamine (NDBA), N-nitrosopyrrolidine (NPYR), N-nitrosodi-n-propylamine (NDPA), N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosomorpholine (NMOR), N-nitrosopiperidine (NPIP), and N-nitrosomethylamine (NMEA) have been reported to have mutagenic effect on the human body (Xie et al. 2010). Many of the nitrosamines are found in water sample of waste treatment plant at the level of 1 or 2 orders of magnitudes that is greater than the permissible limit of tumor risk (Aragôn et al. 2013; Li et al. 2021). Human beings can be exposed to NPYR and NPIP exogenously through the different food sources that contain sodium nitrite as a preservative, including meat, fish and cheese. However, nitrosation of amine precursors endogenously results in a high-level exposure of NPYR, NPIP, and NNN (Li and Hecht 2022) to human beings. Remarkably, larger amount of NPIP can be generated by the nitrosation reaction of piperidine in the presence of nitrite (García et al. 2009a). In the products of meat and spice premixes, concurrent presence of nitrite (in meat, fish and cheese) and piperine (in black pepper) results in the formation of NPIP either by the oxidative cleavage of piperine to piperidine and then nitrosation, or by the direct nitrosation of the existing piperidine. For evaluating the risk results from the formation of N-nitrosamine, it would also be better to evaluate the content of piperidine and piperine in spices (De Mey et al. 2014a). The smaller amount of NPIP is also reported in tobacco smoke. NPIP has been reported mutagenic and genotoxic and may result in severe clastogenicity and chromosome aberrations. It is considered as a contributing agent in different human cancers. The organs targeted by NPIP are mainly larynx, liver, nasal lining, and esophagus. The exposure to NPIP affects different pathways that are involved in cell apoptosis, cell proliferation, and cell cycle regulation (Xie et al. 2010). Due to human exposure to N-nitrosamines, it is important to understand the exact mechanism responsible for their carcinogenesis.
All the N-nitrosamines need metabolic activation to bring their cancer-causing properties. In order to produce electrophile for alkylating the DNA and to initiate the process of carcinogenesis consequently, the metabolic processes are catalysed by the enzyme cytochrome P450 (Li and Hecht 2022). Due to potent carcinogenic nature of N-nitrosamines, some efforts made to prevent the N-nitrosamine formation in meat items are: (1) restriction in the mixing of nitrite up to a limit of 150 mg NaNO2/kg. (2) Usage of nitrate scavenging additives such as alpha-tocopherol, and ascorbate. (3) Reducing the content of biogenic amines by finalizing the starters with less decarboxylase activity or by managing the microbes quality in the raw product of meat. (4) Gamma-irradiation can be utilized as a decontaminant for the removal of N-nitrosamines or their precursors (De Mey et al. 2014a). (5) Dietary antioxidants at the molar ratio of 2:1 with respect to nitrite can inhibit the generation of N-nitrosamines (Karwowska and Kononiuk 2020).
Improper diet and various other risk factors have been determined to raise the cases of different types of tumors (Konishi et al. 1986). It is also reported that the occurrence of tumor differs based on the lifestyle, eating habit, occupation, and religion. These variations are induced due to different concentration of carcinogen in the environment that directs gene mutation. Genetic factors related to neoplastic disorders are determined to be the susceptibility state of tumours caused by mutations in tumour-associated genes. The prevention of cancer is possible if the risk factors can be managed or avoided (Saeki and Sugimachi 2001). Thus, this article on NPIP is the first attempt that aims to review the classification of nitrosamines, chemical and physical properties of NPIP, hazard classes and categories, sources of nitrates and nitrites, estimation of N-nitrosamine exposure, risk factors, overview of carcinogenic NPIP to which human is commonly exposed, exposure concentration of NPIP, mechanism of NPIP formation in food items, mechanism of NPIP carcinogenicity, evaluation of health risk, role of NPIP inducing apoptosis, effect of NPIP on histological lesions, precautions taken while handling NPIP, and removal techniques of NPIP.
Methodology
The literature review is based on the articles and research papers published in English and indexed on different authentic medical and non-medical databases i.e., Pubmed, Web of Science, Wiley Online Library, Science direct, Scopus, NCBI, Google Scholar, World Health Organization, and Centre for Health Security. The keywords used to search the information include risk of nitrosative stress, cancer, nitrite, nitrate, N-nitrosopiperidine, etc. Results of the different database searches are reviewed to collect the matter for this article. About 168 out of 252 articles about NPIP were selected from the aforementioned databases to complete the literature review.
Classification of N-nitroso compounds
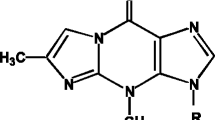
The characterization of N-nitrosamine is based on the N-nitroso functional group (> N–N=O). The International Agency for Research on Cancer (IARC) has categorized (Fig. 1) some of the N-nitrosamine in Group 2A and 2B, namely, N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosomorpholine (NMOR), N-nitrosomethylethylamine (NMEA), N-nitrososarcosine (NSAR), N-nitrosopiperidine (NPIP), N-nitrosodibutylamine (NDBA), and N-nitrosopyrrolidine (NPYR). Compounds belong to these groups are reported to cause cancer in humans whereas, N-nitrosodiphenylamine (NDPhA) and N-nitrosodiphenylamine (NPRO) are not carcinogenic for humans and categorized in Class 3 (Mirvish 2008; Cascella et al. 2018; Park et al. 2018; Xie et al. 2023).
Classes and categories
The hazard classes and categories of NPIP are listed below:
-
1.
Acute toxicity (100%): oral, category 3
-
2.
Carcinogenicity (93.33%): category 2
-
3.
Reactive organic groups present: nitrite, nitrate, nitroso, nitro, azide, hydrazine, azido, diazo, and azo compounds
-
4.
Reactivity profile: found to be reactive with peroxyacids
-
5.
Non-human toxicity values: the non-toxicity values for different animal models are discussed in Table 2.
Sources of nitrates and nitrites
Fertilizers
One of the foremost sources that contribute to the exposure of nitrate to environment is the application of fertilizers. Synthetic or artificial fertilizers include a variety of chemicals such as urea, sodium nitrate, potassium, calcium, and ammonia. Increasing population is also expanding our agricultural needs exponentially. The Asian regions such as India and China have been emerged as the greatest producer as well as consumer of fertilizers in the world. About 60% of world’s fertilizer consumption is observed mostly in East and South Asia. In year 2018, Europe and North America consumed 26.9% of nitrogen fertilizers whereas India in South Asia and China in East Asia utilized 42.3% of nitrogen fertilizers with 36.8% of global population. Oceania and Africa are the regions where consumption of nitrogen fertilizers is found very low and also increasing at a very low pace (Singh and Craswell 2021). The changes in the consumption pattern of nitrogen fertilizers in various areas of the globe have a great impact on nitrate contamination of surface and ground aquatic bodies. Although nitrate-nitrogen leaching from the plant-soil system was affected by soil, climate, and various other components, recently the pollution of nitrate in ground and surface water has been raised as a severe environmental issue in South and East Asia along with different regions of Central and Western Europe and North America (Shukla and Saxena 2019).
The fact that plants are not able to use nitrogen is significant and the utilization of nitrogen may vary from 25 to 85% depending on the agricultural techniques and the varieties of crops. Kohl et al. (1971) reported that 55–60% of nitrogen in the feeding Lake Decatur of the Sangamon River, USA was of fertilizer part. Lee (1970) reported that 3–10 times more run off of nitrogen occurs from the same fertilized area than the unfertilized one. However, Tomlinson (1970) reported that no relation was observed between the fertilizer amount used and concentration of nitrate in case of British rivers. Some studies reported that the application of nitrogen fertilizer tended to raise content of nitrate in vegetables. The application of fertilizer was four times to its original amount that results a higher level of nitrate in spinach (Schupan 1965). During years 2004–2010 when greater amount of nitrogen fertilizers were utilized by the China, the concentration of nitrate-nitrogen (N) was found to be higher in the agro-ecosystem located wells (4.1 ± 0.33 mg L−1) than the wells of forest ecosystem (0.5 ± 0.04 mg L−1). Irrigation or rainfall in combination with immoderate nitrogen fertilizer used in cotton, corn, and wheat fields resulted in higher level of nitrate-nitrogen in the groundwater of agro-ecosystem (Singh and Craswell 2021). Above permitted limits, nitrate nitrogen makes the aquatic system unsuitable for the drinkable use. In surface water where the concentration of nitrogen was limited, the productivity of phytoplankton was directed by the nitrate-nitrogen concentration resulting in eutrophication that leads to harmful algal blooms, biodiversity loss, anoxia and hypoxia that can cause damage to marine environment and fisheries (Bartley et al. 2003).
Due to high solubility and great dispersion into the soil, nitrate is considered to be the most spread contaminant that poses a damaging effect on the standard of agricultural products and drinking water. It forms naturally in the soil system through the microorganism mediated ammonia transformation which is liberated by mineral fertilizer, waste, plant, and by the decomposition of organic fertilizers (Giordano et al. 2021). The nitrate accumulation is also dependent on different physiological, nutritional, and environmental components that are varied from one region to another such as season of fertilization and harvest, vegetation period, crop rotation, cultivation method, day time temperature, light intensity, humidity, soil properties, biological properties of crops and locations (Umar and Iqbal 2007). Many vegetables such as cabbage, celery, broccoli, lettuce, spinach, and radish are reported to have > 1000 mg/kg of nitrate content (Zendehbad et al. 2022). temporal and spatial nitrate nitrogen distribution under cropland in ground water can be evaluated by population per unit area, livestock per unit area, per capita agricultural production, percentage of irrigated area, annual mean temperature, and nitrogen use per unit area. The global consumption of nitrogen fertilizer is found to be increased linearly due to the demand for animal derived food, and cereal food grains (Liu et al. 2017).
Animal wastes
The waste of livestock contains nitrogen in both organic and inorganic form. The fraction of inorganic nitrogen is somewhere equal to the organic nitrogen present in the urine and much more than that. Microorganisms carry out the decomposition of organic nitrogen containing waste into ammonia that further changes to nitrite and then nitrate (Sahoo et al. 2016). Manure produced by livestock commonly returns to the soil and improves its fertility and tilth. Both the United States Environmental Protection Agency and United States Department of Agriculture determined that land application is the only way to utilize the animal waste. However, if the application of manure is not done properly or it is done in excessive amount than the nutrient requirement of the site then the surface water and ground quality get impaired. Approximately 60% of nitrogen is not used that suggests the loss of nitrogen via ammonia immobilization, denitrification, and volatilization in soil (Kumar et al. 2013). The organic components present in waste get decomposed and consume the dissolved oxygen of the aquatic system, resulting in the death of the aquatic fish. Nitrogen and settled solid compounds can kill different forms of aquatic life system. On the other hand, nutrients present in the manure may increase the aquatic plant growth that can disrupt the local water body (Ullah Bhat and Qayoom 2022).
Excessive manure application creates build-up of nutrients in the soil that can seep through soil to water bodies. A prolonged excessive application of waste can also result an imbalance in the chemistry of soil and reduces the yield of growth. Within one year of waste application, some flora and grasses are needed to be planted to take up the excessive nutrients applied to the soil (Kumar et al. 2013). This problem is more severe where farming is widespread, as was the case for poultry and livestock in North America. A 3200 head feedlot results in the formation of 1400 tonnes of nitrogen annually and a 450 kg steer produces about 43 kg of nitrogen per year. This is the amount of waste equivalent to 260,000 people. About 10% of this waste is returned to the land application whereas remaining serves as a problem of environmental pollution (Stanford et al. 1969). Nye (1973) reported that the total nitrogen concentration in runoff is ranged from 50 to 5500 mg/L. Adriano et al. (1971) explained that the waste from the 78 cows was applied efficiently to farmland that increased the concentration of nitrate above 10 mg/L in the subsoil water.
Municipal, industrial, and transport wastes
Industrial and municipal wastes consist of different types of nitrogenous compounds which are directly released to the water bodies (National Research Council 1972). This waste is considered to be the heavy polluter of water bodies, and secondary treatment is able to remove less than half of the nitrate waste. The ammonium ions present in the septic tank may convert to nitrate and may penetrates from the tank. Sludge from the septic tank and treatment plants have to be managed properly as these are observed to be another source of water pollution from nitrate compounds (Englande et al. 2015).
About 50 million tonnes of nitrogen oxides liberated into the environment per year from the different sources such as industrial processes, fossil fuel combustion, and motor vehicles. A small portion of this is returned back to the surface of earth in the form of nitrate (Yahaya et al. 2020). The contamination of aquatic bodies by manure or sewage is determined by the estimation of Fecal Indicator Bacteria. It is crucial to differentiate between the origin of fecal contamination and the animal waste contamination because humans are more prone to the risk caused by fecal contamination such as sewage than the animal waste contamination. Novel methods such as biomarker analysis and PCR quantification can be utilized to check the different microbial sources of contamination. The pig-associated Pig-2-Bac qPCR assays is utilized to detect the sources responsible for pollution from animal fecal matter whereas modified version of HF.183II and BacHum are utilized to check the human waste associated pollution sources. The water pollution by fecal matter leads to exposure of pathogenic microorganism via irrigation, recreation, and drinking water. However, the maintenance of water quality in terms of microbial sources is ignored despite of its significance for the health of a human being (Vrzel et al. 2016).
Point source pollution is the contamination that comes in the waterway from an identifiable, single source such as manure depots, slurry lagoons, and livestock farms with an inappropriate location or building. Large population and discharge from the broken sewer system and septic system contribute to aquatic pollution by introducing nitrate in suburban and urban areas (Nemčić-Jurec and Jazbec 2017). The effects of point source contamination are limited and localized for example: point source lagoon with manure of pig in liquid form affects the nitrate concentration at 36 m of distance from the well. It was determined that the contamination found in the wells is formed on the sandy soil in comparison to the clay soil wells (Ciravolo et al. 1979). Similarly, Richard et al. (1996) reported that the nitrate concentration in the aquatic sample of well located in sandy soil at 6 m of distance from the point source of contamination was quite high i.e., 3.6 mg/L in comparison to nitrate concentration (1.8 mg/l) from the wells located at 60 m distance from the contamination source.
Estimation of N-nitrosamine exposure
Tobacco consumption leads to a larger intake of N-nitrosamines than other sources, with a rate of 21,800 ± 4350 ng/day (Hecht and Hoffmann 1988). N-nitrosamine absorption from food, regardless of form, is the second greatest source of exposure, with consumption levels of 1800 ± 350 ng/day from vegetarian diet and 1900 ± 380 ng/day from westernized diet (Park et al., 2015a). Consuming malt beverages, such as beer, gives a significant amount of N-nitrosamines (1000 ± 200 ng/day), whereas drinking treated water contributes the lowest value (120 ± 24 ng/day).People who follow a western diet and regularly consume tobacco or beer are likely to have daily exposure of < 1% from portable water, 4% from beer intake, 8% from food ingestion, and 88% from tobacco usage. In comparison, those following the western diet but refraining from tobacco and alcohol intake would have 12 times reduced odds of N-nitrosamine exposure.
Risk factors and overview of carcinogenic NPIP to which human is commonly exposed
Humans are exposed to total N-nitrosamine from a variety of sources, including personal care items (1500 ± 750 ng/g), tobacco (16,100 ± 3650 ng/g), beverages and food (6.7 ± 0.8 ng/g), and water (40 ± 10.5 ng/L). Control interventions reduced the concentration of N-nitrosamines in beer by 96%, whereas the level of N-nitrosamines in other sources remained stable (Goff and Fine 1979; Campillo et al. 2011; Gushgari and Halden 2018).
Exposure to different risk factors such as physical, chemical, biological, and genetic may result in the formation of NPIP induced cancer as shown in Fig. 2.
Physical factors
Ionized radiations
It is widely known that ionized radiations induce chromosome aberration or gene mutations. Epidemiological studies related to carcinoma in the case of victims of atomic bomb have shown occurrence of lung cancer, leukemia, etc. in the people. The incidence of leukemia remains after 5–20 years of bomb explosion whereas the cases of lung cancer prevalent even after the 50 years of explosion. According to a theory, carcinogenesis is a multi-stage process propagates as a result of mutation in some genes. In favour of this study, it was determined that these ionized radiations might triggered off any of the steps included in the multiple stages of carcinoma (Saeki and Sgimachi 2001).
Radiation therapy is a widely accepted way which is found useful for the treatment of malignant tumor. Radiotherapy induces damage to nucleic acid indirectly via the formation of reactive oxygen species (ROS) and directly by the ionization (Baskar et al. 2012). However, the ionizing radiations promote the invasion and metastasis of cancer cells by the induction of epithelial-mesenchymal transition (EMT). This process of metastasis works as a barrier to the promising tumor therapy and is related to the incidences of mortality and morbidity of various tumors (Zhou et al. 2017). Reactive oxygen species mediate the biological effects of ionizing radiations by the activation of various transcription factors related to EMT including MAPK, EGFR/PI3K/Akt, G-CSF, Notch, Hedgehog, Wnt, and TGF-β. Cancer cells which undergo EMT show metabolic changes and acquire stemness, although these properties have been debated. These radiations also resulted in the development of cancer stem cell (CSC) properties including regeneration and dedifferentiation and promote metabolism of oncogenic cells by activating the EMT-directing pathways (Qiao et al. 2022). Most of the evidences have shown that the alterations in the metabolism of a cancer cells is related to CSC and EMT phenotype; majorly the IR-directed carcinogenic metabolism seems to be needed for CSC and EMT phenotypes acquisition. These radiations can also bring lot of alterations in tumor microenvironment (TME) which further affect the metastasis and invasion. Oncogenic metabolism, CSC, and EMT are included in providing radio resistance thereby targeting them may increase the radiotherapy efficiency, ultimately preventing metastasis and recurrence of tumor (Lee et al. 2017).
Ultraviolet light (UV)
Ultraviolet radiations act as both the non-specific damaging agent and the mutagen having properties of both tumor promoter and initiator. However, UV also has some beneficial effects as it mediates the formation of endorphins and vitamin D in the skin (Wacker and Holick 2013). The excessive exposure to UV may result in some health risk such as malignancy, wrinkling, pigmentary changes, and atrophy. This light molecularly and epidemiologically is related to three varieties of skin cancer: malignant melanoma, squamous cell carcinoma, and basal cell carcinoma which affect millions of Americans annually. Genetic factors can also be responsible in inducing UV-directed skin problems. The melanocortin 1 receptor (MC1R) gene polymorphism is found to associate with increased risk of cancer, UV sensitivity, and skin fairness (D'Orazio et al. 2013).
Sunlight is a combination of UV A and UV B and each UV type has its distinct effect on the skin. UV A is less active but can induce oxidative stress related free radical damage to biomolecules such as DNA whereas UV B is involved in inflammatory processes and in the generation of photolesions (Dunaway et al. 2018). Besides the photo-dimer formation in genome, UV can also cause mutations by the production of ROS. Nucleotides are more susceptible to the injury caused by free radicals. The oxidation of nucleotide bases promotes mispairing which results in mutagenesis. These types of mutations are observed in skin tumors suggesting the oxidative stress as cancer causing factor. Different pathways for maintenance exist at the cellular level for the inactivation of oxidative species and for the mechanism of DNA repair (Rastogi et al. 2010). The base excision repair pathway is a major one that reverses the damage caused in DNA to avoid any chances of mutagenesis. The initiation of pathway occurs in response of damage specific glycosylates that checks the nucleic acid for other alterations such as oxidized, alkylated, and deaminated base pairs. After the recognition of mutated bases, the enzymes cleave these bases from the phosphodiesterase and sugar backbone by targeting the N-glycosidic bond. This step resulted in the formation of an apurinic/apyrimidinic (AP) site in the nucleic acid and repairs further by the help of complementary strand to determine its fidelity (Chatterjee and Walker 2017).
Moreover, inflammation is a common acute effect shown on the skin with response to UV light. UV B directs a cascade of neuroactive, vasoactive, and cytokines mediators which in combination cause a sunburn and an inflammatory response. If the dosage of UV crosses its threshold limit then keratinocytes activate the pathway of apoptosis and death. The apoptotic keratinocytes can be recognized by the help of pyknotic nuclei and are termed as sunburn cells. The exposure to UV also resulted in the thickening of epidermis which is termed hyperkeratosis. In case of cell injury, UV directs the pathways related to damage in keratinocytes. These signals further activate p53 that changes the physiology of keratinocyte, mediates cell cycle arrest, activate nucleic acid repair, and induces apoptosis if there is a big damage. Thus, these thick layers (basal, spinous, granular, and cornified layer) provide protection against UV by stopping its penetration to the skin (D'Orazio et al. 2013).
Chemical factors
Atmosphere
A lot of interest has been generated in atmospheric particulate matter (PM), specifically PM2.5 due to their harmful effects on the health of a human being. The size of PM2.5 falls in the respiratory limit of humans, so it needs to be controlled as one of the important air pollutants (Hong et al. 2017). Atmospheric PM2.5 commonly comprises of different types of harmful substances such as polychlorinated biphenyls (PCBs), organic nitrogen compounds, PAHs, and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs). Among these, nitrogen containing N-nitrosamines has been classified as human carcinogens which are capable in inducing mutations in humans (IARC 2016). The existence of nitrosamine in PM2.5 includes following steps: (1) first there is a generation of gaseous nitrosamines in the environment, (2) then the gaseous components get converted to aerosol phase, (3) then there is an eventual aerosol growth via accumulation and nucleation stages into PM2.5. This process of formation is identical with the process of formation of secondary organic aerosol (SOA) such as C2–C6 dicarboxylic acid and inorganic aerosol (ammonia, nitrate, and sulphate). The nitrosamines presence has been detected in fogs and clouds at the concentration of 8–500 ng/L and at countryside at the concentration of 497 ng/L that clearly determines its equilibrium between aqueous and gaseous phase. Generally, SOAs are not only formed due to the reaction of volatile organic carbons (VOCs) in the atmosphere, but also due to the reaction of organic compounds, for e.g., about 30% SOA can be formed from the released intermediates of VOCs. The precursor compounds may be the low-volatile and semi-volatile nitrosamines which are linked with PM2.5. The average ratio of N-nitrosamines to PM2.5 is found to be 0.065 ng/mg and this is an insignificant concentration of SOA in particulate matter (Sun et al. 2022).
In contradiction to the statement of non-persistence of NDMA in environment due to their shorter half-life, the latest studies have shown the persistence of NDMA in particulate matter otherwise. This was due to the regeneration through fast time for growth phase and nucleation for vapour nitrosamine conversion to SOA and in situ production of photolyzed nitrosamines. These nitrosamines present in air as dry and wet deposits and as SOA in PM2.5. There are some studies that reported a relationship between the direct depositions of nitrosamines and pollutants in soil and PM. There is an analogous study regarding the effect of atmospheric nitrogen on the quality of surface water. About 20–80% of the nitrogen load (up to 740 ng/L) gets into the Chesapeake Bay watershed (Hong et al. 2017).
Outdoor air pollution
Various types of air contaminants are liberated into the atmosphere from inadequate domestic incineration, municipal waste sites, from mining and related industries. Motor vehicles also add some pollutants to atmosphere in urban areas. Some matter of vehicle exhaust is categorized to group 1 and 2A as these matters are carcinogenic to human (Munsif et al. 2021). Several studies have shown that the occurrence of lung cancer is greater among the urban peoples than the people living in countryside. In East Asia, Russia, Europe, and USA, the emission from agricultural site makes the largest contribution in the particulate matter (PM2.5). This rate of emission stipulates that the role of outdoor contaminants in inducing death could increase by the year 2050 (Dimitrova et al. 2021). Various compounds such as polycyclic aromatic hydrocarbons (PAHs) also raise the chances of cancer, mainly the pulmonary cancer. These compounds can stick to the fine particles of carbon available in the air and perforate our body through breathing. Beside PAHs, various other particles, and environmental pollutants such as nitric oxide are also observed to raise the risk associated with pulmonary cancer and metastasis. Some studies reported increased chances of developing leukemia cancer due to the exposure to exhaust from motor vehicles (Anand et al. 2008).
Indoor air pollution from household combustion
Burning of coal inside the houses for cooking or heating purposes may result in emission of gases and PMs that contain different types of tumor triggering factors such as PAHs, formaldehyde, carbon monoxide, and benzene. These factors have been proved to be carcinogenic to human and categorized in group 1 (Shen et al. 2017). Raising incidences of lung cancer are very much associated with the smoke level inside the household. According to a study, individuals with T-genotype of HIF-1α rs2057482 are more likely susceptible to small cell cancer. Other indoor air pollutant like pesticides and some volatile organic compounds increase the chances of children lymphoma and leukemia. In response to pesticides, adults and children may have increased risk of Wilm’s tumor, germ cell tumor, Ewing’s sarcoma, brain tumor, etc. (Shankar et al. 2019). In utero, exposure to these indoor contaminants is found to be related with testicular cancer. Additionally, dioxin, which is produced from incinerators, has increased the cases of lymphoma and sarcoma, respectively (Anand et al. 2008).
Second-hand smoke
Second-hand smoke and tobacco are considered as the human carcinogen as they attribute to induce pulmonary carcinogenesis in individuals. The cases of mortality from this cancer have been raised with the increasing number of cigarettes smoked and duration of smoking. Various epidemiological investigations have reported the increased chances of having pulmonary cancer after the prolonged exposure to tobacco smoke in the environment and to the second-hand aerosol from electronic cigarettes and tobacco (Cornfield et al. 2009).
Asbestos
Asbestos has shown its importance in thermal and acoustical insulation. It is differentiated into two types: amphiboles and chrysotile, involving tremolite, actinolite, anthophyllite, crocidolite, and amosite fibers. All these types of asbestos are found to have cancer causing nature and may produce mesothelioma and lung cancer. The effects of amphiboles on the peritoneum and pleura are stronger in comparison to chrysotile (Brandi and Tavolari 2020). Various studies are available on workers exposed to asbestos but very less number are available on the health effects of residential and household exposure. The concern in terms of household exposure to the closed ones of asbestos workers arose from the dust of the clothes of a workplace whereas household sources in response to asbestos exposure involved repair, removal, installation, and degradation of asbestos including products. Residential exposure majorly includes manufacturing of asbestos in the nearby areas along with natural exposure from the asbestos erosion (Goswami et al. 2013).
It is difficult to assess the non-occupational exposure to asbestos as the level of this is commonly low, and the type of exposure, frequency and duration is not well defined. The IARC has categorized the all types of asbestos to group I of human carcinogenesis. Environmental Protection Agency (EPA) has categorized asbestos to Group A of human carcinogen. The carcinogenicity of asbestos is related to the fiber length. Intermediate and long length fibers of asbestos (> 5 µm) have been considered to be more tumorigenic than the fibers of short length. Most commonly fibers are of 8 mm in length and these are majorly utilized in India, Africa, and Asia after the China (Shankar et al. 2019).
Metals
Arsenic (As) is an environmental pulmonary carcinogen which usually remains in the form of arsenate and arsenite. The exposure to As occurs due to the inhalation of dust from smelters and copper, gold, and lead ore mines (Jaishankar et al. 2014). Various studies have been conducted in Taiwan, Chile, Bangladesh, and Argentina to identify the presence of higher concentration of As in drinking water for inducing lung cancer. According to IARC and EPA, it is identified as a probable human cancer-causing agent. There are also some evidences that prove the role of beryllium compounds in inducing lung cancer. In various reports, it was found that the incidence of pulmonary carcinogenesis is related to the hexavalent chromium. EPA has not categorized nickel into the group of potential human carcinogenicity. IARC has categorized heavy metal arsenic into group I of carcinogen (Martinez et al. 2011).
The basic source related to the exposure of As is the leakage of inorganic As into the groundwater. Regions with high percentage of As across the world include the Antofagasta province in Northern Chile, West Bengal, and Bangladesh. Occupations that include exposure to As are antifouling paints, lead, and pesticides, production of agrochemicals, pharmaceutical production, glass production, timber manufacturing, and coal-based energy production. The maximum exposure is found among workers of carpentry who works on arsenic pressure treated timber and copper or lead smelters as As is naturally available in these ores. It is utilized as a pesticide for the cotton plants and to cure acute promyelocytic leukemia (Ahmad et al. 2018). The level of As in the sample of urine may be utilized to assess its exposure. As is absorbed in the gastrointestinal tract and detoxified in the liver by glutathione. Glutathione is a natural antioxidant that conjugates with As and excretes through bile. According to IARC, As is found to involve in the cancers of the liver, kidneys, prostrate, bladder, lungs, and the skin in humans. It accumulates in the liver and is responsible for the cause of different vascular diseases such as ischemic heart disease and stroke. Various other processes such as massive alteration in DNA methylation, disturbance of cellular proliferation and signal transduction, generation of free radicals and oxidative stress, genotoxicity, and direct cytotoxicity related to carcinogenesis of As has also been demonstrated but the exact mechanism is not well explained (Barsouk et al. 2021).
Among various heavy metals, focus should also be given to the exposure of cadmium (Cd) in humans. It is a toxic heavy metal and is more abundant as environmental pollutant. It remains present in various water bodies, soil, air, tobacco smoke, and in different food sources of the diet. Although there is a decline in the production of Cd yet its maximum biological half-life and persistence led to different health effects. Many studies have reported the harmful effects of this metal on various organ systems and its toxicity depends on the duration, route and dose of exposure (Buha et al. 2017).
In experimental studies, Cd has induced various biochemical alterations that include aberrant signal transduction and gene expression, E-cadherin (important protein involved in signal transduction and 3-catenin signalling pathway) dysfunction, DNA methylation inhibition, DNA repair disruption, and cell death. It modifies the expression of genes related to carcinogenesis including transcription and translation factor, genes controlling the glutathione and other related protein, heat-shock genes, stress responsive genes (metallothonein), and intermediate early responsive genes such as c-myc, c-jun, and c-fos. At lower concentration, Cd inhibits the repair of DNA that includes base excision repair, mismatch repair, and nucleotide excision repair. It can also affect the process of apoptosis. Its exposure results in a concentration dependent increase of apoptotic cells in the cultured cells. In experimental studies, the increased cell death is due to the increased mRNA levels and p53 protein whereas in other cell line system, Cd directed apoptosis is independent of p53 and is related to ROS. Notably, the induced apoptosis was not found to be useful in providing protection against malignant transformation, however, some researchers have investigated that a very small number of Cd treated cells undergo apoptosis and remaining cells acquire apoptotic resistance. Apoptotic resistance allows the accumulations of early neoplastic or preneoplastic cells and of critical mutations (Barsouk et al. 2021).
The presence of cancer stem cells (CSCs) in cancer tissue is determined to be one of the causes for the failure of treatment and recurrence of tumor. Many studies have shown that under suitable environment, non-CSCs could be transformed to CSCs. The effect of Cd on the formation of CSC lineage in the large population of cancer cells is not fully revealed. The treatment of Cd significantly increases the CSC population in HepG2 and MCF-7 cell lines. These CSCs were recognized by the help of markers such as ALDH1, CD133, CD24, and CD44. Moreover, raised protein expression of p-ERK-1, p-MEK-1, p-Raf-1, and p-Ras and mRNA expression of CD133, ALDH1, and CD44 were also revealed in the Cd treated HepG2 and MCF-7 cell lines. Thus, it was determined that the Cd directs the gene expression related to CSC markers in the liver and breast cancer cell lines and help in the transformation of non-CSC to CSCs (Ju et al. 2017).
Vinyl chloride
The cases of lung cancer have been raised remarkably due to the exposure to the dust of vinyl chloride (VC) and polyvinyl chloride (PVC). However, the rate of exposure for the large number of populations is extremely low (Shankar et al. 2019). VC is a hydrocarbon monomer that is utilized in the production of PVC. PVC does not cause any harm to health and can be easily found in dental or medical appliances, water proof clothes, insulation, window frames, and water pipes. However, a large number of workers working in the plastic production industries have been exposed to VC and to the reactive form of vinyl chloride. Similarly, autoclave workers are also at the risk of VC exposure. About 80,000 workers in US and 40,000 in Europe were exposed to VC before the year 1997 (Lopez et al. 2013). Thioglycolic acid, one of the VC metabolite was detected in the urine samples to check the occupational exposure. The toxicity of VC is found to be disturbed the endothelium of liver which resulted in malignancy, angiosarcoma, and portal hypertension of the liver. The exposure to VC is also associated with hepatocellular carcinoma (HCC), cirrhosis and liver cancer mortality that display its synergistic effect with the alcohol (Cheng et al. 2001). It is also found in cigarette smoke. The exposure to VC can occur through inhalation and after that it gets metabolized by the liver into various carcinogenic and mutagenic compounds such as chloro-ethylene oxide and ethylene dichloride. The by-products such as carbamates induce chromosomal aberrations and DNA breakage in the liver organ. Beside liver cancer, VC has been established to induce cancers of the haematological system, lungs, and brain in humans. The VC exposed workers were seen with HCC and oncogenic mutations in p53 and KRAS, respectively (Barsouk et al. 2021).
Biosolids
Along with residential and industrial sources, nitrosamines can also be generated from the treatment of wastewater. In sludge system, the usage of tertiary and secondary amine based cationic polymers contributes to nitrosamine precursors which form nitrosamines after their nitrosation reaction in the availability of nitrite. Alicyclic and aliphatic nitrosamine (NDBA, NDPA, NPIP, NPYR, NMOR, NDEA, and NDMA) with their concentration range from less than quantification limit to 1057 ng/L were reported in the influent of wastewater. In activated sludge treatment systems, the removal efficiency of nitrosamines in aqueous phase is greater than 60% except in the case of NMOR (40%) whereas it is lower when the concentration of primary effluent is less than 8–15 ng/L (Krauss et al. 2009). This removal efficiency can vary within the same plant over time (0–75%) or between the wastewater treatments plants (0–93%). These variations occur due to the competition of substrate in the complete microbial degradation of nitrosamine at the time of secondary treatment. However, the persistence of nitrosamines in the effluent of wastewater treatment plant (WWTP) is now become a concern as these nitrosamines contaminating the down gradient and downstream resources of drinking water of human beings (Sedlack et al. 2005). The presence of eight different types of N-nitrosamines in biosolids samples from 74 plants of wastewater in the US was investigated. Seven nitrosamines namely NDPhA, NPIP, NPYR, NDBA, NDPA, NMEA, and NDMA were identified in 88% of biosolid samples. Out of seven, five compounds were reported for the first time in the case of biosolid. The NDMA was rarely detected in the amount of 504 ± 417 ng/g dry weight in biosolids whereas NDPhA was majorly found at the concentration of 0.7 ± 147 ng/g, followed by 7–505 ng/g of NDPA and 51–1185 ng/g of NPIP. After determining their frequent presence in national samples and the amount that has been applied as biosolid for soil amendment, more research is required to assess the fate and occurrence of nitrosamines in amended soil samples in the context of crop safety and drinking water (Venkatesan et al. 2014).
Drugs
The reactions of nitrite to form nitrosamine were not restricted to food products. Some researchers observed that the antibiotics and some drugs were also capable in reacting with nitrite to form nitrosamines in large quantities. These drugs include Piperidine,1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]-, tola zaniide, disulfiram, N,N-diethyi-3-pyridinecarboxamide (nikethamide), disulfiram, aminophenazone (aminopyrine), aminopyrine, and oxytetracycline, respectively (Lijinsky and Taylor 1976).
Cosmetics
The formation of N-nitrosamines in cosmetics is arising as a severe problem due to its capability to penetrate the skin. Since 1970s, cosmetic products were contaminated by N-nitrosamines and these cosmetic products were listed under the enforcement action in the USA. N-nitrosamines are highly stable compounds and cannot be destroyed easily unless exposed to nucleophiles (chloride, bromide, thiocyanate, and iodide) and ultraviolet light. Various analytical approaches have been employed to measure the N-nitrosamines in different samples not only to determine their existence but also to evaluate their level with high accuracy and precision. For the detection and separation of N-nitrosamines, liquid chromatography (LC)/MS–MS, GC-tandem mass spectrometry (MS/MS), GC-thermal energy analyser (TEA), and gas chromatography (GC)–mass spectrometry (MS) were utilized. Among all, GC–MS was the most adopted one due to its mass spectral library option that assist in the identification of compound by giving mass spectra from Wiley7n99. Seven different volatile N-nitrosamines (NPYR, NPIP, NDBA, NDPA, NDEA, NMEA, and NDMA) were detected by the help of analytical method in water insoluble cream like cosmetics. Head space-solid phase microextraction (HS-SPME) was found to be appropriate for pre-concentration, clean up, and extraction of N-nitroso compounds from the cream like samples in order to investigate its optimal conditions (Choi et al. 2016).
N-nitroso compounds are teratogenic and mutagenic and their occurrence in cosmetic items is restricted in the European Union since 1992. However, the presence of N-nitrosamines at low level in various cosmetic products is a problem as reported by various studies since 1977 to the present date. It was determined that these restricted compounds were not added deliberately to the cosmetic products. Some of the cosmetic preservatives contain nitro groups in their chemical structure such as bronidox and bronopol which can react with amines to form N-nitrosamine (Lijinsky 1987). The European Scientific Committee on Consumer Safety (SCCS) determined the potential health hazards related to the occurrence of N-nitrosamines in cosmetic products and set a limit of 50 µg/kg for N-nitrosamines in the cosmetic products or in the raw material utilized for the formation of cosmetics. Due to this, the cosmetic industry must restrict or control the impurities in their ingredients which can be utilized in nitrosation reaction. Therefore, taking into account that a single individual utilizes various cosmetic items in a day and few of them utilize various times in a day, analytical control on the formation of N-nitroso compounds in both raw materials and cosmetics is important for the safety purposes (Kanayochukwu et al. 2019).
A new LC–MS technique was utilized to evaluate the levels of restricted N-nitroso compounds at trace levels in cosmetic items. This technique utilizes vortex mixing to result in a cloudy sample that avoids the chances of dispersive solvent in the aqueous extracts. This technique is quite appropriate for the identification of N-nitrosamines in both hydrophilic and lipophilic cosmetic items as per the recommended values set by the European Scientific Committee on Consumer Safety (SCCS). Moreover, this new technique can be utilized for the analysis of cosmetic samples to ensure their quality and consumer safety, respectively (Miralles et al. 2018).
Rubber industry
In rubber industry, exposure to various carcinogens such as N-nitrosamines, rubber fumes, rubber dust, polyaromatic hydrocarbons, aromatic amines, phthalates, β-naphthylamine, and solvents such as benzene is responsible for various types of cancers. The IARC provided various evidences of carcinogenicity such as cancer of non-Hodgkin’s, multiple myeloma, leukaemia, stomach, lung, and urinary bladder as well as increased cases of larynx, oesophagus, and prostate cancers in humans due to the occupational exposure in rubber industry103. In the process of rubber manufacturing, the production of N-nitrosmaines is observed at the vulcanising stage when rubber mixture is heated with morpholino mercapto-benzothiazole, zinc-diethyldithiocarbamate, and tetramethylthiuram disulphide. The mostly observed nitrosamines in rubber industry are NMOR, NPIP, NDEA, and NDMA (Hidajet et al. 2019).
Rubber items, that lead to the production of N-nitrosamines due to the utilization of some accelerators include, pharmaceutical items, pacifiers, windshield washer tubing, tires, radiator hoses, milking inflations, gloves, condoms, baby bottle nipples, athletic shoe soles. Till now, Germany is the only country that leads the globe in elimination and legislation of N-nitroso compounds on the work places. They have listed eight suspected compounds in their “Technical Rules for Dangerous Substances” (TRGS 522). These compounds were used in the rubber industry for producing the carcinogenic nitrosamines. TRGS 522 has also set some restrictions with respect to nitrosamine concentration such as 1.0 µg/m3 for production steps before warehouses and vulcanization and 2.5 µg/m3 for warehouses, production steps, and vulcanization. Slowly, Canada and the US also began to regulate the levels of nitrosamines. Some of the big companies have limited the engineering specifications and have eliminated the usage of nitrosamines in rubber parts. They have also mentioned their specifications needed to a supplier to reveal the list regarding the concentration of nitrosamines present in rubber parts such as PPC, Z5MC, DPTT, NPIP, OTTBS, OTOS, MBSS, DTDM, MBS, NMOR, NRPA, MPTD, NMPA, DBA, TBTD, ZDBC, NDBA, DiBS, NDiPA, TETD, ZDEC, HEXA, CuDMC, TeDMC, TMTD, TMTM, ZDMC, and NDMA (Spiegelhalder and Preussmann 1982).
There are four types of rubber accelerators which result in the generation of reactive intermediates containing secondary amines during the curing process. These rubber accelerators are dithiocarbamates, sulfenamides, sulphur donors, and thiurams (Heideman et al. 2004).
Dithiocarbamates: These are the secondary accelerators which are utilized at low parts per hundred rubber by weight (phr) levels to tweak the cure system in a rubber compound to a desired level of cure. Some of the examples of dithiocarbamates include TDEC, ZDEC, CDMC, BDMC, NDBC, LDMC, SDMC, Z5MC, ZEPC, ZDMC, ZDBC, etc. All these dithiocarbamates have the ability to generate nitrosamines in the availability of nitrosating agents (Alam et al. 2012).
Sulfenamides: These are the primary accelerators which are utilized at low phr levels and can influence not only the cure rate but also the scorch safety of the respective compound. Due to their features, they are named as fast-delayed action accelerators. Three of the sulfenamides, OTOS, OBTS, and MBSS are based on morpholine and help in the production of nitroso-morpholine. However, TBBS, DCBS, and CBS are the three sulfenamides which are not able to direct the production of nitrosamines (Marykutty et al. 2003).
Sulfur donor: These are the accelerators applied to improve compression set and heat aging in rubber formulation. These are unique in nature and can provide mono or di sulphur bond to the rubber compound. Just like the sulfenamides, sulphur donor DTDM is also based on morpholine and can produce nitrosamines (Bornstein and Pazur 2020).
Thiurams: These are the secondary accelerators which function same as dithiocarbamates at low phr levels. However, the thiurams are slow as compared to dithiocarbamates but they can act as both sulphur donor and accelerator. Some of the examples of thiurams are TMTM, TMTD, TETD, TBTB, and DPTT (Alam et al. 2012).
Biological factors
The three common methods applied to eliminate and reduce the nitrosamine production in rubber formulations are: (1) use of inhibitors, (2) use of accelerators that do not produce nitrosamines, (3) use of accelerators that produce non-regulated nitrosamines (Goss et al. 2006).
Diet
Peto et al. (1984) reported that almost 30–35% of mortality cases in the USA are associated with the diet. The contribution of diet in cancer causing death varies on the basis of types of cancers. For example, in 60–70% of colorectal cancer incidences, diet plays a major role. Most of the carcinogens such as dioxins, pesticides, nitrosamines, and nitrates come from cooking, food additives and food. More usage of red meat is also a risk factor for various types of cancers such as oral, prostrate, gastric, breast, bladder, and colorectal cancers. Smoke curing of meat products or charcoal cooking generates different carbon compounds like amino acids and pyrolysates which have strong carcinogenic effect. For example, 2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine (PhIP) is a major mutagen which is found in cooked food and accounts for 20% of total mutagenesis in case of fried beef (John et al. 2012). Among Americans, the consumption of PhIP on daily basis is approximate 280–460 ng/day per individual. Nitrates and nitrites are found in meat products and these compounds stick to myoglobin to inhibit the generation of endotoxin; however, these have been proved to be of carcinogenic nature (Kotopoulou et al. 2022). Long-term exposure to azo dyes and nitrites in the form of preservatives in food items is related to the initiation of carcinogenesis. Furthermore, the presence of bisphenol in the containers of food moves into food and raises the incidence of prostate and breast cancers. Refined sugars, flour, trans fatty acids, and saturated fatty acids in different food items were found to related with different types of cancers. Therefore, food carcinogens were found to be responsible in activating different inflammatory pathways (Anand et al. 2008).
Food
Addition of nitrite as a preserving agent in some meat products may result in the generation of N-nitrosopiperidine (NPIP). This generation occurrs by the oxidative breakdown of amide bond of piperine and subsequent nitrosation of available piperidine. It would be convenient to determine the content of piperidine and piperine in spices to evaluate the damage that occurs by the formation of N-nitrosamines (De Mey et al. 2014b). About 300 various studies of N-nitrosamine contaminated beverages and foods has been cited in a latest report (Gushgari and Halden 2018). The N-nitrosamines levels in sweets, oils, and fats (0–44 ng/g), meat items (0.1–121 ng/g), fish items (0–43.9 ng/g), canned vegetables (0.02–40.5 ng/g), beverages (0.2–45.7 ng/mL), grains (0.2–4.6 ng/g), milk items (0–1.6 ng/g), fruits (8.1 ng/g), rice (1.5 ng/g), drink mixes (0.9 ng/g), tofu (0.2 ng/g) were reported. Food items such as vegetables, fish, meats and sweets, oil and fats were reported to have highest content of N-nitrosamines. Among all N-nitrosamines, NDMA (2.2 ng/g) has the highest concentration across all food sources followed by NDPA (0.02 ng/g), NMEA (0.04 ng/g), NMOR (0.05 ng/g), NPIP (0.5 ng/g), NDEA (0.9 ng/g), and NDBA (1.5 ng/g).
In case of cured meat items, the formation of N-nitrosamine is related to the presence of secondary amines and nitrites which are commonly utilized as colouring and antimicrobial agent. Biogenic amines may give rise to secondary amines by the decarboxylation reaction of free amino acids. This can happen at higher concentration in case of fermented food products since their assemblage was associated with the action of meat enzymes and decarboxylase-positive bacteria during ripening and fermentation. The common biogenic amines are cadaverine (CAD), putrescine (PUT), and tyramine (TYR) which are formed from lysine, putrescine, and tyrosine, respectively (Scanlan 1983). The addition of antioxidants such as alpha-tocopherol and ascorbate was suggested to control biogenic amines. Another way of control includes the selection of starters with lower decarboxylase activity that inhibits the microbial efficiency of raw materials.
Gamma-irradiation is also one of the effective ways for the removal of N-nitrosamines or their precursors. Despite of many precautions, sporadic contamination in various types of meat items by N-nitrosamines is quite common. Among all N-nitroso compounds, NPIP is detected in dry fermented sausages. It is determined that the biogenic amines such as CAD act as a precursor of NPIP. Before nitrosation reaction, this CAD is converted into the alkaloid piperidine. In a model of heated lean meat, the mixing of nitrite and CAD greater than the legal limit, results in the formation of NPIP at a very high processing temperature (Drabik-Markiewicz 2011). Moreover, above mentioned N-nitrosamine is generated more easily when piperidine is mixed to the meat model directly. The excessive growth of Enterobacteriaceae and Enterococci has been responsible to produce PUT (80 mg/kg), CAD (340 mg/kg), and TYR (250 mg/kg). On the other side, sausages contain piperidine which is the major compound of pepper. The role of piperidine and CAD is clear in heated cured meat items whereas the generation of NPIP in dry fermented sausages occurs due to other pH parameters as no heating is involved to this step of production. In general, meat products have a pH value of 5.5 to 6.2 while its range varies from 4.5 to 5.5 in case of dry fermented sausages. This makes meat as a perfect media for the growth of microorganism and the subsequent spoilage may occur due to the availability of vitamins, fats, minerals, proteins, and free water. Since ancient times, salt has been utilized for the storage of meat and other food products. In nineteenth century, it was clear that the nitrite present in saltwater plays a potent function in inhibiting the growth of microorganisms. Recently, sodium nitrate was utilized as an additive in different food items. Processed items of meat comprising nitrite from natural and synthetic sources may result in the formation of N-nitrosamines in the presence of acidic condition of the stomach (Lorenzo et al. 2018). Various researches have been conducted for the evaluation of chronic and acute effects while utilizing nitrite and nitrate as the preservatives in the meat products. It was also determined that variable concentration of nitrite and nitrate compounds can produce different diseases in human and animal models. Different factors such as storage conditions, presence of nitrosamine inhibitors, concentration of nitrosamine precursors, residual amount of nitrite, temperature, and type of cooking methods can also affect the concentration of nitrosamines in meat items (Shakil et al. 2022).
Except for Chile, there is no permissible limit for the level of nitrosamines in the South Africa. In meat products, the maximum limit for NDMA in Chile is 30 µg/kg whereas in US, the acceptable limit of volatile nitrous-amide is 10 µg/kg. According to some reports, humans can tolerate exposure to volatile nitrosamines up to 5–10 µg/kg. The Canadian Food Inspection Agency has decided the concentration of nitrosamines up to 10 µg/kg for NDBA, NDMA, NPIP, NDPA, and NDEA, and 15 µg/kg for NPYR in the products of meat (Johnson et al. 2021). The N-nitrosamines are non-polar molecules having special vapour pressure and lower molecular weight which requires the application of state-of-the-art, sensitive, and accurate device to detect them. Various extraction methods such as supercritical fluid extraction, superheated water extraction, and microwave-directed extraction, which need some complicated instruments, have been utilized. The quantitative estimation of nitrosamine is performed by the help of different analytical methods in all over the world. Among different methods for the estimation of nitrosamines from the food products, gas chromatography/flame ionization detector (GC/FID), nitric oxide analyser (NOA), ultraviolet detectors, thermal energy analyser (TEA) are the broadly utilized techniques. Adaptation limits and high cost of NOA and TEA result in the disposal of NOA and TEA in various laboratories where as low sensitivity and accuracy of GC/FIA have made its application less. GC–MS has been utilized more with non-polar and polar solid phase extraction column due to more sensitivity, specificity, and time saving (Kodamatani et al. 2008).
After considering the large-scale consumption of meat on a daily basis in diet and lack of research related to the assessment of nitrosamines in sample of sausages, it is really important to determine the source of nitrosamines in these items. The effects of various factors such as different brands of nitrosamines, time spent for production, percent of meat in sausages should have been discussed carefully. Among different nitrosamines, the amount of total nitrosamines, NPIP, and NPYR in meat sausages was found higher in chicken sausages. Moradi et al. (2021) analysed the impact of nitrosamines (NPYR and NPIP) present in sausages on human health and stated that these compounds are not responsible in inducing cancer in Iranian population. Further, some biogenic amines such as spermine, spermidine, and putrescine were also found to present in fresh sample of pork in 20–40 mg/kg, < 5 mg/kg, and < 2 mg/kg whereas cadaverine was present in 1 mg/kg and can increase upto 120 mg/kg in fresh meat. The amount of cadaverine and putrescine in raw sample of meat increases due to the action of bacteria which are the consequences of inappropriate processing and storage. The complete opposite situation was observed for spermine and spermidine which present in large amount in raw sample of meat but their level gets lower at the time period of storage. The effect of different biogenic amines on the generation of N-nitrosamine in heated cured lean meat was observed at different temperature and in the absence and presence of sodium nitrite. It resulted in the generation of NPIP and NDMA. Putrescine and spermidine increase the generation of NDMA, but cadeverine and spermine do not play a remarkable role in the generation of this compound. These compounds increase the formation of NPIP. Except for NPYR, no other N-nitrosamines were observed (Drabik-Markiewicz et al. 2011).
Moreover, NPIP was detected in 3 out of 62 chesses samples at the concentration of 2–11 µg/kg whereas less than 1.0 µg/kg in smoked cod. NPIP was found less than 1 µg/kg in fried smoked back rashers, 20–60 µg/kg in mettwurst sausages, 14–50 µg/kg in bologna, 15–50 µg/kg in wieners, and 50 µg/kg in meatloaf. This NPIP was reported in concentration of 0–8.2 µg/kg in different preserved and processed meat products. The concentration of NPIP was observed to increase after cooking. About 1 µg/kg of NPIP level was detected in cooking fat from fried bacon and 0.08–0.25 µg/kg was measured in eight samples of cooked bacon. Dry premixed cures containing sodium nitrite and spices were found to have 300 µg/kg NPIP (IARC 1972).
Water
Nitrosamines also belong to a category of nitrogenous disinfection by-products (N-DBPs) that causes higher health risk due to its frequent occurrence. These are produced when the treatment of drinking water is made with disinfectants likes chlorine dioxide, ozonation, and chlor-amination (Bond et al. 2011).The United States Environmental Protection Agency (USEPA) determined that NDMA at 0.7 ng/L concentration in drinkable water can cause cancer whereas the World Health Organization (WHO) decided a set concentration of 100 ng/L for NDMA. The levels and species of nitrosamines in drinkable water can be affected by several factors such as material of distribution system (rubber gaskets), process of water treatment (disinfectant type), water source (organic precursors), and weather events (temperature). Due to this, the nitrosamines occurrence in drinkable water alters temporally and spatially. The data available regarding the persistence of nitrosamines within the water system is still little but there are some evidences stating that the concentration of NDMA rises with the increase of residence time and pipe length. In the determination of nitrosamine generation in influent water, it is reported that the NDMA is a dominant compound in the water sample ranging from 2.5 to 67.4 ng/L, followed by NPIP and NDEA. There is a decrease in the mean concentration of NPIP, NDEA, and NDMA from influent water sample to treated one. However, no specific variation is reported in the distribution system of water. Nitrosamine exposure through drinking water causes higher chances of cancer risk in children aged 0.75 to 1 year in comparison to adults (Luo et al. 2020).
Disinfection by-products (DBPs) are formed due to the reactions of natural organic matter (NOM) and disinfectants (chloramines and chlorine). Although NPYR, NDPhA, NDPA, NDMA, NDEA have been listed by the US EPA contaminant Candidate List 3 but there are some restrictions that limit the formation of N-nitrosamines in drinkable water. The California Department of Public Health had decided the maximum level 10 ng/L for NDPA, NDEA, and NDMA in drinking water. Health Canada imposed guidelines for the maximum level of NDMA to be 40 ng/L whereas Australia and WHO decided 100 ng/L in drinking water. The occurrence of N-nitrosamines in the drinking water treatment plants (DWTPs) has been broadly reported in Europe, Japan, China, and USA. Based on research, the level of NDMA in treated water and raw water of DWTPs is recorded from sub-ng/L to 10 ng/L, where its concentration in hot tubs, effluent of sewage treatment plants, and in distribution system is increased to 100 ng/L (Park et al. 2015b).
However, some researches have reported that the changes in the level of N-nitrosamines are dependent on the different seasons. During summers, the level in the aquatic samples has ranged from below detection limit (BDL) to 8.5 ng/L for NPYR, BDL to 2.7 ng/L for NDEA, BDL to 9.5 ng/L for NMEA, and BDL to 5.4 ng/L for NDMA. Water samples collected during winter seasons have shown the nitrosamine levels range from BDL to 309 ng/L for NPIP, BDL to 5.2 ng/L for NPyr, BDL to 45 ng/L for NDMA. Both dissolved nitrite and carbon are reported to be linearly correlated with the level of N-nitrosamine in water sample (Li et al. 2021). In early 1970s, N-nitrosamines were observed in water but they were not categorized as a contaminant group till the 2000s (Fan and Lin 2018). The presence of N-nitrosamines in water of river is an indirect risk to human population as these substances cannot be eliminated during the drinking water treatment process (Luo et al. 2020).
The Yangtze River provides drinkable water to about millions of people and the risk of tumor due to the presence of N-nitrosamines mainly after disinfection process have attracted more attention (Liu et al. 2017). According to a report, the tap and finished water of the Yangtze River Delta (YRD) in the Eastern area is majorly contaminated with N-nitroso compounds in comparison to the other regions of China. The levels of NDMA observed in tap and finished water were 27 and 29 ng/L, respectively. The NDMA formulation potential (FP) and NDMA were observed in 1429 and 8789 ng/L of concentration in the effluent gathered from the downstream side of the Yangtze River.
Another research reported the presence of NDMA and NPIP having concentration of 131.1 and 29.5 ng/L in the Huangpu River found in Shanghai City. Other compounds such as NPYR, NDEA, NMEA, and NDMA were observed in moderate amount in the east side river water of Yangtze River Basin (YRB) with 8.5, 2.7, 9.5, 5.4 ng/L of concentration during the hot climate. However, the increased concentration of NPIP (309 ng/L) and NDMA (45 ng/l) were detected in the cold climate. The amount of NDEA was found 311.8 ng/L in chlorinated wastewater of YRB. These results indicated that the wastewater and drinkable water in the YRB are heavily polluted with different types of N-nitrosamines (Chen et al. 2021).
Beer
The existence of N-nitrosamines in beer has been supported by various researches majorly including NDMA followed by NPRO, NPYR, and NPIP. In ancient times, the roasting of malt used to be done in an open fire. The nitrogen oxide gases present in the smoke used to react with amines present in the malt, resulting in the formation of N-nitrosamines. The US Food and Drug Administration (FDA) regulated the standard concentration of NDMA as 5 mg/L for beer. The maximum concentration of NDMA in beer was determined to be 68 mg/L followed by NPIP in the year 1970–1980s. After the year 1980s, it was recommended to decrease the NDMA and NPIP concentrations in beer and malt, particularly by reducing the nitrogen oxides level to reduce the concentration up to 0.057 mg/L (Tricker et al. 1991).
Higher level of NDMA was reported in developing countries in comparison to North Europe and America. In 1990, about 194 Canadians and US beers were surveyed and reported to have 0.074 mg/kg of average level of NDMA. However, the information about nitrosamines in beer is very less, but the usual intake of beer and water is 0.36 L and 2 L, respectively, therefore, it is important to estimate the levels of some major nitroso compounds in beer to determine their levels of risk. A method was used to analyse the water samples which were collected from 11 different reservoirs along with their treatment plants in Taiwan and 10 samples of beer from 6 different brands located in 6 different nations. Out of seven types of N-nitrosamines, the level level of NDMA (10.2 ng/L) was highest in case of drinking water. The concentration of NDMA was observed lower (0.12–0.23 mg/L) than the standard (5 mg/L) whereas NPIP was detected in high amount (4.1–5.3 mg/L) in the beer samples. The evaluation of risk indicated that the damages related with the NDMA were greatest among the other studied N-nitrosamines in case of Taiwan’s samples (Fan and Lin 2018).
Physical activity
According to IARC reports, about 25% of cancer cases are linked with sedentary lifestyle and obesity. The plan of implementing physical activity as a way to prevent tumor was discovered in the twentieth century when some researchers suggested that the rate of fatality among males of varied profession reduced with the increase of physical activity. Mechanism that relates physical activity with the reduced incidence of different types of cancer includes decrease in obesity-related cytokines, pro-inflammatory leptin, sex hormones, insulin-like growth factor, hyperinsulinemia, and systemic inflammation, and significant rise in the level of adiponectin. Additionally, physical activity enhances the function of immune system and diversity and constitution of the gastrointestinal microbiota. Moderate level of physical activity provides protection against cancer but the major alterations in the level of inflammatory markers require physical activity of higher intensity (Jurdana 2021).
The American Cancer Society has reported that the obesity is linked with increased rate of mortality from cancers of liver, gallbladder, prostrate, pancreas, gastric cardia, esophagus, kidney, breast, and colon. Of all death due to these cancers in United States, 20% for women and 14% for men were related to their overweight or obesity. A western life style, diet and increased modernization has been related to a raised number of excess weight peoples in different regions of the globe. Some studies determined that the common denominators between cancer and obesity include inflammation, insulin resistance, adiposity, steroid, sex, leptin, and insulin. Role of signalling pathways such as leptin/JAK/STAT pathway, IGF/insulin/Akt pathway and other inflammatory cascade has also been associated with both cancer and obesity. In hyperglycemia, there is an activation of NF-kB that can link cancer and obesity. Several cytokines from adipocytes such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and leptin get activated in response to hyperglycemia. However, inhibitors of the different signaling pathways can reduce the risk related to obesity-directed cancer has remained unexplained (Anand et al. 2008).
Mutagenic and carcinogenic compounds in food
Four primary compounds are needed to be examined to determine their carcinogenicity to humans. The first are the natural compounds which already persist in food products and are inevitable. For example, the processing of salted fish generates carcinogenic compounds which are unavoidable. The Second are the plant derived products which are avoidable such as contamination of grain with fungal metabolites (aflatoxin) that can be eliminated or reduced using proper strategies of grain storage. The Third category involves the presence of anthropogenic constituents in the food such as, 2,3,7,8-tetracholordibenzo-p-dioxin which unintentionally generates in the manufacturing process of chlorinated hydrocarbons and accumulates in different foodstuffs, resists degradation, and contaminates the environment. The Fourth are the anthropogenic chemicals which are intentionally added to food products, such as colouring agent and saccharin (Abnet 2007).
Nitrosamines, PAHs, and other aromatic amines
Nitrosamines are the substances with cancer causing abilities, persist in various food items, majorly in smoked fish and pickle meat. These are also found in soy sauce, beer and many other items. Research on the harmful reaction of nitrosamine has shown its relation with gastric carcinoma. Polycyclic aromatic hydrocarbons (PAHs) are comprised of condensed aromatic ring systems and released into environment by the partial combustion of organic matters. The major cancer-causing agents in PAH group are benzo[a]anthracene and benzo[a]pyrene (Park et al. 2015a). These substances are commonly found in ham, smoked sausage, tea, fresh vegetables, but also produced by smoking, frying, and baking. These compounds are also found in whisky and brandy. Some mutagenic effects have been reported by meat dishes, smoked fishes, decaffeinated and soluble coffee, and roasted coffee. Heterocyclic aromatic amines are also major mutation causing compounds which are produced by the treatment of protein rich food items at high temperature. These are generated at 300˚C and for that reason; these are observed on the upper surfaces of meat and fish roasted in open fire (Skog et al. 1988). For other protein rich items such as legumes, cheeses, milk, and eggs, heat treatment leads to the generation of mutagen leading to alteration in the colour of food product. Acrylamide is the most probable human carcinogen which is generated by the thermal treatment of starch rich food products such as bread, coffee, chips, crisps, etc. (Lewandowska et al. 2019).
Infections
Globally, about 17.8% of neoplasms are due to results of infections; this percentage is more (25%) in African countries whereas it is lower than 10% in high-income nations. Viruses contribute majorly for generating infection-related carcinomas. Hepatitis C virus (HCV), Hepatitis B virus (HBV), human T-lymphotropic virus 1, Epstein Barr virus, Human papillomavirus (HPV), and Kaposi’s sarcoma-associated herpes viruses are linked with the incidences of liver cancer, B-cell lymphoma, adult T-cell leukemia, Burkitt’s lymphoma, nasopharyngeal, skin, anogenital, Kaposi’s sarcoma, cervical cancer, and Hodgkin’s lymphoma. The HPV causes mutation by inducing the viral gene E7 and E6, whereas HBV causes mutation indirectly by the production of ROS due to chronic inflammation. Moreover, some parasites such as Schistosoma haematobium and Opisthorchis viverrine and bacteria such as Helicobacter pylori may act as a cofactor in inducing carcinogenesis (Williams et al. 2011). The mechanism of infectious agents in inducing cancer is clearly understood. Infection associated inflammation is the main factor in the cancer and all virus-related tumors were determined to activate marker related to inflammation, NF-kB. Helicobacterpylori is also able to activate NF-kB. Thus, the most effective way to treat this condition is to use some agents which can restrict the chronic inflammation (Anand et al. 2008).
Genetic factors
The reasons responsible for the accumulation of carcinogens in individual families were unknown for many years. Recently, a lot of research has been conducted related to the roles of different genes for originating neoplastic diseases. Various genes responsible for the formation of particular tumor (Saeki and Sugimachi 2001) are shown in Fig. 3.
Exposure concentration of NPIP
The occurrence of NPIP in foods and its level of exposure, and related carcinogenicity data including experimental model and route of dosage are listed in Table 3. The dietary exposure to NPIP is reviewed in a number of different food survey studies and is shown in Table 4.
Mechanism of formation of NPIP in food items
Nitrogen is the second most important gas in environment that needs to be fixed by nitrogen cycle before it is taken up by the animals and plants. Nitrogen cycle involves the conversion of N2 to ammonia (NH3) and number of nitrogen oxides (NO). Bacteria catalyse each step of nitrogen cycle including interconversion of NO3−, NO2−, and NO. Microorganisms utilize these substances for the acceptance of terminal electron in the non-availability of oxygen. Nitrate (NO3−), and nitrite (NO2−) are spontaneously present in vegetables and fruits and are consumed by the human beings. Fruits and vegetables mostly receive NO3−, and NO2− from the soil. Excess amount of NO3−, and NO2− is utilized for curing of meat to enhance the quality, appearance, and taste of the meat. Nitrite (NO2−) can also be utilized for protection against different microorganisms such as Clostridium botylinum and to prevent putrefaction and to inhibit lipid peroxidation1 (Atakisi and Merhan 2017; Jain et al. 2020).
The general formula of nitrosamines is R2N–N=O. These are formed at low pH and high temperature in the stomach due to the reaction among alkylureas, amides, amines, and the nitrosonium cation (NO+). These are nitrosating agent formed by the decomposition of nitrogen dioxide (N2O3) and during the thermal processing of meat or from residual nitrite (NO2−) utilized in curing salt. Lower temperature was also found to be responsible in favouring the reactions related to amine nitrosation (Molognoni et al. 2019).
Endogenous NOCs generated by the N-nitrosation processes of amides and amines have been determined as potent genotoxins as they are capable to direct mutations in the nucleic acid. Most of the NOCs such as nitrosoguanidines, nitrosamides, and nitrosamines can produce alkylating agents throughout metabolism. Various nitrosamines such as NSAR, NPRO, NMOR, NPYR, NPIP, NDBA, NDEA, and NDMA are found in food but all of them are not responsible for inducing cancer in human being (Zhao et al. 2007).
Human beings are exposed to two types of NOCs: (1) endogenous nitrosamides which are formed by the reaction of nitrite with the generated products after degradation of amino acids in the stomach and account for the 75% of total NOC exposure; and (2) exogenous nitrosamines which are generated from drugs, occupational environments, diet, and tobacco products. Various pre-clinical and epidemiological researches have been carried out on different animal species explaining the strong relation between the colorectal cancer (CRC) and endogenous NOC. In case of CRC, researchers reported that N-alkyl-NOCs was able to direct transition of nucleic acid bases (GC to AT) in Kras mutated gene in cancer (Cascella et al. 2018).
Moreover, Kuhnle et al. (2007) reported the presence of nitrosyl heme in faeces and in ileum, generated by the nitrosation and nitrosylation, and might promotes the generation of diazoacetate, a very reactive alkylating agent. This agent leads to the generation of O6-carboxymethyl-2′-deoxy-guanosine (O6CMeG), a NOC-nucleic acid adduct. The DNA can also get damage by aldehydes with mutations in human, mammalian, and microorganism cells. In support of this, Peluso et al. (2010) reported the formation of adduct such as 1,N2-malondialdehyde-deoxyguanosine (M1dG) after the reaction of MDA with DNA. This adduct was observed in high levels in case of adenoma as compared to adenoma free subjects. Another aldehyde i.e., 4-hydroxy-2-nonenal (4-HNE) was considered to be weak mutagen but it was the major toxic chemical compound of lipid peroxidation. It interferes with stress directed apoptotic pathway and results necrosis in colon cancer cells by the activation of capase-3. In order to control the pro-carcinogenic effect of heme, a group of antioxidant substances present in vegetables and fruit was suggested to include in daily diet.
The red meat is rich in glycans that contains variety of sialic acid such as N-glycolylneuraminic acid (Neu5G). This compound is not usually present in animal tissues but can be produced by the diet comprising of lamb, beef, and pork. It was reported that the human can metabolically incorporate Neu5Gc on to the cell surface glycol-conjugates. This incorporation of Neu5Gc was somewhere included in the initiation and progression of tumor. It is important to consider that Neu5Gc comprise of glycans that can act as “xeno-autoantigens” and may be targeted by anti-Neu5Gc “Xeno-autoantibodies”. Due to the lack of other epidemiological data, xenosialitis caused by the Neu5Gc containing food products was considered to be the only mechanism that explains the relation of red meat usage with increased chances of colorectal cancer (Cascella et al. 2018).
Enterosalivary circulation of nitrates: nitrates present in the diet are effectively taken up by the gastrointestinal tract. These nitrates mix with endogenously formed nitrate from NOSs by the help of blood stream. Some of these are taken into the tissues and about 25% of them are actively absorbed by the salivary glands and liberated into the oral cavity. Bacteria present in the oral cavity carry out the reduction of nitrate to nitrite that are swallowed along with other nitrates. The nitrates further renters to the enterosalivary loop and nitrite are subjected to further degradation to form nitric oxide (NO) in tissues and blood (EFSA et al. 2020).
Oral nitrogen oxide chemistry: the oral cavity is a complex mixture of ions, proteins, leucocytes, bacteria and various other substances having potential to do reactions with nitric oxides, nitrites, and nitrates. Commensal bacteria do the degradation of nitrate and nitrite. Nitric oxides get oxidized to form nitrite in the availability of oxygen. In an acidic condition, nitrite can protonated to form nitrous acid (HNO2) that form intermediate dinitrogen trioxide (N2O3) and further decomposed into nitrite, nitrosonium ions (NO+) or nitrogen dioxide (NO2) and nitric oxide (NO). The reaction of nitrite under the acidic condition was supported by various reducing agents such as urate, ascorbate, and thiocyanate (Duncan et al. 1995).
The nitrosonium ions can further react with secondary amines (RR′NH) or reduced thiols (RSH) to form N-nitrosamines (RR′NNO) or nitrosothiols (RSNO). Nitric oxide can further react with superoxide (leucocytes product) to generate peroxynitrate (ONOO−). Based on the environmental conditions, peroxynitrate can further isomerized into nitrate or under the acidic condition to nitrogen dioxide and hydroxyl group (OH−). Nitrite and NO can further make reaction with hydrogen peroxide (H2O2) and bacteria to form nitrogen dioxide as shown in Fig. 4. These are not the comprehensive list of reactions but can be mentioned as the reactions involved in the generation of N-nitroso compounds from diet products (Hezel and Weitzberg 2015).
Mechanism of carcinogenicity
Metabolism
The Mechanism of NPIP metabolic activation is shown in Fig. 5. The NPIP is bio-transformed by the help of hepatic P450s along with some additional cytosolic proteins. It undergoes α-hydroxylation and results in the production of unstable α-hydroxy NPIP (3). This unstable compound is being trapped in the form of α-hydroxy NPIP phosphate ester (16) in the presence of ultraviolet radiations. The α-hydroxy NPIP decomposition and voluntary loss of N2 and H2O result in the production of electrophilic intermediate carbocation (8). The carbocation (8) forms the oxonium ion (11) by the intramolecular cyclization and the isomeric carbocation (6) by the 1,2-H shift. The solvolysis reaction result in the production of 5-hydroxypental (9) in equilibrium with 2-hydroxytetrahydropan (12, THP-OH), and reduced form of 1,5-pentadiol (10) and 4-hydroxypentanal (7) in equilibrium with 2-hydroxy-5-methyltrtrahydrofuran (5). The potent oxidized or eliminated product, 4-oxopent-2-enal (4) has also been formed in the resulted mechanism of NPIP α-hydroxylation reaction. This product (4) may be the result of 2-methylfuran metabolism.
Alpha (α-) and beta (β)-hydroxylations of NPIP are also considered as potential metabolic pathway. Their products N-nitroso-4-piperidone (15) and N-nitroso-4-hydroxypiperidine (14) were identified in various in vitro studies related to the hepatic microsomes of rats. N-nitroso-4-hydroxypiperidine (14) and N-nitroso-3-hydroxypiperidine (13) were observed to be the minor products in comparison to 5-hydroxypentanal (9) in the researches related with liver microsomes of guinea pig as shown in Fig. 5. Substitution of methyl at the position of α-carbons of NPIP reduces the formation of tumor in rat administered to NPIP or its analogues in drinking water. The α- and β-subsitutions (hydroxyl and methyl) have no effect on the carcinogenicity induced by NPIP in rats (Li and Hecht 2022).
DNA adducts formed by NPIP metabolism
The α-acetoxy-N-nitrosopiperidine (2) with reaction with 2′-deoxy guanosine (dGuo) results in the formation of 7-(2-oxopropyl)-N1,N2-etheno-dGuo (17) and this occurs by the help of intermediate compound, 4-oxopent-2-enal (4). The unstable hemiaminal precursor was identified either in its original form or in its reduced form and they were characterized as 7-(2-oxopropyl)-NN1,N2-etheno-dGuo (17) and 7-(2-oxopropyl)-5-hydroxy-5,6,7,9-tetrahydro-9-oxo-3-β-d-deoxyribofuranosylimidazo [1,2-a]purine (18) (Li and Hecht 2022) as shown in Fig. 6.
The N2-(3,4,5,6-tetrahydro-2H-pyran-2-yl)-2′-deoxyguanosine [(19) (N2-THP-dGuo)] was observed as the major product in the reaction mixture of dGuo as compared to 17. Likewise, in the reaction of calf thymus DNA with α-acetoxyNPIP (2), N2-THP-dGuo (19) was majorly formed whereas 7-(2-oxopropyl)-N1,N2-etheno-dGuo (17) was formed minimally. Neutral thermal hydrolysis reaction resulted by N2-THP-dGuo results in the release of THP-OH (12) in the DNA hydrolysate (Li and Hecht 2022).
Human DNA adducts upon the metabolic activation of NPIP
Totsuka et al. (2019) reported the investigation on nucleic acid adductome of esophageal carcinoma in Chinese patients. They observed a new pattern of DNA adduct N2-THP-dGuo (19) in response to NPIP in the esophageal carcinoma tissue in the high incidence regions rather than the low-incidence regions. The differences in the results of peripheral blood samples from the two area related to the presence of DNA adduct was statistically significant (p < 0.01). The NPIP exposure was not likely to occur due to drinking and smoking as the samples were collected from the non-drinkers and non-smokers. Vegetables and drinking water may be the potential sources responsible in inducing cancer in these patients. Basically, NPIP directs the transversion of AT to CG in gpt delta transgenic rats. However, the main mutation in the samples of esophageal carcinoma was CG to TA transition as no other mutation was observed to be associated with the esophageal tumor in the region of high incidences. Thus, it was suggested from the obtained results that NPIP induced nucleic acid mutation can be responsible in inducing esophageal carcinogenesis (Li and Hecht 2022).
NPIP causes cancers of the liver and esophagus in the rats. This cyclic N-nitrosamine is identified in the diet and there are sufficient evidences in support of formation of this carcinogen endogenously in humans. Very little information is available regarding the nucleic acid adducts that would be formed upon the metabolic activation of NPIP (Totsuka et al. 2019). In comparison to other N-nitrosmaines, R-hydroxylation of NPIP is the major pathway responsible for its activation. The related reactive intermediates form from the in-vitro R-hydroxylation by the R-acetoxy NPIP hydrolysis. The DNA reactive products that were generated were studied using R-acetoxy NPIP. Some studies reported that 4-oxo-2-pentenal was the hydrolysis product of R-acetoxy NPIP and its reaction with dGuo resulted in the production of 7-(2-oxopropyl)-5,9-dihydro-9-oxo-3-â-D-deoxyribofuranosylimidazo[1,2-a]purine[(7-(2-oxopropyl)-1,N2-ethenodG) (Wong et al. 2005).
Various different products were also produced in these reaction and were recognized as diastereomers of 7-(2-oxopropyl)-5-hydroxy-5,6,7,9-tetrahydro-9-oxo-3-â-Ddeoxyribofuranosylimidazo[1,2-a]purine, the hemiaminal precursors of 7-(2-oxopropyl)-1,N2-ethenodG. The presence of 7-(2-oxopropyl)-1,N2-ethenodG and its hemiaminal precursors in nucleic acid do reaction with cis-4-oxo-2-pentenal and R-acetoxy NPIP that was determined by LC–ESI–MS. These recations suggest a possible pathway with respect to unique carcinogenic properties of NPIP (Liu et al. 1996).
Evaluation of health risks
Acute health effects of NPIP
The short-term health effects are those occur shortly or immediately after exposure to NPIP. The NPIP can damage or irritate eyes with clouded patches vision, reducing vision, damage to the cornea, and inflammation in the pigmented area.
Chronic health effects of NPIP: NPIP and human cancer
Cytotoxic study
The protective effect of isothiocynate solely or in amalgam with vitamin C was investigated against NDBA and NPIP directed oxidative nucleic acid damage by the help of single cell electrophoresis in HepG2 cells.
Indole-3-carbinol (I3C) and Phenethyl isothiocyanate (PEITC) solely showed a poor effect towards NPIP (1 µM, 26–28%) or NDBA (0.1 µM, 26–27%) directed oxidative damage. Allyl isothiocyanate (AITC) solely was also not able to reduce the genotoxic effect induced by NPIP and NDBA. In contrast, AITC, I3C, and PEITC in amalgam with vitamin C showed greater restriction of oxidative damage caused by the NPIP (1 µM, 50, 73, and 63%) or NDBA (0.1 µM, 67, 42, and 32%) in HepG2 cells than the isothiocyanates (ITCs) alone. The ITCs alone or in combination with vitamin C provided protective effect against N-nitroso induced nucleic acid damage possibly by the restriction of cytochrome P450 directed bioactivation. The I3C and PEITC restricted the p-nitrophenol hydroxylation (CYP2E1) activity at 0.1 µM (66–50%) and coumarin hydroxylase (CYP2A6) activity at 0.1 µM (25–37%), respectively. However, PEITC was able to inhibit ethoxysorufin O-deethylation activity at 1 µM concentration (55%). From above results, it was determined that the I3C and PETIC alone or AITC, I3C, and PEITC in combination with vitamin C provide protection against NPIP and NDBA carcinogenic compounds (García et al. 2008a).
Apoptosis is a suicide process that ultimately causes the death of a cell by regulating programs that result in the removal of damaged tissue. HepG2 and HL-60 cells were plated at 1.5 × 106 cells/ml for 24 h, then treated to 10–45 mM, 5–20 mM of NPIP, and 1–3.5 mM, 0.5–2 mM of NDBA for 72 h. HL-60 cells treated with 20 mM NPIP and 2 mM NDBA for 72 h resulted in 74 and 44% apoptosis but greater concentrations of NPIP (25 mM) and NDBA (2.5 mM) were required to achieve a similar proportion of apoptosis in HepG2 cells (70 and 51%). Furthermore, both the extrinsic (caspase-8) and intrinsic (caspase-9) pathways may be involved in N-nitrosamine-induced apoptosis. It is generally known that cytochrome P-450 enzymes metabolize N-nitrosamines. CYP2A6 is a kind of CYP that plays a significant part in the activation process of cyclic N-nitrosamines. Short-chain N-nitrosamines, such as NDMA, are activated by CYP2E1, while longer-chain N-nitrosamines, such as NDBA, are metabolized by CYP1A1 (Fujita and Kamataki 2001). The effect of dipropyl disulphide (DPDS), diallyl disulphide (DADS), and vitamin C was determined against NDBA and NPIP directed apoptosis in hepatoma (HepG2) and leukemia (HL-60) cell line by the help of terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling assay.
DPDS, DADS (1–5 mM), and vitamin C (5–50 mM) did not trigger apoptosis. However, combining DPDS, DADS, and vitamin C reduced apoptosis produced by NDBA and NPIP by around 70% in HL-60 and HepG2 cell lines. Vitamin C reduces the concentration of ROS to the level of control in a dose-dependent manner at the dosage of 5–50 mM whereas DADS increases the levels of ROS induced by NDBA and NPIP at 5 mM concentration in HepG2 (18% increase) and HL-60 (20–40% increase). The DPDS was observed an efficient ROS scavenger at 1 mM concentration in both HepG2 (24% decrease) and HL-60 (25–52% decrease) cell lines. From the above results, it was concluded that the antioxidant ability of DPDS and vitamin C could be responsible for the NDBA- and NPIP-directed apoptosis. However, other mechanism such as induction of phase II and inhibition of phase I enzyme could be responsible for the protective effect of these dietary scavenging antioxidants against NDBA- and NPIP-directed apoptosis in HepG2 and HL-60 cell lines (García et al., 2009b).
Nasal cancer
NPIP was considered to be the potent nasal carcinogen (Lijinsky and Reuber 1981) in rat animal model whereas NPYR was the weak carcinogen for the nasal cavity. This difference in their carcinogenicity was due to the more effective α-hydroxylation of NPIP. The K(M) value of NPIP (13.9–34.7 µM) was lower in comparison to NPYR (484–7660 µM). This was due to the 20–40 times higher catalytic activity [V(max)/K(M)] in nasal microsomes with NPIP as substrate in the presence of CYP2A3 along with the desired role of CYP2G1 (Jeffrey et al. 2006).
N'-nitrosonornicotine (NNN) and N-nitrosopiperidine (NPIP) are powerful carcinogens of the esophagus and nasal cavity in rats, as well as lung carcinogens in mice. Alpha-hydroxylation is thought to be the primary activation mechanism in their carcinogenesis. P450 2A enzymes are vital for nitrosamine alpha-hydroxylation. P450 2A4, 2A5, 2A6, and 2A13 showed considerably lower Km and higher kcat/Km values for NPIP compared to NPYR alpha-hydroxylation (p < 0.05), similar to prior studies with P450 2A3. Taken together, these findings suggest that key P450 2A residues control the catalytic activity of NNN, NPIP, and NPYR alpha-hydroxylation (Wong et al. 2005)
Pulmonary cancer
The NPIP was found to be a causative agent for pulmonary carcinogenesis in mice animal model. The main activation pathway involved in this carcinogenesis was the alpha–hydroxylation and the key enzyme responsible was P450 2As. To understand the catalytic efficiency of different enzymes for carrying out the hydroxylation, a comparative study of human (P450 2A6 and AA13), mouse (P450 2A5 and 2A4), and rat (P450 2A3) was performed. Among all, P450 2As was observed to be the good catalyst for alpha-hydroxylation of NPIP. Other enzymes such as P450 2A13, 2A6, 2A5, and 2A4 were reported with higher Kcat/Km and lower Km values (Wong et al. 2005).
Bronchial and alveolar cancer
Eighty BALB/C strain mice were subjected to 0.2 mmol/kg of NPIP intraperitoneally for 8 weeks and this was continued further for 16 weeks. Among 80 mice, 22 mice of experimental group showed pulmonary tumor as compared to control group. The cancer in the experimental animal groups was originated from the alveolar type II epithelial cells. Additionally, six out of 22 mice were developed the bronchogenic tumor (Xie et al. 2010). The mutation in tumor suppressor gene and oncogene was the initial step in NPIP induced carcinogenesis. The expression of telomerase subunit, ras, c-myc, bcl-2, and p53 was found to increase in all the tumors and pulmonary tissues after the binding of NPIP to nicotine acetylcholine receptors (nAChRs). In conclusion, NPIP was capable in inducing lung cancer with morphological changes during the carcinogenesis by deregulating and enhancing cell invasion, cell migration, cell survival, and cell proliferation. This may be the result of overexpression of cancer related genes or telomerase subunit as shown in the Fig. 7 (Xue et al. 2014).
Liver cancer
From the experimental study, it was determined that the higher intake of NMAMBA, NDMA, and NDEA from plant sources and NPIP from animal sources was related with the development of hepatocellular carcinoma (HCC). Greater consumption of nitrite and NDBA was related with the lower chances to develop HCC and no specific relation was reported for the total NOC and nitrate consumption. The chances of having HCC were greatest among peoples with hepatitis infection and high intake of NOC suggested a synergistic effect of these two factors on the development of HCC. The NDMA and DEA were the common NOCs found in the food and categorized to class 2A of carcinogens (probable carcinogen to humans) by the IARC. These NOCs need activation of metabolism by the help of cytochrome P450 to form an electrophilic alkylating agent that bind to DNA covalently to develop a mutation (Barsouk et al. 2021). The liver had the greater capacity to metabolize NOCs than the extrahepatic tissues. Dose responsive investigational study reported that the NDEA and NDMA can cause liver cancer in experimental animal models. Formation of DNA adduct was observed in both 300 of human lives and animal after exposure to NDEA and NDMA. The NPIP was a less potent carcinogen that belong to class 2B (possible human carcinogen) and found to cause liver cancer in different animals including monkeys via the similar mechanism as described for NDMA and NDEA (Zheng et al. 2021) and is shown in Fig. 8.
Esophageal cancer
The esophageal tumor was found to be common in Cixian, China but the cause of this disorder is remained unidentified. In order to explore the DNA adductome, both non-cancerous and cancerous samples were taken at the Fouth Hospital of Hebei Medical University and Cixian Cancer Hospital in the low-incidence area. N2-(3,4,5,6-Tetrahydro-2H-pyran-2-yl)deoxyguanosine (THP-dG) was the mutation that was observed in the samples of tumor patients (Liang et al. 2017). The NPIP as a precursor of THP-dG has revealed to have great mutagenic activity under the activation of metabolism in the Ames test. A dose dependent rise in the number of mutations during the in vivo mutagenicity test with guanine phosphoribosyl transferase (gpt) was observed in delta rats. The NPIP directed mutations include transversion of A:T to G:C, G:C to A:T, and A:T to C:G transitions in the esophagus and liver of animal tissue. Similar mutation pattern was reported in esophageal tumor patients but they represented a weak correlation with THP-dG levels. Based on the above findings, it was determined that the NPIP was partially responsible in the induction of esophageal tumor in Cixian residents (Totsuka et al. 2019).
Alpha-hydroxylation is the important metabolic pathway that leads to nitrosmaines induced carcinogenesis. The main alpha-hydroxylation products of NPYE and NPIP are 2-hydroxytetrahydrofuran (2-OH-THF) and 2-hydroxytetrahydro-2H-pyran (2-OH-THP), respectively. The NPYR and NPIP (4 µM) were incubated with different concentrations of co-factors and esophageal microsomes. Microsomes converted the NPIP to 2-OH-THP with 40-fold greater velocity than the NPYR to 2-OH-THF. Similar observations were reported with NPYR and NPIP between 4 and 100 µM of substrate concentration. The expression of cytochrome P450 2A3 at low level in rat esophagus acts as a poor catalyst of NPYR alpha-hydroxylation and good catalyst of NPIP alpha hydroxylation (Fig. 9). Thus, the liver microsomes of rat were able to activate NPYR and NPIP whereas esophageal microsomes of rat were able to activate NPIP but not the NPYR (Wong et al. 2003). Other cancers that resulted from exposure to NPIP are listed in Table 4.
Reproductive health effect
The NPIP has induced cancer in the offspring of animals who were exposed during pregnancy. About 3000 of compounds have been identified in the smoke of tobacco so far, the exact chemical composition is based on the tobacco supplied region, type of pesticide applied, and the type of method utilized to process the leaves. Alkaloids are the main compounds reported in tobacco (0.5–5%) and out of them nicotine is the major one (85–95%) with other compounds in the form of numerous metallic compounds, alkali nitrates (0.01–5%), pesticides, O- and N-heterocyclic hydrocarbons, nitriles, amines, ketones, aldehydes, aromatic hydrocarbons, alkanes (0.1–0.4%), carboxylic acid (0.1–0.7%), phytosterols (0.1–2.5%), terpenes (0.1–3%), and polyphenols (0.5–4.5%) (Zhou et al. 2021).
The absorption of nicotine occurs in two ways: unprotonated and protonated. It was determined that the free nicotine/unprotonated nicotine greatly affected by the pH of the product that can cross the cell membranes. Volatile N-nitrosamines (N-nitrosomorpholine, N-nitrosopiperidine, N-nitrosopyrrolidine, and N-nitrosodimethylamine) and N-nitrosamino acid [4-(methylnitrosamino) butyric acids, 3-(methylnitrosamino) propionic acids, and N-nitrososarcosine)] are some other N-nitroso compounds reported in the smoke of tobacco. Some additives such as pesticides, lactones, heavy metals, urethane, benzo[a]pyrene, eugenol, benzyl benzoate, and menthol were also detected in tobacco smoke with different concentrations (Benowitz et al. 2009).
Smokeless tobacco and ovarian function
The ovaries are endocrine glands whose main function is to produce viable amount of ova for steroidogenesis and reproduction. These are also responsible for the generation of growth factors, signalling factors, and transcription factors resulting in successful reproduction, fertilization, and folliculogenesis. The normal functioning of ovaries is under the control of other factors and hormones and can be disturbed by the exogenous substances from the environmental stress and life style habits. Nicotine from the smoke of tobacco is considered to be the hormone mimicking compound. With the increased consumption of tobacco smoke among females in different parts of the globe, it was determined that this consumption is related to the negative outcomes of pregnancies. Due to the occurrence of large number of chemicals in tobacco smoke along with their potential targets, tobacco smoke is considered to have adverse effects on the reproductive system at various stages (Laldinsangi 2022).
Role of NPIP in inducing apoptosis
Cell death can be possible with the help of two mechanisms that include apoptosis and necrosis, each process has characteristics biochemical and morphological features. Necrosis occurs as the cause of acute cellular dysfunction due to some stress in exposure to certain toxic chemicals. It is a passive process related to the rapid depletion of ATP in cells. On a morphological level, necrosis is determined by a rapid increase in rupturing of plasma membrane and cell volume, with cellular contents spilling into the intercellular milieu (Elmore 2007). Another complex process is apoptosis which is determined by the generation of apoptotic bodies, membrane blebbing, internucleosomal DNA fragmentation, chromatin condensation, and cell shrinkage. Apoptosis in response to carcinogens seems to have an important role in the initiation of cancer. Generally, cell apoptosis in exposure to carcinogenic compounds allows the cells with damaged DNA to remove (Wong et al. 2011). However, cell removal of this type gives proliferating and survival signals to the nearby cells with less damaged nucleic acid. This may result in the development of preneoplastic cells which become resistant against the carcinogen induced cell death. N-nitrosodibutylamine (NDBA) and NPIP are the N-nitrosamines which are broadly distributed in the occupational atmosphere and foodstuffs (Fink and Cookson 2005).
A report was done to determine the production of ROS and to evaluate the apoptotic effect of N-nitrosamines in the human leukaemia cell line HL-60. The cells undergoing apoptosis are recognized by (1) poly(ADP-ribose) polymerase (PARP) cleavage, (2) flow cytometric study, and (3) chromatin condensation. The NDBA and NPIP directed morphological changes include apoptotic events in HL-60 cell line. Based on flow cytometric analysis, it is determined that the N-nitrosamines induced apoptosis of cells in a time and concentration based manner. The NDBA is effective as it induces cell apoptosis after 18 h from the starting at 2 mM of concentration than the NPIP at 10 mM concentration. Furthermore, NDBA induces cleavage of PARP at 5 mM of NPIP and 0.5 mM of NDBA after 18 h and 3 h of treatment, respectively (García et al. 2008b). The concentration of ROS is observed to be increased after 0.5 h of N-nitrosamines treatment. Antioxidants such as N-acetylcysteine (NAC) carries the complete inhibition of ROS induced by NDBA and NPIP. The action of NAC is not related to the apoptosis as NAC is not able to block the induced apoptosis by N-nitrosamines. It is concluded that the NDBA and NPIP induce ROS production and apoptosis in HL-60 cells. Some studies showed that if ROS production is reduced by the NAC to control level then it can also able to reduce the carcinogen induced apoptosis. However, it was determined that NDMA and NPYR caused apoptosis in HepG2 cell lines by the activation of intrinsic (caspase 9) and extrinsic pathways (caspase 8). The pathway of apoptosis was dependent on the type of chemical inducer used, and cell type, in accordance with previous studies (García et al. 2009b).
It is broadly reported that N-nitrosamines need metabolic activation with the help of cytochrome P-450 to cause cancer. After activation, they attack and form covlent bond with DNA, resulting DNA adducts. The damaged DNA induces the activation of endonucleases, action of protease, and production of p53 protein which catalyse the DNA fragmentation and leads to apoptosis. The condensation of chromosome could be seen in HepG2 cell lines with the help of fluorescence microscopy after 1 h of NDBA treatment while NPIP was found to be affective after 24 h of treatment. However, single strand break in DNA was observed after 24 h of treatment with both N-nitroso compounds (Li and Hect 2022).
The proteolytic cleavage of PARP was utilized as another marker for determining the NDBA and NPIP-directed apoptosis. The cleavage of PARP was observed in NDBA treated cells whereas the 85 kDa size of PARP fragment was not present in NPIP and etoposide treated cells. The expression of PARP was inhibited after the treatment of cells with 25 and 45 mM of NPIP and after 72 h of incubation with etoposide. Two main pathways involved in eukaryotic cells are intrinsic and extrinsic with caspase-9 and caspase-8 as initiator, respectively. The signal passed from activated caspase-8 is divided into two pathways: the mitochondrial mediated activation of caspase cascade and direct activation of caspase-3. The function of caspase-9 was upstream from caspase 3 and downstream of caspase 8. The caspase 3 further activated the caspase 6 which in turn resulted in the nuclear fragmentation and shrinkage. Both extrinsic and intrinsic pathways are included in the NDBA and NPIP directed apoptosis in HepG2 cell lines (Kominami et al. 2012).
Hashimoto et al. (2012) determined that the 3-amino-1,4-dimethyl-5H-pyrido[4,3- b]indole (Trp-P-1) directs the activation of caspase 9 and 8 in splenocytes of rat. In comparison to other studies, NDBA induced apoptosis (95%) at 3.5 mM concentration after 24 h of incubation whereas 68 mM of NDMA, 50 mM of NPYR, and 45 mM of NPIP was required to obtain more than 50% of apoptosis after 72 h of incubation with the help of TUNEL assay. The variation in the percentage of apoptotic cell with the nitrosamine type suggested that the effect of apoptosis was dependent on the chemical nature of N-nitroso compounds.
Similar observations were recorded in the leukemia HL-60 cell line (García et al. 2009b) whereas HepG2 cells were found to be more resistant with the NDBA and NPIP treatment. Thus, the variations in the apoptotic process directed by N-nitrosamines could be due to the variations in the activities of enzymes in HL-60 and HepG2 cells. The function of ROS as intermediates for the signalling of apoptosis is well determined due to the different antioxidants such as NAC that can block the process of apoptosis. HepG2 cell treated with NPIP showed an increase in ROS generation but in dose-dependent manner and this was not possible with NDBA. These results determined that the initial toxicity due to NDBA was not associated with ROS in HepG2 cells. In a report, García et al. (2009a) reported that the NDBA was able to induce slight time and dose dependent increase in the production of ROS in HL-60 cells. The NAC can reduce the production of ROS directed by NPIP to the levels of control but it did not provide protection to exposed cells from NPIP directed apoptotic event. These outcomes favour a study in which NDBA and NPIP induced apoptosis in leukemia HL-60 cells via ROS-independent pathway. It was determined from the other studies that NAC do not provide protection from apoptosis and ROS are not involved in the regulation of apoptosis. Thus, it was proved that the NDBA and NPIP induce apoptosis via caspase pathway in HepG2 cells but not by ROS (García et al. 2009a).
Effect of NPIP on tissues and on their histological lesions
Esophageal cancerous tissue
There were various studies on the production of esophageal carcinoma by the administration of N-nitroso compound in rats. This cancer can also be induced by these carcinogens in hamster and mice. The esophageal carcinoma caused in response of NPIP in rats was squamous cell cancer. About 95% of cases of carcinoma in males were of epidermoid type and few of them showed horny pearls and keratinization. Animals have shown marked cancer pearls and keratinization or no keratinization in the papilloma or cancer area of the esophagus (Nair and Reddy 2016).
Initial changes observed in the esophageal epithelium after the treatment of NPIP were hyperplastic changes. This type of hyperplasia was always identified after 4 weeks of NPIP administration. It was determined that the papilloma or hyperplasia induced by NPIP in rats may be irreversible and reversible. Papilloma and hyperplasia were considered to be the precursors of bladder tumor induced in response of N-butyl-N-(4-hydroxybutyl) nitrosamine in rats but it can be concluded that the hitogenesis of esophagealtumor in NPIP-treated rats was similar to the urinary bladder cancer in rats. Electron microscopic studies showed many tonofibrils and desmosomes and irregular nuclei in the tumor cells. The nuclear membrane was aggregated and irregular with condensed chromatin in the nucleoplasm. The nucleoli have pars amorpha and fibrillar components, respectively. The cell appearance was very much similar to the cells of urinary bladder tumor that was induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Intranuclear lesions were observed in the tumor cells but their nature was unknown.
Morales et al. (2010) established esophageal cancer cell line obtained from NPIP treated rats. It was possible to determine the nature of intranuclear inclusions and biochemical and morphological characters by in vitro study using these cell lines. Adjacent to squamous cell cancer with many lesions in the epithelium of mucosa in bronchioles such as carcinoma, atypical hypereplasia, and metaplasia was observed in situ. The metaplastic squamous cell lining around the bronchiole was also detected. The lower right mucosa was observed to be lined by squamous metaplasia whereas the upper left one was lined with the help of ciliated columnar. On the other side, atypical squamous cell carcinoma was detected in situ along with the bronchioles surface. The cancerous cells at the basal were sticking out through the basal membrane in situ that resulted in the formation of early invasive carcinoma. Infiltration of squamous cell carcinoma into submucosae and adjacent lymph node metastasis was observed occasionally in NPIP-induced mice. Transmission electron microscopy (TEM) at higher magnification revealed squamous carcinoma cells of irregular size and shape. The nucleus of cell appeared to be irregular, oval, and round shaped. Tonofibrils were recognized in the cytoplasm and desmosomes were detected in an abundant amount between the cells (Xie et al. 2010).
In a study, the effect of alcohol and sodium chloride on NPIP induced esophageal carcinoma in experimental rat model was studied. The histological lesions of esophaus were divided into three types; carcinoma, papilloma, and hyperplasia. Hyperplasia includes thickening of esophageal mucosa due to the multiple layers of epithelial cells with hyperkeratosis. Round cell infiltration of the sub epithelial layer was also observed sometime. The alterations of papillomatous varied from pedunculate polyps to epithelial proliferation with frequent mitotic figures and cellular irregularity in the epithelium of esophagus. These epithelial cells include various cancer pearls in the layer of muscular tissue and were of irregular size and shape (Morales et al. 2010) as listed in Table 5.
Lung cancerous tissues
Light microscopic observation of the NPIP treated mice lung revealed hyperplasia of alveolar epithelial cells along with appearance of reconstituted glands. Lung cancer mostly showed the uniform, closely packed columns of columnar and cubical cells which were supported by a sparse stroma of mature fibrous tissues with some capillary vessels. The arrangement of these cells is of glandula and acini types with some papillary adenomatous type and adenomatous type structures. Few cancer cells were of keratinous type which consist of cubical or oval alveolar shaped cells and filled the alveoli for the formation of keratinous “pearl” and solid nest. The cells of lining were appeared to be of atypical hyperplasia type. The TEM examination of alveologenic tumor identified that the cancer cells were of single polygonal type (Table 5). The development of golgi apparatus, granular endoplasmic reticulum, multivesicular bodies, mitochondria, and cytoplasm were similar with type II alveolar epithelial cells. These type II alveolar cells were identified simply due to the presence of osmiophilic laminated bodies and multivesiculated bodies (Xie et al. 2010).
Liver cancerous tissues
The histological study of the livers of rats treated with TMNPIP and NPIP was extremely outstanding under the light microscope, with the exception of a decrease in periodic acid schiff (PAS)-staining following NPIP treatment. This staining was most abundant at 12th day in the periportal hepatocytes but it become shifted to the hepatic cell cytoplasm in all lobular zones resulting in the appearance of vacuole; this change is mostly marked in the NPIP-treated group. The histological analysis of the liver of the control rats revealed the normal functioning in all aspects, except some hepatocytes with adjoined central veins in which the dispersion of glycogen was observed. The area of glycogen specifically the periportal area is the characteristic of normal cells whereas the remaining glycogen content is being scattered in the cytoplasm in the form of single rosettes. Both kupffer and hepatocyte cells with abnormal quantity of lipid droplet were observed after 12 days of treatment (Rocha-Pereira et al. 2019).
The appearance of rough ER in the parenchymatous region of all lobular zones was scanty and consists of short and single cisternae that gathered around the mitochondria. The smooth ER was found to be proliferated by the 12th day of treatment and this was hyper abundant at the 28th day (Mandl 2023). After 12 days of TMNPIP induction, many of the hepatocytes consisted of a normal amount of glycogen except in some of the parenchymal cells located around the central veins that consist of disposed glycogen area. The alteration and reduction of rough ER was observed after the NPIP exposure whereas TMNPIP treatment was failed to induce any quantitative and morphological changes in this structure (Flaks and Challis 1981) as listed in Table 5.
Precautions taken during the work with human carcinogen NPIP
A charcoal and high efficiency particulate arrestor (HEPA) filters can be utilized to decrease the effect of cancer-causing agents in animal rooms, glove boxes, lab hoods, and air ventilated safety cabinets. The housing of filter should be developed so that the utilized filters can be wrapped into a plastic disposable bag without affecting the cleaning staff. These filters should be immediate transfer to the plastic bag after their removal with proper seal and labelled properly (Montesano et al. 1979).
The collection of waste liquids should be done in the proper containers for their disposal. The lid of the container should be tightly closed and the bottle should be labelled properly. Once the bottle is filled up to the mark then it should be packed in the plastic bag so that its outer surface area is not contaminated. On the other side, the broken glassware utilized in the preparation of carcinogen should be sterilized by the chemical treatment, solvent extraction, and in specially designed incinerators (Montesano et al. 1979).
The application of cosmetics, storage of food, food or beverages containers, eating, drinking, and smoking should be completely restricted in the laboratory. All persons should remove their gloves if they are damaged after the completion of protocol in which carcinogen have been utilized. Afterwards, clean your hands, most probably with liquid detergent and rinse it thoroughly. Proper focus should be given to the methods which were found to be appropriate for the cleaning of skin, based on the contaminant (Montesano et al. 1979).
In animal labs, one has to change their outdoor clothes and should wear a lab suit preferably the disposable and close fitted at wrists and ankles, overshoes, hair covering, and gloves. Clothes should be changed regularly and discarded right away if any contamination occurs. In chemical labs, gown and gloves should wear but these things do not provide full protection. So, respirators or fitted mask should wear when working with some gases and particulates (Montesano et al. 1979).
Procedures related with purification and synthesis should be utilized under aseptic conditions inside a ventilated hood. All practices should be done with extreme care and the vapour generated during the procedure should be removed. It is recommended to take expert advice when new fume cupboard are installed. It is advisable to decrease the air extraction rate so that carcinogenic powder does not blowout the hood. The boxes of gloves should be placed under the negative air pressure. The changes in the air flow should be adequate to prevent the occurrence of vapours of volatile carcinogen (Montesano et al. 1979).
The preparation of infant milk should be done by using the water with low concentration of nitrates. If this type of water is not present then use of cow milk and breast feeding is advisable. Vegetables with lower level of nitrates should be considered for the preparation of baby foods. If nitrate containing vegetables are utilized then desired food processing safety measures should be followed. The usage of nitrate and nitrite in food items as preservative should be reduced to a minimum level. The levels of nitrate in drinkable water should preferably be lower than 45 mg/L of tentative limit as per the Intetional Standards for Drinking Water (Karwowska and Kononiuk 2020).
Handling and storage
The NPIP is not compatible with certain oxidizing agents like fluorine, bromine, chlorine, nitrates, chlorates, permanganates, peroxides, and perchlorates. So, store it tightly in a closed container under aseptic condition and in a cool area away from the light. Ignition sources such as open flames and smoking should be banned where NPIP is stored, handled, or used in a manner that could cause an explosion hazard and potential fire (Tricker and Preussmann 1991).
Removal techniques for nitrosamine forming nitrates
Different sophisticated methods have been applied for the removal of nitrates. These methods which lead to the production of nitrosamines are discussed below:
Biological denitrifications
Biological remediation of N-based contaminants and nitrate (NO3−) produces the N2 that can be recycled back into the atmosphere. Both prokaryotic (archaea and bacteria) and eukaryotic (algae, plant, and fungi) biological entities have the nitrogen metabolism capacity that result in the uptake of NO3− from the atmosphere and change it into various forms. The reduction of nitrate by various biological means can result in different by-products of nitrogen such as N2, N2O, NO, NH3, and NO2, based on the environmental condition and organism respectively. Biological denitrification is the process that depends on the enzymatic reactions carried out by the organisms. These organisms have different proteins that help in the reduction of oxidized nitrogen into different various forms. In biological system, the removal of NO3− from the aquatic body can be dissimilatory, where the oxidized form of nitrogen is reduced to less oxidized form through catabolism or can be assimilatory, where the nitrogen is added into the biomass based on the environmental condition and the organisms (Table 6).
Aquatic plants like water spinach (Ipomoea aquatic), water hyacinth (Eichhorniacrassipes), and water lettuce (Pistia stratiotes) needs high nitrogen concentration and can uptake 50 mg/L of nitrogen, hence their cultivation is recommended for the phytoremediation of NO3− to different sites. Aquatic plants are fast grower (1 to 12 days) in comparison to the terrestrial plants and some types can sequester upto 90% NO3− from the NO3− contaminated water bodies and lower down the concentration level to 10 mg/L that is near to the acceptable limit. Photosynthetic nitrogen demanding microorganism such as cyanobacteria and microalgae are the potential biological agents that can remove 100 mg/L of NO3− from the ground and drinking water with lower concentration of metal. Heterotrophic denitrifiers utilize chemical and natural organic carbon sources for energy to perform denitrification reaction in the wastewater with high concentration of NO3− i.e., 1000 mg/L. Another category involves autotrophic denitrifiers that need inorganic electron donors such as sulphide and hydrogen as their energy source and inorganic carbon forms to carry the denitrification process with limit of 500 mg/L. These two types of denitrifiers such as autotrophic and heterotrophic denitrifiers can exist together in the wastewater system and can facilitate 95% of denitrification process of 1000 mg/L of NO3− to N2 (Moloantoa et al. 2022).
Electrodialysis
In electrodialysis (ED) process, there is transfer of ions from the less concentrated solution to more concentrated one by the help of direct electric current. The ED selectively removes the undesirable ions from the water through a semi permeable membrane. An ED needs a pressurized water supply (345–578 kPa) along with pre-treatment. In reversed form electrodialysis process, the electrodes change their polarity about 2 to 4 times to change the directions of ion movement. This reversed process reduces the chemical and scaling usage as compared to the conventional ED process and is utilized for the formation of drinkable water from the nitrate containing water. The efficiency to remove NO3− by RO and ED was quite same. NitRem, a selective process to remove nitrate was developed based on ED19. This process was effective in removing 50 mg/L to 25 mg/L of NO3− from the water sample. This process is attractive as it involves the NO3− removal without the incorporation of any chemicals. In order to remove 100 to 50 mg/L of nitrate, the cost of RO and EDR were same (Archna et al. 2012).
Ion-exchange chromatography
This process is utilized for the remediation of groundwater in different parts across the globe. A strong base anion exchange resin is utilized for the exchange of bicarbonate and chloride ions with NO3− from the polluted aquatic sample. The ion exchange technology can show the affinity of ion exchange in the following sequence: bicarbonate < chloride < nitrate < phosphate. The resins were developed with carbon atoms that are arranged around the ammonium functional groups to raise the selectivity of NO3−. The reusability of spent resins makes this process economically attractive for the treatment of water. These resins are usually applied to remove the anionic pollutant in the treatment of drinking water. Resins are commonly composed of a polyacrylic or polystyrene divinylbenzene cross-linked polymer matrix that is rich with amine groups of quaternary structure in order to provide positive charge at the exchange sites. These resins are further categorized based on the functional group. Type I resin include tributylamine (TBA), tripropylamine (TPA), triethylamine (TEA), and trimethylamine (TMA) whereas Type II resins include dimethylethanolamine (DMEA). In column and bench scale experiments, anion exchange resins application was related to the availability of cancer-causing nitrosamines (NDBA, NDPA, NDEA, and NDMA) in treated water. Many resins were successful to remove high level (< 2000 ng/L) of nitrosamines at initial rinsing with the lab water. Pre-formed monochloramine or free chlorine in feed water directs the generation of nitrosamines. Resins were capable in releasing the different types of nitrosamines or their precursors based on the functional groups. These observations helped in the pre-treatment and proper utilization of anion exchange resins for the drinkable water treatment (Flowers and Singer 2013).
Reverse osmosis
It is a physical process that requires semipermeable membrane to carry out its application. Water is forced with a pressure across a semi-permeable membrane that allow water molecule to pass and dissolved material to retain in the membrane. This process is utilized for the removal of multiple contaminants at the same time such as organic constituents and ionic particles. This process can achieve the water efficiency to 85% but needs higher intake of energy. This process is the most expensive one and can be cost-effective in case of low water demand or multiple contaminants treatment. This process requires proper review of pre-treatment and water characteristics in order to protect membrane from damage. Pre-treatment involve removal of suspended particles from the water. This can be achieved by passing the water across a series of filter before the stage of reverse osmosis. After the stage of RO, all the activated ions will be removed from the water. This resulted in the fluctuation of pH unless controlled. Treatment plant utilizing this process has to provide post-treatment alkalinity and pH adjustment for the stabilization of water (Matei and Racoviteanu 2021).
Minimizing nitrate leaching
Although number of technologies are available for the decontamination of NO3− polluted water but those are very costly. Therefore, it is recommended to reduce the leaching of NO3− from non-agricultural as well as agricultural activities. In developed countries, the NO3− reducing approach is majorly dependent on the pattern of land use. Any change such as reduction in fertilizer, change in cropping system, conversion of grassland, and conversion from agriculture to non-agricultural use would not be acceptable in India and other developing nations where the main challenge is to maximize the production of food with no increase in cultivation land. In developing countries, the main focus will be on increasing the fertilizer use. By adopting the below mentioned improved methods, leaching of NO3− can be reduced:
-
Substitute the inorganic fertilizers with organic ones by adopting integrated nutrient management facilities
-
Matching the fertilizer application with the plant need by utilizing an appropriate split application
-
Application of slow release fertilizers such as coated fertilizers and urea-aldehyde polymeric compounds
-
Application of urease and nitrification inhibitors
-
Safe chemicals should be applied
-
Selection of right cropping system
-
Establishment of monitoring networks and information systems
-
Education of farmers in particular and the population in general (Rao and Puttanna 2000).
Cationic cellulose nanopaper membranes
Another possible candidate that can fulfil the requirement of NO3− removal is nano-fibrillated cellulose (CNF) that can be simply processed and upgraded into nanopapers. These are utilized in different membrane applications but the main disadvantage of these is moderate affinity and permeance to pollutant that reduce their usability. For the removal of NO3− using CNF by adsorption, positively charged functional groups such as ammonium ions need to be fixed onto the cellulosic nanofibrils surface. Cationic CNFs with quaternary ammonium functional charges were applied as non-exchange nanopapers. The adsorption and permeance capacity of nitrate ions to these nanopapers was quite low. These disadvantages can be overcome by raising the ammonium group concentration on the CNF surface and by using the thinner nanopaper (Mautner et al. 2017).
Electrocoagulation/electroflotation unit
Electro-Coagulation-Floatation (ECF) process is recognized for reduction in the sludge generation, easy operation, and no requirement of chemicals. This process directs the formation of coagulants via the electro dissolution of aluminium or iron composed anode. The production of metal ions takes place at the anode side and release of hydrogen gas occurs from cathode. The hydrogen gas is helpful in the floatation of flocculated materials out of the water. The maximum removal of NO3− was achieved at 0.4 to 3.2 mA cm−2 of current density by aluminium–aluminium arrangement of electrode as anode–cathode under optimal conditions of pH and time. A continuous utilization of ECF reactor led to the removal of NO3− from 36 to 96% at detention time of 10−30 min. The current efficiency and mean energy consumption were 160% and 2.66 kWh g-1 NO3−.
Conclusion and future perspectives
There are enough evidences in support of hypothesis that determines susceptibility of humans to NPIP induced carcinogenesis and other related disorders. Thus, continuous exposure to low level of NPIP compounds from different sources would be a risk factor for various diseases in human. Some preventive measures need to be implemented to minimize the biological, chemical, and physical exposure to known carcinogen NPIP and for the formation of environmental interventions to minimize the incidences of diseases and the social, personal, and economic burden related to it. In the future, the carcinogenicity mechanism will need to be understood from a genetic standpoint, and its relationship with risk factors will need to be understood from a gene abnormality perspective. Further, a better understanding between the metabolism of NPIP and nucleic acid adduct formation is required to provide the help for relevant cancer and prevention. The patterns of exposure and metabolic activation capacities need to be assessed in humans through the use of molecular epidemiology for the construction of better risk management.
References
Abnet CC (2007) Carcinogenic food contaminants. Cancer Investig 25:189–196. https://doi.org/10.1080/07357900701208733
Adriano CC, Pratt PF, Bishop SE (1971) Fate of inorganic forms of N and salt from land disposed manures from dairies. Int Natl Symp Livestock Wastes. ASAE St Joseph MI. 243-6
Ahmad SA, Khan MH, Haque M (2018) Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Healthc Policy 11:251–261. https://doi.org/10.2147/RMHP.S153188
Alam MN, Mandal SK, Debnath SC (2012) Effect of zinc dithiocarbamates and thiazole-based accelerators on the vulcanization of natural rubber. Rubber Chem Technol 85(1):120–131. https://doi.org/10.5254/1.3672434
Ali MA, Poortvliet E, Strömberg R, Yngve A (2011) Polyamines in foods: development of a food database. Food Nutr Res 14:55. https://doi.org/10.3402/fnr.v55i0.5572
Al-Kaseem M, Al-Assaf Z, Karabeet F (2014) Determination of seven volatile N-nitrosamines in fast food. Pharmacol Pharm 5:195–203. https://doi.org/10.4236/pp.2014.52026
Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 25:2097–2116. https://doi.org/10.1007/s11095-008-9661-9
Aragón M, Marcé RM, Borrull F (2013) Determination of N-nitrosamines and nicotine in air particulate matter samples by pressurised liquid extraction and gas chromatography-ion trap tandem mass spectrometry. Talanta 115:896–901. https://doi.org/10.1016/j.talanta.2013.07.010
Archna, Sharma SK, Sobti RC (2012) Nitrate removal from groundwater: a review. J Chem 9:1667–1675. https://doi.org/10.1155/2012/154616
Atakisi E, Merhan O (2017) Nitric oxide synthase and nitric oxide involvement in different toxicities. In: Nitric oxide synthase-simple enzyme-complex roles. IntechOpen. https://doi.org/10.5772/intechopen.68494
Atawodi SE, Maduagwu EN, Preussmann R, Spiegelhalder B (1991) Potential of endogenous formation of volatile nitrosmaines from Nigerian vegetables and spices. Cancer Lett 57:219–222. https://doi.org/10.1016/0304-3835(91)90160-j
Barsouk A, Thandra KC, Saginala K, Rawla P, Barsouk A (2021) Chemical risk factors of primary liver cancer: an update. Hepat Med 12:179–188. https://doi.org/10.2147/HMER.S278070
Bartley R, Henderson A, Prosser IP, Hughes AO, McKergow LA, Lu H, Brodie J, Bainbridge Z, Roth CH (2003) Patterns of erosion and sediment and nutrient transport in the Herbert River catchment. Queensland. Consultancy Report. CSIRO Land and Water. 59
Baskar R, Yap SP, Chua KLM, Itahana K (2012) The diverse and complex roles of radiation on cancer treatment: therapeutic target and genome maintenance. Am J Cancer Res 2:372–382
Bennett G (2004) Sax’s dangerous properties of industrial materials, 11th edn., Richard J. Lewis. John Wiley & Sons, Inc., Hoboken. NJ. J Hazard Mater. https://doi.org/10.1016/J.JHAZMAT.2004.12.010
Benowitz NL, Hukkanen J, Jacob P III (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192:29–60. https://doi.org/10.1007/978-3-540-69248-5_2
Bernado P, Patarata L, Lorenzo JM, Fraqueza MJ (2021) Nitrate is nitrate: the status quo of using nitrate through vegetable extracts in meat products. Foods 10:3019. https://doi.org/10.3390/foods10123019
Bond T, Huang J, Templeton MR, Graham N (2011) Occurrence and control of nitrogenous disinfection by-products in drinking water–a review. Water Res 45:4341–4354. https://doi.org/10.1016/j.watres.2011.05.034
Bornstein D, Pazur RJ (2020) The sulfur reversion process in natural rubber in terms of crosslink density and crosslink density distribution. Polym Test 88:106524. https://doi.org/10.1016/j.polymertesting.2020.106524
Brandi G, Tavolari S (2020) Asbestos and intrahepatic cholangiocarcinoma. Cells 9:421. https://doi.org/10.3390/cells9020421
Buha A, Wallace D, Matovic V, Schweitzer A, Oluic B, Djordjevic V (2017) Cadmium exposure as a putative risk factor for the development of pancreatic cancer: three different lines of evidence. BioMed Res Int 2017:1981837. https://doi.org/10.1155/2017/1981837
Campillo N, Viñas P, Martínez-Castillo N, Hernández-córdoba M (2011) Determination of volatile nitrosamines in meat products by microwave-assisted extraction and dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. J Chromatogr A 1218(14):1815–1821. https://doi.org/10.1016/j.chroma.2011.02.010
Cascella M, Bimonte S, Barbieri A, Del Vecchio V, Caliendo D, Schiavone V, Fusco R, Granata V, Arra C, Cuomo A (2018) Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): an overview on the current state of knowledge. Infect Agents Cancer 13:3. https://doi.org/10.1186/s13027-018-0174-9
Chatterjee N, Walker GC (2017) Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58:235–263. https://doi.org/10.1002/em.22087
Chen Y, Chen W, Huang H, Zeng H, Tan L, Pang Y, Ghani J, Qi S (2021) Occurrence of N-nitrosamines and their precursors in the middle and lower reaches of Yangtze river water. Environ Res 195:110673. https://doi.org/10.1016/j.envres.2020.110673
Cheng TJ, Huang YF, Ma YC (2001) Urinary thioglycolic acid levels for vinyl chloride monomer-exposed polyvinyl chloride workers. J Occup Environ Med 43:934–938. https://doi.org/10.1097/00043764-200111000-00002
Choi NR, Kim YP, Ji WH, Hwang G-S, Ahn YG (2016) Identification and quantification of seven volatile N-nitrosamines in cosmetics using gas chromatography/chemical ionization-mass spectrometry coupled with head space-solid phase microextraction. Talanta 148:69–74. https://doi.org/10.1016/j.talanta.2015.10.045
Ciravolo TG, Martens DC, Hallock DL, Collins ER, Kornegay ET, Thomas HR (1979) Pollutant movement to shallow ground water tables from anaerobic swine waste lagoons. J Environ Qual 8:126–130. https://doi.org/10.2134/jeq1979.00472425000800010027x
Cornfield J, Haenszel W, Cuyler Hammond E, Lilienfeld AM, Shimkin MB, Wynder EL (2009) Smoking and lung cancer: recent evidence and a discussion of some questions. Int J Epidemiol 38:1175–1191. https://doi.org/10.1093/ije/dyp289
De Mey E, De Maere H, Dewulf L, Paelinck H, Sajewicz M, Fraeye I, Kowalska T (2014a) Assessment of N-nitrosopiperidine formation risk from piperine and piperidine contained in spices used as meat product additives. Eur Food Res Technol 238:477–484. https://doi.org/10.1007/s00217-013-2125-4
De Mey E, De Maere H, Goemaere O, Steen L, Peeters M-C, Derdelinckx G, Paelinck H, Fraeye I (2014b) Evaluation of N-nitrosopiperidine formation from biogenic amines during the production of fermented sausages. Food Bioprocess Technol 7:1269–1280. https://doi.org/10.1007/s11947-013-1125-5
Dimitrova A, Marois G, Kiesewetter G, Samir KC, Rafaj P, Tonne C (2021) Health impacts of fine particles under climate change mitigation, air quality control, and demographic change in India. Environ Res Lett 16:054025. https://doi.org/10.1088/1748-9326/abe5d5
D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T (2013) UV radiation and the skin. Int J Mol Sci 14:12222–12248. https://doi.org/10.3390/ijms140612222
Drabik-Markiewicz G, Dejaegher B, De Mey E, Kowalska T, Paelinck H, Vander Heyden Y (2011) Influence of putrescine, cadaverine, spermidine or spermine on the formation of N-nitrosamine in heated cured pork meat. Food Chem 126:1539–1545. https://doi.org/10.1016/j.foodchem.2010.11.149
Dunaway S, Odin R, Zhou L, Ji L, Zhang Y, Kadekaro AL (2018) Natural antioxidants: multiple mechanisms to protect skin from solar radiation. Front Pharmacol 9:392. https://doi.org/10.3389/fphar.2018.00392
Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N (1995) Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1:546–551. https://doi.org/10.1038/nm0695-546
EFSA Panel on Contaminants in the Food Chain (CONTAM), Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, Grasl-Kraupp B, Hoogenboom LR, Leblanc JC, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Wallace H, Bampidis V, Cottrill B, Frutos MJ, Furst P, Parker A, Binaglia M, Christodoulidou A, Gergelova P, Guajardo IM, Wenger C, Hogstrand C (2020) Risk assessment of nitrate and nitrite in feed. EFSA J 18:e06290. https://doi.org/10.2903/j.efsa.2020.6040
Ellen G, Egmond E, Van Loon JW, Sahertian ET, Tolsma K (1990) Dietary intakes of some essential and non-essential trace elements, nitrates, nitrite and N-nitrosamines, by Dutch adults: estimated via a 24-hour duplicate portion study. Food Addit Contam 7:207–221. https://doi.org/10.1080/02652039009373885
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Englande AJ Jr, Krenkel P, Shamas J (2015) Wastewater treatment & water reclamation. Reference Module in Earth Systems and Environmental Sciences. https://doi.org/10.1016/B978-0-12-409548-9.09508-7
Fan CC, Lin TF (2018) N-nitrosamines in drinking water and beer: detection and risk assessment. Chemosphere 200:48–56. https://doi.org/10.1016/j.chemosphere.2018.02.025
Ferysiuk K, Wójciak KM (2020) Reduction of nitrite in meat products through the application of various plant-based ingredients. Antioxidants 9:711. https://doi.org/10.3390/antiox9080711
Fink SL, Cookson BT (2005) Apoptosis, pyrotosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907–1916. https://doi.org/10.1128/IAI.73.4.1907-1916.2005
Flaks B, Challis BC (1981) A comparative electron microscope study of early changes in rat liver induced by N-nitrosopiperidine and 2,2’,6,6’-tetra-methyl-N-nitrosopiperidine. Carcinogenesis 2:385–394. https://doi.org/10.1093/carcin/2.5.385
Flowers RC, Singer PC (2013) Anion exchange resins as a source of nitrosamines and nitrosamine precursors. Environ Sci Technol 47:7365–7372. https://doi.org/10.1021/es4003185
Fujita K, Kamataki T (2001) Screening of organosulfur compounds as inhibitors of human CYP2A6. Drug Metab Dispos 29(7):983–989
García A, Haza AI, Arranz N, Rafter J, Morales P (2008a) Protective effects of isothiocyanates alone or in combination with vitamin C towards N-nitrosodibutylamine or N-nitrosopiperidine-induced oxidative DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. J Appl Toxicol 28:196–204. https://doi.org/10.1002/jat.1270
García A, Morales P, Arranz N, Delgado E, Rafter J, Haza AI (2008b) Induction of apoptosis and reactive oxygen species production by N-nitrosopiperidineN-nitrosodibutylamine in human leukemia cells. J Appl Toxicol 28:455–465. https://doi.org/10.1002/jat.1295
García A, Morales P, Rafter J, Haza AI (2009a) N-nitrosopiperidine and N-nitrosodibutylamine induce apoptosis in HepG2 cells via the caspase dependent pathway. Cell Biol Int 33:1280–1286. https://doi.org/10.1016/j.cellbi.2009.08.015
García A, Morales P, Arranz N, Delgado ME, Rafter J, Haza AI (2009b) Antiapoptotic effects of dietary antioxidants towards N-nitrosopiperidine and N-nitrosodibutylamine-induced apoptosis in HL-60 and HepG2 Cells. J Appl Toxicol 29:403–413. https://doi.org/10.1002/jat.1426
Giordano M, Petropoulos SA, Rouphael Y (2021) The fate of nitrogen from soil to plants: influence of agricultural practices in modern agriculture. Agriculture 11:944. https://doi.org/10.3390/agriculture11100944
Goff EU, Fine DH (1979) Analysis of volatile N-nitrosamines in alcoholic beverages. Food Cosmet Toxicol 17(6):569–573. https://doi.org/10.1016/0015-6264(79)90115-9
Goss LC, Monthey S, Issel HM (2006) Review and the latest update of N-nitrosmaines in the rubber industry; the regulated, the potentially regulated, and compounding to eliminate nitrosamine formation. Rubber Chem Technol 79:541–552. https://doi.org/10.5254/1.3547950
Goswami E, Craven V, Dahlstrom DL, Alexander D, Mowat F (2013) Domestic asbestos exposure: a review of epidemiologic and exposure data. Int J Environ Res Public Health 10:5629–5670. https://doi.org/10.3390/ijerph10115629
Gushgari AJ, Halden RU (2018) Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 210:1124–1136. https://doi.org/10.1016/j.chemosphere.2018.07.098
Haas H, Mohr U, Krüger FW (1973) Comparative studies with different doses of N-nitrosomorpholine, N-nitrosopiperidine, N-nitrosomethylurea, and dimethylnitrosamine in Syrian golden hamsters. J Natl Cancer Inst 51:1295–1301. https://doi.org/10.1093/jnci/51.4.1295
Hashimoto N (2012) Expression of COX2 and p53 in rat esophageal cancer induced bu reflux of duodenal contents. Int Sch Res Notices 2012:914824. https://doi.org/10.5402/2012/914824
Hecht SS, Hoffmann D (1988) Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 9:875–884. https://doi.org/10.1093/carcin/9.6.875
Heideman G, Datta RN, Noordermeer J, van Baarle B (2004) Activators in accelerated sulphur vulcanization. Rubber Chem Technol 77(3):512–541. https://doi.org/10.5254/1.3547834
Hezel MP, Weitzberg E (2015) The oral microbiome and nitric oxide homoeostatsis. Oral Dis 21:7–16. https://doi.org/10.1111/odi.12157
Hidajat M, McElvenny DM, Mueller W, Ritchie P, Cherrie JW, Darnton A, Agius RM, Kromhout H, de Vocht F (2019) Job-exposure matrix for historical exposures to rubber fumes and n-nitrosamines in the British rubber industry. Occup Environ Med 76:259–267. https://doi.org/10.1136/oemed-2018-105182
Hong Y, Kyung Kim H, Sang B-I, Kim H (2017) Simple quantification method for N-nitrosamines in atmospheric particulates based on facile pretreatment and GC-MS/MS. Environ Pollut 226:324–334. https://doi.org/10.1016/j.envpol.2017.04.017
IARC (1972) Monographs on the evaluation of the carcinogenic risk of chemicals to humans. World Health Organization, International Agency for Research on Cancer, Geneva
IARC (2016) Agents classified by the IARC monographs. International Agency for Research on Cancer, 1–133
Jain D, Chaudhary P, Varshney N, Janmeda P (2020) Carcinogenic effects of N-nitroso compounds in the environment. Environ Conserv J 21:25–41. https://doi.org/10.36953/ECJ.2020.21304
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Jeffrey AM, Latropoulos MJ, Williams GM (2006) Nasal cytotoxic and carcinogenic activities of systemically distributed organic chemicals. Toxicol Pathol. https://doi.org/10.1080/01926230601042494
John EM, Stern MC, Sinha R, Koo J (2012) Meat consumption, cooking practices, meat mutagens and risk of prostrate cancer. Nutr Cancer 63:525–537. https://doi.org/10.1080/01635581.2011.539311
Johnson GE, Dobo K, Gollapudi B, Harvey J, Kenny J, Kenyon M, Lynch A, Minocherhomji S, Nicolette J, Thybaud V, Wheeldon R, Zeller A (2021) Permitted daily exposure limits for noteworthy N-nitrosamines. Environ Mol Mutagen 62:293–305. https://doi.org/10.1002/em.22446
Ju H, Arumugam P, Lee J, Song JM (2017) Impact of environmental pollutant cadmium on the establishment of a cancer stem cell population in breast and hepatic cancer. ACS Omega 2:563–572. https://doi.org/10.1021/acsomega.6b00181
Jurdana M (2021) Physical activity and cancer risk. Actual knowledge and possible biological mechanisms. Radiol Oncol 55:7–17. https://doi.org/10.2478/raon-2020-0063
Kanayochukwu Nduka J, Ijeoma Kelle H, OmocheOdiba I (2019) Review of health hazards and toxicological effects of constituents of cosmetics. In: Poisoning in the modern world—new tricks for an old dog? IntechOpen. https://doi.org/10.5772/intechopen.84590
Karwowska M, Kononiuk A (2020) Nitrates/nitrites in food-risk for nitrosative stress and benefits. Antioxidants 9:241. https://doi.org/10.3390/antiox9030241
Ketkar MB, Fuhst R, Preussmann R, Mohr U (1983) The carcinogenic effect of nitrosopiperidine administered in the drinking water of Syrian golden hamsters. Cancer Lett 21:219–224. https://doi.org/10.1016/0304-3835(83)90210-0
Kodamatani H, Yamazaki S, Saito K, Amponsaa-Karikari A, Kishikawa N, Kuroda N, Tomiyasu T, Komatsu Y (2008) Highly sensitive method for determination of N-nitrosamines using high-performance liquid chromatography with online UV irradiation and luminol chemiluminescence detection. J Chromatogr A 1216:92–98. https://doi.org/10.1016/j.chroma.2008.11.025
Kohl DH, Sharer GB, Commoner B (1971) Fertilizer nitrogen: contribution to nitrate in surface water in a corn belt watershed. Science 74:1331–1334. https://doi.org/10.1126/science.174.4016.1331
Kominami K, Nakabayashi J, Bagai T, Tsujimura Y, Chiba K, Kimura H, Miyawaki A, Sawasaki T, Yokota H, Manabe N, Sakamaki K (2012) The molecular mechanism of apoptosis upon caspase-8 activation: quantitative experimental validation of a mathematical model. Biochim Et Biophys Acta (BBA) Mol Cell Res 1823:1825–1840. https://doi.org/10.1016/j.bbamcr.2012.07.003
Konishi N, KitahoriYmShimoyama T, Takahashi M, Hiasa Y (1986) Effects of sodium chloride and alcohol on experimental esophageal carcinogenesis induced by N-nitrosopiperidine in rats. Jpn J Cancer Res 77:446–451
Kotopoulou S, Zampelas A, Magriplis E (2022) Risk assessment of nitrite and nitrate intake from processed meat products: results from the Hellenic national nutrition and health survey (HNNHS). Int J Environ Res Public Health 19:12800. https://doi.org/10.3390/ijerph191912800
Koujitani T, Yasuhara K, Onodera H, Takagi H, Tamura T, Hirose M, Mitsumori K (2002) The utility of N-nitrosamines as initiators for a 26-week rat two-stage nasal carcinogenesis model. J Toxicol Pathol 15:39
Krauss M, Longrée P, Dorusch F, Ort C, Hollender J (2009) Occurrence and removal of N-nitrosamines in wastewater treatment plants. Water Res 43:4381–4391. https://doi.org/10.1016/j.watres.2009.06.048
Kuhnle GGC, Story GW, Reda T, Mani A (2007) Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radic Biol Med 43:1040–1047. https://doi.org/10.1016/j.freeradbiomed.2007.03.011
Kumar RR, Park BJ, Cho JY (2013) Application and environmental risks of livestock manure. J Korean Soc Appl Biol Chem 56:497–503. https://doi.org/10.1007/s13765-013-3184-8
Laldinsangi C (2022) Toxic effects of smokeless tobacco on female reproductive health: a review. Curr Res Toxicol 3:100066. https://doi.org/10.1016/j.crtox.2022.100066
Lee GF (1970) Lutrophication. Occasional Paper No. 2, University of Wisconsin, Water Resources Center, Madison, pp 277–293
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS (2017) Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer 16:10. https://doi.org/10.1186/s12943-016-0577-4
Lewandowska AM, Rudzki M, Rudzki S, Lewandowski T, Laskowska B (2019) Environmental risk factors for cancer: review paper. Ann Agric Environ Med 26:1–7. https://doi.org/10.26444/aaem/94299
Li Y, Hecht SS (2022) Metabolic activation and DNA interactions of carcinogenic N-nitrosamines to which humans are commonly exposed. Int J Mol Sci 23:4559. https://doi.org/10.3390/ijms23094559
Li K, Ricker K, Tsai FC, Hsieh CYJ, Osborne G, Sun M, Marder ME, Elmore S, Schmitz R, Sandy MS (2021) Estimated cancer risks associated with nitrosamine contamination in commonly used medication. Int J Environ Res Public Health 18:9465. https://doi.org/10.3390/ijerph18189465
Liang H, Fan J-H, Qiao Y-L (2017) Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 14:33–41. https://doi.org/10.20892/j.issn.2095-3941.2016.0093
Lijinsky W (1987) Carcinogenicity and mutagenicity of N-nitroso compounds. Mol Toxicol 1:107–119
Lijinsky W, Taylor HW (1976) Carcinogenicity test of two unsaturated derivatives of N-nitrosopiperidine in Sprague-Dawley rats. J Natl Cancer Inst 57:1315–1317. https://doi.org/10.1093/jnci/57.6.1315
Lijinsky W, Reuber MD (1981) Carcinogenic effect of nitrosopyrrolidine, nitrosopiperidine and nitrosohexamethyleneimine in Fischer rats. Cancer Lett 12(1–2):99–103. https://doi.org/10.1016/0304-3835(81)90044-6
Lin K, Shen ZY, Lu SH, Wu YN (2002) Intake of volatile N-nitrosamines and their ability to exogenously synthesize in the diet of inhabitants from high-risk area of esophageal cancer in southern China. Biomed Environ Sci 15:277–282
Liu Z, Young-Sciame R, Hecht SS (1996) Liquid chromatography-electrospray ionization mass spectrometric detection of an ethenodeoxyguanosine adduct and its hemiaminal precursors in DNA reacted with α-Acetoxy-N-nitrosopiperidine and cis-4-Oxo-2-pentenal. Chem Res Toxicol 9(4):774–780. https://doi.org/10.1021/tx950206r
Liu J, Jiang LH, Zhang CJ, Li P, Zhao TK (2017) Nitrate-nitrogen contamination in groundwater: spatiotemporal variation and driving factors under cropland in Shandong Province, China. IOP Conf Ser Earth Environ Sci 82:012059. https://doi.org/10.1088/1755-1315/82/1/012059
Lopez V, Chamoux A, Tempier M, Thiel H, Ughetto S, Trousselard M, Naughton G, Dutheil F (2013) The long-term effects of occupational exposure to vinyl chloride monomer on microcirculation: a cross-sectional study 15 years after retirement. BMJ Open 3:e002785. https://doi.org/10.1136/bmjopen-2013-002785
Lorenzo JM, Munekata PE, Dominguez R, Pateiro M, Saraiva JA, Franco D (2018) Chapter 3-Main groups of microorganisms of relevance for food safety and stability: general aspects and overall description. In: Barba FJ, Sant’Ana AS, Orlien V, Koubaa M (eds) Innovative technologies for food preservation, pp 53–107. https://doi.org/10.1016/B978-0-12-811031-7.00003-0
Luo Q, Bei E, Liu C, Deng Y-L, Miao Y, Qiu Y, Lu W-Q, Chen C, Zeng Q (2020) Spatial, temporal variability and carcinogenic health risk assessment of nitrosamines in a drinking water system in China. Sci Total Environ 736:139695. https://doi.org/10.1016/j.scitotenv.2020.139695
Ma L, Hu L, Feng X, Wang S (2018) Nitrates and nitrite in health and disease. Aging Dis 9:938–945. https://doi.org/10.14336/AD.2017.1207
Mandl J (2023) Glycogen-endoplasmic reticulum connection in the liver. Int J Mol Sci 24(2):1074. https://doi.org/10.3390/ijms24021074
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011:431287. https://doi.org/10.1155/2011/431287
Marykutty CV, Mathew G, Mathew E, Thomas S (2003) Studies on novel binary accelerator system in sulfur vulcanization of natural rubber. J Appl Polym Sci 90(12):3173–3182. https://doi.org/10.1002/app.13023
Masuda M, Mower HF, Pignatelli B, Celan I, Friesen MD, Nishino H, Ohshima H (2000) Formation of N-nitrosamine and N-nitramines by the reaction of secondary amines with peroxynitrite and other reactive nitrogen species: comparison with nitrotyrosine formation. Chem Res Toxicol 13:301–308. https://doi.org/10.1021/tx990120o
Matei A, Racoviteanu G (2021) Review of the technologies for nitrates removal from water intended for human consumption. IOP Conf Ser Earth Environ Sci 664(2021):012024. https://doi.org/10.1088/1755-1315/664/1/012024
Mautner A, Kobkeatthawin T, Bismarck A (2017) Efficient continuous removal of nitrates from water with cationic cellulose nanopaper membranes. Resour Effic Technol 3:22–28. https://doi.org/10.1016/j.reffit.2017.01.005
Miralles P, Chisvert A, Salvador A (2018) Determination of N-nitrosamines in cosmetic products by vortex-assisted reversed-phase dispersive liquid–liquid microextraction and liquid chromatography with mass spectrometry. J Sep Sci 41:3143–3151. https://doi.org/10.1002/jssc.201800388
Mirvish SS (1995) Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposure to NOC. Cancer Lett 93:17–48. https://doi.org/10.1016/0304-3835(95)03786-V
Mirvish SS (2008) Chapter 18 Methods for the determination of N-nitroso compounds in food and biological fluids. Compr Anal Chem 51:653–684. https://doi.org/10.1016/S0166-526X(08)00018-4
Mohr U, Reznik G, Reznik-Schüller H (1974) Carcinogenic effects of N-nitrosomorpholine and N-nitrosopiperidine on European Hamster (Cricefuscricefus) 2. J Natl Cancer Inst 53:231–237. https://doi.org/10.1093/jnci/53.1.231
Moloantoa KM, Khetsha ZP, van Heerden E, Xastillo JC, Cason ED (2022) Nitrate water contamination from industrial activities and complete denitrification as a remediation option. Water 14:799. https://doi.org/10.3390/w14050799
Molognoni L, Daguer H, Motta GE, Merlo TC, Lindner JDD (2019) Interactions of preservatives in meat processing: formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res Int. https://doi.org/10.1016/j.foodres.2019.108608
Montesano R, Bartsch H, Boyland E, Della Porta G, Fishbein L, Griesemer RA, Swan AB, Tomatis L (1979) Handling chemical carcinogens in the laboratory: problems of safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer. 15
Moradi S, Shariatifar N, Akbari-adergani B, MolaeeAghaee E, Arbameri M (2021) Analysis and health risk assessment of nitrosamines in meat products collected from markets, Iran: with the approach of chemometric. J Environ Health Sci Eng 19:1361–1371. https://doi.org/10.1007/s40201-021-00692-z
Morales P, Arranz N, Haza AI (2010) Apoptosis induced by N-nitrosamines in two cancer cell lines. J Environ Prot 1:314–323. https://doi.org/10.4236/jep.2010.13037
Munsif R, Zubair M, Aziz A, Nadeem Zafar M (2021) Industrial air emission pollution: potential sources and sustainable mitigation. Environmental Emissions. IntechOpen. https://doi.org/10.5772/intechopen.93104
Nair DV, Reddy AG (2016) Laboratory animal models for esophageal cancer. Vet World 9:1229–1232. https://doi.org/10.14202/vetworld.2016.1229-1232
National Center for Biotechnology Information, NCBI (2022) PubChem Compound Summary for CID 7526, N-Nitrosopiperidine
National Center for Biotechnology Information (2023) PubChem Compound Summary for CID 7526, N-Nitrosopiperidine
National Research Council (1972) Accumulation of nitrate. The National Academies Press, Washington, p 48
Nemčić-Jurec J, Jazbec A (2017) Point source pollution and variability of nitrate concentrations in water from shallow aquifers. Appl Water Sci 7:1337–1348. https://doi.org/10.1007/s13201-015-0369-9
Nye JC (1973) Nitrogenous compounds in the environment. Draft Report, Washington, DC, US Environmental Protection Agency, Hazardous Materials Advisory Committee, pp 95–109
Österdahl BG (1988) Volatile nitrosamines in foods on the Swedish market and estimation of their daily intake. Food Addit Contam 5:587–595. https://doi.org/10.1080/02652038809373722
Park J-E, Seo J-E, Lee J-Y, Kwon H (2015) Distribution of seven N-nitrosamines in food. Toxicol Res 31:279–288. https://doi.org/10.5487/TR.2015.31.3.279
Park SH, Pafhye LP, Wang P, Cho M, Kim J-H, Huang C-H (2015) N-nitrosodiethylamine (NDMA) formation potential of amine-based water treatment polymers: effects of in situ chloramination, breakpoint chlorination, and pre-oxidation. J Hazard Mater 282:133–140. https://doi.org/10.1016/j.jhazmat.2014.07.044
Park S-J, Jeong M-J, Park S-R, Choi JC, Choi H, Kim M (2018) Release of N-nitrosamines and N-nitrosatables substances from baby bottle teats and rubber kitchen tools in Korea. Food Sci Biotechnol 27:1519–1524. https://doi.org/10.1007/s10068-018-0373-6
Peluso M, Srivatanakul P, Munnia A, Jedpiyawongse A, Ceppi M, Sangrajrang S, Piro S, Boffetta P (2010) Malondialdehyde-deoxyguanosine adducts among workers of a Thai industrial estate and nearby residents. Environ Health Perspect 118:55–59. https://doi.org/10.1289/ehp.0900907
Peto R, Gray R, Brantom P, Grasso P (1984) Nitrosamine carcinogenesis in 5120 rodents: chronic administration of sixteen different concentrations of NDEA, NDMA, NPYR and NPIP in the water of 4440 inbred rats, with parallel studies on NDEA alone of the effect of age of starting (3, 6 or 20 weeks) and of species (rats, mice or hamsters). IARC Sci Publ 57:627–665
Qiao L, Chen Y, Liang N, Xie J, Deng G, Chen F, Wang X, Liu F, Li Y, Zhang J (2022) Targeting epithelial-to-mesenchymal transition in radioresistance: crosslinked mechanisms and strategies. Front Oncol 12:775238. https://doi.org/10.3389/fonc.2022.775238
Rao EVSP, Puttanna K (2000) Nitrates, agriculture, and environment. Curr Sci 79:1163–1168
Rastogi RP, Richa N, Kumar A, Tyagi MB, Sinha RP (2010) Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids 2010:592980. https://doi.org/10.4061/2010/592980
Richards RP, Baker DB, Creamer NL, Kramer JW, Ewing DE, Merryfield BJ, Wallrabenstein LK (1996) Well water quality, well vulnerability and agricultural contamination in the Midwestern United States. J Environ Qual 25:389–402. https://doi.org/10.2134/jeq1996.00472425002500030002x
Rocha-Pereira C, Silva V, Costa VM, Silva R, Garcia J, Gonçalves-Monteiro S, Duarte-Araújo M, Santos-Silva A, Coimbra S, Dinis-Oliveira RJ, Lopes C, Silva P, Long S, Sousa E, de Lourdes Bastos M, Remião F (2019) Histological and toxicological evaluation, in rat, of a P-glycoprotein inducer and activator: 1-(propan-2-ylamino)-4-propoxy-9H-thioxanthen-9-one (TX5). EXCLI J 18:697–722. https://doi.org/10.17179/excli2019-1675
Saeki H, Sugimachi K (2001) Carcinogenic risk factors. JMAJ 44:245–249
Sahoo PK, Kim K, Powell MA (2016) Managing groundwater nitrate contamination from livestock farms: implication for nitrate management guidelines. Curr Pollut Rep 2:178–187. https://doi.org/10.1007/s40726-016-0033-5
Scanlan RA (1983) Formation and occurrence of nitrosamines in food. Cancer Res 43:2435s–2440s
Schuphan W (1965) The nitrate content of spinach (Spinaciasleracea, L) in relation to methaemoglobinaemia in children. Z Erndhr Wiss 5:207–209. https://doi.org/10.1007/BF02021227
Sedlak DL, Deeb RA, Hawley EL, Mitch WA, Durbin TD, Mowbray S, Carr S (2005) Sources and fate of nitrosodimethylamine and its precursors in municipal wastewater treatment plants. Water Environ Res 2005:32–39. https://doi.org/10.2175/106143005x41591
Sen NP, Schwinghamer LA, Donaldson BA, Miles WF (1972) N-nitrosodimethylamine in fish meal. J Agric Food Chem 20:1280–1281. https://doi.org/10.1021/jf60184a013
Shakil MH, Trisha AT, Rahman M, Talukdar S, Kobun R, Huda N, Zzaman W (2022) Nitrites in cured meats, health risk issues, alternatives to nitrites: a review. Foods 11:3355. https://doi.org/10.3390/foods11213355
Shankar A, Dubey A, Saini D, Singh M, Prasad CP, Roy S, Bharati SJ, Rinki M, Singh N, Seth T, Khanna M, Sethi N, Kumar S, Mohan A, Guleria R, Rath GK (2019) Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res 8:S31–S49. https://doi.org/10.21037/tlcr.2019.03.05
Shen G, Preston W, Ebersviller SM, Williams C, Faircloth JW, Jetter JJ, Hays MD (2017) Polycyclic aromatic hydrocarbons in fine particulate matter emitted from burning kerosene, liquid petroleum gas, and wood fuels in household cookstoves. Energy Fuels 31(3):3081–3090. https://doi.org/10.1021/acs.energyfuels.6b02641
Shukla S, Saxena A (2019) Global status of nitrate contamination in groundwater: its occurrence, health impacts, and mitigation measures. In: Hussain C (ed) Handbook of environmental materials management. Springer, Cham, pp 869–888
Siddiqi MA, Tricker AR, Kumar R, Fazili Z, Preussmann R (1991) Dietary sources of N-nitrosmaines in a high-risk area for oesophageal cancer-Kasmir, India. IARC Sci Publ 1991:210–213
Singh B, Craswell E (2021) Fertilizers and nitrate pollution of surface and ground water: an increasingly pervasive global problem. SN Appl Sci 3:518. https://doi.org/10.1007/s42452-021-04521-8
Skog KI, Johansson MA, Jägerstad MI (1988) Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence, and intake. Food Chem Toxicol 36:879–896. https://doi.org/10.1016/s0278-6915(98)00061-1
Spiegelhalder B, Preussmann R (1982) Nitrosamines and rubber. IARC Sci Publ 1982(41):231–243
Standford G, England CB, Taylor AW (1969) Fertilizer use and Water quality. US Dept Agric 19:41–168
Sun H, Chen H, Yao L, Chen J, Zhu Z, Wei Y, Ding X, Chen J (2022) Sources and health risks of PM2.5-bound polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in a North China rural area. J Environ Sci (china) 95:240–247. https://doi.org/10.1016/j.jes.2020.03.051
Tomlinson TF (1970) Trends in nitrate concentrations in English rivers in relation to fertilizer. Water Treat Exam 19:277–293
Totsuka Y, Lin Y, He Y, Ishino K, Sato H, Kato M, Nagai M, Elzawahry A, Totoki Y, Nakamura H, Hosoda F, Shibata T, Matsuda T, Matsushima Y, Song G, Meng F, Li D, Liu J, Qiao Y, Wei W, Inoue M, Kikuchi S, Nakagama H, Shan B (2019) DNA adductome analysis identifies N-nitrosopiperidine involved in the Etiology of esophageal cancer in Cixian, China. Chem Res Toxicol 32:1515–1527. https://doi.org/10.1021/acs.chemrestox.9b00017
Tricker AR, Preussmann R (1991) Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat Res 259:277–289. https://doi.org/10.1016/0165-1218(91)90123-4
Tricker AR, Pfundstein B, Theobald E, Preussmann R, Spiegelhalder B (1991) Mean daily intake of volatile N-nitrosamines from foods and beverages in West Germany. Food Chem Toxicol 29:729–732. https://doi.org/10.1016/0278-6915(91)90180-f
Ullah Bhat S, Qayoom U (2022) Implications of sewage discharge on freshwater ecosystems. Sewage—recent advances, new perspectives and applications. IntechOpen. https://doi.org/10.5772/intechopen.100770
Umar AS, Iqbal M (2007) Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron Sustain Dev 27:44–57. https://doi.org/10.1051/agro:2006021
Venkatesan AK, Pycke BFG, Halden RU (2014) Detection and occurrence of N-nitrosamines in archived biosolids from the targeted national sewage sludge survey of the U.S. environmental protection agency. Environ Sci Technol 48:5085–5092. https://doi.org/10.1021/es5001352
Vrzel J, Vuković-Gačić B, Kolarević S, Gačić Z, Kračun-Kolarević M, Kostić J, Aborgiba M, Farnleitner A, Reischer G, Linke R, Paunović M, Ogrinc N (2016) Determination of the sources of nitrate and the microbiological sources of pollution in the Sava River Basin. Sci Total Environ 573:1460–1471. https://doi.org/10.1016/j.scitotenv.2016.07.213
Wacker M, Holick MF (2013) Sunlight and vitamin D. Dermatoendocrinol 5:51–108. https://doi.org/10.4161/derm.24494
Ward MH, Jones RR, Brender JD, de Kok TM, Weyer PJ, Nolan BT, Villanueva CM, van Breda SG (2018) Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15:1557. https://doi.org/10.3390/ijerph15071557
Wasserman AE, Fiddler W, Dofrr RC, Osman SF, Dooley CJ (1972) Dimethylnitrosamine in frankfurters. Food Cosmet Toxicol 10:681–684. https://doi.org/10.1016/s0015-6264(72)80148-2
Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ (2011) HPV-DNA integration and carcinogenesis: putative role for inflammation and oxidative stress. Future Virol 6:45–57. https://doi.org/10.2217/fvl.10.73
Wong HL, Murphy SE, Wang M, Hecht SS (2003) Comparative metabolism of N-nitrosopiperidine and N-nitrosopyrrolidine by rat liver and esophageal microsomes and cytochrome P450 2A3. Carcinogenesis 24:291–300. https://doi.org/10.1093/carcin/24.2.291
Wong HL, Murphy SE, Hecht SS (2005) Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N’-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem Res Toxicol 18:61–69. https://doi.org/10.1021/tx0497696
Wong RSY (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Xie XY, Shen J, Xu LY, Li EM, Shen ZY (2010) Bronchogenic and alveologenictumors in mice induced by N-nitrosopiperidine. Biochem Cell Biol 88:775–782. https://doi.org/10.1139/O10-019
Xie Y, Geng Y, Yao J, Ji J, Chen F, Xiao J, Hu X, Ma L (2023) N-nitrosamines in processed meats: exposure, formation, and mitigation strategies. J Agric Food Res 13:100645. https://doi.org/10.1016/j.jafr.2023.100645
Xue J, Yang S, Seng S (2014) Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers 6:1138–1156. https://doi.org/10.3390/cancers6021138
Yahaya A, Babatunde D, Olaniyan LWB, Agboola O (2020) Application of chromatographic techniques in the analysis of total nitrosamines in water. Heliyon 6:e03447. https://doi.org/10.1016/j.heliyon.2020.e03447
Yamamoto M, Iwata R, Ishiwata H, Yamada T, Tanimura A (1984) Determination of volatile nitrosamine levels in foods and estimation of their daily intake in Japan. Food Chem Toxicol 22:61–64. https://doi.org/10.1016/0278-6915(84)90054-1
Yurchenko S, Mölder U (2007) The occurrence of volatile N-nitrosamines in Estonian meat products. Food Chem 100:1713–1721. https://doi.org/10.1016/j.foodchem.2005.10.017
Zendehbad M, Mostaghelchi M, Mojganfar M, Cepuder P, Loiskandl W (2022) Nitrate in groundwater and agricultural products: intake and risk assessment in northeastern Iran. Environ Sci Pollut Res 29:78603–78619. https://doi.org/10.1007/s11356-022-20831-9
Zhang Q, Jin L, Zhang F, Yao K, Ren Y, Zhang J, Zhang Q, He Q, Wan Y, Chi Y (2018) Analysis of 7 volatile N-nitrosamines in Chinese Sichuan salted vegetables by gas chromatography-tandem mass spectrometry coupled to modified QuEchERS extraction. Food Control 98:342–347. https://doi.org/10.1016/j.foodcont.2018.11.047
Zhao YL, Garrison SL, Gonzalez C, Thweatt WD, Marquez M (2007) N-nitrosation of amines by NO2 and NO: a theoretical study. J Phys Chem A 111:2200–2205. https://doi.org/10.1021/jp0677703
Zheng J, Daniel CR, Hatia RI, Stuff J, Abdelhakeem AA, Rashid A, Hassan MM (2021) Dietary N-nitroso compounds and risk of hepatocellular carcinoma: a US-based study. Hepatology 74:3161–3173. https://doi.org/10.1021/jp0677703
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y, Yao Y, Li D (2017) The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Mol Cancer 16:52. https://doi.org/10.1186/s12943-017-0624-9
Zhou Y, Chen J, Dong Y, Shen J, Tian M, Yang Y, Song L, Li J (2021) Maternal tobacco exposure during pregnancy and allergic rhinitis in offspring: a systematic review and meta-analysis. Medicine (Baltimore) 100(34):e26986. https://doi.org/10.1097/MD.0000000000026986
Acknowledgements
The authors would like to warmly thank Vice-Chancellor of Banasthali Vidyapith, Rajasthan for providing excellent research facilities and also acknowledge the Bioinformatics Center, Banasthali Vidyapith supported by DBT and DST for providing computation and networking support through the FIST and CURIE programs at the Department of Bioscience and Biotechnology, Banasthali Vidyapith, Rajasthan. The authors are also thankful to Department of Chemistry, Mohanlal Sukhadia University for providing computational facilities and infrastructure.
Funding
This study did not receive any specific grant from public, commercial, or non-profit funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhary, P., Singh, D. & Janmeda, P. A comprehensive review on various carcinogenic aspects of N-nitrosopiperidine (NPIP). Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-10000-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-10000-w