Abstract
Carbazole alkaloids characterized by a heterocyclic aromatic basic skeleton are known from different organisms but do not represent a biogenetically homogenous group. The majority, comprising more than 330 derivatives, is derived from 3-methylcarbazole as common precursor and is designated as phytocarbazoles. They are nearly exclusively known from the four closely related plant genera Bergera (part of Murraya s. l.), Clausena, Glycosmis, and Micromelum of the family Rutaceae. Derived from anthranilic acid and malonyl-CoA the tricyclic basic skeleton is formed via a prenylated 2-quinolone intermediate. The following steps are speculated to involve the formation of 2-prenylindole and cyclization of the prenyl side chain to generate 3-methylcarbazole. Apart from different oxygenations and oxidations of the basic skeleton additional prenylations and geranylations contribute to the great structural diversity of phytocarbazoles which are grouped together according to their C13-, C18-, and C23-basic structures. Of taxonomic significance are the different oxidations of the characteristic C-3 methyl group leading to 3-formyl- and 3-carboxyl derivatives particularly accumulated in Clausena and Micromelum species. Predominant prenylation at C-5 is typical for Glycosmis and Micromelum, whereas in Clausena prenylation at different positions can contribute to an infrageneric grouping. Geranylation represents a characteristic biogenetic trend of Bergera. A wide variety of biological activities ranges from significant antimicrobial, antiprotozoal, and insecticidal properties to anti-inflammatory, antioxidative, antiplatelet aggregative, and anti-HIV activities. Of particular interest is the cytotoxicity of phytocarbazoles against various cancer cell lines, where some derivatives turned out to act as cell cycle inhibitors and apoptosis inducers. Especially the C23 derivative mahanine induced different cell-signaling pathways suggesting that it represents a multi-targeted and multi-functional compound that works on an array of different cancer types and has the potential to inhibit tumor growth in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbazole alkaloids are characterized by a tricyclic aromatic basic skeleton consisting of a central pyrrole ring fused with two benzene rings. Carbazole itself was originally isolated from the anthracene fraction of coal tar (Graebe and Glaser 1872). As shown in Fig. 1 several naturally occurring alkaloids are known to contain such a heterocyclic system but do not represent a biogenetically homogenous group. They were found in bacteria, myxomycetes, fungi, sponges, tunicates, and in the related plant families Apocynaceae and Loganiaceae. However, the vast majority of carbazoles, comprising more than 330 derivatives (Tables 1, 2, 3, 4, 5, 6), was shown to be derived from 3-methylcarbazol (1) as common precursor. This type of carbazoles, designated as phytocarbazoles (Chakraborty 1977), was nearly exclusively isolated from the Citrus (Rutaceae) family, where they are known only from the four closely related genera Bergera (part of Murraya s. l.), Clausena, Glycosmis, and Micromelum of the subfamily Aurantioideae (Bhattacharyya and Chakraborty 1987; Chakraborty and Roy 1991; Knölker and Reddy 2002, 2008; Schmidt et al. 2012).
The first three derivatives girinimbine (92), murrayanine (27), and mahanimbine (211), were isolated from the well-known South Asian Curry-leaf Tree, Bergera koenigii L. (published as Murraya koenigii (L.) Spreng.) by Chakraborty et al. (1964, 1965, 1966), respectively (Fig. 2). They exhibited antifungal properties against some human pathogenic fungi and initiated the search for further active derivatives within that class of alkaloids (Das et al.1965). Later, various phytocarbazoles have shown to exhibit significant antimicrobial (Chakraborty et al. 1995a, b; Ramsewak et al. 1999; Pacher et al. 2001; Ma et al. 2005; Nagappan et al.2011; Maneerat et al. 2012b; Tatsimo et al. 2015) and antiprotozoal properties (Bringmann et al. 1998b; Yenjai et al. 2000; Adebajo et al. 2006; Pieroni et al. 2012). In addition, anti-inflammatory (Shen et al. 2014; Xia et al. 2015; Lv et al. 2015; Nalli et al. 2016), antiplatelet aggregation (Wu and Huang 1992; Wu et al. 1996c, 1998a), antioxidant (Ramsewak et al. 1999; Tachibana et al. 2001, 2003), and anti-HIV activities (Meragelman et al. 2000; Kongkathip et al. 2005; Wang et al. 2005) were also reported. A matter of particular interest was their cytotoxicity against various cancer cell lines (Itoigawa et al. 2000; Ito et al. 2000, 2012; Cui et al. 2002; Roy et al. 2004, 2005; Sinha et al. 2006; Nagappan et al. 2011; Samanta et al. 2013; Das et al. 2014; Bhattacharya et al. 2014; Tatsimo et al. 2015). The bioactivities and the intricate structures of these alkaloids, sometimes leading to complex molecules, have already attracted considerable synthetic interest. They served as a stimulus for the development of new strategies for the construction of the skeleton as well as for successful total syntheses of a number of derivatives (e.g. Bringmann et al. 1998a; Knölker and Reddy 2002; Lebold and Kerr 2007; Schmidt et al. 2012; Qiu et al. 2013; Hesse et al. 2014; Börger et al. 2014; Dai et al. 2015; Markad and Argade 2016).
Apart from synthetic-chemical and pharmaceutical considerations the formation of phytocarbazoles awaked also biological interest. This biogenetic trend represents an important chemotaxonomic criterion apparently restricted to the Rutaceae family, where it separates the tribe Clauseneae from the Citreae within the subfamily Aurantioideae (Kong et al. 1986, 1988a, b; Samuel et al. 2001; Bayer et al. 2009; But et al. 2009; Shivakumar et al. 2016). With regard to the limited distribution the frequently cited report on the isolation of the phytocarbazole ekeberginin (160) from Ekebergia senegalensis A. Juss. of the Meliaceae family (Lontsi et al.1985) appears dubious. It might be explained by a confusion with Clausena anisata (Willd.) Hook.f. ex Benth., a well-known and widely distributed medicinal plant, superficially showing morphological similarities in leaves and inflorescence. Another exception is the recently published occurrence of the furocarbazoles 155 and 171 in Lonicera quinquelocularis Hard. of the Caprifoliaceae (Khan et al. 2016). However, regarding the chemical make-up of that family and the biogenetic trends already known from the genus Lonicera, the formation of phytocarbazoles is surprising and should be reconfirmed with properly identified plant material.
Although the chemistry of different types of carbazoles was comprehensively reviewed by Knölker and Reddy (2002, 2008), Schmidt et al. (2012), and some of their pharmacological values were summarized therein, a biologically orientated interpretation of the structural diversity and distribution of phytocarbazoles as well as their various activities is missing. In the course of our broad-based phytochemical comparison in Rutaceae, particularly within the subfamily Aurantioideae, distinct accumulation trends towards different types of phytocarbazoles, coumarins, quinolones, acridones, quinazolines, flavonoids, or amides were found in different genera and species as well as in different plant parts (Greger et al. 1996; Riemer et al. 1997; Vajrodaya et al. 1998; Grassi 1998; Lukaseder 2000; Lukaseder et al. 2009; Pacher et al. 2001; Herdits-Riemer 2002; Pemmer 2002). In accordance with previous reports (Wu 1991; Wu et al. 1991, 1996a, e; Ito et al. 1997) geographical and seasonal variation of carbazole profiles were also detected in different collections and even individuals of the same species. Of special biological interest was the discovery of the stress induced formation of carbazole phytoalexins suggesting an important ecological role of that type of alkaloids (Pacher et al. 2001).
In order to get an overview about the great variability of phytocarbazoles and to provide a quick visual reference guide for the different structures, all naturally occurring derivatives are grouped together in Tables 1, 2 and 3 according to their C 13-, C 18-, and C 23-basic skeleton. In addition, di- and trimeric derivatives are shown in Tables 4 and 5, respectively, whereas phytocarbazoles linked with other biogenetic moieties are presented in Table 6.
Structural relationships
To date not all steps in the biosynthesis of phytocarbazoles are confirmed experimentally, but the pathway depicted in Fig. 3 is now widely accepted. According to that anthranilic acid and malonyl-CoA form a 2-quinolone intermediate, which is prenylated at the nucleophilic C-3 position. The next steps are speculated to involve decarboxylation and the formation of 2-prenylindole (Lee et al. 1996; Schmidt et al. 2012). Since prenylation of indole at position C-2 is only rarely reported it should be pointed out that 2-dehydroprenylindole, previously only known as a synthetic product (Lee et al. 1996), was isolated from the roots of Glycosmis macrophylla (Blume) Miquel (=G. sapindoides Lindley) (Lukaseder 2000) and G. parviflora (Sims) Little (Bacher 1999; Pacher 2005). The following formation of the aromatic ring C is interpreted as cyclization product with the dehydroprenyl side chain (Fig. 3). A similar formation of a methylated aromatic ring involving a prenyl group with a conjugated diene system was already assumed for the quinolone derived phenaglydon isolated from G. cyanocarpa (Blume) Spreng. (Wurz et al. 1993). With regard to the still unconfirmed biosynthetic steps of phytocarbazole formation the alternative accumulation of pyranoquinolones in infected leaves of G. parviflora supports a common biosynthetic origin (Pacher et al. 2001).
2-Hydroxylation of 3-methylcarbazole (1) was suggested to play a key role in the biogenesis of many phytocarbazoles (Kureel et al. 1970b). Biomimetic studies have shown that hydroxylations are preferably attached at C-2 and C-1 position (Roy et al. 1982a). This is also suggested by the stress-induced formation of carbalexins A–C (12, 17, 14) together with 2-hydroxy-3-methylcarbazole (4) in G. parviflora (Pacher et al. 2001). As shown in the structural overview (Tables 1, 2, 3, 4, 5, 6), hydroxylations and methoxylations are found at almost each position of the two aromatic rings A and C with a noteworthy exception: they are generally missing at C-4, where in murrayaquinones A–E (9, 130, 247, 246, 129), koenigine-quinones A, B (20, 23), pyrayaquinones A–C (121, 131, 248), bismurrayaquinone A (269), and bikoeniquinone (291) only an oxygen atom is attached as part of a 1–4 quinoid system. In five derivatives (53, 88, 271, 285, 289) methoxylation is also shown to be linked to nitrogen.
Of taxonomic significance are the different oxidations of the characteristic C-3 methyl group leading either to the many 3-formylcarbazoles of the C 13, C 18, and C 23 series (Tables 1B, 2B, 3B), or to corresponding 3-carboxylcarbazoles (Tables 1C, 2C, 3C) (Fig. 7). By contrast, only a few derivatives are reported with a 3-hydroxymethyl group (79, 80, 88, 180, 181, 317) or the corresponding methyl- (81) and ethylether (82, 83). The co-occurrence of the 3-hydroxymethyl carbazole mukoline (80) and its 3-formyl congener mukolidine (33) in the roots of B. koenigii suggested a biogenetic connection (Roy et al. 1982b). Taking into account the partial conversion of koenoline (79) to the corresponding formyl derivative murrayanine (27) on standing at room temperature, the rare reports on 3-hydroxymethyl derivatives might be explained by their labile nature (Fiebig et al. 1985). The loss of the characteristic carbon atom at position 3, interpreted as product of oxidative decarboxylation (Chowdhury et al. 1987), results in the two 3-hydroxycarbazoles murrastanine A (77) and clausenawalline D (78), as well as in carbazole itself (75) and clausine V (76). The linkages between C-3 and C-5 in the dimeric clausenawalline B (293) and between C-3 and N in murrastifoline B (320) may also be explained by a foregoing 3-decarboxylation of the corresponding monomeric units. The incorporation of an additional carbon atom into position C-8 of the basic skeleton leads to the rare C 14-derivatives 84–87 and the dimers 272 and 292 with an 8-formyl group. If this additional carbon in murrayakonine C (217) is represented either by the methyl or by the formyl group, remains unclear. A specific enzymatic activity was discussed for the formation of the unique N-formyl- (89) and N-carbethoxy-3-methylcarbazole (90) (Kedderis et al. 1986; Chakrabarty et al. 1997).
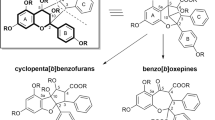
Apart from different oxygenations and oxidations of the 3-methylcarbazole basic skeleton shown in Table 1 prenylations and geranylations play a major role in structural diversification (Tables 2, 3), most likely catalyzed by specific prenyltransferases (Winkelblech et al. 2015). The prenyl moieties are found at each position of the two aromatic rings A and C, but are preferably linked to positions C-1 and C-8 (Tables 2, 3, 4). Exceptions are a twofold insertion of a prenyl group each in the rings A and C, leading to the C 23 carbazoles clausenawalline C (264) and clauszoline A (265), and the N-geranylation in karapinchamine A (254). Moreover, from the fruit pulp of B. koenigii the four dimeric bisgerayafolines A–D (307–310) were isolated, which deviate by the insertion of C-15 farnesyl groups (Uvarani et al. 2013, 2014). Unlike the co-occurring coumarins, where prenyl groups are frequently linked in a “reverse” way via their C-3′ (e.g. dentatine, nordentatine, clausarin), in phytocarbazoles they are solely connected in a “regular” way with C-1′. As in the co-occurring coumarins, quinolones, acridones, and flavonoids, prenyl groups give rise to the formation of pyrano and, to a lesser extent, furano carbazoles. The six- and five-membered carbazolelactones (Ito et al. 1998; Wu et al. 1998b), grouped together in Table 2D, can be interpreted as result of esterification between the 3-carboxyl group and the hydroxyl groups at 2′- or 1′-position, respectively, of the prenyl group. Consequently, lactonization with the hydroxyl group at C-3′ can be assumed for the formation of the seven-membered ring of clauemarazole C (198) and the related derivatives 186–190 with an additional ether bridge. Transformations of the geranyl side chain in the C 23 series contribute to a great variety of complex derivatives generating various ring systems (Fig. 4; Table 3A, B).
The formation of dimeric phytocarbazoles in plants was already expected by biomimetic studies (Roy et al. 1982a). The first derivatives bismurrayafolines A, B (311, 275) (Furukawa et al. 1983), and murrafoline A (303) (McPhail et al. 1983) were isolated from the roots of “Murraya” euchrestifolia Hayata.Footnote 1 In the meantime 61 bis-carbazoles have been isolated most likely representing products of oxidative dimerisation (Wu et al. 1991). They are grouped together in Table 4 according to the various combinations of monomeric units: in the symmetrical dimers two identical monomers are linked to each other via the corresponding positions at C-1, C-2, C-3, C-8, and C-8′ (Table 4A), whereas in the asymmetrical dimers different monomers are attached to almost each position (Table 4B). The N-linked dimers are presented separately in Table 4C. They are additionally characterized by frequent combinations with the C-3 methyl group of the second monomer. Two structurally unique trimeric phytocarbazoles, murratrines A (328) and B (329), were recently isolated from the leaves and stems of the Chinese “Murraya” (sect. Bergera) tetramera C. C. Huang together with 15 dimers (Lv et al. 2015) (Table 5).
The phytocarbazoles shown in Table 6 contain building blocks originating from different biosynthetic pathways. Carbazomarin A (332) from the roots of Clausena excavata Burm. f. is characterized by an incorporation of a pyranocoumarin moiety (Wu et al. 1996b). The double named “murrayanin” (334) from the aerial parts of B. koenigii, collected in Yunnan province (P. R. China), contains a phenylpropanyl unit linked to koenigine (99) (Wang et al. 2003). This building block was also found to be linked to claulansine J (51), forming claulansine K (333), which was isolated from the fruit peels of C. lansium (Lour.) Skeels, known as “Wampee” in China (Deng et al. 2014). In a subsequent investigation 333 was isolated as a mixture of two enantiomers in equal amounts (Du et al. 2015). The glycosmisines A (331) and B (330) from the stem bark of Glycosmis pentaphylla (Retz.) DC. deviate by an incorporation of a prenylindol moiety (Chen et al. 2015) (see “Appendix”).
Biological activities
Antimicrobial properties
The nitrogen-containing aromatic heterocyclic system of carbazoles possessing desirable electronic and charge-transport properties represents the structural basis for many biological activities (Zhang et al. 2010). Preliminary bioassays with phytocarbazoles for antimicrobial properties were carried out initially by Chakraborty and co-workers using the standard agar-cup assay method with nutrient agar for bacterial strains and Sabouraud’s medium for fungal strains. Although the activities were only moderate in comparison to standard antibiotics used in practice, considerable activities against the human pathogenic fungi Microsporum gypseum, Trichophyton rubrum, Candida albicans and the Gram-positive bacterium Nocardia asteroides were shown for murrayanine (27), girinimbine (92), and mahanimbine (211) (Das et al. 1965) (Fig. 2). Comparing the minimum inhibition zones of some related derivatives glycozolinine (7) exhibited the highest activity, especially against M. gypseum and T. rubrum (Chakraborty et al. 1975). The 2-methoxylated glycozolidol (15) from the roots of G. pentaphylla showed more activity against Gram-positive bacteria than against Gram-negative ones (Bhattacharyya et al. 1985). In a disc diffusion assay against different bacteria activities were observed for the C 23 derivatives murrayanol (242) and pyrayafoline D (=isomahanine) (244) from leaves of B. koenigii (Nutan et al.1998). Minimum inhibitory concentration (MIC) values against both types of bacteria and fungi were determined for clausenalene (19) from the stem bark of C. heptaphylla (Bhattacharyya et al. 1993) and for clausenal (41) from the leaves (Chakraborty et al. 1995a). The latter revealed pronounced activities against both Gram-positive (Bacillus subtilis, Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa), and fungi (T. rubrum, C. albicans) with MIC-values ranging from 3 to 25 µg/ml. Similar values against these organisms were obtained for clausenol (10) from the stem bark of C. anisata (Chakraborty et al. 1995b), and 6,7-dimethoxy-1-hydroxy-3-methylcarbazole (21) from the leaves of B. koenigii (Chowdhury et al. 2001) (Table 7). Comparative bioassays with stem bark carbazoles from B. koenigii revealed the strongest activity for girinimbine (92) against the Gram-positive S. aureus (MIC = 3.17 µg/ml), whereas the newly described dimeric carbazole 271 was the most potent compound against the Gram-negative E. coli (MIC = 25 µg/ml) and Proteus vulgaris (MIC = 6.25 µg/ml) as well as against the fungi Apergillus niger and C. albicans (MIC = 25 µg/ml) (Rahman and Gray 2005) (Table 7).
A bioassay guided fractionation of the leaf extract of B. koenigii resulted in the three active C23-derivatives mahanimbine (211), mahanine (214), and murrayanol (242) when tested against Candida kruseii, C. parapsilasis, E. coli, S. aureus and Streptococcus pyogenes. The most active compound was 214 with MIC100-values of 100 µg/ml against C. kruseii, C. parapsilasis, and E. coli, but 25 µg/ml against S. aureus and S. pyogenes, whereas 211 was least active with an MIC100 of 50 µg/ml against S. aureus and S. pyogenes, and no activity against the other organisms tested. These differences were speculated to be attributed to the free phenolic OH-group and the inhibition of topoisomerase I and II activities (Ramsewak et al. 1999). Similar results of leaf carbazoles from B. koenigii were later reported by Nagappan et al. (2011). The activities of compounds 214, 211, and its isomer (+) mahanimbicine (239b) were tested against antibiotic resistant bacterial strains involving the evaluation of the diameter of inhibition zone, minimum inhibition concentration (MIC), and minimum bactericidal concentration. In spite of the similar structures selective activities were shown for the three derivatives with the highest MIC value at 12.5 µg/ml for 214, and the lowest at 175 µg/ml for 211 against Streptococcus pneumoniae.
The C13-derivatives 7-methoxymukonal (46) and methyl carbazole-3-carboxylate (60), isolated from rhizomes and roots, respectively, of Clausena excavata exhibited significant antifungal activities against C. albicans with IC50-values at 2.8 µg/ml for 46 and 9.5 µg/ml for 60. In addition, the latter showed moderate activities (MIC = 50 µg/ml) against Mycobacterium tuberculosis (Sunthitikawinsakul et al. 2003). Higher anti-tuberculosis activities were reported for lansine (43), 3-formyl-6-methoxycarbazole (30), micromeline (162), and 3-formylcarbazole (25) from the stem bark of Micromelum hirsutum Oliv. Their MIC90 values against the M. tuberculosis strain H37Rv were 14.3, 15.6, 31.5, and 42.3 µg/ml, respectively (Ma et al. 2005). Based on these results a comparison of the activities of a series of naturally occurring and synthetic carbazoles informed about structure–activity relationships and the in vitro cytotoxicities against Vero cells (Choi et al. 2006, 2008). Due to the anti-periodontopathogenic activity against the Gram negative Porphyromonas gingivalis, the compounds 43, 30, and glycozolidal (44) deserve some interest for use in the treatment of periodontal diseases (Rodanant et al. 2015). 7-Hydroxyheptaphylline (134) and the dimeric clausenawallines B (293), and E (294), isolated from the roots of C. wallichii Oliv. showed pronounced antibacterial activity against the S. aureus strain TISTR 1466 with an MIC value at 8 µg/ml. Compound 134 was even more active against the methicillin-resistent strain SK1 with an MIC at 4 µg/ml, whereas 293 was weaker with 16 µg/ml. By contrast, all three derivatives were less active against the Gram-negative E. coli TISTR 780 and Salmonella typhimurium TISTR 292 with MIC values at 128 µg/ml for 293 and 294, and 64 µg/ml for 134 (Maneerat et al. 2012b). Six phytocarbazoles from the roots of Clausena harmandiana (Pierre) Guillaumin were tested for their activity against the oomycete Pythium insidiosum, the causative agent of the granulomatous disease pythiosis. In a disc diffusion assay clausine L (64) and especially clausine K (59) exhibited higher inhibition of the mycelial growth compared to the commercial antifungal agents terbinafine and itraconazole (Sriphana et al. 2013). Pronounced antibacterial activities against Shigella flexneri 2a and S. aureus ATCC 25923 (MIC = 64 µg/ml) as well as against different strains of Vibrio cholerae (MIC = 32–128 µg/ml) were reported for murrayamine A (95) isolated from C. anisata (Tatsimo et al. 2015). A reinvestigation of the antimicrobial activities of carbazoles from B. koenigii exhibited prominent IC50 values at 3.4 and 10.9 µmol for girinimbine (92) and 1-hydroxy-7-methoxy-8-prenyl-3-formylcarbazole (170), respectively, against Bacillus cereus IIIM 25, and moderate activities at 11.7, 17.0, and 20.9 µmol for murrayamine J (260), koenimbine (94), and koenigicine (100), respectively, against S. aureus ATCC 29213 (Nalli et al. 2016).
Activities against phytopathogenic fungi
In contrast to the activities reported for murrayanine (27), girinimbine (92), and mahanimbine (211) against human pathogenic fungi their antibiotic action against some plant pathogenic fungi was not promising (Das et al. 1965). These findings were also confirmed in the author’s laboratory by biotests against the facultative phytopathogenic fungi Cladosporium herbarum (Pers.) Link and Curvularia eragrostidis Boedijn. In bioautography on TLC plates sprayed with a conidial suspension of C. herbarum no activities were observed for girinimbine (92) and mahanimbine (211), but clear inhibition spots were shown for siamenol (117) from the leaves and fruits of “Murraya” (=Bergera) siamensis Craib and for murrayafoline A (3) from the underground parts. More detailed information about the different activities were obtained with germtube inhibition tests against C. eragrostidis showing EC50 values at 0.7 µg/ml for 117, and 2 µg/ml for 3 (Pemmer, 2002) (Table 7). The fungicidal activity of 3 was compared with that of three further 1-oxygenated derivatives, the demethylated 1-hydroxy-3-methylcarbazole (2), and the synthetic 1-O-prenylated and 1-O-glycoyslated 3-methylcarbazoles. Growth inhibition areas against the phytopathogenic fungus Cladosporium cucumerinum Ell. et Arth. at dose of 12.5 µg exhibited the highest activity for 3 (213 mm2) followed by 2 (205 mm2) and the 1-O-prenylated derivative (95 mm2). No inhibition zone was observed for the 1-O-glycosylated derivative (Cuong et al. 2008).
As shown in the literature and in broad-based phytochemical analyses in the author’s laboratory phytocarbazoles of the genus Glycosmis are mainly accumulated in the stem bark and to a lesser extent in the roots, but are rarely found in the leaves (Kamaruzzman and Chakraborty 1989; Greger et al. 1992). However, wounding, UV-irradiation, and especially infection with the fungus Botrytis cinerea resulted in an accumulation of phytocarbazoles in the leaves of G. parviflora and G. pentaphylla. Since the isolated carbalexins A, B, C (12, 17, 14) and 2-hydroxy-3-methylcarbazole (4) displayed pronounced antifungal activity in bioautographic tests on TLC plates against C. herbarum, they were regarded as a new class of phytoalexins (Pacher et al. 2001). In view of these results the previously published glycozolidol (15) (Greger et al. 1992) was also suggested to be formed after induction. This hypothesis could later be confirmed by reinvestigation of small samples of the corresponding (10 years-old!) hebarium material exhibiting an accumulation of 15 only in infested leaves, whereas intact leaves of the same individual did not show any carbazole. Comparing the structures of 12, 14, and 17 with 4 it was tempting to suggest that their antifungal properties were due to the free hydroxy group at C-2. However, the very weak activity of the co-occurring glycoborinine (114) containing such a hydroxy group but an additionally condensed pyran ring in aromatic ring A demonstrated that other substitutions also play an important role in bioactivity (Fig. 5) (Pacher et al. 2001).
Stress induced carbazole phytoalexins from Glycosmis species (Pacher et al. 2001)
Antiprotozoal activities
A series of natural and synthetic carbazoles were tested for activity against Plasmodium falciparum. 1-Hydroxy-3-methylcarbazole (2), isolated from M. euchrestifolia, exhibited promising antiplasmodial activity in vitro with an IC50 value at 7.62 µg/ml, whereas the 1-O-methylated derivative murrayafoline A (3) were virtually inactive. Interestingly, the synthetic 1,4-diacetoxy-3-methylcarbazole gave the highest activity in this series with an IC50 at 1.79 µg/ml. The results suggested that either the free phenolic hydroxy group of 3 is not required for the high activity or that the ester functionalities are cleaved by P. falciparum (Bringmann et al. 1998b). Similar antiplasmodial activities were reported for different carbazoles from Clausena species: IC50 values at 3.2–6.4 and 5.5–10.7 µg/ml were determined for heptaphylline (131) and clausine H (74), respectively (Yenjai et al. 2000), and at 2.46 µg/ml for the dimeric clausenawalline A (273) (Maneerat et al. 2011). Significant activities against the P. falciparum strain K1 were determined for mukonal (28), clauszoline K (32) and glycoborinine (114) with IC50 values at 4.03, 3.46, and 3.41 µg/ml, respectively (Auranwiwat et al. 2014), and a MIC value at 6.74 µg/ml for glycosinine (29) (Sripisut and Laphookhieo 2010). The leaf extract of B. koenigii exhibited activity against Trichomonas gallinae. A comparison between eight isolated and four structurally modified carbazoles provided information about structure–activity relationships. The C 18-derivatives girinimbine (92) and mukoenine A (=girinimbilol) (91) were the most active with IC50 values at 1.08 and 1.20 µg/ml, respectively. Concerning the different basic skeletons of the derivatives the order of activity was C 18 > C 23 > C 13. Insertion of a 7-OH group into mahanimbine (211) reduced the activity by about 29 and 27% at 24 and 48 h, respectively. Interestingly, acetylation of the OH-group in 91 and mahanimbinol (209) improved their activities from 1.20 and 4.00 to 0.60 and 1.08 µg/ml, respectively (Adebajo et al. 2006). The anti-trichomonal activity of 3-formylcarbazole (25), isolated from the stem bark of C. lansium, was determined with LC50 and LC90 values at 3.6 and 243.9 µg/ml, respectively, after 24 h, and at 9.7 and 41.4 µg/ml after 48 h (Adebajo et al. 2009). Lansine (43) was investigated for its activity against Leishmania donovani, the causative agent of visceral leishmaniasis. The synthesis of a series of analogues, and the rational modifications made at positions C-2, C-3, C-6, and N allowed to elucidate plausible structure–activity relationships with respect to their antileishmanial activity. Six derivatives demonstrated improved potency against L. donovani promastigotes in comparison to 43, and four also significantly enhanced activity against axenic amastigotes (Pieroni et al. 2012).
Insecticidal activities
Mosquitocidal assays against larvae of Aedes aegyptii exhibited high activity for the three carbazoles mahanimbine (211), mahanine (214), and murrayanol (242). The two compounds 214 and 242, possessing a free phenolic OH group, had an LD100 (24 h) at 12.5 µg/ml, while 211 showed 100% mortality at 100 µg/ml. Compound 211 showed 20% mortality at 6.25 µg/ml, while 214 and 242 showed 49 and 69% mortality, respectively, at 1 µg/ml (Ramsewak et al. 1999).
Cytotoxic activities
In the search for new naturally occurring cytotoxic antitumor compounds many carbazole alkaloids turned out to act as cell cycle inhibitors or apoptosis inducers (Shaikh et al. 2015). Initially, bioassays have focused on phytocarbazoles isolated from B. koenigii and the closely related M. euchrestifolia which were tested against cultured nasopharyngeal carcinoma (KB) cells. Significant cytotoxicity was observed for koenoline (79) and murrayanine (27) from the roots of B. koenigii with ED50 values at 4.0 and 26 µg/ml, respectively (Fiebig et al. 1985), and for murrayamine A (95) and (+) mahanine (214) from the leaves of M. euchrestifolia at 3.0 and 3.3 µg/ml, respectively (Wu 1991). Among the six pyranocarbazoles 94, 214, 215, 277, 305, and 308 from the fruit pulp of B. koenigii an increased cytotoxicty of mahanine (214) was reported against human cervical cancer (HeLa), stomach adenocarcinoma (AGS), and colorectal adenocarcinoma (HCT-116) cells, showing only weak activity against normal mouse embryonic fibroblasts (NIH3T3). In this connection the interactive behavior of the six carbazoles with calf thymus DNA was also evaluated. It was found that pyranocarbazoles containing either prenyl or geranyl moieties possessed more DNA binding properties than the parent compound suggesting that the cyclic planar part can intercalate with DNA while the prenyl or geranyl residue interacts externally with the minor groove of DNA. The higher binding constants of 214 also indicated stronger interaction between 214 and DNA (Uvarani et al. 2014). In a broad-based screening of 20 carbazoles against a panel of about 60 tumor cell lines murrayaquinone A (9) from the roots of M. euchrestifolia had a broad cytotoxic profile against KB, melanoma (SK-MEL-5), colon carcinoma (Colo-205), and ileocecal adenocarcinoma (HCT-8) cells with ED50 values at 5.18, 2.58, 3.85, and 5.50 µg/ml, respectively (Itoigawa et al. 2000). Another cytotoxic carbazole quinone, clausenaquinone A (24), previously isolated from the stem bark of C. excavata, also exhibited significant activity against HCT-8, malignant melanoma (RPMI7951), and rhabdomyosarcoma (TE671) cell lines with IC50 values at 0.92, 0.22, and 3.82 µg/ml, respectively (Wu et al. 1994). These results suggest that carbazole quinone derivatives have potential as cytotoxic agents with cell line selectivity (Table 8).
As shown in Table 8 different phytocarbazoles were tested against a panel of cancer cell lines. Apart from the active carbazole quinones 9 and 24, the majority of active derivatives are characterized by a 3-formyl and 2-hydroxy substitution. Increased cytotoxicity was observed by C-1-prenylation in 7-methoxyheptaphylline (135) against four different cell lines compared to methoxymukonal (46) (Chaichantipyuth et al. 1988), and by C-4-prenylation in clausine D (160) compared to O-demethylmurrayanine (26) (Jiang et al. 2014). On the other hand, unprenylated 46 exhibited a high IC50 value at 1.74 µg/ml against KB cells compared to the weak activities of the related C-1-prenylated heptazoline (136) (Thongthoom et al. 2010) and heptaphylline (132) (Sripisut et al. 2012) at 23.54 and 26.31 µg/ml, respectively (Table 8). Dramatic differences in activity against small cell lung cancer (NCI-H187) and KB cells were shown between the three C-1-prenylated heptaphylline derivatives 132, 134, and 135, differing only in C-7-substitution: heptaphylline (132) and its 7-methoxy derivative 135 exhibited strong cytotoxicity against NCI-H187 cells with IC50 values at 1.32 and 1.68 µmol, respectively, and at 1.61 and 2.75 µmol against KB cells. By contrast, the 7-hydroxylated 134 showed about 8- to 17-fold weaker activity at 14.59 and 27.9 µmol (Songsiang et al. 2011). Significant cytotoxic effects against breast cancer (MCF-7) cells were determined for lansine (43) and glycozolidal (44) from the roots of C. lansium both with an IC50 value at 0.78 µg/ml, which is higher active than the standard drug doxorubicin with 1.25 µg/ml. Also strongly active were the co-occurring mafaicheenamines A (183), E (196), and murrayanine (27) with IC50 values at 2.96, 3.1, and 3.76 µg/ml, respectively (Maneerat and Laphookhieo 2010; Maneerat et al. 2012a). A selective cytotoxic activity of clausine TY (73) from the bark of C. excavata was reported against T-lymphoblastic leukemia (CEM-SS) (IC50 = 8.2 µg/ml) (Taufiq-Yap et al. 2007), and a moderate activity of clausamine E (176) against promyelocytic leukemia (HL-60) cells (Ito et al. 2009). Using cell-based small molecule screening an anti-proliferative effect of murrayafoline A (3) on colon cancer cells was detected. It was shown that it suppressed Wnt/β-catenin signaling by promoting the degradation of β-catenin (Choi et al. 2010). The two recently described carbazoles murrastinine C (166) and murrayatanine A (222), isolated from the leaves and stems of B. koenigii collected in Malaysia, exhibited potent cytotoxic activity via the colorimetric (MTT) assay against HL-60 and HeLa cells, with CD50 values less than 20 µg/ml (Tan et al. 2015). Significant activity of murrayamine A (95) against HeLa cells was recently reported with an LC50 value at 3.26 µg/ml showing no toxicity to Vero cells (Tatsimo et al. 2015).
In continuation of their screening for antitumor-promoting agents in the family Rutaceae Ito et al. (2000) reported on potent inhibitory effects of phytocarbazoles from C. anisata. The activity was determined by assaying the capability to inhibit the expression of early antigen of Epstein-Barr virus (EA-EBV) in Raji cells which was induced by the tumor promoter phorbol 12-myristate 13-acetate (PMA). In the structure activity relationship analysis of nine active carbazoles prenylation at C-4 was shown to play an important role for inhibitory activity and especially ekeberginine (161) might be valuable as antitumor promoter in chemical carcinogenesis. Using the same test system, four carbazoles from the stem bark of G. pentaphylla (syn.: G. arborea (Roxb.) DC) showed weak dose-dependent inhibitory effects: glybomines B (113) and C (112) with an open prenyl group at C-5 were more effective at all concentrations than glycoborinine (114) and glycozolidine (16) (Ito et al. 2004). Kok et al. (2012) reported on the strong effect of girinimbine (92) on EA-EPV activation and the viability of Raji cells.
Cell cycle inhibition and apoptosis induction
In the course of a screening for new cell cycle inhibitors and apoptosis inducers Cui et al. (2002) examined more than thousand Chinese medicinal plants and found that C. dunniana H. Levl. exhibited strong activity in inducing apoptosis and inhibiting cell cycle at the G2/M phase on mouse tsFT210 cells. Bioassay-guided fractionation of the stem extract led to the isolation of the four 3-methylcarbazoles murrayafoline A (3), girinimbine (92), mahanimibine (211), and bicyclomahanimbine (226). Their activities were assayed by flow cytometry accompanied with morphological observation of the cells and their nuclei by light and fluorescent microscopy. Induction of apoptosis was observed in all four alkaloids with MIC values at 1.56, 25, 20, and 30 µg/ml for 3, 92, 211, and 226, respectively, while pronounced cell cycle inhibition in M-phase was determined for 3 with an MIC value 0.78 µg/ml (Cui et al. 2002). The antiproliferative and apoptosis inducing mechanisms of girinimbine (92) were later determined in-depth with colorectal carcinoma (HCT-15) (Wang et al. 2008) and human liver cancer (HepG2) cells (Syam et al. 2011). Among thirteen carbazoles from the bark of M. euchrestifolia murrayafoline A (3) and murrayazolinine (224) exhibited significant growth suppression in human promyelocytic leukemia (HL)-60 cells due to apoptosis mediated by the activation of the caspase-9/caspase-3 pathway (Ito et al. 2012). From C. vestita D. D. Tao eleven carbazoles were evaluated for their growth inhibitory activities against human liver cancer (HepG2). The various IC50 values revealed that the activities were significantly affected by structural modifications of the compounds showing the highest values for clauszoline K (32) at 4.23 µmol, 2-hydroxy-3-methylcarbazole (4) at 14.4 µmol, and clausine E (=clauszoline I) (61) at 15.8 µmol. Since clausine E (61) showed almost no cytotoxic effect against the normal liver cell line LO2 compared to 32 and 4, its potential anti-tumor activity and underlying mechanism as a novel anti-cancer drug was further investigated against HeLa, glioblastoma (T98G), nasopharyngeal carcinoma (CNE2), and hormon-independent breast cancer (MDA-MB-231). As a result clausine E (61) showed significant anti-tumor activity against all four cancer cell lines through its ability to induce cell cycle arrest in the S and G2/M phases (Lin et al. 2012). By contrast, a dramatic loss of activity against five human cancer cell lines was observed by 1-O-methylation in mukonine (62) (Liger et al. 2007). A reinvestigation of the anti-tumor activity of glycoborinine (114) (Ito et al. 2004), aimed at finding out the cell-signaling pathway related to its activity against human liver cancer (HepG2). It was shown that 114 could induce HepG2 apoptosis through the mitochondrial-dependent pathway, which might provide a promising approach to cure liver cancer (Yang et al. 2014). The cytotoxic effects of the recently described glycosmisines A (331) and B (330), and the dimeric biscarbalexine A (267) from the stem bark of G. pentaphylla were determined against HepG-2, lung adenocarcinoma (A549), and hepato cellular carcinoma (HuH-7) using the colorimetric (MTT) assay: glycosmisine A (331) inhibited cell proliferation in a dose-dependent manner with IC50 values at 50.30, 43.68, and 30.60 µmol in HepG-2, A549, and HuH-7 cells, respectively, and glycosmisine B (330) at 62.89, 57.10, and 62.87 µmol. Similar IC50 values at 60.06, 56.06, and 73.16 µmol were determined for biscarbalexine A (267) after 48 h of incubation. The three carbazoles also induced apoptosis in the same cell lines as evidenced by changes in the morphological features and their dose-dependent accumulation of a sub-G1 population (Chen et al. 2015).
Mahanine as inducer of different cell-signaling pathways
Mahanine (214) was identified as potent apoptosis-inducing agent in human promyelocytic leukemia (HL-60) cells. At a concentration of 10 µmol it caused a complete inhibition of cell proliferation and the induction of apoptosis in a time dependent manner. The cell death was characterized with changes in nuclear morphology, DNA fragmentation, activation of caspase like activities, and release of cytochrome c into cytosol (Roy et al. 2004). In a following study the authors investigated in-depth the various mechanisms induced by 214 on the activation of the apoptotic pathway in human leukemia U937 cells. They concluded that the cytotoxic effect of 214 can be compared with that of many anti-cancer drugs which induce apoptosis in tumor cells by permeabilizing mitochondrial membranes (Roy et al. 2005). In addition to 214 the isomeric pyrayafoline D (244) and the dimeric murrafoline I (299), isolated from the leaves of B. koenigii, were also shown to induce apoptosis in HL-60 cells. It was found that these compounds also induced the loss of mitochondrial membrane potential and the subsequent activation of caspase-9/caspase-3 (Ito et al. 2006). Of special interest was the anti-proliferative and apoptosis-inducing activity of 214 on prostate cancer. Sinha et al. (2006) showed that it was active both on androgen-responsive (LNCaP) and androgen-independent (PC3) cells and did not affect normal hepatocytes, cardiomyocytes, and skeletal muscle cells. Moreover, the concentration used were clearly below that used to induce apoptosis in the leukemic cell lines U937 and HL-60 mentioned above. In a following study the same authors showed that 214 can reverse the epigenetically silenced tumor suppressor gene RASSF1A (Ras association domain family member 1) in human prostate cancer cells (Jagadeesh et al. 2007). The involvement of various apoptotic pathways in the mahanine-induced cell death was investigated in the acute lymphoid (MOLT-3) and chronic myeloid (K562) leukemic cells using both in vitro and in vivo models. The results suggested that 214 is a multi-targeted or multi-functional compound that works on an array of different cancer types and has the potential to inhibit tumor growth in vivo (Bhattacharya et al. 2010) (Fig. 6). The authors also investigated the oxidative stress-mediated dysfunction of the chaperone protein Hsp90 by 214 as a possible strategic therapeutic in pancreatic cancer (Sarkar et al. 2013). To recognize structure–activity correlation the functional groups of 214 were chemically modified and the anti-proliferative activity of these derivatives was determined in 19 different cancer cell lines from 7 different types of cancer. Mahanine (214) showed enhanced apoptosis compared to its dehydroxylated derivative mahanimbine (211) indicating significant contribution of the C-7-OH-group. O-Methylated mahanine (215)- and N-methylated mahanimbine-treated cells exhibited apoptosis only at higher concentrations, suggesting additional contribution of the –NH group (Fig. 6).
Using biophysical techniques it was demonstrated that 214 interacts through strong association with the phosphate backbone of DNA, but is unable to induce any conformational change in DNA, hence suggesting the possibility of being a minor groove binder (Samanta et al. 2013; Uvarani et al. 2014). It was also reported that 214 synergistically increase cisplatin-mediated apoptosis in cervical cancer cells (HeLa, SiHa), thereby reducing the concentration of the toxic cisplatin by ~5–8 folds. Moreover, 214 potentially deactivated the signal transducer and activator of transcription 3 (STAT3) by suppressing its phosphorylation (Das et al. 2014). Based on extensive studies it was suggested that 214 is a novel mitochondrial complex-III inhibitor of the electron transfer chain with potent anti-glioblastoma multiforme activity both in in vitro and in vivo model triggering cell death by inducing cell cycle arrest through reactive oxygen species. Since it showed high therapeutic potential in several other cancer models 214 is regarded as a potential agent for future chemotherapy (Bhattacharya et al. 2014). Later, Bhattacharya et al. (2016) showed that 214 is involved in the cellular cross-talk between the tumor suppressor protein PTEN (phosphatase and tensin homolog) and the two protein complexes mTORC1 and mTORC2 (mammal target of rapamycin complex 1 and 2), having a central role in cell growth and proliferation. In a more recent paper 214 and pyrayafoline D (=isomahanine) (244) were shown to be cytotoxic to oral squamous cell carcinoma (CLS)-354 cells, triggering apoptosis via caspase-dependent and -independent mechanisms and inhibition of autophagic flux (Utaipan et al. 2017).
Anti-inflammatory activities
From twentyfive phytocarbazoles isolated from the roots of C. lansium five were assayed for their in vitro anti-inflammatory effects on superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB (formyl-L-methionyl-L-leucyl-l-phenylalanine/cytochalasin B). The results showed that O-demethylmurrayanine (26), methyl carbazole-3-carboxylate (60), murrayanine (27), and glycozolidal (=O-methyllansine) (44) exhibited strong inhibition of superoxide anion generation with IC50 values ranging from 3.5 to 4.6 µmol, while compounds 26, 27, and clausine D (160) inhibited elastase release with IC50 values at 6.9, 3.9, and 2.0 µmol, respectively (Shen et al. 2014). From twentyfour carbazoles from the stems of C. emarginata four displayed anti-inflammatory activity as measured by nitric oxide (NO) production in lipopolysaccharide (LPS)-induced microglia BV2 cells. Clausine K (59) exhibited the highest IC50 value at 4.61 µmol followed by clausine O (45) with 6.37, murrayanine (27) with 9.94, and clauemarazole E (158) with 10.91 µmol (Xia et al. 2015). A similar IC50 value at 6.13 µmol was reported for claulansine R (83) isolated from the stems of C. lansium (Du et al. 2015). Consistent with the traditional use of M. tetramera for the treatment of inflammation-related diseases five dimeric carbazoles with anti-inflammatory activities were isolated from leaves and stems. The inhibitory effects of murradines B (284), H (315), chrestifoline A (287), bismurrayafolinol (317), and 3,3′-[oxybis(methylene)]bis(9-methoxy-9H-carbazole (271) on LPS-induced NO production in BV2 microglia cells displayed IC50 values ranging from 11.2 to 19.3 µmol (Lv et al. 2015). In a more recent study the anti-inflammatory activities of carbazoles from leaves and stems of B. koenigii were evaluated against the LPS-induced inflammatory mediators tumor necrosis factor (TNF)-α and interleukin (IL)-6. In the in vitro experiments murrayakonine A (302), O-methylmurrayamine A (96), and murrayanine (27) were proven the most potent derivatives with IC50 values at 10, 9.4, and 7 µmol, respectively, against TNF-α, and with 8.4 µmol for 96 and 27 against IL-6 (Nalli et al. 2016). In a further investigation of twigs of C. lansium only 3-formyl-6-methoxycarbazole (30) was significantly active in inhibiting LPS-induced monocyte secretion of TNF-α. It showed comparable efficacy to the control dexamethasone (Rodanant et al. 2015).
Antiplatelet aggregation activities and vasorelaxing effects
Wu and Huang (1992) reported for the first time on the antiplatelet activity of phytocarbazoles from the stem bark of C. excavata. The aggregation of rabbit platelets induced by arachidonic acid (AA) showed 53 and 37% inhibition by the two 4-prenylated derivatives clausine D (160) and clausine F (173), respectively, at 1 µg/ml. Clausenaquinone A (24) showed 100% inhibition at 10 µg/ml, induced by AA (100 µmol) (Wu et al. 1994). In a further extensive investigation the same authors isolated seventeen carbazoles from the stembark. Most of them showed significant antiplatelet aggregative activity and vasorelaxing effect. Especially clausine E (61) exhibited 58.3 and 89.3% inhibition of rat aorta phasic and tonic contraction induced by norepinephrine (3 µmol), together with 87.0% inhibition of tonic contraction induced by K+ (80 mmol) + Ca2+ (1.9 mmol) (Wu et al. 1996c). Girinimbine (92), isolated from M. euchrestifolia, inhibited AA-, collagen-, U46619 (a synthetic analog of prostaglandin PGH2)-, and PAF (platelet-activating factor)-induced aggregation of rabbit platelets and ATP release in a concentration-dependent manner with IC50 values at 9.1, 16.7, 60, and 71.9 µmol, respectively. Compound 92 also inhibited cyclooxygenase activity as reflected by the attenuation of prostaglandin E2 (PGE2) formation (Ko et al. 1994). From the acetone extract of the leaves and roots of M. euchrestifolia bioassay-guided fractionation showed that twelve carbazoles have antiplatelet aggregative activity. Interestingly, murrayafoline A (3), murrayamine M (263), and (+) mahanine (214) promoted platelet aggregation or lysis at high dose, but they also exhibited antiplatelet aggregation activity at low concentration. These results might explain the use in Chinese medicine in that the dose and content variation in a prescription produced different, promotive or inhibitive, effects on therapy (Wu et al. 1998a).
Antioxidative properties
Antioxidant bioassays on phytocarbazoles were first conducted by Ramsewak et al. (1999) by analysis of model liposome oxidation using fluorescence spectroscopy. From the three leaf carbazoles mahanimbine (211), mahanine (214), and murrayanol (242) of B. koenigii the two latter showed only weak antioxidant activity. This was interpreted to be partially due to the precipitation of these compounds in the buffer solution used in the assay. However, compound 211 displayed 49% inhibition of lipid peroxidation after 21 min at 33.1 µg/ml. Tachibana et al. (2001) evaluated the antioxidant properties of the five leaf carbazoles euchrestine B (241), bismurrayafoline E (278), mahanine (214), mahanimbicine (239), and mahanimbine (211) on the basis of the oil stability index (OSI) (Nakatani et al. 2001) and their radical scavenging ability against 2,2-diphenyl-1-picrylhydrazyl (DPPH). The OSI values of compounds 241 and 214 were higher than those of α-tocopherol or butylated hydroxytoluene (BHT) and were assumed to contribute to the high value of the CH2Cl2 crude extract of B. koenigii. The DPPH radical scavenging activity increased with the number of hydroxyl groups in the order ascorbic acid (AA) > 278 > 241, 214 and α-tocopherol > BHT > 239, 211 (Tachibana et al. 2001). In a following study the same authors compared the activities of twelve carbazoles for antioxidative properties measuring OSI values and DPPH radical scavenging activity. Among nine monomeric carbazoles the three derivatives euchrestine B (241), mahanine (214), and pyrayafoline D (244), characterized by free phenolic OH groups, showed significantly higher activity. Free phenolic OH groups are also present in the four dimeric derivatives bismahanine (304), bispyrayafoline (277), mahabinine A (=8,10′-[3,3′,11,11′-tetrahydro-9,9′-dihydroxy-3,3′,5,8′-tetramethyl-3,3′-bis(4-methyl-3-pentenyl)]bipyrano[3,2-a]carbazole) (306), and bismurrayafoline E (278), but the activity of compound 278 was clearly weaker. This discrepancy might be explained by the fact that the OH-group of 278 is hindered by the ortho-substituent, whereas the OH-groups of 304, 277, and 306 are located at the outer part of the molecule. In comparison to the monomeric derivatives the dimeric carbazoles showed a tendency to raise DPPH radical scavenging activity (Tachibana et al. 2003). Kok et al. (2012) reported on the antioxidant activity of girinimbine (92) isolated from B. koenigii. It was shown to be comparable with that of α-tocopherol when measured with the ferric thiocyanate (FTC) method and was able to inhibit superoxide generation in the phorbol 12-myristate 13-acetate (PMA)-induced promyelocytic HL-60 cells. However, in cotrast to α-tocopherol 92 failed to scavenge the DPPH-free radical.
Anti-HIV activities
The lipophilic extract of the aerial parts of “Murraya” (=Bergera) siamensis showed anti-HIV activity in the XTT-tetrazolium assay. Bioassay-guided fractionation led to the three phytocarbazoles siamenol (117), mahanimbine (211), and mahanimbinol (=mahanimbilol) (209). The newly described C-6-prenylated siamenol (117) exhibited the highest activity (EC50 = 2.6 µg/ml), reaching 50–60% maximum protection in the XTT-tetrazolium assay. Compound 209 was less active with an EC50 value at 8.6 µg/ml, whilst 211 was shown to be inactive (Meragelman et al. 2000). The crude extract from the underground parts of C. excavata was found to inhibit HIV-1 activity by the syncytium assay. Beside a limonoid and a pyranocoumarin the three carbazoles glycosinine (=O-methylmukonal) (29), 3-formyl-2,7-dimethoxycarbazole (47), and clausine K (=clauszoline J) (59) were shown to contribute to the activity. The results showed that compound 29 strongly exhibited anti-HIV-1 activity with an EC50 value at 12 µmol and an IC50 value at 680 µmol for inhibition of tetrazolium conversion to formazan. Compounds 47 and 59 exhibited activities with EC50 values at 29 and 34 µmol, and IC50 values at 232 and 54 µmol, respectively (Kongkathip et al. 2005). The two C-5-prenylated carbazoles glybomine B (113) and glycoborinine (114) from the twigs and leaves of G. montana were tested for in vitro inhibitory effects against HIV replication in C8166 lymphocytes. They demonstrated only weak to moderate anti-HIV activity with IC50 values at 9.73 and 4.47 µg/ml, respectively (Wang et al. 2005).
Anti-hyperlipidemic and anti-hyperglycemic activities
In the search for new pancreatic lipase inhibitors from plants 63 extracts from 21 different plants were screened for their inhibitory activity in vitro. All leaf extracts (CH2Cl2, EtOAc, MeOH) from B. koenigii exhibited antilipase activity greater than 80% which was shown to be correlated with the presence of carbazoles. Bioactivity guided fractionation led to the isolation of the four carbazoles mahanimbine (211), koenimbine (94), koenigicine (100), and clauszoline K (32) with IC50 values of 17.9, 168.6, 428.6, and <500 µmol, respectively (Birari et al. 2009). The most active mahanimbine (211) when given orally (30 mg/kg/day) significantly lowered the body weight gain as well as plasma total cholesterol and triglyceride levels in rats (Birari et al. 2010). The leaf extract of B. koenigii was also shown to decrease hyperglycemia and insulin resistance in dexamethasone-treated mice (Pandey et al. 2014). Based on preliminary evaluations of carbazoles on glucose uptake and GLUT4 (glucose transporter type 4) translocation in L6-GLUT4myc myotubes the activity of the four derivatives koenimbine (94), O-methylmurrayamine A (96), koenigicine (=koenidine) (100), and mahanimbine (211) were investigated in streptozotocin-induced diabetic mice. Koenigicine (100) was identified as a metabolically stable antidiabetic compound, when evaluated in a rodent type 2 model and showed a considerable reduction in the postprandial blood glucose profile with an improvement in insulin sensitivity (Patel et al. 2016). Mahanimbine (211) was shown to prevent high-fat diet-induced hyperlipidemia and fat accumulation in adipose tissue and liver along with the restricted progression of systemic inflammation and oxidative stress. Since adiposity and inflammation are major factors responsible for insulin resistance, benefits of compound 211 can be extended in improvement of insulin sensitivity (Jagtap et al. 2016).
Melanogenesis inhibition
The EtOAc fraction of the methanolic leaf extract of Sri Lankan B. koenigii was shown to significantly inhibit melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells with an IC50 value at 11.6 µg/ml. Fourteen carbazoles were isolated from this fraction and tested for their inhibitory properties. Among eight active derivatives koenimbine (94) showed the highest activity with an IC50 value of 1.2 µmol. A high inhibition (IC50 = 1.4 µmol) was also determined for mahanimbine (211), but a considerable cytotoxic effect was observed at 10 µmol. Mahanimbicine (239) and murrayamine E (230) inhibited melanogenesis with IC50 values at 2.2 and 2.9 µmol (Nakamura et al. 2013).
Hepatoprotective effects
From the stems of C. emarginata twentyfour carbazoles were isolated and assayed for their hepatoprotective effects. The six derivatives claulansine I (156), clausine F (173), clauszoline E (150), methyl 6-methoxycarbzole-3-carboxylate (65), methyl carbazole-3-carboxylate (60), and O-demethylmurrayanine (26) displayed activities against DL-galactosamine-induced toxicity in hepatocytic precursor (WB-F344) cells (Xia et al. 2015). Moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in human liver cancer (HepG2) cells was determined for the five derivatives claulansines N (34), P (189), Q (82), glycozoline (8), and methyl carbazole-3-carboxylate (60) from the stems of C. lansium (Du et al. 2015).
Miscellaneous properties
Mahanimbine (211), mahanine (214), and mahanimbicine (239) were investigated for their efficacy in healing subcutaneous wounds. Compound 239 showed the highest rate of collagen deposition with well-organized collagen bands, formation of fibroblasts, hair follicle buds and with reduced inflammatory cells compared to wounds treated with the compounds 214 and 211 (Nagappan et al. 2012). In an antimutagenic assay (+) mahanine (214) inhibited approximately 99% of the mutation induced by heterocyclic amines such as Trp-P-1 (tryptophan pyrolysis product 1) with an IC50 value of 5.2 µmol (Nakahara et al. 2002). Murrayafoline A (3) was shown to inhibit the proliferation of vascular smooth muscle cells and hence, may be a good candidate for the management of and protection from atherosclerosis and vascular restenosis (Han et al. 2015). Clausine Z (36) isolated from the leaves and stems of C. excavata exhibited potent inhibition of CDK5 (cyclin-dependent kinase 5), an essential kinase in sensory pathways, in a filter plate assay with an IC50 value at 0.11 µmol and showed protective effects on cerebellar granule neurons in vitro (Potterat et al. 2005). Claulansine F (143) from the stems of C. lansium was shown to promote neuritogenesis in PC12 cells via the extra cellular signal-regulated kinase (ERK) pathway (Ma et al. 2013). In a more recent study this working group demonstrated that compound 143 also initiated neuronal differentiation with a significant increase of TuJ-1 (neuron-specific β-III tubulin antibody) positive cells and TuJ-1 protein levels. They also found that 143 promoted the maturity and sustainability of neurons by increasing MAP2 (microtubule-associated protein)-positive cells and MAP2 protein levels (Huang et al. 2016). Hyperphosphorylation of tau protein is thought to play an important role in the etiology of Alzheimer’s disease. A possible new therapeutic strategy was discussed by reducing the phosphorylation by activation of phospho-tau phosphatase (PP2A) by carbazoles, e.g. 6,7-dimethoxy-1-hydroxycarbazole (21), from B. koenigii (Manimekalai et al. 2015).
Chemotaxonomy
The ability to produce and accumulate phytocarbazoles appears to be restricted to the four genera Murraya, Clausena, Glycosmis, and Micromelum of the family Rutaceae, grouped together in the tribe Clauseneae of the subfamily Aurantioideae. Within the genus Murraya there is a clear morphological and chemical dichotomy leading to the recognition of the two sections Murraya and Bergera (Kong et al. 1986; Wu et al. 1996e). Species of the section Murraya (e.g. M. paniculata (L.) Jack, M. exotica L., M. gleniei Thw. ex Oliver, etc.) are characterized by 8-prenylated coumarins, polyoxygenated flavonoids, and 3-prenylindols, whereas those of the section Bergera (e.g. M. koenigii (L.) Spreng., M. euchrestifolia Hayata, M. siamensis Craib., etc.) clearly deviate by the formation of many different phytocarbazoles at present unknown in section Murraya (Kong et al. 1988a; Reisch et al. 1994; Pemmer 2002). According to phylogenetic analyses of the subfamily Aurantioideae based on non-coding plastide DNA sequences the classical subdivision into tribes Clauseneae and Citreae is only justified if the genus Murraya (exclusive the species segregated as Bergera) is transferred to the tribe Citreae (Samuel et al. 2001; But et al. 2009). The reinstatement of Bergera as a separate genus is also supported by pollen morphology (Mou and Zhang 2009) and analysis of chloroplast genomes (Shivakumar et al. 2016). In view of the chemical characters the genus Micromelum takes a somewhat intermediate position containing carbazoles as well as 8-prenylated coumarins and polyoxygenated flavonoids (Bowen and Perera 1982; Kong et al. 1988b; Grassi 1998). Regarding the available plastid DNA data of the tribe Clauseneae, Micromelum forms a weakly supported clade with Glycosmis, whereas Bergera shows close relationships to Clausena (Samuel et al. 2001; But et al. 2009).
Broad-based phytochemical comparisons within the tribe Clauseneae exhibited a predominant formation of carbazoles in the genera Clausena (Herdits-Riemer 2002) and Bergera (Pemmer 2002), while in Glycosmis (Vajrodaya 1998) and Micromelum (Grassi 1998) they represented only minor constituents. Generally, carbazoles are mainly found in stem bark and roots but occur also as major compounds in the leaves of Bergera species (listed as Murraya in Tables 1, 2, 3, 4, 5, 6 according to literature). The ability to produce carbazoles appears to have reached its peak in B. koenigii which has already yielded around 80 different derivatives (Nalli et al. 2016). Structurally simple C13-carbazoles preferentially occur in the roots while the more complex C18- and C23-derivatives accumulate in the stem bark. However, in Bergera large amounts of C23-derivatives (e.g. mahanimbine (211) and mahanine (214)) were also isolated from the leaves and fruits. A remarkable seasonal variation of carbazole profiles was observed in the leaves of B. (“Murraya”) euchrestifolia by different cyclizations of the geranyl chain of the C23-derivatives cyclomahanimbine (=murrayazolidine) (220), murrayazoline (227), murrayamine F (250)), and the formation of the dimers bis-7-hydroxygirinimbines B (301) and A.Footnote 2 While the C18-pyranocarbazole girinimbine (92) was detected in all seasons, the C23-derivatives 220, 227, and 250 were found only in spring (May), and the dimer 301 only in winter (February). Variations were also found in autumn (November) by the oxidation of the 3-methyl group leading to the murrayamines J (260), N (261), M (263) and the oxidation of one methyl group of the dimethylpyran rings leading to the murrayamines K (104) and I (105) (Wu et al. 1996e). In spite of these seasonal variations and the differences observed between geographical provenances (Pemmer 2002; Herdits-Riemer 2002), some general trends become apparent from the present overview which are obviously of chemotaxonomic significance.
The carbazoles of the genus Glycosmis are characterized by the common trend towards 5-prenylation, such as in glycomaurrol (109), glycomaurin (111), glybomine C (112), glycoborinine (114), and glycosmisine A (331), as well as by the mostly unoxidised C-3 methyl group (Kumar et al. 1989; Rahmani et al. 1998; Vajrodaya 1998; Lukaseder 2000; Pacher et al. 2001; Ito et al. 2004; Wang et al. 2005; Yang et al. 2012; Chen et al. 2015). With respect to these common trends the accumulation of the 3-formylcarbazole murrayanine (27) and the dimeric bisisomahanine (279) together with murrayafoline A (3) in G. stenocarpa (Cuong et al. 2004) appears “unusual” for Glycosmis, but suggests relations to Bergera or Clausena. The trend towards 5-prenylation is also typical for the genus Micromelum, where, however, the carbazoles deviate by frequent oxidations of the C-3 methyl group leading to formyl derivatives such as 3-formyl-6-methoxycarbazole (30), lansine (43), the double named micromeline (162) (cf. Lamberton et al. 1967), as well as to the 3-carboxyl derivatives methyl carbazole-3-carboxylate (60), the newly named “microfalcatine” (178), and clauraila C (179) (Kong et al. 1988b; Grassi 1998; Bacher 1999; Ma et al. 2005) (Fig. 7).
To determine chemotaxonomic relevant characters in the genus Clausena and to detect species-specific chemical profiles a broad-based UV-HPLC comparison was carried out with different collections from Thailand. In this connection the HPLC profiles of 19 samples of the widepread C. excavata showed that carbazoles are accumulated mainly in the stem bark and roots but were only occasionally detected in small amounts in the leaves (Herdits-Riemer 2002). In contrast to Glycosmis the carbazoles showed a predominant trend towads oxidation of the C-3 methyl group leading to the formation of many 3-formyl and 3-carboxyl derivatives. Mostly small amounts of structurally simple derivatives such as 3-formylcarbazole (25), glycosinine (29), O-demethylmurrayanine (26), 7-methoxymukonal (=2-hydroxy-3-formyl-7-methoxycarbazole) (46), 3-formyl-2,7-dimethoxycarbazole (47) as well as methyl carbazole-3-carboxylate (60), clausines E (61), and L (64) were shown to be widespread in the stem bark and roots. Only the 3-carboxylcarbazole clausine K (=clauszoline J) (59) was frequently accumulated in bigger amounts. A characteristic trend of the stem bark of all collections was the accumulation of the 1-prenylated heptazoline (136) and the structurally related 1-geranylated clauszoline F (257) as major constituents. In addtion, clauszoline B (167), a pyranocarbazole closely related to 136, was found in bigger amounts in collections from N-Thailand (Herdits-Riemer 2002). Higher quantities of plant material led to extraction of further closely related unprenylated derivatives (Wu et al. 1996c, d). In view of this common biogenetic trend the preferred formation of the 4-prenylated carbazoles clausines D (160) and F (173) in the stem bark (Wu and Huang 1992) and the related lactones clausevatines D (201), E (203), F (204), G (205), and clausamine A (199) in the roots (Wu et al. 1998b) appears “irregular” for that species.
Due to its wide use as medicinal plant C. anisata was already subject of many phytochemical analyses, especially in Africa. Apart from the “unusual” 7- and 8-prenylated carbazoles clausanitin (164) and atanisatin (168) (Okorie 1975), not found in subsequent investigations, the profiles were characterized by a predominant trend towards 4-prenylation such as in ekeberginine (161) (Ngadjui et al. 1989; Songue et al. 2012), clausamines A (199), B (200), C (202), D (174), E (176), F (175), G (177), clausine F (173) (Ito et al. 1998, 2000), furanoclausamines A (207), B (206) (Ito et al. 2009), and murrayamine H (108) (Tatsimo et al. 2015).
C. lansium, known as “wampee” in China or “mafaicheen” in Thailand, is a popular fruit tree in Southeast Asia. Since it is also used in traditional Chinese medicine a number of phytochemical investigations have already been carried out. As in other Clausena species the root extract was shown to contain mainly simple C13-formyl derivatives such as 3-formylcarbazole (25), O-demethylmurrayanine (26), murrayanine (27), 3-formyl-6-methoxycarbazole (30), 3-formyl-1,6-dimethoxycarbazole (38), lansine (43), glycozolidal (44). A more specific trend is the formation of 2-prenylated derivatives related to indizoline (157), found in the roots, twigs, and stems. They are additionally characterized by oxidations and cyclizations of the prenyl unit leading to the mafaicheenamines A (183), B (185), C (193), D (192), E (196) (Maneerat and Laphookhieo 2010; Maneerat et al. 2012a), claulansines A (186), B (187), C (184), D (197), E (195), I (156), M (159), P (189), clausenalines B (191), C (194), claulamine E (182), 1′-O-methylclaulamine B (188), and 1′-acetylclaulamine B (190) (Li et al. 1991; Liu et al. 2012; Shen et al. 2012, 2014; Du et al. 2015). Regarding this characteristic biogenetic capacity it is tempting to assume a close relationship to C. emarginata C. C. Huang, showing a very similar carbazole profile predominated by the 2-prenylated derivatives clauemarazoles E (158), C (198), the mafaicheenamines C (193), D (192), E (196), claulamine E, and clauslansine I (156) (Xia et al. 2015). Generally, phytocarbazoles of Clausena species accumulate mainly in the stem bark, where prenylation at position C-1 frequently leads to the formation of derivatives of heptaphylline (132) and girinimbine (92) as major constituents (Fig. 7). However, additional prenylations at C-4 in C. anisata or C-2 in C. lansium apparently reflect species-specific biogenetic trends. Further comparisons of different geographical provenances of the morphologically similar C. harmandiana (Pierre) Guillaumine, C. heptaphylla (Roxb.) Wight & Arn., and C. indica (Dalz.) Oliv. will have to show whether these chemical trends can contribute to a better species delimitation.
Of great taxonomic significance is the formation of geranylated derivatives. With the exception of mukoenine B (=clausenatine A) (255) and clauszoline F (256), isolated from C. excavata, the C23-carbazoles shown in Table 3 are only known from species of the genus Bergera (part of Murraya s. l.). Most of them are additionally characterized by an unoxidized C-3 methyl group (Fig. 7). Regarding these biogenetic trends the carbazoles reported for C. dunniana Lév, containing mahanimbine (211) and bicyclomahanimbine (226) (Cui et al. 2002), don’t fit to the chemical profiles of Clausena, but show great similarities with those of the genus Bergera. The formation of the unique trimeric carbazoles murratrines A (328) and B (329) in M. tetramera C. C. Huang together with a series of dimeric derivatives deserves special taxonomic interest (Lv et al. 2015). Due to its preferred accumulation of carbazoles it should be removed from the genus Murraya. Although no C23-carbazoles have as yet been isolated structural similarities of the monomeric subunits as well as morphological characters suggest close relationship with B. (“Murraya”) euchrestifolia.
References
Adebajo AC, Ayoola OF, Iwalewa EO, Akindahunsi AA, Omisore NOA, Adewunmi CO, Adenowo TK (2006) Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 13:246–254
Adebajo AC, Iwalewa EO, Obuotor EM, Ibikunle GF, Omisore NO, Adewunmi CO, Obaparusi OO, Klaes M, Adetogun GE, Schmidt TJ, Verspohl EJ (2009) Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J Ethnopharmacol 122:10–19
Auranwiwat C, Laphookhieo S, Trisuwan K, Pyne SG, Ritthiwigrom T (2014) Carbazole alkaloids and coumarins from the roots of Clausena guillauminii. Phytochem Lett 9:113–116
Bacher M (1999) Strukturaufklärung von Sekundärmetaboliten aus tropischen Rutaceen und Meliaceen. Ph.D. thesis, University of Vienna (herein the NMR data of the newly described derivatives 117, 118, 178, 179, 181, 237)
Bayer RJ, Mabberley DJ, Morton C, Miller CH, Sharma IK, Pfeil BE, Rich S, Hitchcock R, Sykes S (2009) A molecular phylogeny of the orange subfamily (Rutaceae: Aurantioideae) using nine cpDNA sequences. Am J Bot 96:668–685
Bhattacharya K, Samanta SK, Tripathi R, Mallick A, Chandra S, Pal BC, Shaha C, Mandal C (2010) Apoptotic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and Bid protein and via mitochondrial pathways. Biochem Pharmacol 79:361–372
Bhattacharya K, Bag AK, Tripathi R, Samanta SK, Pal BC, Shaha C, Chi Mandal (2014) Mahanine, a novel mitochondrial complex-III inhibitor induces GO/G1 arrest through redox alteration-mediated DNA damage response and regresses glioblastoma multiforme. Am J Cancer Res 4:629–647
Bhattacharya K, Maiti S, Mandal C (2016) PTEN negatively regulated mTORC2 formation and signaling in grade IVglioma via Rictor hyperphosphorylation at Thr1135 and direct the mode of action of an mTORC1/2 inhibitor. Oncogenesis 5:e227 (1–14)
Bhattacharyya P, Chakraborty A (1984) Mukonal, a probable biogenetic intermediate of pyranocarbazole alkaloids from Murraya koenigii. Phytochemistry 23:471–472
Bhattacharyya P, Chakraborty DP (1987) Carbazole alkaloids. In: Herz W, Grisebach H, Kirby GW, Tamm C (eds) Progress in the chemistry of organic natural products, vol 52. Springer, Wien, New York
Bhattacharyya P, Chowdhury BK (1984) Clausenapin: a new carbazole alkaloid from Clausena heptaphylla Wt. & Arn. Chem Ind 301
Bhattacharyya P, Chowdhury BK (1985a) Glycozolidal, a new carbazol alkaloid from Glycosmis pentaphylla. J Nat Prod 48:465–466
Bhattacharyya P, Chowdhury BK (1985b) 2-Methoxy-3-methylcarbazole from Murraya koenigii. Indian J Chem 24B:452
Bhattacharyya L, Roy SK, Chakraborty DP (1982) Structure of the carbazole alkaloid isomurrayazoline from Murraya koenigii. Phytochemistry 21:2432–2433
Bhattacharyya P, Chakraborty A, Chowdhury BK (1984) Heptazolicine, a carbazole alkaloid from Clausena heptaphylla. Phytochemistry 23:2409–2410
Bhattacharyya P, Chakrabarty PK, Chowdhury BK (1985) Glycozolidol, an antibacterial carbazole alkaloid from Glycosmis pentaphylla. Phytochemistry 24:882–883
Bhattacharyya P, Jash SS, Chowdhury BK (1986) A biogenetically important carbazole alkaloid from Murraya koenigii Spreng. Chem Ind 246
Bhattacharyya L, Chatterjee SK, Roy S, Chakraborty DP (1989) Murrayazolinol—a minor carbazole alkaloid from Murraya koenigii Spreng. J Indian Chem Soc 66:140–141
Bhattacharyya P, Biswas GK, Barua AK, Saha C, Roy IB, Chowdhury BK (1993) Clausenalene, a carbazole alkaloid from Clausena heptaphylla. Phytochemistry 33:248–250
Bhattacharyya P, Maiti AK, Basu K, Chowdhury BK (1994) Carbazole alkaloids from Murraya koenigii. Phytochemistry 35:1085–1086
Birari R, Roy SK, Singh A, Bhutani KK (2009) Pancreatic lipase inhibitory alkaloids of Murraya koenigii leaves. Nat Prod Commun 4:1089–1092
Birari R, Javia V, Bhutani KK (2010) Antiobesity and lipid lowering effects of Murraya koenigii (L.) Spreng leaves extracts and mahanimbine on high fat diet induced obese rats. Fitoterapia 81:1129–1133
Bordner J, Chakraborty DP, Chowdhury BK, Ganguli SN, Das KC, Weinstein B (1972) The crystal structure of murrayazoline (mahanimbidine, curryangin). Experientia 28:1406–1407
Börger C, Schmidt AW, Knölker HJ (2014) First total synthesis of chrestifoline-B and (±)-chrestifoline-C, and improved synthetic routes to bismurrayafoline-A, bismurrayafolinol and chrestifoline-D. Org Biomol Chem 12:3831–3835
Bowen IH, Perera KPWC (1982) Alkaloids, coumarins, and flavonoids of Micromelum ceylanicum. Phytochemistry 21:433–437
Bringmann G, Tasler S, Endress H, Peters K, Peters EM (1998a) Synthesis of mukonine and seven further 1-oxygenated carbazole alkaloids. Synthesis 1501–1505
Bringmann G, Ledermann A, Holenz J, Kao MT, Busse U, Wu HG, François G (1998b) Antiplasmodial activity of mono- and dimeric carbazoles. Planta Med 64:54–57
But PPH, Poon AWS, Shaw PC, Simmons MP, Greger H (2009) Contribution of molecular cladistics to the taxonomy of Rutaceae in China. J Syst Evol 47:144–150
Chaichantipyuth C, Pummangura S, Naowsaran K, Thanyavuthi D, Anderson JE, Mclaughlin JL (1988) Two new bioactive carbazole alkaloids from the root bark of Clausena harmandiana. J Nat Prod 51:1285–1288
Chakrabarty M, Nath AC, Khasnobis S, Chakrabarty M, Konda Y, Harigaya Y, Komiyama K (1997) Carbazole alkaloids from Murraya koenigii. Phytochemistry 46:751–755
Chakraborty DP (1966) Glycozoline, a carbazole derivative from Glycosmis pentaphylla (Retz.) DC. Tetrahedron Lett 7:661–664
Chakraborty DP (1969) Glycozoline, a carbazole derivative, from Glycosmis pentaphylla. Phytochemistry 8:769–772
Chakraborty DP (1977) Carbazole alkaloids. In: Herz W, Grisebach H, Kirby GW (eds) Fortschritte der Chemie organischer Naturstoffe, vol 34. Springer, Wien, New York
Chakraborty DP, Das BP (1966) Glycozolidine, a new carbazole derivative from Glycosmis pentaphylla. Sci Cult 32:181–182
Chakraborty DP, Roy S (1991) Carbazole alkaloids III. In: Herz W, Kirby GW, Steglich W, Tamm C (eds) Progress in the chemistry of organic natural products, vol 57. Springer, Wien, New York
Chakraborty DP, Barman BK, Bose PK (1964) On the structure of girinimbine, a pyranocarbazole derivative, isolated from Murraya koenigii Spreng. Sci Cult 30:445
Chakraborty DP, Barman BK, Bose PK (1965) On the constitution of murrayanine, a carbazole derivative isolated from Murraya koenigii Spreng. Tetrahedron 21:681–685
Chakraborty DP, Das KC, Bose PK (1966) Structure of mahanimbine, a pyranocarbazole derivative from Murraya koenigii Spreng. Sci Cult 32:83–84
Chakraborty DP, Das KC, Islam A (1970) Chemical taxonomy. XXIII. Heptazoline, a new carbazole alkaloid from Clausena heptaphylla Wt. and Arn. J Indian Chem Soc 47:1197–1198
Chakraborty DP, Das KC, Chowdhury BK (1971) Structure of murrayacine. J Org Chem 36:725–727
Chakraborty DP, Ganguly SN, Maji PN, Mitra AR, Das KC, Weinstein B (1973) Murrayazolinine, a carbazole alkaloid from Murraya koenigii Spreng. Chem Ind 322–323
Chakraborty DP, Bhattacharyya P, Islam A, Roy S (1974a) Chemical taxonomy. XXXV. Structure of murrayacinine, a new carbazole alkaloid from Murraya koenigii Spreng. Chem Ind 165
Chakraborty DP, Bhattacharyya P, Islam A, Roy S (1974b) Chemical taxonomy. XXXVI. Heptazolidine, a carbazole alkaloid from Clausena heptaphylla. Chem Ind 303
Chakraborty DP, Das KC, Das BP, Chowdhury BK (1975) On the antibiotic properties of some carbazole alkaloids. Trans Bose Res Inst 38:1–4
Chakraborty DP, Bhattacharyya P, Roy S, Bhattacharyya SP, Biswas AK (1978) Structure and synthesis of mukonine, a new carbazole alkaloid from Murraya koenigii. Phytochemistry 17:834–835
Chakraborty A, Saha C, Podder G, Chowdhury BK, Bhattacharyya P (1995a) Carbazole alkaloid with antimicrobial activity from Clausena heptaphylla. Phytochemistry 38:787–789
Chakraborty A, Chowdhury BK, Bhattacharyya P (1995b) Clausenol and clausenine—two carbazole alkaloids from Clausena anisata. Phytochemistry 40:295–298
Chakraborty M, Saha S, Mukhapadhyay S (2009) Murrayakoeninol—a new carbazole alkaloid from Murraya koenigii (Linn) Spreng. Nat Prod Commun 4:355–358
Chakravarty AK, Sarkar T, Masuda K, Shiojima K (1999) Carbazole alkaloids from roots of Glycosmis arborea. Phytochemistry 50:1263–1266
Chakravarty AK, Sarkar T, Masuda K, Takey T, Doi H, Kotani E, Shiojima K (2001) Structure and synthesis of glycoborine, a new carbazole alkaloid from the roots of Glycosmis arborea: a note on the structure of glycozolicine. Indian J Chem 40:484–489
Chakthong S, Bindulem N, Raknai S, Yodwaree S, Kaewsanee S, Kanjana-Opas A (2016) Carbazole-pyranocoumarin conjugate and two carbazole alkaloids from the stems of Clausena excavata. Nat Prod Res 30:1690–1697
Chen Y, Tang C, Wu Y, Mo SS, Wang S, Yang GZ, Mei ZN (2015) Glycosmisines A and B: isolation of two new carbazole-indole-type dimeric alkaloids from Glycosmis pentaphylla and an evaluation of their antiproliferative activities. Org Biomol Chem 13:6773–6781
Choi TA, Czerwonka R, Fröhner W, Krahl MP, Reddy KR, Franzblau SG, Knölker HJ (2006) Synthesis and activity of carbazole derivatives against Mycobacterium tuberculosis. Chem Med Chem 1:812–815
Choi TA, Czerwonka R, Forke R, Jäger A, Knöll J, Krahl MP, Krause T, Reddy KR, Franzblau SG, Knölker HJ (2008) Transition metals in organic synthesis—part 83: synthesis and pharmacological potential of carbazoles. Med Chem Res 17:374–385
Choi H, Gwak J, Cho M, Ryu MJ, Lee JH, Kim SK, Kim YH, Lee GW, Yun MY, Cuong NM, Shin JG, Song GY, Oh S (2010) Murrayafoline A attenuates the Wnt/β-catenin pathway by promoting the degradation of intracellular β-catenin proteins. Biochem Biophys Res Commun 391:915–920
Choudhury BK, Chakraborty DP (1971) Mukoeic acid, the first carbazole carboxylic acid from a plant source. Phytochemistry 10:1967–1970
Chowdhury BK, Mustapha A, Garba M, Bhattacharyya P (1987) Carbazole and 3-methylcarbazole from Glycosmis pentaphylla. Phytochemistry 26:2138–2139
Chowdhury BK, Jha S, Bhattacharyya P, Mukherjee M (2001) Two new carbazole alkaloids from Murraya koenigii. Indian J Chem 40B:490–494
Cui CB, Yan SY, Cai B, Yao XS (2002) Carbazole alkaloids as new cell cycle inhibitor and apoptosis inducers from Clausena dunniana Levl. J Asian Nat Prod Res 4:233–241
Cuong NM, Hung TQ, Sung TV, Taylor WC (2004) A new carbazole alkaloid from Glycosmis stenocarpa roots. Chem Pharm Bull 52:1175–1178
Cuong NM, Wilhelm H, Porzel A, Arnold N, Wessjohann L (2008) 1-O-Substituted derivatives of murrayafoline A and their antifungal properties. Nat Prod Res 22:1428–1432
Dai J, Ma D, Fu C, Ma S (2015) Gram scale total synthesis of 2-hydroxy-3-methylcarbazole, pyrano[3,2-a]carbazole and prenylcarbazole alkaloids. Eur J Org Chem 5655–5662
Das KC, Chakraborty DP, Bose PK (1965) Antifungal activity of some constituents of Murraya koenigii Spreng. Experientia 21:340
Das R, Bhattacharya K, Samanta SK, Pal BC, Mandal Chi (2014) Improved chemosensitivity in cervical cancer to cisplatin: synergistic activity of mahanine through STAT3 inhibition. Cancer Lett 351:81–90
Deng HD, Mei WL, Wang H, Guo ZK, Dong WH, Wang H, Li SP, Dai HF (2014) Carbazole alkaloids from the peels of Clausena lansium. J Asian Nat Prod Res 16:1024–1028
Du YQ, Liu H, Li CJ, Ma J, Zhang D, Li L, Sun H, Bao XQ, Zhang DM (2015) Bioactive carbazole alkaloids from the stems of Clausena lansium. Fitoterapia 103:122–128
Fiebig M, Pezzuto JM, Soejarto DD, Kinghorn AD (1985) Koenoline, a further cytotoxic carbazole alkaloid from Murraya koenigii. Phytochemistry 24:3041–3043
Furukawa H, Wu TS, Ohta T (1983) Bismurrayafoline-A and -B, two novel “dimeric” carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 31:4202–4205
Furukawa H, Yogo M, Ito C, Wu TS, Kuoh CS (1985a) New carbazolequinones having dimethylpyran ring system, from Murraya euchrestifolia. Chem Pharm Bull 33:1320–1322
Furukawa H, Wu TS, Kuoh CS (1985b) Structures of murrafoline-B and -C, new binary carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 33:2611–2613
Furukawa H, Wu TS, Ohta T, Kuoh CS (1985c) Chemical constituents of Murraya euchrestifolia Hayata. Structures of novel carbazolequinones and other new carbazole alkaloids. Chem Pharm Bull 33:4132–4138
Furukawa H, Wu TS, Kuoh CS (1985d) Dihydroxygirinimbine, a new carbazole alkaloid from Murraya euchrestifolia. Heterocycles 23:1391–1393
Furukawa H, Ito C, Yogo M, Wu TS (1986) Structures of murrayastine, murrayaline, and pyrayafoline; three new carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 34:2672–2675
Furukawa H, Ito C, Wu TS, McPhail AT (1993) Structure elucidation of murrafolines, six novel binary alkaloids isolated from Murraya euchrestifolia. Chem Pharm Bull 41:1249–1254
Ganguly SN, Sarkar A (1978) Exozoline, a new carbazole alkaloid from the leaves of Murraya exotica. Phytochemistry 17:1816–1817
Graebe C, Glaser C (1872) Ueber Carbazol. Chem Ber 5:12–15
Grassi P (1998) Vergleichende phytochemische Untersuchungen in der Gattung Micromelum (Rutaceae). Diploma-thesis, University of Vienna
Greger H, Hofer O, Kählig H, Wurz G (1992) Sulfur containing cinnamides with antifungal activity from Glycosmis cyanocarpa. Tetrahedron 48:1209–1218
Greger H, Zechner G, Hofer O, Vajrodaja S (1996) Bioactive amides from Glycosmis species. J Nat Prod 59:1163–1168
Han JH, Kim Y, Jung SH, Lee JJ, Park HS, Song GY, Cuong NM, Kim YH, Myung CS (2015) Murrayafoline A induces a G0/G1-phase arrest in platelet-derived growth factor-stimulated vascular smooth muscle cells. Korean J Physiol Pharmacol 19:421–426
Herdits-Riemer B (2002) Vergleichende phytochemische Untersuchungen in der Gattung Clausena (Rutaceae). Organspezifische und geographische Differenzierung. Ph.D. thesis, University of Vienna
Hesse R, Kataeva O, Schmidt AW, Knölker HJ (2014) Synthesis of prenyl- and geranyl-substituted carbazole alkaloids by DIBAL-H promoted reductive pyran ring opening of dialkylpyrano[3,2-a]carbazoles. Chem Eur J 20:9504–9509
Huang JY, Ma YZ, Yuan YH, Zuo W, Chu SF, Liu H, Du GH, Zhang DM, Chen NH (2016) Claulansine F promoted the neuronal differentiation of neural stem and progenitor cells through Akt/GSK-3ß/ß-catenin pathway. Eur J Pharmacol 786:72–84
Ito C, Furukawa H (1990) New carbazole alkaloids from Murraya euchrestifolia Hayata. Chem Pharm Bull 38:1548–1550
Ito C, Furukawa H (1991) Structure of murranimbine, a novel dimeric carbazole alkaloid from Murraya euchrestifolia. Chem Pharm Bull 39:1355–1357
Ito C, Wu TS, Furukawa H (1987) Three new carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 35:450–452
Ito C, Wu TS, Furukawa H (1988) New carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 36:2377–2380
Ito C, Wu TS, Furukawa H (1990) Novel binary carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 38:1143–1146
Ito C, Nakagawa M, Wu TS, Furukawa H (1991a) New carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 39:1668–1671
Ito C, Nakagawa M, Wu TS, Furukawa H (1991b) New carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 39:2525–2528
Ito C, Okahana N, Wu TS, Wang ML, Lai JS, Kuoh CS, Furukawa H (1992a) New carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull 40:230–232
Ito C, Kanbara H, Wu TS, Furukawa H (1992b) Murrayamine-C from Murraya euchrestifolia. Phytochemistry 31:1083–1084
Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H (1993) Alkaloidal constituents of Murraya koenigii. Isolation and structural elucidation of novel binary carbazolequinones and carbazole alkaloids. Chem Pharm Bull 41:2096–2100
Ito C, Ohta H, Tan HTW, Furukawa H (1996) Constituents of Clausena excavata. Isolation and structural elucidation of seven new carbazole alkaloids and a new coumarin. Chem Pharm Bull 44:2231–2235
Ito C, Katsuno S, Ohta H, Omura M, Kajiura I, Furukawa H (1997) Constituents of Clausena excavata. Isolation and structural elucidation of new carbazole alkaloids. Chem Pharm Bull 45:48–52
Ito C, Katsuno S, Ruangrungsi N, Furukawa H (1998) Structures of clausamine-A, -B, -C, three novel carbazole alkaloids from Clausena anisata. Chem Pharm Bull 46:344–346
Ito C, Katsuno S, Itoigawa M, Ruangrungsi N, Mukainaka T, Okuda M, Kitagawa Y, Tokuda H, Nishino H, Furukawa H (2000) New carbazole alkaloids from Clausena anisata with antitumor promoting activity. J Nat Prod 63:125–128
Ito C, Itoigawa M, Sato A, Hasan CM, Rashid MA, Tokuda H, Mukainaka T, Nishino H, Furukawa H (2004) Chemical constituents of Glycosmis arborea: three new carbazole alkaloids and their biological activity. J Nat Prod 67:1488–1491
Ito C, Itoigawa M, Nakao K, Murata T, Tsuboi M, Kaneda N, Furukawa H (2006) Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine 13:359–365
Ito C, Itoigawa M, Aizawa K, Yoshida K, Ruangrungsi N, Furukawa H (2009) γ-Lactone carbazoles from Clausena anisata. J Nat Prod 72:1202–1204
Ito C, Itoigawa M, Nakao K, Murata T, Kaneda N, Furukawa H (2012) Apoptosis of HL-60 leukemia cells induced by carbazole alkaloids isolated from Murraya euchrestifolia. J Nat Med 66:357–361
Itoigawa M, Kashiwada Y, Ito C, Furukawa H, Tachibana Y, Bastow KF, Lee KH (2000) Antitumor agents 203. Carbazole alkaloid murrayaquinone A and related synthetic carbazolequinones as cytotoxic agents. J Nat Prod 63:893–897
Jagadeesh S, Sinha S, Pal BC, Battacharya S, Banerjee PP (2007) Mahanine reverses an epigenetically silenced tumor suppressor gene RASSF1A in human prostate cancer cells. Biochem Biophys Res Commun 362:212–217
Jagtap S, Khare P, Mangal P, Kondepudi KK, Bishnoi M, Bhutani KK (2016) Effect of mahanimbine, an alkaloid from curry leaves, on high-fat diet-induced adiposity, insulin resistance, and inflammatory alterations. BioFactors. doi:10.1002/biof.1333(1-12)
Jash SS, Biswas GK, Bhattacharyya SK, Bhattacharyya P, Chakraborty A, Chowdhury BK (1992) Carbazole alkaloids from Glycosmis pentaphylla. Phytochemistry 31:2503–2505
Jiang HY, Zhang WJ, You CX, Yang K, Fan L, Feng JB, Chen J, Yang YJ, Wang CF, Deng ZW, Yin HB, Du SS (2014) Two new cytotoxic constituents from the Clausena lansium (Lour.) Skeels. Phytochem Lett 9:92–95
Joshi BS, Gawad DH (1974) Isolation and structure of indizoline, a new carbazole alkaloid from Clausena indica. Indian J Chem 12:437–440
Joshi BS, Kamat VN, Saksena AK, Govindachari TR (1967) Structure of heptaphylline, a carbazole alkaloid from Clausena heptaphylla Wt. & Arn. Tetrahedron Lett 41:4019–4022
Joshi BS, Kamat VN, Gawad DH (1970) On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron 26:1475–1482
Joshi BS, Kamat VN, Gawad DH, Govindachari TR (1972) Structure and synthesis of heptaphylline. Phytochemistry 11:2065–2071
Kamaruzzman RS, Chakraborty DP (1989) Mupamine from Glycosmis pentaphylla. Phytochemistry 28:677–678
Kedderis GL, Rickert DE, Pandey RN, Hollenberg PF (1986) 18 O-studies of the peroxidase-catalyzed oxidation of N-methylcarbazole. J Biol Chem 261:15910–15914
Khan D, Khan S, Badshah S, Ali H, Ullah H, Muhammad Z, Woodward S (2016) New furocarbazole alkaloids from Lonicera quinquelocularis. Nat Prod Res 30:74–78
Knölker HJ, Reddy KR (2002) Isolation and synthesis of biologically active carbazole alkaloids. Chem Rev 102:4303–4427
Knölker HJ, Reddy KR (2008) The chemistry and biology of carbazoles. In: Cordell GA (ed) The alkaloids, vol 65. Academic Press, Amsterdam
Ko FN, Lee YS, Wu TS, Teng CM (1994) Inhibition of cyclooxygenase activity and increase in platelet cyclic AMP by girinimbine isolated from Murraya euchrestifolia. Biochem Pharmacol 48:353–360
Kok YY, Mooi LY, Ahmed K, Sukari MA, Mat N, Rahmani M, Ali AM (2012) Anti-tumour promoting activity and antioxidant properties of girinimbine isolated from the stem bark of Murraya koenigii S. Molecules 17:4651–4660
Kong YC, Cheng KF, Ng KH, But PPH, Li Q, Yu SX, Chang HT, Cambie RC, Kinoshita T, Kan WS, Waterman PG (1986) A chemotaxonomic division of Murraya based on the distribution of the alkaloids yuehchukene and girinimbine. Biochem Syst Ecol 14:491–497
Kong YC, But PPH, Ng KH, Cheng KF, Chang KL, Wong KM, Gray AI, Waterman PG (1988a) The biochemical systematics of Merrillia; in relation to Murraya, the Clauseneae and the Aurantioideae. Biochem Syst Ecol 16:47–50
Kong YC, But PPH, Ng KH, Li Q, Cheng KF, Waterman PG (1988b) Micromelum: a key genus in the chemosystematics of the Clauseneae. Biochem Syst Ecol 16:485–489
Kongkathip B, Kongkathip N, Sunthitikawinsakul A, Napaswat C, Yoosook C (2005) Anti-HIV-1 constituents from Clausena excavata: Part II. Carbazoles and a pyranocoumarin. Phytother Res 19:728–731
Kumar V, Reisch J, Wickramasinghe A (1989) Glycomaurin and glycomaurrol, new carbazole alkaloids from Glycosmis mauritiana (Rutaceae) bark. Aust J Chem 42:1375–1379
Kumar V, Vallipuram K, Adebajo AC, Reisch J (1995) 2,7-Dihydro-3-formyl-1-(3′-methyl-2′-butenyl)carbazole from Clausena lansium. Phytochemistry 40:1563–1565
Kureel SP, Kapil RS, Popli SP (1969a) Terpenoid alkaloids from Murraya koenigii Spreng. II. The constitution of cyclomahanimbine, bicyclomahanimbine, and mahanimbidine. Tetrahedron Lett 3857–3862
Kureel SP, Kapil RS, Popli SP (1969b) New alkaloids from Murraya koenigii Spreng. Experientia 25:790–791
Kureel SP, Kapil RS, Popli SP (1970a) Terpenoid alkaloids from Murraya koenigii Spreng. IV. Structure and synthesis of mahanimbinine. Experientia 26:1055
Kureel SP, Kapil RS, Popli SP (1970b) Two novel alkaloids from Murraya koenigii Spreng: mahanimbicine and bicyclomahanimbicine. Chem Ind 29:958
Lamberton JA, Price JR, Redcliffe AH (1967) Micromelin, a new coumarin from Micromelum minutum (Forst. f.) Seem (family Rutaceae). Aust J Chem 20:973–979
Lebold TP, Kerr MA (2007) Total synthesis of eustifolines A–D and glycomaurrol via a divergent Diels–Alder strategy. Org Lett 9:1883–1886
Lee V, Cheung MK, Wong WT, Cheng KF (1996) Studies on the acid-catalyzed dimerization of 2-prenylindoles. Tetrahedron 52:9455–9468
Li WS, McChesney JD, El-Feraly FS (1991) Carbazole alkaloids from Clausena lansium. Phytochemistry 30:343–346
Liger F, Popowycz F, Besson T, Picot L, Galmarini CM, Joseph B (2007) Synthesis and antiproliferative activity of clausine E, mukonine, and koenoline bioisosteres. Bioorg Med Chem 15:5615–5619
Lin W, Wang Y, Lin S, Li CX, Zhou C, Wang SG, Huang H, Liu PQ, Ye G, Shen XY (2012) Induction of cell cycle arrest by the carbazole alkaloid clauszoline-I from Clausena vestita D. D. Tao via inhibition of the PKCδ phosphorylation. Eur J Med Chem 47:214–220
Liu H, Li CJ, Yang JZ, Ning N, Si YK, Li L, Chen NH, Zhao Q, Zhang DM (2012) Carbazole alkaloids from the stems of Clausena lansium. J Nat Prod 75:677–682
Lontsi D, Ayafor JF, Sondengam BL, Connolly JD, Rycroft DS (1985) Ekeberginine, a new carbazole alkaloid from Ekebergia senegalensis (Meliaceae). Tetrahedron Lett 26:4249–4252
Lukaseder B (2000) Vergleichende HPLC-Analysen in der Gattung Glycosmis (Rutaceae). Isolierung und Identifizierung charakteristischer Inhaltsstoffe. Diploma-thesis, University of Vienna
Lukaseder B, Vajrodaya S, Hehenberger T, Seger C, Nagl M, Lutz-Kutschera G, Robien W, Greger H, Hofer O (2009) Prenylated flavanones and flavanonols as chemical markers in Glycosmis species (Rutaceae). Phytochemistry 70:1030–1037
Lv HN, Wen R, Zhou Y, Zeng KW, Li J, Guo XY, Tu PF, Jiang Y (2015) Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J Nat Prod 78:2432–2439
Ma CY, Case RJ, Wang YH, Zhang HJ, Tan GT, Hung NV, Cuong NM, Franzblau SG, Soejarto DD, Fong HHS, Pauli GF (2005) Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med 71:261–267
Ma YZ, Ning N, He WB, Li JW, Hu JF, Chu SF, Chen NH (2013) Claulansine F promotes neuritogenesis in PC12 cells via the ERK signaling pathway. Acta Pharmacol Sin 34:1499–1507
Maneerat W, Laphookhieo S (2010) Antitumor alkaloids from Clausena lansium. Heterocycles 81:1261–1269
Maneerat W, Ritthiwigrom T, Cheenpracha S, Prawat U, Laphookhieo S (2011) Clausenawallines A and B, two new dimeric carbazole alkaloids from the roots of Clausena wallichii. Tetrahedron Lett 52:3303–3305
Maneerat W, Ritthiwigrom T, Cheenpracha S, Laphookhieo S (2012a) Carbazole alkaloids and coumarins from Clausena lansium roots. Phytochem Lett 5:26–28
Maneerat W, Ritthiwigrom T, Cheenpracha S, Promgool T, Yossathera K, Deachathai S, Phakhodee W, Laphookhieo S (2012b) Bioactive carbazole alkaloids from Clausena wallichii roots. J Nat Prod 75:741–746
Manimekalai R, Salomi MV, Shoba G (2015) Insiliko docking analysis of carbazole alkaloids from Murraya koenigii against PP2A. Int J Pharm Bio Sci 6(B):913–928
Markad SB, Argade NP (2016) Biomimetic collective total synthesis of bioactive carbazole alkaloids indizoline, mafaicheenamine A, claulamine A, claulansine A, and the proposed claulamine E. J Org Chem 81:5222–5227
McPhail AT, Wu TS, Ohta T, Furukawa H (1983) Structure of ±murrafoline, a novel biscarbazole alkaloid from Murraya euchrestifolia. Tetrahedron Lett 24:5377–5380
Meragelman KM, McKee TC, Boyd MR (2000) Siamenol, a new carbazole alkaloid from Murraya siamensis. J Nat Prod 63:427–428
Mester I, Reisch J (1977) Constituents of Clausena anisata (Willd.) Oliv. (Rutaceae), II. Isolation and structure of mupamine, a new carbazole alkaloid. Liebigs Ann Chem 1725–1729
Mou FJ, Zhang DX (2009) Pollen morphology supports the reinstatement of Bergera (Rutaceae). Nord J Bot 27:298–304
Mukherjee M, Mukherjee S, Shaw AK, Ganguly SN (1983a) Mukonicine, a carbazole alkaloid from leaves of Murraya koenigii. Phytochemistry 22:2328–2329
Mukherjee S, Mukherjee M, Ganguly SN (1983b) Glycozolinine, a carbazole derivative from Glycosmis pentaphylla. Phytochemistry 22:1064–1065
Nagappan T, Ramasamy P, Abdul Wahid ME, Chandra Segaran T, Vairappan CS (2011) Biological activity of carbazole alkaloids and essential oil of Murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules 16:9651–9664
Nagappan T, Chandra Segaran T, Abdul Wahid ME, Ramasamy P, Vairappan CS (2012) Efficacy of carbazole alkaloids, essential oil and extract of Murraya koenigii in enhancing subcutaneous wound healing in rats. Molecules 17:14449–14463
Nakahara K, Trakoontivakorn G, Alzoreky NS, Ono H, Onishi-Kameyama M, Yoshida M (2002) Antimutagenicity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromelum minutum. J Agric Food Chem 50:4796–4802
Nakamura S, Nakashima S, Oda Y, Yokota N, Fujimoto K, Matsumoto T, Ohta T, Ogawa K, Maeda S, Nishida S, Matsuda H, Yoshikawa M (2013) Alkaloids from Sri Lankan curry-leaf (Murraya koenigii) display melanogenesis inhibitory activity: Structures of karapinchamines A and B. Bioorg Med Chem 21:1043–1049
Nakatani N, Tachibana Y, Kikuzaki H (2001) Establishment of a model substrate oil for antioxidant activity assessment by oil stability index method. J Am Oil Chem Soc 78:19–23
Nalli Y, Khajuria V, Gupta S, Arora P, Riyaz-Ul-Hassan S, Ahmed Z, Ali A (2016) Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org Biomol Chem 14:3322–3332
Narasimhan NS, Paradkar MV, Chitguppi VP (1968) Structures of mahanimbin and koenimbin. Tetrahedron Lett 5501–5504
Narasimhan NS, Paradkar MV, Kelkar SL (1970) Alkaloids of Murraya koenigii: Structures of mahanine, koenine, koenigine, and koenidine. Indian J Chem 8:473–474
Ngadjui BT, Ayafor JF, Sondengam BL, Connolly JD (1989) Quinolone and carbazole alkaloids from Clausena anisata. Phytochemistry 28:1517–1519
Nutan MTH, Hasnat A, Rashid MA (1998) Antibacterial and cytotoxic activities of Murraya koenigii. Fitoterapia 69:173–175
Nutan MTH, Hasan CM, Rashid MA (1999) Bismurrayafoline E: a new dimeric carbazole alkaloid from Murraya koenigii. Fitoterapia 70:130–133
Okorie DA (1975) A new carbazole alkaloid and coumarins from roots of Clausena anisata. Phytochemistry 14:2720–2721
Pacher T (2005) Phytochemical analyses within the two tropical plant genera Glycosmis (Rutaceae) and Stemona (Stemonaceae) in special consideration of the formation of phytoalexins and artifacts. Ph.D. thesis, University of Vienna
Pacher T, Bacher M, Hofer O, Greger H (2001) Stress induced carbazole phytoalexins in Glycosmis species. Phytochemistry 58:129–135
Pandey J, Maurya R, Raykhera R, Srivastava MN, Yadav PP, Tamrakar AK (2014) Murraya koenigii (L.) Spreng. ameliorates insulin resistance in dexamethasone-treated mice by enhancing peripheral insulin sensitivity. J Sci Food Agric 94:2282–2288
Patel OPS, Mishra A, Maurya R, Saini D, Pandey J, Taneja I, Raju KSR, Kanojiya S, Shukla SK, Srivastava MN, Wahajuddin M, Tamrakar AK, Srivastava AK, Yadav PP (2016) Naturally occurring carbazole alkaloids from Murraya koenigii as potential antidiabetic agents. J Nat Prod 79:1276–1284
Pemmer J (2002) Charakteristische Stoffausstattungen von Bergera (Rutaceae) und ihre antifungalen Aktivitäten. Diploma-thesis, University of Vienna
Peng WW, Zeng GZ, Song WW, Tan NH (2013) A new cytotoxic carbazole alkaloid and two new other alkaloids from Clausena excavata. Chem Biodivers 10:1317–1321
Pieroni M, Girmay S, Sun D, Sahu R, Tekwani BL, Tan GT (2012) Synthesis and structure-activity relationships of lansine analogues as antileishmanial agents. Chem Med Chem 7:1895–1900
Potterat O, Puder C, Bolek W, Wagner K, Ke C, Ye Y, Gillardon F (2005) Clausine Z, a new carbazole alkaloid from Clausena excavata with inhibitory activity on CDK5. Pharmazie 60:637–639
Prakash D, Raj K, Kapil RS, Popli SP (1980) Chemical constituents of Clausena lansium: part I. Structure of lansamide I and lansine. Indian J Cem 19B:1075–1076
Qiu Y, Ma D, Fu CL, Ma SM (2013) An efficient Au-catalyzed synthesis of isomukonidine, clausine L, mukondine, glycosinine, mukonal, and clausine V from propadienyl methyl ether. Org Biomol Chem 11:1666–1671
Rahman MM, Gray AI (2005) A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry 66:1601–1606
Rahmani M, Ling CY, Sukari MA, Ismail HBM, Meon S, Aimi N (1998) 7-Methoxyglycomaurin: A new carbazole alkaloid from Glycosmis rupestris. Planta Med 64:780
Rama Rao AV, Bhide KS, Mujumdar RB (1980) Mahanimbinol. Chem Ind 697–698
Ramsewak RS, Nair MG, Strasburg GM, DeWitt DL, Nitiss JL (1999) Biologically active carbazole alkaloids from Murraya koenigii. J Agric Food Chem 47:444–447
Reisch J, Goj O, Wickramasinghe A, Herath HMTB, Henkel G (1992) Carbazole alkaloids from seeds of Murraya koenigii. Phytochemistry 31:2877–2879
Reisch J, Adebajo AC, Aladesanmi AJ, Adesina KS, Bergenthal D, Meve U (1994) Chemotypes of Murraya koenigii growing in Sri Lanka. Planta Med 60:295–296
Riemer B, Hofer O, Greger H (1997) Tryptamine derived amides from Clausena indica. Phytochemistry 45:337–341
Rodanant P, Surarit R, Lapookhieo S, Kuvatanasuchati J (2015) In vitro evaluation of the antibacterial and anti-inflammation activities of Clausena lansium. Songklanakarin J Sci Technol 37:43–48
Roy S, Bhattacharyya P, Chakraborty DP (1974) 3-Methylcarbazole from Clausena heptaphylla. Phytochemistry 13:1017
Roy S, Guha R, Ghosh S, Chakraborty DP (1982a) Biomimetic hydroxylation studies on carbazole alkaloids. Indian J Chem 21B:617–618
Roy S, Bhattacharyya L, Chakraborty DP (1982b) Structure and synthesis of mukoline and mukolidine, two new carbazole alkaloids from Murraya koenigii Spreng. J Ind Chem Soc 59:1369–1371
Roy MK, Thalang VN, Trakoontivakorn G, Nakahara K (2004) Mechanism of mahanine-induced apoptosis in human leukemia cells (HL-60). Biochem Pharmacol 67:41–51
Roy MK, Thalang VN, Trakoontivakorn G, Nakahara K (2005) Mahanine, a carbazole alkaloid from Micromelum minutum, inhibits cell growth and induces apoptosis in U937 cells through a mitochondrial dependent pathway. Br J Pharmacol 145:145–155
Ruangrungsi N, Ariyaprayoon J, Lange GL, Organ MG (1990) Three new carbazole alkaloids isolated from Murraya siamensis. J Nat Prod 53:946–952
Saha C, Chowdhury BK (1998) Carbazoloquinones from Murraya koenigii. Phytochemistry 48:363–366
Samanta SK, Dutta D, Roy S, Bhattacharya K, Sarkar S, Dasgupta AK, Pal BC, Chhab Mandal, Chit Mandal (2013) Mahanine, a DNA minor groove binding agent exerts cellular cytotoxicity with involvement of C-7-OH and –NH functional group. J Med Chem 56:5709–5721
Samuel R, Ehrendorfer F, Chase MW, Greger H (2001) Phylogenetic analyses of Aurantioideae (Rutaceae) based on non-coding plastid DNA sequences and phytochemical features. Plant Biol 3:77–87
Sarkar S, Dutta D, Samanta SK, Bhattacharya K, Pal BC, Li JP, Datta K, Chhab Mandal, Chit Mandal (2013) Oxidative inhibition of Hsp90 disrupts the super-chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int J Cancer 132:695–706
Schmidt AW, Reddy KR, Knölker HJ (2012) Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem Rev 112:3193–3328
Shaikh MS, Karpoormath R, Thapliyal N, Rane RA, Palkar MB, Faya AM, Patel HM, Alwan WS, Jain K, Hampannavar GA (2015) Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anticancer Agents Med Chem 15:1049–1065
Shen DY, Chao CH, Chan HH, Huang GJ, Hwang TL, Lai CY, Lee KH, Thang TD, Wu TS (2012) Bioactive constituents of Clausena lansium and a method for discrimination of aldose enantiomers. Phytochemistry 82:110–117
Shen DY, Chan YY, Hwang TL, Juang SH, Huang SC, Kuo PC, Thang TD, Lee EJ, Damu AG, Wu TS (2014) Constituents of the roots of Clausena lansium and their potential anti-inflammatory activity. J Nat Prod 77:1215–1223
Shen DY, Kuo PC, Huang SC, Hwang TL, Chan YY, Shieh PC, Ngan NT, Thang TD, Wu TS (2017) Constituents from the leaves of Clausena lansium and their anti-inflammatory activity. J Nat Med 71:96–104
Shi XJ, Ye G, Tang WJ, Zhao WM (2010) A new coumarin and carbazole alkaloid from Clausena vestita D. D. Tao. Helv Chim Acta 93:985–990
Shivakumar VS, Appelhans MS, Johnson G, Carlsen M, Zimmer EA (2016) Analysis of whole chloroplast genomes from the genera of the Clauseneae, the curry tribe (Rutaceae, Citrus family). Mol Phylogenet Evol 10:10. doi:10.1016/j.ympev.2016.12.015
Sim KM, Teh HM (2011) A new carbazole alkaloid from the leaves of Malayan Murraya koenigii. J Asian Nat Prod Res 13:972–975
Sinha S, Pal BC, Jagadeesh S, Banerjee PP, Bandyopadhaya A, Bhattacharya S (2006) Mahanine inhibits growth and induces apoptosis in prostate cancer cells through the deactivation of Akt and activation of caspases. Prostate 66:1257–1265
Songsiang U, Thongthoom T, Boonyarat C, Yenjai C (2011) Claurailas A–D, cytotoxic carbazole alkaloids from the roots of Clausena harmandiana. J Nat Prod 74:208–212
Songue JL, Kouam Dongo E, Mpondo TN, White RL (2012) Chemical constituents from stem bark and roots of Clausena anisata. Molecules 17:13673–13686
Sriphana U, Thongsri Y, Prariyachatigul C, Pakawatchai C, Yenjai C (2013) Clauraila E from the roots of Clausena harmandiana and antifungal activity against Pythium insidiosum. Arch Pharm Res 36:1078–1083
Sripisut T, Laphookhieo S (2010) Carbazole alkaloids from the stems of Clausena excavata. J Asian Nat Prod Res 12:614–617
Sripisut T, Cheenpracha S, Ritthiwigrom T, Prawat U, Laphookhieo S (2012) Chemical constituents from the roots of Clausena excavata and their cytotoxicity. Rec Nat Prod 6:386–389
Sunthitikawinsakul A, Kongkathip N, Kongkathip B, Phonnakhu S, Daly JW, Spande TF, Nimit Y, Rochanaruangrai S (2003) Coumarins and carbazoles from Clausena excavata exhibited antimycobacterial and antifungal activities. Planta Med 69:155–157
Syam S, Abdul AB, Sukari MA, Mohan S, Abdelwahab SI, Wah TS (2011) The growth suppressing effects of girinimbine on Hepg2 involve induction of apoptosis and cell cycle arrest. Molecules 16:7155–7170
Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N (2001) Antioxidative activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem 49:5589–5594
Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N (2003) Comparison of antioxidative properties of carbazole alkaloids from Murraya koenigii leaves. J Agric Food Chem 51:6461–6467
Tan SP, Ali AM, Nafiah MA, Awang K, Ahmad K (2015) Isolation and cytotoxic investigation of new carbazole alkaloids from Murraya koenigii (Linn.) Spreng. Tetrahedron 71:3946–3953
Tatsimo SJN, Tamokou JDD, Lamshöft M, Mouafo FT, Lannang AM, Sarkar P, Bag PK, Spiteller M (2015) LC-MS guided isolation of antibacterial and cytotoxic constituents from Clausena anisata. Med Chem Res 24:1468–1479
Taufiq-Yap YH, Peh TH, Ee GCL, Rahmani M, Sukari MA, Ali AM, Muse R (2007) A new cytotoxic carbazole alkaloid from Clausena excavata. Nat Prod Res 21:810–813
Thongthoom T, Songsiang U, Phaosiri C, Yenjai C (2010) Biological activity of chemical constituents from Clausena harmandiana. Arch Pharm Res 33:675–680
Utaipan T, Athipornchai A, Suksamrarn A, Jirachotikoon C, Yuan X, Lertcanawanichakul M, Chunglok W (2017) Carbazole alkaloids from Murraya koenigii trigger apoptosis and autophagic flux inhibition in human oral squamous cell carcinoma cells. J Nat Med 71:158–169
Uvarani C, Sankaran M, Jaivel N, Chandraprakash K, Ata A, Mohan PS (2013) Bioactive dimeric carbazole alkaloids from Murraya koenigii. J Nat Prod 76:993–1000
Uvarani C, Jaivel N, Sankaran M, Chandraprakash K, Ata A, Mohan PS (2014) Axially chiral biscarbazoles and biological evaluation of the constituents from Murraya koenigii. Fitoterapia 94:10–20
Vajrodaya S (1998) Comparative phytochemical analyses within the genus Glycosmis (Rutaceae-Citroideae). Ph.D. thesis, University of Vienna
Vajrodaya S, Bacher M, Greger H, Hofer O (1998) Organ-specific chemical differences in Glycosmis trichanthera. Phytochemistry 48:897–902
Wang YS, He HP, Shen YM, Hong X, Hao XJ (2003) Two new carbazole alkaloids from Murraya koenigii. J Nat Prod 66:416–418
Wang JS, Zheng YT, Efferth T, Wang RR, Shen YM, Hao XJ (2005) Indole and carbazole alkaloids from Glycosmis montana with weak anti-HIV and cytotoxic activities. Phytochemistry 66:697–701
Wang SL, Cai B, Cui CB, Yan SY, Wu CF (2008) Study on induction of apoptosis by girinimbine in HCT-15 cell in vitro. Chin J Pharm Anal 28:176–181
Whiting DA, Begley MJ, Clarke DG, Crombie L (1970) X-ray structure of dibromocannabicyclol structure of bicyclomahanimbine. J Chem Soc Chem Commun 1547–1548
Winkelblech J, Fan A, Li SM (2015) Prenyltransferases as key enzymes in primary and secondary metabolism. Appl Microbiol Biotechnol 99:7379–7397
Wu TS (1991) Murrayamine-A,-B,-C and (+)-mahanine, carbazole alkaloids from Murraya euchrestifolia. Phytochemistry 30:1048–1051
Wu TS, Huang SC (1992) Clausine-D and –F, two new 4-prenylcarbazole alkaloids from Clausena excavata. Chem Pharm Bull 40:1069–1071
Wu TS, Wang ML, Lai JS, Ito C, Furukawa H (1991) Binary carbazole alkaloids from Murraya euchrestifolia. Phytochemistry 30:1052–1054
Wu TS, Huang SC, Lai JS, Teng CM, Ko FN, Kuoh CS (1993) Chemical and antiplatelet aggregative investigation of the leaves of Clausena excavata. Phytochemistry 32:449–451
Wu TS, Huang SC, Wu PL, Lee KH (1994) Structure and synthesis of clausenaquinone A. A novel carbazolequinone alkaloid and bioactive principle from Clausena excavata. Bioorg Med Chem Lett 4:2395–2398
Wu TS, Wang ML, Wu PL, Jong TT (1995a) Two carbazole alkaloids from leaves of Murraya euchrestifolia. Phytochemistry 40:1817–1819
Wu TS, Wang ML, Wu PL (1995b) Murrayamine-O and -P, two cannabinol-skeletal carbazole alkaloids from Murraya euchrestifolia. Tetrahedron Lett 36:5385–5388
Wu TS, Wang ML, Wu PL, Ito C, Furukawa H (1996a) Carbazole alkaloids from the leaves of Murraya euchrestifolia. Phytochemistry 41:1433–1435
Wu TS, Huang SC, Wu PL (1996b) Carbazole-pyranocoumarin dimer and binary carbazole alkaloid from Clausena excavata. Tetrahedron Lett 37:7819–7822
Wu TS, Huang SC, Wu PL, Teng CM (1996c) Carbazole alkaloids from Clausena excavata and their biological activity. Phytochemistry 43:133–140
Wu TS, Huang SC, Wu PL (1996d) Carbazole alkaloids from stem bark of Clausena excavata. Phytochemistry 43:1427–1429
Wu TS, Wang ML, Wu PL (1996e) Seasonal variations of carbazole alkaloids in Murraya euchrestifolia. Phytochemistry 43:785–789
Wu TS, Huang SC, Wu PL (1997) Pyrano- and furocarbazole alkaloids from the root bark of Clausena excavata. Heterocycles 45:969–973
Wu TS, Chan YY, Liou MJ, Lin FW, Shi LS, Chen KT (1998a) Platelet aggregation inhibitor from Murraya euchrestifolia. Phytother Res 12:S80–S82
Wu TS, Huang SC, Wu PL (1998b) Lactonic carbazole alkaloids from the root bark of Clausena excavata. Chem Pharm Bull 46:1459–1461
Wu TS, Huang SC, Wu PL, Kuoh CS (1999) Alkaloidal and other constituents from the root bark of Clausena excavata. Phytochemistry 52:523–527
Wurz G, Hofer O, Greger H (1993) Structure and synthesis of phenaglydon, a new quinolone derived phenanthridine alkaloid from Glycosmis cyanocarpa. Nat Prod Lett 3:177–182
Xia HM, Yang GQO, Li CJ, Yang JZ, Ma J, Zhang D, Li Y, Li L, Zhang DM (2015) Clauemarazoles A–G, seven carbazole alkaloids from the stems of Clausena emarginata. Fitoterapia 103:83–89
Yan SY, Cui CB, Cai B, Qu GX, Yao XS (2001) New carbazole alkaloid from Clausena dunniana Levl. Chin J Med Chem 11:345–346
Yang GZ, Wu Y, Chen Y (2012) Alkaloids from the stems of Glycosmis pentaphylla. Helv Chim Acta 95:1449–1454
Yang H, Tian ST, Wu RY, Chen Y, Mei ZN, Wang CY, Yang GZ (2014) Glycoborinine induces apoptosis through mitochondrial pathway in H3pG2 cells. J Asian Nat Prod Res 16:991–999
Yenjai C, Sripontan S, Sriprajun P, Kittakoop P, Jintasirikul A, Tanticharoen M, Thebtharanonth Y (2000) Coumarins and carbazoles with antiplasmodial activity for Clausena harmandiana. Planta Med 66:277–279
Zhang FF, Gan LL, Zhou CH (2010) Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorg Med Chem Lett 20:1881–1884
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix

Rights and permissions
About this article
Cite this article
Greger, H. Phytocarbazoles: alkaloids with great structural diversity and pronounced biological activities. Phytochem Rev 16, 1095–1153 (2017). https://doi.org/10.1007/s11101-017-9521-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-017-9521-5