Abstract
Gmelina asiatica is one of medicinal plants that is famous in traditional medicines. It is known as Asian bushbeech under the family Lamiaceae. Gmelina asiatica is widely used in Indian folklore to treat many illnesses and disorders, such as treatment of jaundice, hemorrhoids, dysuria, arthritis, edema, liver diseases, neurological disorders, fever, heart diseases, dandruff, skin infections, acne, diabetes mellitus, catarrh of the bladder, syphilis, as antiseptic, astringent, demulcent, contraceptive and blood purifier. As well as, there are various reports on the pharmacological activities of this plant that scientifically support some of its traditional uses. These activities have been shown to include anticancer, anti-inflammatory, antioxidant, antihyperglycemic, antipyretic, nematicidal, anxiolytic, neuroprotective, anti-microbial, hepatoprotective, nephroprotective and analgesic activity. Gmelina asiatica is rich in furofuran lignans and flavonoids and contains many other secondary and primary metabolites, but only a few studies have been conducted to identify and isolate its phytoconstituents. The current review aims to provide the published information on Gmelina asiatica, its features, traditional uses, ethnobotanical uses by different tribes, pharmacological activities, and reported phytoconstituents, from 1961 to September 2023, which was collected from books and online databases such as Scopus, Google Scholar, PubMed, Science Direct, SpringerLink, and Wiley Online Library.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gmelina asiatica Linn. is an ornamental flowering (Chowdhuri and Deka 2019) woody shrub (Jayasingam et al. 1992), typically standing at a height of 2–4 m (de Kok 2012). At times, it can even resemble a small tree, but usually does not exceed 10 m in height (Chen and Gilbert 1994; Kannan et al. 2012). The plant is characterized by its straggling, climbing, and semi-evergreen nature with numerous branches and spines (Kannan et al. 2012; Rajesh et al. 2013). However, it can also occasionally take on a decumbent form (Girija and Ravindran 2011) and be deciduous (de Kok 2012). Initially, it classified under Bentham and Hooker’s system within the tribe Viticeae of the subfamily Viticoideae, under the family Verbenaceae, Gmelina asiatica then underwent a taxonomic shift. Phylogenetic analyses, forming the basis of the Angiosperm Phylogeny Group (APG) classification, led to the relocation of the subfamily Viticoideae to Lamiaceae. As a result, Gmelina asiatica is now recognized as a member of the family Lamiaceae (Kooiman 1975; Kannan et al. 2012; Vignesh 2020; Zhao et al. 2021).
Synonyms and vernacular names
Synonyms
Gmelina parvifolia Roxb., Gmelina asiatica f. lobata Moldenke, G. attenuata H. R. Fletcher, G. paniculata H. R. Fletcher (de Kok 2012).
International names according to the place or language name
English: Asian Bushbeach (Rajesh et al. 2013), Small Cashmere tree (Khare 2007), Asiatic beechberry (Sivapalan and Sanmugarajah 2019). Chinese: Ya zhou shi zi (Chen and Gilbert 1994; de Kok 2012). Cambodia: Köncaang (de Kok 2012), Agnchagn (Top et al. 2004). Peninsular Malaysia (Malay language): Bulang, Bulongan (de Kok 2012). Thailand: Jo mae (Neamsuvan et al. 2015). Philippines (Cebuano language): Banganga (de Kok 2012). Laos: Phoung nou (de Kok 2012). Indonesia: in Javanese language: Wareng, in West Timor (Dawan language): Hau bako, in Alor: Lombaul (de Kok 2012). Sri Lanka (Singhalese language): Demata (de Kok 2012).
Indian common names
Hindi: Badhara (Kannan et al. 2012), Bhedaira, Bhdara (Srivastava 1967). Sanskrit: Vikarini (Kannan et al. 2012), Gopabhandra (Rajesh et al. 2013), Biddari (Sivapalan and Sanmugarajah 2019). Telugu: Adavi Gummudu (Silvia and Satyanaraya 2014), Nelagummudu (Rajesh et al. 2013), Challa Gummudu (Kannan et al. 2012). Oriya (Odia): Gombhari, Gopogombhari, Nomdano (Jeeva et al. 2019). Tamil: Nilakumil, Kumil, Mulkumizh (Sivapalan and Sanmugarajah 2019). Bengali: Bhadra (Jeeva et al. 2019). Malayalam: Kumil (Kannan et al. 2012), Kumilamaram (Jeeva et al. 2019), Cherukumizh (Vignesh 2020). Varanasi local language: Nagphool (Kusuma and Joshi 2010).
Ayurveda and Siddha names
Ayurveda: Gambhaari, Gopabhadra, Vikarini (Khare 2007), Kshudragambhari (Murugeswaran et al. 2016). Siddha: Kumizham (Khare 2007).
Taxonomy
Gmelina asiatica, a species that originated in India, was used by Linnaeus to identify the genus Gmelina in 1753. Linnaeus classified this genus under the class ‘Didynamia Angiospermea’ without specification of its family. In 1806 the genus Gmelina was placed in the family Verbenaceae by de Jussieu. Further taxonomic refinements occurred as the family Verbenaceae underwent divisions into tribes and subfamilies. In 1829 and 1895, the genus Gmelina was successively included in the tribe Viticeae and the subfamily Viticoideae. More detailed classification emerged in 1895 when the genus Gmelina was divided into sections based on species characteristics by Briquet. Gmelina asiatica found its place under the section Microstromatae. Subsequent to phylogenetic analyses, several subfamilies, including Viticoideae, were transferred from Verbenaceae to the family Lamiaceae. Then, based on molecular studies, Gmelina and two other genera (Cornutia and Premna) have been removed from the subfamily Viticodeae and together they formed another subfamily called Premnoideae under the family Lamiaceae. Thus, Gmelina asiatica is now a part of the family Lamiaceae (Munir 1984; Li et al. 2016; Zhao et al. 2021).
Ecology and distribution
G. asiatica can live in a variety of habitats, showcasing remarkable adaptability. It flourishes in different soil types, including sandy, clay, and poor soils (de Kok 2012). This resilient species can be found in both deciduous and evergreen forests, as well as dry thorn forests (de Kok 2012; Rajulu et al. 2021). Notably, it extends its presence to swamp shrubland and swamp forests in Cambodia and New Guinea (Giesen 2018). The adaptability of G. asiatica is further emphasized by its ability to thrive in diverse environments such as desert areas, coastal lands, barren regions, along roadsides, and in secondary and disturbed lands (de Kok 2012; Azhagumurugan and Rajan 2014b).
Its widespread presence is evident in various regions, including India, Sri Lanka (Ceylon), Thailand (east to the north), North Vietnam, south China, Peninsular Malaysia (de Kok 2012), New Guinea, Cambodia (Giesen 2018), Myanmar (Burma) (Collett and Hemsley 1890), Laos (Kato et al. 2008), Borneo, Java, Mauritius, Bengal, Sumatra and Philippine Islands (Lam 1919).
Description
Leaves: opposite, simple, papery leaves, occasionally alternate. The leaf morphology can take on shapes such as ovate, obovate, elliptical, or deltoid. The leaf size ranges from 5 to 50 (− 130) mm long, and 5–33 (− 60) mm wide. The leaf margin is entire to 3–5-lobed. Apex may be acuminate, acute, rounded, or obtuse. Base is cuneate or rounded. It has 3 or 4 pairs of veins and the nerves are covered with fine hairs. The upper side of the leaf (adaxis) is dark green, glossy, usually hairless, with a leathery texture. The lower side of the leaf (abaxis) is typically without hairs, sometimes covered by villi, involving glands (Lam 1919; Chen and Gilbert 1994; de Kok 2012; Kannan et al. 2012). Leaf stalk (petiole): it is 0.5–3 cm long, glabrous or pubescent (de Kok 2012). Inflorescence: usually terminal, pendulous, and downy. The flowers are also pendulous. The morphology of inflorescence is either simple raceme or compound raceme (panicle) (Chen and Gilbert 1994; de Kok 2012; Kannan et al. 2012). Bracts and bracteoles are easily detached and leaf-like (Chen and Gilbert 1994; de Kok 2012). Sepals (Calyx): consists of 4 to 5 lobes, pubescent, with numerous glands. The size of this calyx is up to 0.4 × 0.6 cm maximum. Petals (Corolla): The corolla is yellow and long. It consists of 4 lobes and it is 2-lipped, the front lip consists of 3 lobes where the middle one is longer than the other two, while the back lip has just one lobe. It has some glands and is covered by hairs. Fruits (Drupes): without hairs and has a yellow color when ripe (Chen and Gilbert 1994; de Kok 2012). Heartwood: light pinkish red (Anjaneyulu et al. 1975). Roots: brown with Superficial cracks (Krishnan and Gopi 2015). In addition, the anatomical studies of leaves and stems of G. asiatica has been reported by Florence and Domettila (2016). Figure 1 shows pictures of different parts of the plant.
Adulteration and substitution
While there are no reported adulterants for Gmelina asiatica, the roots of Gmelina arborea are documented by the ICMR to be adulterated with those of Gmelina asiatica, possibly due to the easier harvesting from the shrub-like G. asiatica compared to the tree-like G. arborea. Additionally, G. asiatica may offer better medicinal properties (Babu et al. 2010; Krishnan and Gopi 2015; Vignesh and Sumitha 2021).
Materials and methods
In the current review, a detailed report on Gmelina asiatica medicinal plant has been prepared based on related literature available from 1961 to 2023. All the inputs were gathered from books and electronic databases, such as Scopus, Google Scholar, PubMed, Science Direct, SpringerLink, and Wiley Online Library. The published articles related to Gmelina asiatica have been carefully reviewed to collect all information available on this plant, its traditional and tribal uses, pharmacological activities which have been proven through in vitro and in vivo studies, its phytochemicals and the gaps which are needed to be filled. The structures presented in this report have been drawn using ChemDraw software, and ChatGPT was used to edit and improve language.
Results and discussion
Traditional uses
Gmelina asiatica holds significant importance in traditional medicinal practices due to its diverse therapeutic applications. Revered in folklore medicine, every part of the plant is recognized for its medicinal benefits. Despite its extensive use by traditional healers and individuals knowledgeable about herbal remedies, G. asiatica remains classified as an Anukta Dravya, signifying an “undocumented or extra-pharmacopeial medicinal plant” (Kusuma and Joshi 2010; Vignesh 2020).
Traditional medicine and formulations
G. asiatica finds application in Ayurvedic, Unani, Siddha, and Khmer traditional medicine, featuring prominently in various formulations (Kannan et al. 2012; Ali et al. 2014; Kapur 2016; Chassagne et al. 2017). Cambodian Khmer traditional therapists utilize Gmelina asiatica to treat liver diseases (Chassagne et al. 2017). In Siddha formulations, the powder of G. asiatica roots is internally administered for joint pain, while the leaves are included in external applications to alleviate headaches (Wilson et al. 2007; Esakkimuthu et al. 2021). Notably, an Ayurvedic drug, patented in 2005, incorporates G. asiatica to treat and repair the uncommon type of mullerian dysgenesis (Patil and Wadekar 2021). The Unani medicine formulation, Habb-e-Asgand, designed to address conditions such as gout, lumbago, arthritis, joint pain, and liver protection, features Gmelina asiatica stem as a key component (Ali et al. 2014). In general, traditionally, G. asiatica is used in the treatment of various ailments, including hemorrhoids, painful urination, neurological disorders, burning sensation, edema (Murugeswaran et al. 2016), arthritis (Choudhary et al. 2015), jaundice, pyrexia, diabetes mellitus, infections of the bladder (Jeeva et al. 2019), scalp and skin infections like dandruff and acne (Mahendra 2015).
Furthermore, G. asiatica serves as a blood purifier and has historically been employed in treating sexually transmitted diseases (STDs) such as syphilis and gonorrhea (Khare 2007; Florence and Regini Balasingh 2016). It is also reputed for their effectiveness in addressing leucorrhea, an abnormal genital discharge in females. Moreover, this plant possesses anti-diarrheal properties, and is known to alleviate symptoms of anxiety, depression, yawning, and lethargy (Rathnam and Mudaliar 2002). Furthermore, the root bark of Gmelina asiatica is therapeutically valued for treating congenital heart disease in traditional medicine (Ray and Saini 2021). The details of ethnomedicinal uses of various parts of G. asiatica plant are listed in Table 1. Additionally, Gmelina asiatica plant is also found in the Military Medical Museum as this specimen was collected from Sri Lanka and was accompanied by medical notes often attributed to medical expertise or indigenous people. These notes emphasize the significance of the plant’s leaves and roots in relieving swelling around the neck caused by infection of the lymph nodes (Kandamalai) (Cooper 1842).
Uses of G. asiatica by tribal communities
Saora tribe in Andra Pradesh employs the peel of Gmelina asiatica’s fruit topically to treat wounds and dandruff (Jeeva et al. 2019). In Chittoor villages, the Yanadi tribe uses a paste made from the fruits of Gmelina asiatica and the Soapnut tree to eliminate dandruff, applying it to the scalp before washing (Ganesh and Sudarsanam 2013).
Tribes in Tamil Nadu utilize the aerial parts of G. asiatica for addressing jaundice and liver problems, while other tribes in the same state consider it as an antipyretic to lower body temperature (Silvia and Satyanaraya 2014). Paliyar tribe in Virudhunagar district, Tamil Nadu, recommends Gmelina asiatica for dermatological ailments and uses its fruit juice as an ear drop for relieving ear pain (Bose et al. 2014; Mutheeswaran et al. 2021). In Theni district, Tamil Nadu, the Paliyar and Muthuvar tribes concoct a paste by boiling coconut oil and Gmelina asiatica fruits to combat dandruff (Jeyaprakash et al. 2011). Local tribes in the Eastern Ghats of Andra Pradesh use the fruits of Gmelina asiatica to treat eczema (Jeevan Ram et al. 2004), leprosy, dandruff, and toothache (Venkaiah et al. 2020). They use the stem bark in the form of a paste to treat dandruff and root juice for patients with gonorrhea (Murthy et al. 2020).
The local tribes of Telangana employ the leaves of Gmelina asiatica to stop nosebleeds and treat epistaxis (Suthari et al. 2018). The Irulas tribe in Tamil Nadu traditionally used the fruits of Gmelina asiatica as a substitute for soap (Ragupathy and Newmaster 2009). Additionally, indigenous groups in South India utilize G. asiatica for bacterial and viral throat infections, diabetes, and cough (JU et al. 2019). In some provinces of Sri Lanka, traditional practitioners rely on Gmelina asiatica to treat snakebites (Dharmadasa et al. 2016). It is also known that the Portuguese use this plant to get rid of all toxins in the body (Rathnam and Mudaliar 2002). The herbal preparations and their methods of use are summarized in Table 2.
Pharmacological activity
Studies have systematically investigated the biological activities of the G. asiatica plant in alignment with its traditional applications.
Antipyretic activity
Ikram et al. (1987) reported the antipyretic activity of G. asiatica roots. The residues of chloroform, hexane, and water fractions of ethanolic extract were administered (150 mg/kg p.o.) to male and female rabbits with yeast-induced fever. A comparison with aspirin as a standard reference revealed a noteworthy antipyretic effect in the chloroform and hexane extracts, demonstrating no observable toxicity at the administered dose.
Anxiolytic and neuroprotective activity
Kamboj (2015) conducted an assessment of the anxiolytic properties of G. asiatica using the Elevated Plus Maze (EPM) test. Animal subjects were administered a methanolic extract of the leaves (400 mg/kg). The results revealed a significant increase in both the number of rodents entering the open arm and the duration spent in this arm, compared to the control group. This heightened activity in the open arm, as opposed to the closed arm, signifies the anxiolytic effects of G. asiatica leaves. Moreover, these findings suggest a potential neuroprotective role for this plant.
Antiulcer effect
A study to investigate the antiulcer activity of G. asiatica plant was conducted by Girija and Ravindhran (2014) using three models (pylorus ligation-, aspirin-, and cold stress-induced ulcer albino mice models). The aqueous and methanolic extracts of roots, leaves, and stems as well as the powder derived from these parts were evaluated at both low (100 mg/kg p.o) and high doses (400 mg/kg p.o). A standard comparison was made using ranitidine (10 mg/kg i.p). The findings of this study revealed the effectiveness of G. asiatica roots and leaves in both the treatment and prevention of gastric ulcers. This effect was attributed to the reduction in acid secretion and an augmentation of the protective mucous lining in the stomach.
Antidiabetic activity
Kasiviswanath et al. (2005) investigated the hypoglycemic activity of G. asiatica root. The study employed normal and alloxan-induced diabetic rats distributed among nine groups. Ethanolic extract at different dosages of 100, 250, and 500 mg/kg were orally administered to the rats and compared with tolbutamide at a dose of 40 mg/kg p.o as a standard drug. A dose-dependent reduction in blood glucose level was observed among rats treated with the G. asiatica root extract. Notably, the study reported that the antidiabetic activity of G. asiatica surpassed that of the standard tolbutamide in diabetic rats, and no signs of toxicity were observed at therapeutic doses.
Anti-inflammatory activity
The anti-inflammatory activity of G. asiatica has been studied in vivo by Syed et al. (1997) and Merlin et al. (2009a), both utilizing the albino rat model. In the study conducted by Merlin et al., four methods with different agents were employed to assess the anti-inflammatory activity. The reports from these studies collectively indicate that Gmelina asiatica possesses significant anti-inflammatory effects, demonstrating efficacy in both acute and chronic inflammation, as succinctly detailed in Table 3.
Furthermore, the anti-inflammatory effect of G. asiatica was also evaluated in vitro by Kiruba et al. (2014). The study utilized albumin denaturation inhibition method, human erythrocyte membrane stabilization test, and inhibition of proteinase enzyme assay to investigate the in vitro anti-inflammatory effect of aqueous extract of G. asiatica. Aspirin served as the standard reference. The in vitro study exhibited the ability of this plant to suppress the denaturation of protein, inhibit red blood cells hemolysis and protect against proteinase activity. Thus, these findings indicate the potential anti-inflammatory activity of G. asiatica plant.
Antimicrobial activity
The pharmacological properties of Gmelina asiatica include demonstrated antibacterial and antifungal activities, as reported by various researchers who employed different parts of the plant and varied extraction solvents in their studies. In these investigations, antibacterial standards such as kanamycin, ampicillin, and amikacin, along with antifungal standards griseofulvin, climbazole, and ketoconazole, were employed. The reports revealed that Gmelina asiatica extracts exerted an inhibitory effect against a spectrum of bacteria, including Pseudomonas aeruginosa, Escherichia coli, Bacillus subtilis, Salmonella typhi, Staphylococcus aureus, Bacillus pumilus, Streptococcus faecalis, Micrococcus luteus, Streptococcus pneumoniae, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Actinomyces howelli, Bacillus circulans, and Streptococcus pyogenes. Additionally, these extracts demonstrated efficacy against various fungi, such as Candida albicans, Aspergillus niger, and Trichoderma viride as presented in Table 4. These results support the plant’s traditional use as an antiseptic agent and in wound healing (Merlin et al. 2009a).
Mosquito larvicidal activity
Muthukumaran et al. (2015) demonstrated the mosquito larvicidal activity of the aqueous extract of G. asiatica leaves and the silver nanoparticles synthesized from the same extract. The study targeted Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus, significant vectors of human diseases. Various concentrations of the aqueous extract (50, 100, 150, 200, and 250 ppm) were tested and the LC50 and the LC90 were determined. The results showed the effectiveness of G. asiatica extract in larvae mortality compared to the control group.
Similarly, Florence and Solomon (2016) reported the larvicidal activity of G. asiatica leaves against Culex quinquefasciatus and Aedes aegypti by exposing the species to different concentrations of four distinct extracts of G. asiatica leaves. These findings suggest the potential of G. asiatica as a natural insecticide, holding promise in the control of mosquito-borne illnesses such as malaria, dengue, and lymphatic filariasis.
Nematicidal effect
Two studies were carried out by Azhagumurugan et al. in 2014 and 2015 to investigate the nematicidal effect of G. asiatica leaves against Meloidogyne incognita, a significant species of root-knot nematode notorious for infesting a wide range of plants and causing crop destruction. The host plants for the nematode, namely the tomato plant and black gram plant, were utilized, and various concentrations of acetone extract from G. asiatica leaves were tested. These concentrations were subsequently compared with the control group by assessing different parameters. The results from these studies underscored the potential nematicidal effect of G. asiatica, emphasizing its agricultural significance and the prospect of employing it to safeguard crops from the detrimental impact of agricultural pests (Azhagumurugan and Rajan 2014a; Azhagu Murugan and Rajan 2015).
Nephroprotective potential
The nephroprotective activity of G. asiatica plant has been investigated in vitro using gentamicin-induced cytotoxicity in Vero cell line. To assess its potential for nephroprotection, the aqueous extract (50 µl of a 500 mg/ml solution) was applied, with Vitamin E serving as the reference compound in the experiment. The inhibition of nephrotoxicity was evaluated by two methods; the MTT assay and epifluorescence microscopy technique. The MTT assay demonstrated a dose-dependent response in cell survival following treatment with the G. asiatica extract. Moreover, the epifluorescence staining assay revealed a green color under the microscope, indicating the viability of treated cells. These findings collectively suggest the potential of the G. asiatica plant to act as a cytoprotective agent (Kiruba et al. 2014).
Hepatoprotective potential
Merlin and Parthasarathy (2011) assessed the hepatoprotective potential of G. asiatica extracts in the context of carbon tetrachloride (CCl4)-induced hepatotoxicity in Wistar albino rats and mice. Chloroform and ethanolic extracts of G. asiatica aerial parts were administered orally at a dose of 400 mg/kg per day for 5 days. A standard reference, silymarin at 50 mg/kg, was also administered orally for 5 days. Liver biochemical tests and histological study were conducted to examine the liver protective effect of both plant extracts and the standard. Following treatment with either plant extracts or silymarin, there was a significant decrease in the elevated levels of alanine aminotransferase (ALT), aspartate transaminase (AST), bilirubin, and alkaline phosphatase (ALP). Notably, the ethanolic extract exhibited a more pronounced effect compared to the chloroform extract. These results, corroborated by the histological study, confirm the hepatoprotective activity of G. asiatica extracts against CCl4-induced hepatotoxicity in rats.
Antioxidant activity
In vitro and in vivo studies have been conducted by different researchers to study the antioxidant activity of different parts of G. asiatica plant, as elaborated in Table 5. In vitro assessments utilized methods such as free radical scavenging test, reducing power assay, lipid peroxidation inhibition assay, and total antioxidant capacity method. For in vivo antioxidant activity evaluation, levels of reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and hydroperoxides were assessed in CCl4-treated Wistar albino rats. The reports revealed that G. asiatica has a significant antioxidant effect which could be the reason of many other therapeutic activities related to this plant.
Anticancer activity and cytotoxic potential
Various researchers have undertaken studies to investigate the anticancer activity of the G. asiatica plant. These investigations encompassed both in vitro assessments against human breast cancer and cervical cancer, with a singular in vivo study targeting lymphoma, as summarized in Table 6. The studies utilized different plant parts, including roots, leaves, and aerial parts. The collective results indicate that G. asiatica exhibits a noteworthy antiproliferative effect and cytotoxic activity. These findings suggest the potential of G. asiatica as a valuable candidate for further exploration and development as an anticancer agent.
Toxicity studies of G. asiatica plant
Toxicity studies on G. asiatica were conducted by various researchers, examining different parts of the plant (roots, stems, barks, leaves, and aerial parts), and utilizing diverse animal models such as rats, mice, and rabbits. Various extracts were employed and a range of doses was tested, as summarized in Table 7. The studies were performed according to OECD guidelines.
Phytochemistry
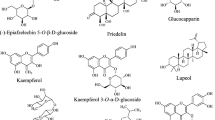
The examination of phytochemistry plays a crucial role in identifying the potential medicinal applications of plants, as certain secondary metabolites can indicate their suitability for specific medical fields. In the case of G. asiatica, extensive studies have utilized diverse solvents to scrutinize the phytoconstituents across its various parts, aiming to forecast its therapeutic value. Preliminary phytochemical screening, extensively detailed in Table 8, has been a cornerstone of many of these investigations. Identification of these phytoconstituents predominantly relied on the GC–MS technique, as elucidated in Table 9. Notably, a singular study employed LC–MS (UHPLC–HRMS) for a more nuanced analysis, also detailed in Table 9. While these analytical approaches have provided valuable insights, the isolation of compounds from G. asiatica has been somewhat limited, as summarized in Table 10. These isolated compounds predominantly include flavonoids, lignans, and a few other compounds, as showed in Figs. 2 and 3. On the other hand, Figs. 4 and 5 present the structures of compounds identified through LC–MS and GC–MS, respectively.
The initial attempts to analyze the components of this plant focused on seed oils, employing chromatographic techniques in 1961 and 1965 (Gunstone and Sykes 1961; Gunstone and Qureshi 1965). These investigations unveiled a rich composition, including high levels of saturated fatty acids such as palmitic and stearic acids (Gunstone and Qureshi 1965), cis-11-eicosenoic, oleic, linoleic, ricinoleic acids (Gunstone and Sykes 1961) alongside the presence of sitosterol (Nair and Subramanian 1975).
Conclusion and future perspective
G. asiatica, an invaluable medicinal plant, holds a significant place in traditional medicine due to its numerous therapeutic benefits. Local practitioners and healers have long utilized its properties to address various ailments, contributing to its esteemed status in folklore medicine. However, despite its extensive use, scientific efforts to validate these traditional uses have been limited. Moreover, there is a crucial need for in-depth studies on G. asiatica to unlock its full potential. Current gaps in research include the insufficient exploration of its phytoconstituents, the validation of their therapeutic values, and a comprehensive understanding of their mode of action. Addressing these gaps will not only contribute to the scientific understanding of this plant but also pave the way for harnessing its valuable therapeutic properties. Given the increasing demand for more effective medicines to combat diverse diseases, dedicating attention to the systematic study of G. asiatica is imperative. Through rigorous scientific investigation, we can capitalize on the medicinal benefits of this plant, offering new and improved treatment options.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- APG:
-
Angiosperm phylogeny group
- AST:
-
Aspartate transaminase
- BHT:
-
Butylated hydroxytoluene
- CAT:
-
Catalase
- CBC:
-
Complete blood count
- CCL4 :
-
Carbon tetrachloride
- CMC-Na:
-
Sodium carboxymethyl cellulose
- DPPH:
-
2,2-Diphenylpicrylhydrazyl
- EPM:
-
Elevated plus maze
- FTC:
-
Ferric thiocyanate
- GAE:
-
Gallic acid equivalent
- G. arborea :
-
Gmelina arborea
- G. asiatica :
-
Gmelina asiatica
- g.b.w:
-
Gram of body weight
- GC–MS:
-
Gas chromatography-mass spectrometry
- GSH:
-
Glutathione
- IC50 :
-
Half maximal inhibitory concentration
- i.p.:
-
Intraperitoneal injection
- MCF-7:
-
Michigan cancer foundation-7
- MDA-MB-231:
-
MD Anderson-metastatic breast 231
- MIC:
-
Minimum inhibitory concentration
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OECD:
-
Organisation for economic co-operation and development
- P.O.:
-
Per oral
- ppm:
-
Parts per million
- QE:
-
Quercetin equivalent
- SOD:
-
Superoxide dismutase
- STDs:
-
Sexually transmitted diseases
- TBA:
-
Thiobarbituric acid
- UHPLC–HRMS:
-
Ultra-high-performance liquid chromatography-high resolution mass spectroscopy
References
Ali M, Khan SA, Chang PS, Haque R, Bhatia K, Ahmad S (2014) Habb-e-Asgand, polyherbal Unani formulation, protects liver and antioxidative enzymes against paracetamol induced hepatotoxicity. Pharm Biol 52:506–515
Anjaneyulu ASR, Rao AM, Rao VK, Row LR (1975) The lignans of Gmelina asiatica. Phytochemistry 14:824. https://doi.org/10.1016/0031-9422(75)83052-4
Apparanantham T, Chelladurai V, Subramanian V (1982) Some tribal folk medicines of point calimere (Kodikkarai) in Tamil Nadu. Bull Med Ethnobot Res 3:173–177
Azhagu Murugan C, Rajan M (2015) Growth changes of black gram, Vigna mungo infected with root knot nematode, Meloidogyne incognita treated with leaf extract of Nilakkumil, Gmelina asiatica. Acta Parasitologica Globalis 6:107–111
Azhagumurugan C, Rajan M (2014a) Effect of leaf extract of Nilakumil, (Gmelina asiatica) against the root knot Nematode (Meloidogyne incognita). Res J Recent Sci 3:264–266
Azhagumurugan C, Rajan M (2014b) GC-MS analysis of methanalic leaf extract of Nilakkumil, Gmelina asiatica. Afr J Basic Appl Sci 6:153–158
Babu K, Parimala G, Sidhan V (2010) Micromorphological studies on Gmelina arborea and Clerodendrum serratum. Phcog J 2:137–141
Balijepalli MK, Tandra S, Pichika MR (2010) Antiproliferative activity and induction of apoptosis in estrogen receptor-positive and negative human breast carcinoma cell lines by Gmelina asiatica roots. Pharmacogn Res 2:113–119
Bose MFJN, Aron S, Mehalingam P (2014) An ethnobotanical study of medicinal plants used by the Paliyars aboriginal community in Virudhunagar district, Tamil Nadu. India Indian J Tradit Knowl 13:613–618
Chassagne F, Deharo E, Punley H, Bourdy G (2017) Treatment and management of liver diseases by Khmer traditional healers practicing in Phnom Penh area, Cambodia. J Ethnopharmacol 202:38–53. https://doi.org/10.1016/j.jep.2017.03.002
Chen S-l, Gilbert MG (1994) Verbenaceae. In: Raven PH, Wu Z (eds) Flora of China. Science Press, Beijing; Missouri Botanical Garden Press, St. Louis, pp 1-49
Choudhary M, Kumar V, Malhotra H, Singh S (2015) Medicinal plants with potential anti-arthritic activity. J Intercult Ethnopharmacol 4:147–179
Chowdhuri TK, Deka K (2019) Biodiversity and conservation of ornamental crops. In: Rajasekharan PE, Rao VR (eds) Conservation and utilization of horticultural genetic resources. Springer, Singapore, pp 139–216
Collett H, Hemsley WB (1890) On a collection of plants from upper Burma and the Shan States. Bot J Linn Soc 28:1–150. https://doi.org/10.1111/j.1095-8339.1890.tb01452.x
Cooper D (1842) Notes and memoranda on the properties and uses of plants, collected from the herbarium of the medical officers of the army: Kept at the Army Medical Museum, Fort Pitt, Chatham. Lancet 39:164–167. https://doi.org/10.1016/S0140-6736(02)76517-3
de Kok R (2012) A revision of the genus Gmelina (Lamiaceae). Kew Bull 67:293–329
Dharmadasa RM, Akalanka GC, Muthukumarana PRM, Wijesekara RGS (2016) Ethnopharmacological survey on medicinal plants used in snakebite treatments in Western and Sabaragamuwa provinces in Sri Lanka. J Ethnopharmacol 179:110–127. https://doi.org/10.1016/j.jep.2015.12.041
Esakkimuthu S, Mutheeswaran S, Elankani P, Pandikumar P, Ignacimuthu S (2021) Quantitative analysis of medicinal plants used to treat musculoskeletal ailments by non-institutionally trained siddha practitioners of Virudhunagar district, Tamil Nadu, India. J Ayurveda Integr Med 12:58–64. https://doi.org/10.1016/j.jaim.2018.11.005
Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HHS (1975) Potential value of plants as sources of new antifertility agents I. J Pharm Sci 64:535–598. https://doi.org/10.1002/jps.2600640404
Florence A, Domettila C (2016) Anatomical studies on leaf and stem of Gmelina asiatica L.: an ethnomedicinal important plant. J Pharmacogn Phytochem 5:115–119
Florence A, Jeeva S (2015) FTIR and GC-MS spectral analysis of Gmelina asiatica L. Leaves JSRR 5:125–136
Florence A, Jeeva S (2016a) Chemical composition of essential oil from the leaves of Gmelina asiatica L. J Med Plants Stud 4:8–10
Florence A, Jeeva S (2016b) In vitro anticancer activity of Gmelina asiatica L. leaf against human breast cancer cell line (MCF-7). Int J Pharm Sci Res 7:2116
Florence A, Regini Balasingh G (2016) Phytochemical analysis of Gmelina asiatica L. leaves. Int J Chem Stud 4:78–82
Florence AR, Balasingh GSR (2016) In vitro antibacterial activities of crude leaf extracts of Gmelina asiatica L. World J Pharm Res 5:536–549
Florence AR, Solomon J (2016) Larvicidal activity of Gmelina asiatica L. leaf extracts against Aedes aegypti and Culex quinquefasciatus. Ann Biol Res 7:12–20
Ganesh P, Sudarsanam G (2013) Ethnomedicinal plants used by Yanadi tribes in Seshachalam biosphere reserve forest of Chittoor district, Andhra Pradesh India. Int J Pharm Life Sci 4:3073–3079
Giesen W (2018) Tropical freshwater swamps (mineral soils). In: Finlayson CM, Milton GR, Prentice RC, Davidson NC (eds) The wetland book: II: distribution, description, and conservation. Springer, Netherlands, pp 199–226
Girija S, Ravindhran R (2011) Identification of antioxidant potential of Gmelina asiatica. Biosci Biotech Res Asia 8:845–848
Girija S, Ravindhran R (2014) Studies on pharmacognostic, acute toxic and antiulcer effect of Gmelina asiatica Linn. (Doctoral dissertation). University of Madras
Girija S, Ravindran R (2011) Screening for qualitative phytochemicals of Gmelina asiatica. Herb Tech Ind 21:74–76
Gunstone F, Qureshi MI (1965) Glyceride studies. Part IV. The component glycerides of ten seed oils containing linoleic acid. J Am Oil Chem Soc 42:961–965
Gunstone F, Sykes P (1961) Vegetable oils. IX.—application of reversed-phase chromatography to the analysis of seed oils. J Sci Food Agric 12:115–123
Hassan Mohammad M, Kanagasabai V, Nandini M, Prabhu K, Rao M, Kalaivannan J, Janaki C (2021) The Gc Ms analysis of ethyl acetate extract of one herbal plant, ‘Gmelina asiatica’. Nat Vol Essent Oils 8:6827–6836
Ikram M, Gul Khattak S, Naeemuddin Gilani S (1987) Antipyretic studies on some indigenous Pakistani medicinal plants: II. J Ethnopharmacol 19:185–192. https://doi.org/10.1016/0378-8741(87)90040-7
Janarny G, Ranaweera KKDS, Gunathilake KDPP (2021) Antioxidant activities of hydro-methanolic extracts of Sri Lankan edible flowers. Biocatal Agric Biotechnol 35:102081. https://doi.org/10.1016/j.bcab.2021.102081
Jayasingam T, Balasubramaniam S, Vivekanantharajah S (1992) Vegetation survey of the Wasgomuwa National Park: reconnaissance. Vegetatio 101:171–181. https://doi.org/10.1007/BF00033200
Jeeva S, Florence A, Sujin RM (2019) Therapeutic biology of Gmelina asiatica Linn. In: Mohan VR, Doss A, Tresina PS (eds) Ethnomedicinal plants with therapeutic properties. Apple Academic Press Inc, Palm Bay, pp 113–123
Jeevan Ram A, Bhakshu LM, Venkata Raju RR (2004) In vitro antimicrobial activity of certain medicinal plants from Eastern Ghats, India, used for skin diseases. J Ethnopharmacol 90:353–357. https://doi.org/10.1016/j.jep.2003.10.013
Jeyaprakash K, Ayyanar M, Geetha K, Sekar T (2011) Traditional uses of medicinal plants among the tribal people in Theni District (Western Ghats), Southern India. Asian Pac J Trop Biomed 1:S20–S25
Ju SK, Mj KC, Semotiuk AJ, Krishna V (2019) Indigenous knowledge on medicinal plants used by ethnic communities of South India. Ethnobot Res Appl 18:1–112
Kamboj S (2015) Pharmacognostic and anti-anxiety studies on leaves of Gmelina asiatica (Linn.). In: Global summit on herbals and natural remedies. Med Aromat Plants, Chicago, pp 103
Kannan R, Prasant K, Babu U (2012) Botanical pharmacognosy of stem of Gmelina asiatica Linn. Anc Sci Life 31:190–193
Kapur M (2016) Disorders of childhood and treatments. Psychological perspectives on childcare in Indian indigenous health systems. Springer, India, pp 129–143
Kasiviswanath R, Ramesh A, Kumar KE (2005) Hypoglycemic and antihyperglycemic effect of Gmelina asiatica Linn. in normal and in alloxan induced diabetic rats. Biol Pharm Bull 28:729–732
Kato M, Kosaka Y, Kawakita A, Okuyama Y, Kobayashi C, Phimminith T, Thongphan D (2008) Plant–pollinator interactions in tropical monsoon forests in Southeast Asia. Am J Bot 95:1375–1394
Khare CP (2007) Gmelina asiatica Linn. In: Khare CP (ed) Indian medicinal plants: an illustrated dictionary. Springer, New York, pp 1–1
Kiruba AK, Brindha P (2014) In vitro studies on nephroprotective efficacy of cynodon dactylon and Gmelina asiatica. Asian J Pharm Clin Res 7:111–120
Kooiman P (1975) The occurrence of iridoid glycosides in the Verbenaceae. Acta Bot Neerl 24:459–468
Krishnan V, Gopi M (2015) Micromorphological characterisation of two simulating root drugs: Gmelina arborea Roxb. and Gmelina asiatica L. (Verbenanceae). Indian J Med Healthc 4:1–5
Ksirri R, Bhanukiran K, Maity S, Maiti P, Hemalatha S (2023) Evaluation of anticancer activity of Gmelina asiatica leaves, in-vitro and in-silico studies. J Biomol Struct Dyn 5:1–16
Kusuma G, Joshi V (2010) Nomenclature of Anukta Dravya. Anc Sci Life 29:17–23
Lam HJ (1919) The Verbenaceæ of the Malayan archipelago: together with those from the Malayan peninsula, the Philippines, the Bismark-archipelago, and the Palau-, Marianne-and Caroline-islands. M. de Waal
Li B, Cantino PD, Olmstead RG, Bramley GLC, Xiang C-L, Ma Z-H, Tan Y-H, Zhang D-X (2016) A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Sci Rep 6:34343. https://doi.org/10.1038/srep34343
Mahendra C (2015) Insignificant activity of extracts of Gmelina asiatica and Ipomoea digitata against skin pathogens. J Pharm Negat Results 6:27–32
Mahendra C, Gowda D, Vijayakumar M, Babu U (2015) Anti-dandruff activity of supercritical fluid extracts of Rosemarinus officinalis and Gmelina asiatica. Indo Am J Pharm Res 5:1463–1467
Merlin N, Parthasarathy V (2010) Potential antitumour activity of Gmelina asiatica aerial parts against Dalton Ascites Lymphoma in mice. Asian J Chem 22:3193–3199
Merlin N, Parthasarathy V (2011) Antioxidant and hepatoprotective activity of chloroform and ethanol extracts of Gmelina asiatica aerial parts. J Med Plant Res 5:533–538
Merlin N, Parthasarathy V, Manavalan R, Devi P, Meera R (2009a) Phyto-physico chemical evaluation, anti-inflammatory and anti microbial activities of aerial parts of Gmelina asiatica. Asian J Res Chem 2:76–82
Merlin N, Parthasarathy V, Manavalan R, Kumaravel S (2009b) Chemical investigation of aerial parts of Gmelina asiatica Linn. by GC-MS. Pharmacogn Res 1:152–156
Merlin N, Parthasarathy V, Santhoshkumar T (2010) Induction of apoptosis in human breast cancer cell line MCF-7 by phytochemicals from Gmelina asiatica. Afr J Biotechnol 9:4451–4456
Munir AA (1984) A taxonomic revision of the genus Gmelina l. (Verbenaceae) in Australia. J Adelaide Bot Gard 7:91–116
Murthy KSR, Rani SS, Karuppusamy S, Lalithamba A, Pullaiah T (2020) Tree flora of Andhra Pradesh, India. In: Khasim SM, Long C, Thammasiri K, Lutken H (eds) Medicinal plants: biodiversity, sustainable utilization and conservation. Springer, Singapore, pp 33–86
Murugeswaran R, Rajendran A, Ahamed K, Arunachalam C, Venkatesan K, Thomas B (2016) Potential medicinal plants used in Ayurvedic system of medicine and their diversity in Southern Western Ghats of Coimbatore District, Tamil Nadu. India J Ayurvedic Herb Med 2:136–145
Mutheeswaran S, Mariappan A, Ragavendran K, Porchezhiyan V, Elankani P, Al-Dhabi NA, Arasu MV, Ignacimuthu S (2021) Quantitative ethnobotany of Paliyar tribe in Sathuragiri hills, Virudhunagar district, Tamil Nadu India. Adv Tradit Med. https://doi.org/10.1007/s13596-021-00609-z
Muthukumaran U, Govindarajan M, Rajeswary M, Hoti SL (2015) Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol Res 114:1817–1827. https://doi.org/10.1007/s00436-015-4368-4
Nair AGR, Subramanian SS (1975) Quercetagetin and other flavones from Gmelina arborea and G. asiatica. Phytochemistry 14:1135–1136. https://doi.org/10.1016/0031-9422(75)85211-3
Neamsuvan O, Kama A, Salaemae A, Leesen S, Waedueramae N (2015) A survey of herbal formulas for skin diseases from Thailand’s three southern border provinces. J Herb Med 5:190–198. https://doi.org/10.1016/j.hermed.2015.09.004
Parekh J, Jadeja D, Chanda S (2005) Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk J Biol 29:203–210
Patil K, Wadekar R (2021) Herbal drug patenting. In: Mandal SC, Chakraborty R, Sen S (eds) Evidence based validation of traditional medicines: a comprehensive approach. Springer, Singapore, pp 555–588
Ragupathy S, Newmaster SG (2009) Valorizing the ‘Irulas’ traditional knowledge of medicinal plants in the Kodiakkarai Reserve Forest. India J Ethnobiol Ethnomed 5:10. https://doi.org/10.1186/1746-4269-5-10
Rajesh N, Silvia S, Preethi K, Kumar E, Satyanarayana T (2013) Pharmacognostic standardization of stem of Gmelina asiatica Linn. J Chem Pharm Sci 1:187–192
Rajulu MBG, Suryanarayanan TS, Murali TS, Thirunavukkarasu N, Venkatesan G (2021) Minor species of foliar fungal endophyte communities: do they matter? Mycol Prog 20:1353–1363. https://doi.org/10.1007/s11557-021-01740-6
Rathnam V, Mudaliar KSM (2002) Siddha materia medica (medicinal plants division). Indian Medicine—Department of Homeopathy, Chennai
Ray S, Saini MK (2021) Cure and prevention of cardiovascular diseases: herbs for heart. Clin Phytosci 7:64. https://doi.org/10.1186/s40816-021-00294-0
Satyanarayana T, Katyayani B, Latha EH, Routhu K, Prasad YD (2007) Phytochemical studies on roots of Gmelina asiatica Linn. Pharmacogn Mag 3:156
Shibu A, Pandian SS, Dhanam S (2012) Antibacterial activity of the leaf, stem and root powders of Gmelina asiatica L. Biosci Biotech Res Asia 9:297–304
Silvia N, Satyanaraya T (2014) Phytochemical and antioxidant studies on methanolic extract of Gmelina asiatica Linn. stem. Int J Pharmacog Phytochem Res 6:276–281
Sivapalan SRS, Sanmugarajah V (2019) Dictionary of medicinal plants-scientific names, family and selected vernacular (English, Sinhala, Sanskrit and Tamil) names. Book Publisher International
Srivastava J (1967) Botanical studies of some Ayurvedic and Yunani drugs “Bidhara.” Q J Crude Drug Res 7:1051–1058
Sudhakar M, Rao CV, Rao P, Raju D (2006) Evaluation of antimicrobial activity of Cleome viscosa and Gmelina asiatica. Fitoterapia 77:47–49
Suthari S, Vatsavaya SR, Majeti NVP (2018) Ethnobotanical explorations in telangana, the youngest state in union of india: a synoptic account. In: Ozturk M, Hakeem KR (eds) Plant and human health, volume 1: ethnobotany and physiology. Springer International Publishing, Cham, pp 65–123
Syed IT, Gopalakrishnan S, Hazeena BV (1997) Biochemical modes of action of Gmelina asiatica in inflammation. Indian J Pharmacol 29:306–309
Top N, Mizoue N, Kai S, Nakao T (2004) Variation in woodfuel consumption patterns in response to forest availability in Kampong Thom Province, Cambodia. Biomass Bioenergy 27:57–68
Venkaiah M, Rao JP, Prameela R (2020) Biodiversity of Medicinal plants in the Eastern Ghats of Northern Andhra Pradesh, India. In: Khasim SM, Long C, Thammasiri K, Lutken H (eds) Medicinal plants: biodiversity, sustainable utilization and conservation. Springer, Singapore, pp 3–20
Vignesh RM, Sumitha VR (2021) A comparative pharmacognostic phytochemical and pharmacological evaluation of Gmelina arborea Roxb. and Gmelina asiatica Linn. (Doctoral dissertation). University of Kerala
Vignesh RMSV (2020) Morpho-anatomical appraisal of Gmelina asiatica Linn.: a pharmacobotanic approach for quality control of raw drug material. Int J Botany Stud 5:300–303
Vikneshwaran D, Viji M, Rajalakshmi K (2008) Ethnomedicinal plants survey and documentation related to Paliyar community. Ethnobot Leafl 12:1108–1115
Vinitha M, Prasanna G (2019) Phytochemical screening and in vitro antimicrobial activity of Gmelina asiatica L. BARK JETIR 6:604–616
Wilson E, Rajamanickam G, Vyas N, Agarwal A, Dubey G (2007) Herbs used in siddha medicine for arthritis–a review. Indian J Tradit Knowl 6:678–686
Zhao F, Chen Y-P, Salmaki Y, Drew BT, Wilson TC, Scheen A-C, Celep F, Bräuchler C, Bendiksby M, Wang Q (2021) An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biol 19:1–27
Acknowledgements
The author Rasha Ksirri acknowledges Indian Institute of Technology (Banaras Hindu University), Varanasi, India, Damascus University, Syria and Indian Council for Cultural Relations (ICCR), India for providing a scholarship. We extend our gratitude to Dr. V. Chelladurai, Professor (Retired) from the Department of Botany, Medicinal Plant Survey for Siddha, for his assistance in identifying the plant and providing the accompanying pictures.
Funding
No funding was received for the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
RK: design, literature review, writing, and editing-original manuscript. MK: reviewed, and provided critical comments. KB: reviewed, edited, and provided critical comments, SH: design, supervision, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ksirri, R., Khazem, M., Bhanukiran, K. et al. Gmelina asiatica: exploring traditional uses, pharmacological insights, and phytoconstituents—a comprehensive review (1961–2023). Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09951-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09951-x