Abstract
Elms (Ulmus spp.) have long been appreciated for their environmental tolerance, landscape and ornamental value, and the quality of their wood. Although elm trees are extremely hardy against abiotic stresses such as wind and pollution, they are susceptible to attacks of biotic stressors. Over 100 phytopathogens and invertebrate pests are associated with elms: fungi, bacteria and insects like beetles and moths, and to a lesser extent aphids, mites, viruses and nematodes. While the biology of the pathogen and insect vector of the Dutch elm disease has been intensively studied, less attention has been paid so far to the defence mechanisms of elms to other biotic stressors. This review highlights knowledge of direct and indirect elm defences against biotic stressors focusing on morphological, chemical and gene regulation aspects. First, we report how morphological defence mechanisms via barrier formation and vessel occlusion prevent colonisation and spread of wood- and bark-inhabiting fungi and bacteria. Second, we outline how secondary metabolites such as terpenoids (volatile terpenoids, mansonones and triterpenoids) and phenolics (lignans, coumarins, flavonoids) in leaves and bark are involved in constitutive and induced chemical defence mechanisms of elms. Third, we address knowledge on how the molecular regulation of elm defence is orchestrated through the interaction of a huge variety of stress- and defence-related genes. We conclude by pointing to the gaps of knowledge on the chemical and molecular mechanisms of elm defence against pest insects and diseases. An in-depth understanding of defence mechanisms of elms will support the development of sustainable integrated management of pests and diseases attacking elms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elms are a large and important group of trees that have been closely associated with humans for at least 5000 years. Among many other uses of elm wood and bark, their timber has been used for agricultural equipment, ship building and furniture making, their leaves for fodder, and their trunk and crown for supporting grape vines growing up around the tree, while elm bark has been used as medical or skin care product and as an emergency food in times of famine (Richens 1983; Heybroek 2015). Because of their remarkable tolerance to a broad range of climates and soils and their majestic architecture, they were among the most widely planted urban ornamental and shade trees in Europe and North America until the mid of the twentieth century. The outbreak of the Dutch elm disease (DED) in the early 1900s, one of the most devastating tree diseases ever, decimated millions of elm trees worldwide with American and European elm species being especially susceptible. Huge international efforts have been undertaken since for elm conservation and breeding with the most successful strategy using Asian elm species as the source of resistance genes (Mittempergher and Santini 2004). Today, there is a revival of interest in the elm because of newly bred elm cultivars that may be resistant to the disease (Buiteveld et al. 2015; Martin et al. 2015).
Elms are deciduous and semi-deciduous trees of the genus Ulmus L. (Ulmaceae). Most species can grow up to 30 or 40 m, and life spans up to 400 years are known. The genus originated in Asia, but spread across North America and Europe. Due to cultivation elms occur today throughout the whole temperate world. With approximately 45 species, elms are one of the world’s major groups of tree species (Richens 1983; Wiegrefe et al. 1994).
Fungi, bacteria and insects like beetles and moths are the major biotic stressors of elms, with aphids, mites, viruses, nematodes and parasitic plants such as mistletoe, also having an effect (Stipes and Campana 1981; Richens 1983). Among hundreds of insect pests and diseases, three important elm-specific ones are known: (1) DED caused by the ascomycete Ophiostoma ulmi (Buisman) Nannf. and the more aggressive species O. novo-ulmi Brasier which are vectored by elm bark beetles (Scolytus spp.), (2) the elm yellows (EY) caused by phytoplasms (Sticklen and Sherald 1993; Mittempergher 2000) and (3) the elm leaf beetle (ELB). Elm species show a great variability in their morphological and physiological characteristics which render several species resistant to diseases like DED and EY and pest insects like ELB (Miller 2000).
Current knowledge of tree defence against biotic stressors is dominated by information about economically valuable pines and spruces used in forestry plantation and fast-growing angiosperm trees including birch and the closely related poplars (Tuzun and Bent 2006; Eyles et al. 2009; Ralph 2009; Novriyanti et al. 2010; Kolosova and Bohlmann 2012). Elms have received less attention, and most of the morphological and chemical features of elm defence and their regulation at the genetic level are still rather poorly described. Some information is mentioned in previous reviews about tree defence, in particular in the context of morphological and chemical defence mechanisms against DED (Shigo 1984; Blanchette and Biggs 1992; Pearce 1996; Veluthakkal and Dasgupta 2010) or of indirect defence against ELB (Hilker and Meiners 2011). Thus, the current information on elm defence is often focused on a distinct biotic stressor or a specific methodological approach, while a comprehensive overview on the state of research on elm defence against various biotic stressors is almost lacking.
In this review, we provide an overview of the current knowledge on (1) morphological, (2) chemical and (3) molecular aspects of elm defence against various biotic stressors, including DED and ELB, but also beyond these stressors. The comparison of knowledge on defence should elucidate defence pathways that are used in common. Linking these different aspects shall identify the gaps in elm research and encourage more integrative approaches in future research on the multiple defence mechanisms of this major group of trees. We searched for experimental, analytical and descriptive studies that investigated elm responses to attack by herbivores and pathogens. All types of attack to living elms were considered including ovipositional damage. We also included studies which simulated herbivory to mimic attack by an insect herbivore. Various literature data bases (Google Scholar, the ISI Web of Knowledge, PubMed) were used to retrieve the relevant publications. We searched for the terms ‘elm’ or ‘Ulmus’ in combination with ‘pathogen’, ‘disease’, ‘herbivore‘, ‘insect’ ‘damage’, ‘defence’ and ‘resistance’. Reference sections of papers were also scanned for additional studies.
Many plant pathologists cited in this review generally use the term ‘resistance’ to refer to the protection from disease caused by biotic agents that activate the host plant`s physical or chemical response (Kloepper et al. 1992). In the context of this article the term resistance and inducible resistance (IR) refers to any mechanism that negatively affects the preference for (or performance on) the plant attacked by an herbivore or pathogen (Karban and Baldwin 1997; van Dam and Heil 2011).
Biotic stressors: major pests and diseases of elms
Among hundreds of insect pest species and diseases associated with elms (reviewed in detail by Stipes and Campana 1981), DED, EY and ELB are the most serious elm specific ones, but other herbivores and pathogens are also important.
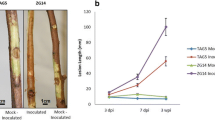
The DED pandemics, named after their first discovery in Holland, developed in the second half of the twentieth century into one of the most devastating tree diseases known. O. ulmi and the more aggressive strain O. novo-ulmi massively reduced European and North American elm populations during the past century. These vascular fungi, transmitted from diseased to healthy trees by Scolytus and Hylurgopinus bark beetle-vectors induce vessel cavitation rather than blocking of the vascular system while the tree responses, such as gum accumulation and tyloses, block the vessels of the tree. The leaves wilt and cause death of the trees sometimes within a few weeks (Sticklen and Sherald 1993).
Verticillium wilt is another fungus-caused wilt disease which is a common problem on elms in North America. The soilborn fungi V. albo-atrum (Rheinke & Berth) or V. dahliae (Kleb.) that are responsible for this disease may not only affect elm, but numerous other herbaceous and woody plant species (Rauscher et al. 1974). Further infectious and worldwide distributed elm diseases include cankers caused by several fungi and the elm black leaf spot disease caused by the fungus Gnomonia ulmea (Stipes and Campana 1981).
Phytoplasms, which are parasitic phloem-restricted bacteria, cause the elm phloem necrosis, better known as EY, which is a very aggressive disease. Phytoplasms are spread by insect-vectors such as phloem-feeding Hemiptera, among them leafhopper, planthopper and psyllid species. Infection and death of the phloem result in an undersupply of water and nutrients and thus, kill the tree. EY is epidemic and several elms native to North America and Asia were highly susceptible to EY, while it was much less severe in the European elms (Mittempergher 2000; Sinclair et al. 2000).
Many chewing defoliator insects (e.g. beetles such as Chrysomelidae, Scarabaeidae or caterpillars of moths and sawflies), leaf sap-sucking insects (bugs, leafhoppers, cicadas and aphids), and wood-boring insects (caterpillars of moths, beetles such as Scolytidae, Curculionidae or Cerambycidae) feed on elms worldwide. According to a list compiled in 1942, worldwide 585 insect species are associated with elm through feeding, breeding, ovipositing and hibernating (Stipes and Campana 1981). In European forests, 106 insect pests are associated with the genus Ulmus L. Two-thirds of the pest species are beetles and moths (Klimetzek 1993). Elms can survive heavy infestation of beetles and moth caterpillars during one or even more seasons. However, biotic attacks such as these presumably can weaken the elm’s defence and render them more susceptible to other diseases.
Among beetle species specialised on elm, the most serious one—in addition to the DED-transmitting bark beetle vector species—is Xanthogaleruca luteola (Müller) (Coleoptera: Chrysomelidae), the ELB. The ELB was accidentally introduced to the USA and Australia and is there responsible for fatal defoliation of elms owing to the absence of any specialist predators and parasitoids. Both larvae and adults may heavily damage a tree. In Europe, the indigenous ELB are often heavily predated by the chalcidoid egg parasitoid wasp Oomyzus gallerucae, a species which can parasitise 50 to 90 % of the eggs of an ELB population (Kielbaso and Kennedy 1983; Dahlsten et al. 1994; Kwong and Field 1994), so enabling elms to survive ELB infestation. The Japanese beetle, Popillia japonica (Newman) (Coleoptera: Scarabaeidae) is a general feeder on about 250 host plants, including elm. In Japan, where the beetle is native, it is controlled by natural predators, whereas in the USA it is a serious pest.
Among moth species that are pests of elm, larvae of the spring and fall cankerworm (Paleacrita vernata (Peck) and Alsophila pometaria (Harris); Lepidoptera: Geometridae) may attack elm, but can also feed on a variety of other trees. In North America cankerworms commonly appear as destructive populations. Similarly, the gypsy moth (Lymantria dispar (L.); Lepidoptera: Lymantriidae) is a major forest defoliator in North America and Europe. The caterpillars can completely defoliate an entire elm tree in one season (Stipes and Campana 1981).
Larvae of the sawfly Fenusa ulmi Sundevall (Hymenoptera: Tenthredinidae) mine elm leaves; this species is a common pest in the USA and Canada. The only other known elm leaf-mining sawfly is Anafenusa shinoharai (Smith and Altenhofer 2011).
In addition to elm-infesting insect species, mites can also cause severe damage of elms. According to Weidhaas (1979), several spider mite species (at least eight) attack elm leaves and suck upon leaf cell contents. Leaf injury caused by spider mites usually leads to premature leaf fall.
Elms do not only need to cope with pest species living aboveground, but also need to defend against root feeders. More than 15 genera of nematodes are known to endo- or ectoparasitically suck cell contents out of elm root tissue and thus reduce tree growth (Stipes and Campana 1981). The fungal pathogen Fusarium solani induces rots of elm root cuttings (Schreiber 1967).
Morphological defence of elm
Constitutive morphological defence
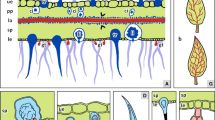
Elms, like most plant species, have evolved a combination of constitutive and induced defence mechanisms. Physical barriers including bark, tough leaves and trichomes represent the first effective constitutive barrier of elms against insects and fungal pathogens (Lucas et al. 2000; Bosu and Wagner 2008).
The outer bark consists of mostly lignified and suberised cells. Furthermore the tendency of Ulmaceae to accumulate calcium carbonate and silicic acid crystals results in characteristic membrane incrustation of cell lumina (parenchyma) in the affected wood (cited in Hegnauer 1973, pp. 545–553).
Leaves of most elm species have bulbous glandular trichomes and hairlike non-glandular trichomes, similar to many other vascular plants (Bosu and Wagner 2007, 2008). In general, leaf trichomes can contribute to plant defence in different ways. Non-glandular trichomes can physically obstruct the movements of herbivorous arthropods over the plant surface or prevent herbivores from reaching the surface with their mouthparts. Glandular trichomes function as important chemical barriers against herbivores by the production and accumulation of terpenoids, flavonoids, sugars and defensive proteins (Glas et al. 2012; Tian et al. 2012). Nothing is known about chemicals in elm trichomes, but skin irritation (personal observation K. Büchel) and the taxonomic relationship of elm to other families in the Urticales such as the Urticaceae strongly hint at the presence of secondary compounds in elm leaf trichomes. Future studies are recommended to investigate secondary compounds produced by elm leaf trichomes, and to evaluate their role in elm resistance against biotic stressors.
Higher trichome density on the foliage of elm species but not leaf toughness may be associated with reduced herbivory of the ELB (Miller and Ware 1999; Bosu and Wagner 2008). Dix et al. (1996) evaluated spring cankerworm (Lepidoptera: Geometridae) preferences for elm leaves with low trichome density. Leaf trichome density has been correlated with insect avoidance also in other trees (Soetens et al. 1991; Gange 1995). Further studies have focused on the variation in leaf traits such as leaf water content, leaf protein content (Young and Hall 1986), (water stress induced changes in) trichome density and leaf nutritional quality (Bosu and Wagner 2007); all these parameters are implicated in the resistance to ELB.
DED resistant trees do not only flush earlier than susceptible ones (Ghelardini and Santini 2009), but also differ from susceptible trees especially in the anatomical structure of the vascular system. Short and narrow vessels restrict fungal growth within a tree. They reduce the probability of embolism and allow an easier and faster occlusion of vessels by gums and tyloses which cause early isolation of the infection (Sinclair et al. 1975; Martin et al. 2013). Resistant elms show smaller pit membrane diameters, smaller pit aperture areas, and lower pit membrane abundance per vessel (Martin et al. 2009). A recent study on a Dutch elm hybrid species with some tolerance to DED observed large leaves, high net photosynthetic rate and some specific leaf vascular traits that may contribute to reduced fungal growth (Durkovic et al. 2013). How leaf vascular traits can affect the spread of disease will be outlined in the following section.
Inducible morphological (histochemical) defence
Induced resistance (IR) of elms by inoculations with fungi was tested and proven to be a valuable defence method since the 1980s (Myers and Strobel 1983; Sutherland et al. 1995; Solla and Gil 2003). In general, the success of induced resistance (IR) in protecting a tree against pathogen attack depends on the genetic constitution of the tree, its health and environmental conditions (Hubbes 2004). The effectiveness of IR is dependent on the timely expression of the morphological and chemical resistance mechanisms causing incompatibility in host-pathogen interactions and isolating the pathogen in rapid time. Therefore the regulation of IR becomes a critical determinant of the effectiveness of plant defence.
Barrier zone formation is an important non-specific and inducible morphological defence mechanism that can prevent colonisation by most wood- and bark-inhabiting fungi and bacteria. The production of these unique cells separates infected xylem tissue from non-infected living cambium allowing formation of new healthy tissue. The barrier zone protects living tissue from damage by the pathogen or diffusion of fungal toxins (Tippett and Shigo 1981; Shigo 1984). In U. americana barrier zones were formed of parenchyma cells and fibers in contrast to Populus balmifera (only fibers) and Prunus pensylvanica (only parenchyma cells).
When elm species resistant to DED are exposed to the DED-eliciting fungus, they form more axial parenchyma which is full of starch grains and enriched with polyphenolic compounds including lignin and suberin. This response has previously been described as tissue browning (Bonsen et al. 1985; Martin et al. 2005). Shigo et al. (1986) and Martin et al. (2008) have suggested a function of starch in host–pathogen interactions. On the one hand starch is an easily assimilable source of carbohydrates for the fungus and thus, could favour fungal spread, but on the other hand starch is required as a source of energy for the tree to establish the induced defence responses. During the maturation of cells in barrier zones, much of the starch is replaced by polyphenolic compounds; these cells persist up to several years after their formation (Tippett and Shigo 1981). Both lignin and suberin represent efficient barriers against pathogens. Phenylpropanoids are known to re-enforce cell walls (Mandal and Mitra 2007). They may act as protectors against cell wall degradation, as shown for U. americana (Jones et al. 2012). The fact that barrier zones produced after O. ulmi inoculation form later (after 22 days) in elms than in non-host species like Populus balmifera and P. pensylvanica (after 10 days) and the fact that barrier zones form discontinuously in elms and continuously in the non-hosts may contribute to elm susceptibility to DED (Rioux et al. 1995; Rioux and Ouellette 1991).
Infected elms form suberised tyloses that are distributed within or very near the barrier zones. DED-resistant elms are able to quickly and efficiently induce more tyloses than susceptible elms, so preventing the spread of O. ulmi by filling xylem vessels (Elgersma 1973). The structures of tyloses and their walls are well characterised and often include thick, inner suberised walls (mature tyloses) and pectic external layers (Rioux et al. 1995).
Vessel occlusion, a common response in plant defence, represents a further mechanism of compartmentalisation (blocking the spread of vascular pathogens) and has been studied in DED infested elms in detail (Sticklen et al. 1991; Ouellette et al. 2004). Vessel occlusion is caused by pectic substances within the xylem of elm trees invaded by vascular pathogens, and several types of occlusion by tyloses and/or deposition of mucilage in gels/gums occur (Gardner et al. 1983; Rioux et al. 1998; Beckman 2000; Eynck et al. 2009; Rajput et al. 2009). In earlier studies, no general consensus was reached as to the origin of the occlusion products. Often deposition of pectic substances, singly or mixed with further compounds such as lignins and suberin, proceeds occlusion.
The size of vessels and intervessel pits plays an important role in the spread of the DED pathogen, the transport of toxins, and the tree´s ability to prevent colonisation by the fungal disease. It is suggested that the smaller the earlywood vessels, the more resistant elm species are to DED. The rapidity with which compartmentalisation occurs, probably determines resistance. Vascular blocking is a slower and more difficult process in large diameter vessels than in small diameter vessels (Elgersma 1970; Sinclair et al. 1975; Martin et al. 2013).
With respect to morphological defences induced by biotic stressors, pathogen-induced H2O2 production in plants is thought to play a role in cell wall reinforcing processes (lignification). Furthermore, infection-induced H2O2 production is involved in killing invading pathogens, in triggering programmed plant cell death during the hypersensitive response that restricts the spread of infection, and in inducing defence genes (Kuzniak and Urbanek 2000). In vitro bioassays demonstrated that H2O2 inhibits the growth of O. novo-ulmi, but the further role that H2O2 production plays in inducible elm defence responses is presently unknown (De Rafael et al. 2001). Oliveira et al. (2012) observed in in vitro U. minor plants increased H2O2 production, membrane degradation in leaves, increased activity of major reactive oxygen species (ROS) and scavenging enzymes (catalase, peroxidase, superoxide dismutase) after inoculation with O. novo-ulmi subsp. americana. Peroxidases are known to be involved in the cell wall reinforcement during plant responses to pathogens; they are involved in polymerisation of saccharides and phenols which leads to stable vascular-occluding gels (Crews et al. 2003). The cambium region of both healthy and diseased elm trees shows very strong peroxidase activity, but in infected trees the activity was also found in fibres and vessels (Gagnon 1968). H2O2 is produced in elm mostly during the first day after infection, suggesting that the oxidative burst occurs early after infection as already described for other plant species. The function of ROS in plant defence against pathogens has been intensively studied (reviewed by e.g. Lamb and Dixon 1997).
Additional extraneous substances in vessel lumina have received much attention in later studies on DED-infested elms and other plant species affected by other fungal wilt diseases (Ouellette et al. 2004). The so-called alveolar network with associated coating layers accumulating on vessel walls was observed to be connected with fungal cells and to occasionally contain opaque matter. The compact coating and bands of opaque matter were clearly shown to be different from tyloses, and did not label for chitin, cellulose or pectin, but for DNA, which most likely originates from the pathogen. Therefore, recent studies have suggested a role for these coatings and opaque matter in pathogenesis rather than in plant defence, where it might play a role in the initial infection stages but also in recurrent infections at a time when host resistance mechanisms are ineffective. The fact that the alveolar network rarely occurs in U. pumila, which is very resistant to DED, supports this suggestion (reviewed in Ouellette et al. 2011).
Chemical defence of elm
The chemistry of the elm has been studied to only a limited extent (Table 1). Most knowledge of biological activity of secondary compounds in elms originates from medicinal research. Yet, in comparison with other plant species, elms are remarkably poor in their content of medically important substances, and their leaves can be eaten without harm. The bark and leaf extracts of elms (e.g. U. wallichiana and U. davidiana) have long been used in oriental medicine to treat inflammation, edema, mastitis, and to accelerate fracture repair (Richens 1983; Schütt et al. 1995). The mucilaginous inner bark of slippery elm (U. rubra) has been used as a remedy in North America for centuries. It is the only elm pharmaceutical that has survived modern scrutiny and is produced commercially to treat throat irritation (Watts and Rousseau 2012). Recent studies have shown that elm glycoproteins may have anti-cancer and anti-aging properties, and that flavonoid-C-glucoside compounds display osteoprotective effects (Jung et al. 2007; Hartmann et al. 2011; Sharan et al. 2011; Kim et al. 2012).
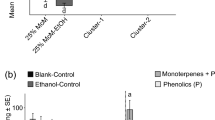
Although hardly any medically important substances from elm are known, many secondary compounds are produced by elm, including terpenes, phenolics and alkaloids in the leaves, and triterpenes, phytosterols, free fatty acids and suberins with smaller amounts of glycoproteins in the bark. Furthermore, elm trees produce polysaccharide-containing mucilage in the bark (Beveridge et al. 1971; Paluch et al. 2006; Hartmann et al. 2011). However, there is a lack of knowledge as to which role these compounds play in the defence of elms against biotic stressors. Prominent groups of chemicals known to be involved in elm defence are terpenoids (volatile terpenoids, mansonones and triterpenoids) and phenolics (lignans, scopoletin, flavonoids). These chemical defence metabolites can be constitutively synthesised in the bark and leaves, or can be induced by biotic stressors. Many elm secondary compounds act directly as toxins, repellents or anti-nutrients for herbivores, or as inhibitory substances against microbial infections, whereas others act indirectly as anti-herbivore devices via the attraction of predators or parasitoids of herbivorous insects.
Terpenoids
Terpenoids synthesised by the isoprenoid pathway form the most abundant and structurally diverse group of plant secondary metabolites (Cheng et al. 2007; Gershenzon and Dudareva 2007). In elms their ecological function was demonstrated in induced direct and indirect defence (see below).
Volatile terpenoids
Terpenoids are constitutively present in small amounts in the odour of undamaged elm leaves. The blend of volatile leaf compounds of healthy trees qualitatively and quantitatively differs from that of ELB-infested plants as shown for the field elm U. minor. Feeding-damaged elms are known to emit more than 40 compounds (Wegener et al. 2001) with a six-fold increase in the total amount of terpenoids (mono- and sesquiterpenes) and up to a 58-fold increase in the amount of the sesquiterpenoid (E)-β-caryophyllene (Büchel et al. 2011). Little is known about the role of constitutively emitted terpenoids in elm, but the role of herbivore-induced terpenoids as volatile signal in indirect defence in elms is well-investigated (see ‘Chemical ecology’). Emission of volatile terpenoids of U. americana wood including (−)-β-pinene, (−)-α-cubebene, (+)-spiroaxa-5,7-diene and (+)-δ-cadinene was up-regulated after inoculation with O. novo-ulmi; these volatiles are attractive to the elm bark beetle Hylurgopinus rufipes (Byers et al. 1980; McLeod 2005). While nothing is known about the protective role of these volatile bark terpenoids, emitting these semiochemicals is detrimental to the tree, as they are attractive to the beetle vector of DED and so ultimately increase fungal infection (McLeod 2005).
Sesquiterpenoid phytoalexins
Different sesquiterpenes in elm are classed as phytoalexins, antimicrobial compounds whose biosynthesis is induced in plants upon infection by phytopathogens. In common with other species of the Malvales, roots and heartwood of Ulmus constitutively contain quinone sesquiterpenes which are accumulated after stress induction in young wood (cited in Hegnauer 1990, p. 658).
The accumulation of mansonones as an integral component of IR against DED was first reported by Elgersma and Overeem (1971) in U. hollandica. Mansonones are a group of highly oxidised sesquiterpenoids, mainly sesquiterpene o-quinones that were originally isolated from the West African tree Mansonia altissima and have since been identified in many other plant species (Bettòlo et al. 1965; Chen et al. 1990). In elms, different mansonones (A, C–I) were isolated from the sapwood of U. americana and U. glabra (Dumas et al. 1983; Burden and Kemp 1984), and other elm species in response to infection by O. ulmi (Elgersma and Overeem 1971; Duchesne et al. 1986). Their accumulation is correlated with resistance to aggressive strains of the fungus O. ulmi in susceptible U. americana after seedlings were first inoculated with a non-aggressive isolate of O. ulmi (Jeng et al. 1983; Duchesne et al. 1985, 1990). The effect of mansonones on the fungi includes inhibition of growth, ion leakage, cell wall disruption, aggregation of ribosomes, and the accumulation of electron-dense material in the mitochondria (Dumas et al. 1986; Wu et al. 1989). The antioxidative activities of elm mansonones in root bark of U. davidiana evaluated by measuring their inhibitory effect on lipid peroxidation of rat liver microsomes (Kim et al. 1996) may protect elm cells from the toxic effects of ROS which are often found in stressed tissues (Kuzniak and Urbanek 2000),
Further quinone sesquiterpenes including cadalene- and 1,2,3,4-tretrahydrocadalene derivatives and lacinilene were also detected constitutively in elm heartwood (cited in Hegnauer 1990, p. 658). Cadalene derivatives and lacinilene are characteristic wood components in the Section Madocarpus (U. laciniata, U. glabra, U. carpinifolia, U. rubra) (Rowe et al. 1972; cited in Hegnauer 1973, p. 584). Inoculation of Wych elm (U. glabra) with the fungus O. ulmi induces accumulation of a series of antifungal cadalene derivatives like (−)-7-hydroxycalamenene and 7-hydroxycadalene (Burden and Kemp 1984). It is known that mansonones can easily be produced through oxidation of these compounds (Strunz et al. 1989). However, interestingly DED infested young twigs of U. glabra first produced the mansonones and later the related cadalene derivatives (cited in Hegnauer 1990, p. 658). Cadalene derivatives very likely play a role as phytoalexins (or precursors) in elm defence, as demonstrated to date only for Gossypium (cotton) spp. defence against herbivorous insects and phytopathogens (cited in Hegnauer 1989, p. 146; Dubery and Slater 1997).
Triterpenes and sterols
Elm bark extracts are mainly composed of triterpenes and sterols (up to 60 %), and biological activity was demonstrated in medicine where elm bark extracts had anticancer effects (Hartmann et al. 2011). Sterols play important roles in all plants as membrane components and hormones. One type of steroid with much more restricted taxonomic distribution, the phytoecdysteroids, mimics arthropod hormones and play a defensive role by disrupting moulting and other developmental and physiological processes with lethal consequences (Slama 1979). In elm, sterols including ß-sitosterol (Baker and Norris 1967; Dumas et al. 1983), stigmasterol and stigmastenone (Martin-Benito et al. 2005) have been identified in elm bark extracts. The pathogen-inducible conversion of the known membrane sterol ß-sitosterol to stigmasterol has been shown to promote plant disease susceptibility (Griebel and Zeier 2010). In general, terpenoids in elms are considered to be major defence compounds against pathogens and herbivores even if knowledge about their role in direct defence is limited.
Many triterpenoids were detected in root or bark extracts of several elm species, among them the recently identified lupenol, alnulin, ilexol, moretenol and betulin (Martin et al. 2004; Wang et al. 2006). Wegener (2002) identified several triterpenoids including β-amyrin, friedelin, and epifriedelinol in U. minor leaf extracts that had experienced ELB feeding or egg deposition, or treatment by jasmonic acid (JA). These substances were constitutively present, and their concentrations were not enhanced by the treatments. They seem to play a role as toxins against herbivores as shown for β-amyrin and other triterpenes, and may act by compromising the digestion of essential sterols by herbivorous insects (Gershenzon and Croteau 1991). In elms, the significance of triterpenoids as feeding stimulants or deterrents for the elm bark beetle S. multistriatus remains controversial. In U. americana, a pentacyclic triterpene serves as feeding stimulant for the elm bark beetle (Baker and Norris 1967). Martin-Benito et al. (2005) indicated an inverse relationship between the total triterpene content in the bark of elms and elm suitability for bark beetles. They identified various triterpenes and sterols among elm species. ß-Amyrin which showed high concentrations in some elm species including U. laevis and U. glabra (less preferred by bark beetles) was absent or present in only low concentrations in U. minor and U. pumila (preferred by bark beetles) (see Sacchetti et al. 1990; Webber and Kirby 1983 for studies on preferences); it may be involved in deterring Scolytus beetles. Pajares (2004) suggests that the high specificity in host selection behaviour of elm bark beetles results from the combined effect of the presence of host feeding stimulants and the absence of feeding deterrents. As feeding stimulants identified to date are not specific to elms, host selection is probably determined by the absence of non-host specific feeding deterrents.
Interestingly current year bark contains mainly aliphatic hydrocarbons, whereas 2–4-year-old bark contains mainly triterpenoids, which may result from adaptation of different stages of the tree to different attackers (Martin et al. 2004). Both compound groups are characteristic constituents of plant epicuticular waxes with important water repellent and protection functions (Baker 1982). The high triterpenoid content in birch (Betula sp.) bark was implicated in resistance to mountain hare (Lepus timidus) feeding (Laitinen et al. 2004).
Phenolics
Phenolic compounds are synthesised via the phenylpropanoid pathway, and many compounds including flavonoids, lignans, tannins, and coumarins are an ubiquitous feature of inducible defence in woody species, although their exact role in plant defence remains unclear. The fact that the phenylpropanoid pathway is involved in elm defence against DED is demonstrated by the increasing activity of the pathway`s key enzyme, phenylalanine-ammonia-lyase (PAL), 42–72 h after infection by the DED pathogen. In DED resistant U. pumila, but not in susceptible U. minor suspension cultures, the pathogen induces a large increase in PAL activity (Corchete et al. 1993). Nasmith et al. (2008a) reported higher in vivo targeted PAL expression in leaf midribs of DED-susceptible U. americana during fungal colonization by O. novo-ulmi with the highest level of expression at day 7. Expression of PAL was correlated with the accumulation of suberin, lignin and other phenolic compounds in O. novo-ulmi infected callus cultures of U. americana (Aoun et al. 2009). Inhibition of PAL reduces flavonoid content and decreases tissue browning in cultured elm tissue (Jones et al. 2012). Phenolics in U. americana accumulating after DED infection are mainly composed of catechins, the individual units that make up condensed tannins. Condensed tannins detected at the later stages of infection in callus tissues were proposed to serve as building blocks in the synthesis of lignin-like molecules (Aoun et al. 2009).
Scopoletin, a coumarin phenolic, is known as major secondary compound of elms. Its induced accumulation in response to pathogen infection has been mainly investigated in several members of the Solanaceae family, but scopoletin has also been shown to possess antibacterial and antifungal properties in many other plant species (Gnonlonfin et al. 2012). DED resistant U. pumila cell cultures accumulate more scopoletin than DED susceptible U. campestris cultures. In in vitro bioassays, scopoletin shows a direct antifungal activity against O. ulmi spore germination, but the role for scopoletin in limiting the spread of the pathogen in elm has yet to be demonstrated (Valle et al. 1997). De Rafael et al. (2001) detected differentially elicited scopoletin accumulation among various elm cultures. In U. minor leaves, scopoletin was found to be induced by ELB feeding and egg deposition, as well as by treatment with JA. In contrast to furanocumarins, scopoletin does not have the ability to intercalate into double stranded DNA and is considered to be more effective against generalist herbivorous insects than against specialists (Wegener 2002; Gnonlonfin et al. 2012).
Further phenolic compounds were isolated from wood of U. thomasii including the lignan thomasic acid with a content of 0.2 %, but nothing is known about their role in plant defence (Seikel et al. 1968). In contrast to lignin, the structurally diverse lignans are not ubiquitously distributed in all higher terrestrial plants. Nevertheless, the wood of many tree species contains lignans, and those have been reported to be involved in constitutive and induced defence against fungal attack (Naoumkina et al. 2010).
Flavonoids
Numerous flavonoids including the flavonols, quercetin, kaempferol, rutin and myricetin, the anthocyanidins delphinidin and cyanidin, and various leucoanthocyanidins, catechins and condensed tannins were identified in elm species worldwide (Bate-Smith and Richens 1973; Hegnauer 1973). Flavonoid identification in Ulmus was proposed for chemosystematic classification of the genus to distinguish artificial or natural interspecific hybrids. Early investigations identified glycosides of quercetin as major compounds (Santamour 1972). Subsequent investigations identified more than 30 foliar flavonoids in six North American elm species, whereby American elms comprise two distinct groups, one that produces the two flavonols, kaempferol and quercetin, and one that produces myricetin in addition (Sherman and Giannasi 1988).
In elms (and in other plants) the most investigated and abundant flavonol is quercetin. The role of quercetin in plant defence ranges from being a beneficial antioxidant scavenging ROS to being a damaging prooxidant depending on concentration and free radical source. The pro-oxidant quercetin develops its toxicity after its metabolic activation to quinoidal radicals and contributes to pathogen resistance via H2O2 burst (Jia et al. 2010; Metodiewa et al. 1999). In rapid IR of silver birch (Betula pendula), levels of lipophilic flavonoids increase after feeding by gypsy moth larvae, while quantities of several glycosides of quercetin decrease (Martemyanov et al. 2012).
Flavonoids are generally considered to contribute to resistance against pathogens. In Scots pine (Pinus sylvestris), flavonoids occur constitutively in phloem tissues and have been related to reaction efficiency against the bark beetle associated fungi. The low molecular phenolic flavonoid (+)-catechin and the phenolic chlorogenic acid were demonstrated as constitutively present compounds of Salix spp. and Picea ssp. with a suggested role in resistance against pathogens (Witzell and Martin 2008). Despite the identification of many flavonoids, there is a lack of studies on elms demonstrating a direct role of their flavonoids in defence.
Among the flavonoids, the class of condensed tannins represents the most abundant secondary metabolites typically found in woody plants. Tannins can defend leaves against insects by deterrence and toxicity, and their induction by herbivory has been reported for several tree species. Tannins are often referred to as anti-digestive protein-binding agents, but there is a lack of studies on herbivorous insects demonstrating the ability of tannins to decrease protein utilization. More recent studies supposed that the deterrent and toxic activity of tannins towards insects is due to oxidative stress caused by auto-oxidation or enzymatic oxidation of tannins. Such effects depend especially on the interaction between the plant-specific tannin and specific pH conditions in different parts of the digestive tract of the herbivore species (Barbehenn and Constabel 2011; Salminen and Karonen 2011). Osier and Lindroth (2001) showed that in P. tremuloides phenolic glycosides rather than condensed tannins act as constitutively present defensive compounds against the gypsy moth. Ulmus species contain mainly condensed tannins instead of hydrolysable tannins. European elm leaves contain more tannins than the twigs, and tannins may constitute over 4 % of their dry mass (cited in Hegnauer 1973, p. 547). In Alaska paper birch (Betula resinifera), condensed tannins were shown to significantly contribute to delayed IR. Previous defoliation of these birch trees prepares the plant for future attack; defence mechanisms of previously defoliated trees were induced more rapidly and more strongly by subsequent herbivore attack (Bryant et al. 1993).
Mucilage
Mucilage is one important biochemical component typically present in the inner bark of slippery elm U. rubra, but is also present in leaves and bark of other elm species (Anderson 1934; Gill et al. 1946; citations in Hegnauer 1973, p. 546; Hough et al. 1950). The inner bark of U. rubra contains around 7 % mucilage, mainly composed of galactose, rhamnose, galacturonic acid and 3-O-methylgalactose (Beveridge et al. 1971). The polymeric nature of mucilage is composed of polar glycoprotein and dense polysaccharide coatings, which provide its characteristic viscosity and gelling properties (Watts and Rousseau 2012). These pectic polysaccharides are produced by many plants in different organs such as roots, seeds, foliar and inner bark in high concentrations and are assumed to play a role in water and food storage and seed germination (Malviya et al. 2011; Yang et al. 2012). Their role in wound responses and plant defence against pathogens and parasitic plants has also been demonstrated for Zea mays and Vicia sativa (Crews et al. 2003; Pérez-de-Luque et al. 2006). However, in elm species, vessel occlusion by such pectic substrates is a common response and seems to improve elm resistance to wilt disease by limiting their spread through the tree´s vascular system (see “Morphological defence of elm” section).
Alkaloids
Alkaloids are toxic defensive compounds to herbivorous vertebrates as well as to arthropods, having no role in primary plant metabolism. However, the Ulmaceae do not belong to the alkaloid-rich plant families such as the Solanaceae or Papaveraceae where the alkaloid synthesis is a central part of the chemical defence (Mithöfer and Boland 2012). There are no confirmed reports of alkaloids in Ulmaceae although alkaloids were mentioned for U. pumila (cited in Hegnauer 1973, p. 552). Nevertheless, there may be other yet-to-be discovered classes of defence compounds in elms. Paluch et al. (2006) analysed leaf extracts of Asian elm species for differences in lipid, phenolic, and terpene diversity to link with susceptibility to Japanese beetle feeding damage. Asian elm species (closely associated to the U. davidiana complex), which are known to be more resistant to DED, EY and the elm leaf miner (Miller 2000), show a larger diversity of leaf chemicals than other elm species. However, compound diversity may not necessarily be an advantage, because elms with greater diversity of leaf lipids are more susceptible to infestation by Japanese beetles and gypsy moths (Paluch et al. 2006).
Chemical ecology: indirect defence of elm
Elms have played a prominent role in research on indirect defence against insect eggs. The first study demonstrating indirect plant defence against insect eggs was on the European field elm (U. minor), where egg deposition by the ELB induced volatiles that attract the egg parasitoid O. gallerucae, a wasp specialised on ELB eggs (Meiners and Hilker 1997, 2000). During the past two decades knowledge about indirect defence strategies of plants has grown continuously. Most studies concentrated on indirect plant defence via the emission of plant volatiles—so-called synomones—that are induced by feeding activity of herbivorous arthropods and attract predators or parasitoids of the herbivores (Arimura et al. 2009; Dicke and Baldwin 2010).
Yet, more and more studies have revealed that egg deposition by herbivorous insects also induces indirect plant defence. So far, plant defence induced by insect egg deposition has been shown for more than 20 plant species that range from herbaceous species to trees, from gymnosperms to angiosperms, from C3- to C4-plants, and from mono- to dicotyledonous plants (Hilker and Meiners 2010, 2011; reviewed by Hilker and Fatouros 2015).
The elm–ELB–O. gallerucae tritrophic system is characterised by a high species specificity of the elm’s defence response and the close relationship of the leaf beetle, its egg parasitoid and the tree. Neither ELB egg deposition on the leaves of the mountain elm (U. glabra), nor egg deposition by the related leaf beetle Galeruca tanaceti on field elm resulted in the emission of synomones that were attractive to O. gallerucae. Only ELB eggs laid on field elm induced the emission of leaf volatiles that were attractive to the egg parasitoid (Meiners et al. 2000). ELB shows even specialisation within its host plant taxon Ulmus and strongly prefers to feed and oviposit on leaves of some European and American Ulmus spp. and their hybrids (Miller and Ware 1994). Prior to egg deposition, female beetles scratch the lower leaf surface by gnawing shallow grooves in the epidermis and then glue eggs in place with an oviduct secretion into those grooves. It is important for the induction of the indirect defence reaction that the elicitor which induces the elm´s response to eggs contacts the cells exposed by the epidermal scratching. The elicitor itself is most likely a proteinaceous compound released with the oviduct secretion; treatment of oviduct secretion with a proteinase destroys its elicitor activity (Hilker and Meiners 2011). The ovipositional wounding of the leaf surface prior to egg deposition or artificial application of oviduct secretion onto an undamaged leaf per se do not cause the release of the attractive volatiles from elm leaves (Meiners et al. 2000).
Treatment with JA or methyl jasmonate (MeJA) also elicited the emission of attractive volatiles in field elms, but the volatile patterns differed quantitatively and qualitatively from those of elms induced by egg deposition and beetle feeding activity (Wegener et al. 2001; T. Meiners unpublished data).
The induction of elm leaf volatiles attractive to egg parasitoids was demonstrated on a time scale of a few hours after egg deposition and up to 5 days later. This time period exactly matches the development time of the eggs (Hilker and Meiners, 2006; T. Meiners unpublished data). Furthermore, induction of leaf volatiles mediated by ELB egg deposition was shown to occur locally in leaves with eggs and systemically in leaves that were egg-free, but adjacent to the leaves with eggs. The systemic signal extended acropetally along the elm tree to a height of at least 2 m above the egg-infested leaves (Meiners and Hilker 2000; T. Meiners unpublished data).
The blend of egg-induced elm leaf volatiles attracting egg parasitoids consisted mainly of GLVs including (Z)-3-hexenyl acetate and terpenoids like (E,E)-α-farnesene, (E)-β-caryophyllene and (E)-4,8-dimethyl-1,3,7-nonatriene. These substances were attractive to the egg parasitoids both in lab and in field studies and therefore probably play a crucial role in indirect elm defence responses (Büchel et al. 2011, 2012; Wegener et al. 2001). These terpenoids are ubiquitous compounds in most blends of higher plants and play a significant role in indirect defence also in other tritrophic systems (Vet and Dicke 1992; Colazza et al. 2004).
In the USA and Australia where no O. gallerucae are present naturally, elms are sometimes almost completely defoliated by the ELB indicating how strongly these trees can benefit from indirect defence via infestation-induced volatile emissions. Attraction of the egg parasitoids reduces the future number of larvae that would further damage the plant.
Another form of indirect defence in elm is the avoidance of DED by early flushing. During the flight peak of the DED vector, early flushing trees were better able to defend themselves against pathogen infections by producing “latewood”—a later stage of wood development with has a greater proportion of fibers which are less suitable for spore germinations (Ghelardini and Santini 2009; Santini and Faccoli 2015).
Little is known in elms about the costs of defence. The production of volatile terpenoids for indirect defence of field elms against ELB egg deposition seems to proceed without major photosynthetic costs since no difference in photosynthetic activity was observed when field elms were induced by egg deposition (T. Meiners unpublished data). However, in other tree and crop species it was shown that photosynthetic activity is locally and systemically reduced in response to insect egg deposition on leaves (Schroeder et al. 2005; Velikova et al. 2010). Interestingly transcription of photosynthesis-related genes in elm was also reduced after insect egg deposition (Büchel et al. 2011). However, the expression of defence traits in response to herbivore attack requires major changes both in primary and secondary metabolism, and plants invest a large amount of resources to produce volatile isoprenoids for defence against biotic stressors (Schwachtje and Baldwin 2008; Fineschi and Loreto 2012).
Molecular regulation of elm defence
During the last decade a rapid advancement in our understanding of the molecular biology of plant defences has taken place. Numerous studies have addressed the processes that trigger plant responses to biotic stressors such as herbivorous insects or pathogens (reviewed e.g. by; Mithöfer and Boland 2012; Smith and Clement 2012; Stam et al. 2014; Maag et al. 2015). Most molecular research on elm has been performed in relation to the DED pathogen, including the elicitors that induce host defence, the characteristics of the fungal strain and its population dynamics (Sticklen and Sherald 1993; reviewed in Bernier et al. 2015). Induction of resistance by the injection of fungal elicitors is viewed as a new biological approach against DED, although there is little knowledge about the molecular processes behind it.
While genetic transformation of elms was first developed more than 20 years ago (Bolyard et al. 1991; Fenning et al. 1996; Gartland et al. 2000), molecular investigations of the defence mechanisms of elms and the genes and pathways involved started only a few years ago (Table 2). There are only two studies of transgenic elms encoding antimicrobial peptides for enhanced resistance against DED (Gartland et al. 2005; Newhouse et al. 2007). The time consuming method of genetic transformation and conflicts with anti-GMO (genetically modified organism) organizations are reasons why genetically modified elms have not yet been employed in research on elm defence. Many studies have noted that elms have proved to be problematic for molecular work, due to the release of mucilaginous compounds that impede DNA or RNA isolation and downstream analysis (Loureiro et al. 2007; Nasmith et al. 2008a; b; noted in Büchel et al. 2011).
The first transcript expression analysis of Ulmus stress-related genes showed increased expression of PAL, chitinase, and polygalacturonase inhibiting protein (PGIP) during DED disease development in leaf midrib, root and bark of DED-resistant U. pumila in comparison to DED-susceptible U. americana. These three groups of genes are supposed to act in DED resistance (Nasmith et al. 2008a). PAL is involved in phytoalexin, lignin and flavonoid synthesis, while PGIPs inhibit fungal polygalacturonases and as a consequence reduce fungal damage to the cell wall (De Lorenzo et al. 2001).
The availability of the first tree genome to be sequenced (Populus trichocarpa) has enabled efforts to identify genes and pathways involved in angiosperm tree defence (Tuskan et al. 2006; Muchero et al. 2014). In the meantime “next generation sequencing” has allowed the publication of an increasing number of other tree genomic sequences, mainly those of commercial fruit trees, including e.g. apple, eucalypt, citrus (Velasco et al. 2010; Myburg et al. 2011; Xu et al. 2013).
Despite their high economic importance prior to DED and the massive reduction of elms by DED, only three large scale gene expression studies are known for elm. In one study of elms, using tissue cultures of U. americana inoculated with O. novo-ulmi, 314 unique transcripts were identified. After differential screening and RT-qPCR analyses, transcripts connected to the phenylpropanoid pathway, the compartmentalisation process, and phytoalexin production were shown to be up-regulated in response to fungal infection (Aoun et al. 2010). Another, much larger EST database containing information on 52,823 unique transcripts from the leaves of the field elm (U. minor), represents the largest genome resource for the elms to date (Büchel et al. 2012). Comparative in silico analysis among different treatments including MeJA treatment, ELB feeding and egg laying, ELB feeding only and ELB eggs only (by artificial transfer of egg clutches), revealed increased abundance of defence- and stress-related elm gene transcripts after egg laying and feeding of the ELB. Many further transcripts with a potential relevance in egg-induced defences involved in processes like signal transduction, transport, and primary metabolism were detected (Büchel et al. 2012). Recently, a total of 58.429 putative unigenes were identified in three U. minor genotypes that showed contrasting levels of tolerance to DED and were exposed to several biotic and abiotic stresses (Perdiguero et al. 2015). Some pathways were identified as relevant according to their implications in response to pathogens like phenylalanine metabolism, alpha-linolenic acid metabolism, biosynthesis of cutin, suberine and wax, flavonoids and T-cell receptor signalling pathways.
The classic assumptions that plant resistance to herbivore attack is principally determined by its secondary metabolism has already been put in doubt by newer transcriptomic and proteomic studies demonstrating that the timing and localization of plant response is critical to the manifestation of resistance. Of the hundreds of genes whose transcript levels were altered during plant–herbivore or plant–pathogen interaction substantial involvement of the primary metabolism was demonstrated for several plant species (Schwachtje and Baldwin 2008). A preliminary proteomic study on field elms demonstrated that levels of elm leaf proteins involved in primary metabolism like energy metabolism (succinyl CoA-ligase) and sugar- and amino acid metabolism (UDP-glucose-dehydrogenase (UGDH), arginase) increase after ELB feeding or after egg deposition accompanied by feeding activity. Further putative proteins with increased quantities in U. minor leaves after ELB feeding or egg deposition were involved in the synthesis of the phytohormone ethylene (S-adenosylmethionine synthase) (K. Büchel unpublished data). All these proteins are closely associated with defence mechanisms, e.g. through enhanced cell wall biosynthesis (UGDH, Karkonen et al. 2005) and enhanced amino acid metabolism activity, which was demonstrated to enhance resistance against necrotrophic fungal pathogens (arginase, Brauc et al. 2012).
Here, we highlight those sequences of the above-mentioned two large-scale elm gene expression studies which were upregulated in high abundance in response to ELB infestation or DED. The list includes transcripts encoding enzymes belonging to different branches of the phenylpropanoid and shikimate pathways, and to different classes of pathogen-related (PR) proteins, proteinase inhibitors (PI), and proteins involved in phytohormone signalling (Table 2). In particular, PR proteins seem to be a prominent feature of the defence profile of elms inducible by ELB and DED, among them PR 1–3, PR 6, peroxidases (PR 9) and PR 10 proteins. PR genes and proteins are known to be involved in host–pathogen interactions in many tree species. PR 1 genes were induced by DED (and by other pathogens or salicylic acid (SA) treatment), but the mode of action of PR 1 proteins towards DED is unknown (Veluthakkal and Dasgupta 2010). Chitinases (PR 3) transcripts were among the most up-regulated transcripts in field elm after ELB feeding and were induced at a similar point in time (48–72 h) after U. americana calli inoculation with the DED fungus. Chitinases play a direct role in plant defence by hydrolysing chitin and degrading microbial cell wall components, often coordinated with the induction of glucan endo-1,3-ß-glucosidases (PR 2). Transformation of an elm chitinase gene of resistant U. americana into bentgrass (Agrostis palustris) causes disease resistance in the transformed plant against the brown patch fungus Rhizoctonia solani (Chai et al. 2002). Transcripts encoding genes of a Kunitz-like proteinase inhibitor (PR 6) were strongly induced in DED infected elm calli. Further upregulated sequences had sequence similarity to genes coding for proteinase inhibitor I (PR 6). This protease inhibitor participates in defence of plants against herbivorous insects and pathogens. Further DED upregulated sequences showed sequence similarity to genes coding for S-norcoclaurine synthase (PR 10), which catalyses the first committed step in the biosynthesis of benzylisoquinoline alkaloids, a large and diverse group of secondary metabolites found in several plant families. Transcript abundance of major latex proteins (PR 10) were strongly induced by ELB egg-laying. Although PR 10 proteins were induced by both biotic stressors (ELB and DED) in various plant tissues, the biological function remains to be elucidated.
Increased transcripts of compartmentalisation-associated proteins are consistent with the high accumulation of the respective proteins in compartmentalisation processes in DED infected elms. Sieve element occluding proteins upregulated in ELB infested elm are possibly involved in sieve cell occlusion after wounding. In DED infested elm calli, transcripts had sequence similarity to genes coding for proteins that may be involved in the production of isoflavonoids (isoflavone reductase-like protein), anthocyanin pigments (O-methyltransferase), and lignans (phenylcoumaran benzylic ether reductase), which could also be associated with the compartmentalisation process against pathogens. Phenylcoumaran benzylic ether reductase is the most abundant protein in the secondary xylem of P. trichocarpa, strongly associated with phenylpropanoid biosynthesis in lignifying cells (Vander Mijnsbrugge et al. 2000). Yet, genes directly involved in lignin and suberin biosynthesis were neither (identified to be) up-regulated in response to DED nor to ELB infestation (Aoun et al. 2010). Using Fourier transform-infrared spectroscopy, as a complementary method to transcriptome analysis, Martin et al. (2007) demonstrated that levels of lignin increased earlier in inoculated xylem tissues of elms that were more resistant to DED than in tissue of susceptible elm species.
Further defence- and stress-related transcripts which were present in high abundance in leaves after ELB egg laying coded for a key enzyme involved in JA synthesis (LOX = lipoxygenase), and proteins involved in JA, SA and auxin signalling (JAZ = Jasmonate ZIM-domain protein; NPR1 = non-expresser of PR genes; auxin signalling F-box 2). These proteins are intimately associated with plant defence or disease development. A phospholipase protease (patatin-like protein) known to be involved in oxylipin biosynthesis contributed in Arabidopsis mutants to plant cell death and pathogen resistance (La Camera et al. 2009). Almost all of the elm transcripts that were upregulated in U. americana in response to DED and reported to have sequence similarities to defence related proteins (Aoun et al. 2010) were also found in the much larger database on U. minor induced by ELB activity (Büchel et al. 2012). It is a challenging future task to elucidate how the expression of genes encoding these defence related proteins is regulated and how protein activity is mediated in response to DED and ELB attack.
Conclusions and future challenges
Elms show several morphological and chemical defences against attackers which are regulated by a variety of stress- and defence-related genes. In spite of increasing information on how elms can successfully defend against biotic stress, this review also shows the many gaps of our knowledge on the compounds and genes playing a role in the defence of elms against pests and diseases.
Prominent groups of chemicals known to be involved in elm defence are terpenoids (volatile terpenoids, mansonones and triterpenoids) and phenolics (lignans, scopoletin, flavonoids). These defence chemicals are constitutively synthesised in the bark and leaves, but their production is also inducible by biotic stressors. Many elm secondary compounds act directly as toxins, repellents or anti-nutrients for herbivores, or as inhibitory substances against microbial infections. Others act indirectly as anti-herbivore devices via the attraction of predators or parasitoids of herbivorous insects. For many secondary compounds produced by elms, there is also a lack of knowledge as to which role these compounds play in the defence of elms against biotic stressors. Nothing is known about chemicals in elm leaf trichomes, and future studies are recommended to investigate secondary compounds produced by elm leaf trichomes, and to evaluate their role in elm resistance against herbivores.
Molecular investigations of defence mechanisms of elms, the genes and the pathways involved only started a few years ago. The molecular regulation of elm defence is orchestrated through the interaction of a huge variety of stress- and defence-related genes. The list of sequences which are upregulated in high abundances in response to ELB or DED infestation includes transcripts encoding enzymes belonging to different branches of the phenylpropanoid and shikimate pathways, and to different classes of pathogen-related (PR) proteins, proteinase inhibitors (PI), and proteins involved in phytohormone signalling. A detailed and statistical comparison of the expression levels of genes in response to fungal infection of the elm trunk by DED and to herbivore attack of elm leaves by ELB may provide insights into tissue- and aggressor-specific responses of elms to stress. Currently, we do not know whether infection of elm by DED would also affect defence responses to ELB attack, and vice versa.
While knowledge on elm responses to DED is increasing, no knowledge is available on elm responses to subsequent or simultaneous infection of elm by various phytopathogens. Plant–pathogen interactions are also essential for primary physiological processes like plant–water relation, mineral and salt absorption, stomatal regulation, gaseous exchange, photosynthesis. Hence, it also needs more basic studies on pathogen effects on these primary processes in elms to comprehensively understand the regulation of elm responses to biotic (and abiotic) stresses.
Moreover, little is known about the costs of defence in elms. Investment in defence against biotic stressors is expected to be adjusted to the extent of the current stress and/or to the risk of future stress which might be “estimated” by cues providing hints on future stress (e.g. insect eggs as indicator for larval feeding damage). However, answering the question whether it pays off for elms to invest more in defence rather than in regrowth after herbivory will need future studies.
Mainly during the last decade, elm research concentrated in particular on the breeding of DED resistant elm hybrids, on elm conservation, and on DED pest management. More elms than previously assumed survived DED, and new DED resistant elm hybrid cultivars, which are the result of crosses with resistant Asian species, have been released on the market. However, their value as a replacement for native elm species has yet to be proved. The timely expression of the morphological and chemical resistance mechanisms causing incompatibility in host-pathogen interactions and isolating the pathogen in rapid time is a critical determinant of the effectiveness of plant defence. Studies on the regulation of the timely expression of IR against DED and other pathogens should be performed and the knowledge generated should be used in elm breeding programs. Breeding of plants responding earlier or more efficiently to insect attack has been suggested, and the proof–of-concept has been shown in some cases (Kappers et al. 2005; Degenhardt et al. 2009; Xiao et al. 2012). The knowledge on defence traits against the ELB indicates that breeding for resistance against the ELB might be a viable and promising option.
Today, a changing trend of pest and disease management can be observed that leads away from sanitation and pesticide application towards enhancing IR by abiotic and biotic (e.g. microbes and microbial products) activators and using biological agents and cultural control methods. For example, application of plant hormones such as SA or inoculation of elms with a non-aggressive pathogen strain of DED as elicitor of IR has been demonstrated in elm and in some cases successfully enhanced the resistance to O. novo-ulmi (Hubbes 2004; Martín et al. 2012).
This review indicates that the defences of elms against DED and ELB are polygenic. Moreover, there is intraspecific variability among different DED tolerant genotypes. For instance, narrow earlywood vessel diameters have been associated with high tolerance to DED, but not all resistance genotypes have narrow vessels. The knowledge of heritable resistance factors, both constitutive and inducible, that provide tolerance to a genotype is very important for breeding purposes. When designing the most appropriate crossings among tolerant genotypes to obtain progenies with enhanced resistance, these resistance factors should be further investigated by next generation sequencing tools coupled with bioinformatics. A key improvement derived from the knowledge of resistance mechanisms and the involved genes would be the development of early screening techniques which reduce the time currently needed for selection and breeding of resistant elms (about 5 years for screening and 10 years for completing one breeding cycle). Research combining different disciplines like forest entomology, plant physiology, plant pathology, ecology, chemistry and molecular biology is required to understand the complex mechanisms of elm defence against biotic (and abiotic) stressors and to use this knowledge as valuable tool in sustainable pest management. The acquired knowledge might help bringing back the magnificent elm species into our cities and landscapes.
Abbreviations
- ELB:
-
Elm leaf beetle
- EY:
-
Elm yellows
- DED:
-
Dutch elm disease
- IR:
-
Induced resistance
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- PAL:
-
Phenylalanine-ammonia-lyase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
References
Anderson E (1934) The mucilage from slippery elm bark. J Biol Chem 104:163–170
Aoun M, Rioux D, Simard M, Bernier L (2009) Fungal colonization and host defense reactions in Ulmus americana callus cultures inoculated with Ophiostoma novo-ulmi. Phytopathology 99:642–650
Aoun M, Jacobi V, Boyle B, Bernier L (2010) Identification and monitoring of Ulmus americana transcripts during in vitro interactions with the Dutch elm disease pathogen Ophiostoma novo-ulmi. Physiol Mol Plant Pathol 74:254–266
Arimura G, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923
Baker EA (1982) Chemistry and morphology of plant epicuticular waxes. Academic Press, London
Baker JE, Norris DM (1967) A feeding stimulant for Scolytus multistriatus (Coleoptera: Scolytidae) isolated from the bark of Ulmus americana. Ann Entomol Soc Am 60:1213
Barbehenn RV, Constabel CP (2011) Tannins in plant–herbivore interactions. Phytochemistry 72:1551–1565
Bate-Smith EC, Richens RH (1973) Flavonoid chemistry and taxonomy in Ulmus. Biochem Syst Ecol 1:141–146
Beckman CH (2000) Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol Mol Plant Pathol 57:101–110
Bernier L, Aoun M, Bouvet GF, Comeau A, Dufour J, Naruzawa ES, Nigg M, Plourde KV (2015) Genomics of the Dutch elm disease pathosystem: are we there yet? iForest 8:149
Bettòlo GBM, Casinovi CG, Galeffi C (1965) A new class of quinones: sesquiterpenoid quinones of Mansonia altissima chev. Tetrahedron Lett 6:4857–4864
Beveridge RJ, Jones JKN, Lowe RW et al (1971) Structure of slippery elm mucilage (Ulmus Fulva). J Polym Sci Part C Polym Symp 36:461–466
Blanchette RA, Biggs AR (1992) Defense mechanisms of woody plants against fungi. Springer, Berlin
Bolyard MG, Hajela RK, Sticklen MB (1991) Microprojectile and Agrobacterium-mediated transformation of pioneer elm. J Arboric 17:34–37
Bonsen KJM, Scheffer RJ, Elgersma DM (1985) Barrier zone formation as a resistance mechanism of elms to Dutch elm disease. IAWA Bull 6:1–77
Bosu PP, Wagner MR (2007) Effects of induced water stress on leaf trichome density and foliar nutrients of three elm (Ulmus) species: implications for resistance to the elm leaf beetle. Environ Entomol 36:595–601
Bosu PP, Wagner MR (2008) Anatomical and nutritional factors associated with susceptibility of elms (Ulmus spp.) to the elm leaf beetle (Coleoptera: Chrysomelidae). J Econ Entomol 101:944–954
Brauc S, De Vooght E, Claeys M et al (2012) Overexpression of arginase in Arabidopsis thaliana influences defence responses against Botrytis cinerea. Plant Biol 14:39–45
Bryant JP, Reichardt PB, Clausen TP et al (1993) Effects of mineral nutrition on delayed inducible resistance in Alaska paper birch. Ecology 74:2072–2084
Büchel K, Malskies S, Mayer M et al (2011) How plants give early herbivore alert: Volatile terpenoids attract parasitoids to egg-infested elms. Basic Appl Ecol 12:403–412
Büchel K, McDowell E, Nelson W et al (2012) An elm EST database for identifying leaf beetle egg-induced defense genes. BMC Genom 13:242
Buiteveld JB, Van Der Werf B, Hiemstra JA (2015) Comparison of commercial elm cultivars and promising unreleased Dutch clones for resistance to Ophiostoma novo-ulmi. iForest 8:158
Burden RS, Kemp MS (1984) Sesquiterpene phytoalexins from Ulmus glabra. Phytochemistry 23:383–385
Byers JA, Svihra P, Koehler CS (1980) Attraction of elm bark beetles to cut limbs on elm. J Arboric 6:245–246
Chai BL, Maqbool SB, Hajela RK et al (2002) Cloning of a chitinase-like cDNA (hs2), its transfer to creeping bentgrass (Agrostis palustris Huds.) and development of brown patch (Rhizoctonia solani) disease resistant transgenic lines. Plant Sci 163:183–193
Chen C-M, Chen Z-T, Hong Y-L (1990) A mansonone from Helicteres angustifolia. Phytochemistry 29:980–982
Cheng AX, Lou YG, Mao YB et al (2007) Plant terpenoids: biosynthesis and ecological functions. J Integr Plant Biol 49:179–186
Colazza S, McElfresh JS, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30:945–964
Corchete MP, Diez JJ, Valle T (1993) Phenylalanine ammonia-lyase activity in suspension cultures of Ulmus pumila and U. campestris treated with spores of Ceratocystis ulmi. Plant Cell Rep 13:111–114
Crews LJ, McCully ME, Canny MJ (2003) Mucilage production by wounded xylem tissue of maize roots—time course and stimulus. Funct Plant Biol 30:755–766
Dahlsten DL, Rowney DL, Tait SM (1994) Development of integrated pest management programs in urban forests: the elm leaf beetle (Xanthogaleruca luteola (Müller)) in California, USA. For Ecol Manag 65:31–44
De Lorenzo G, D’Ovidio R, Cervone F (2001) The role of polygacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol 39:313–335
De Rafael MA, Valle T, Babiano MJ et al (2001) Correlation of resistance and H2O2 production in Ulmus pumila and Ulmus campestris cell suspension cultures inoculated with Ophiostoma novo-ulmi. Physiol Plant 111:512–518
Degenhardt J, Hiltpold I, Köllner TG et al (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106:13213–13218
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dix ME, Cunningham RA, King RM (1996) Evaluating spring cankerworm (Lepidoptera: Geometridae) preference for Siberian elm clones. Environ Entomol 25:58–62
Dubery IA, Slater V (1997) Induced defence responses in cotton leaf disks by elicitors from Verticillium dahliae. Phytochemistry 44:1429–1434
Duchesne LC, Jeng RS, Hubbes M (1985) Accumulation of phytoalexins in Ulmus americana in response to infection by a nonaggressive and an aggressive strain of Ophiostoma ulmi. Can J Bot 63:678–680
Duchesne LC, Hubbes M, Jeng RS (1986) Mansonone E and F accumulation in Ulmus pumila resistant to Dutch elm disease. Can J For Res 16:410–412
Duchesne LC, Jeng RS, Hubbes M et al (1990) Accumulation of mansonones E and F in elm callus cultures inoculated with Ophiostoma ulmi. Trees Struct Funct 4:187–190
Dumas MT, Strunz GM, Hubbes M et al (1983) Isolation and identification of six mansonones from Ulmus americana infected with Ceratocystis ulmi. Experientia 39:1089–1090
Dumas MT, Straunz GM, Hubbes M et al (1986) Inhibition of Ceratocystis ulmi by mansonones A, C, D, E, F, and G isolated from Ulmus americana. Eur J For Pathol 16:217–230
Durkovic J, Canova I, Lagana R et al (2013) Leaf trait dissimilarities between Dutch elm hybrids with a contrasting tolerance to Dutch elm disease. Ann Bot 111:215–227
Elgersma DM (1970) Length and diameter of xylem vessels as factors in resistance of elms to Ceratocystis ulmi. Neth J Plant Pathol 76:179–182
Elgersma DM (1973) Tylose formation in elms after inoculation with Ceratocystis ulmi, a possible resistance mechanism. Neth J Plant Pathol 79:218–220
Elgersma DM, Overeem JC (1971) The relation of mansonones to resistance against dutch elm disease and their accumulation, as induced by several agents. Neth J Plant Pathol 77:168–174
Eyles A, Bonello P, Ganley R et al (2009) Induced resistance to pests and pathogens in trees. New Phytol 185:893–908
Eynck C, Koopmann B, Karlovsky P et al (2009) Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 99:802–811
Fenning TM, Tymens SS, Gartland JS et al (1996) Transformation and regeneration of English elm using wild-type Agrobacterium tumefaciens. Plant Sci 116:37–46
Fineschi S, Loreto F (2012) Leaf volatile isoprenoids: an important defensive armament in forest tree species. iForest 5:13–17
Gagnon C (1968) Peroxidase in healthy and diseased elm trees investigated by the benzidine histochemical technique. Can J Bot 46:1491–1494
Gange AC (1995) Aphid performance in an alder (Alnus) hybrid zone. Ecology 76:2074–2083
Gardner JM, Feldman AW, Stamper DH (1983) Role and fate of bacteria in vascular occlusions of citrus. Physiol Plant Pathol 23:295–309
Gartland JS, McHugh AT, Brasier CM et al (2000) Regeneration of phenotypically normal English elm (Ulmus procera) plantlets following transformation with an Agrobacterium tumefaciens binary vector. Tree Physiol 20:1063
Gartland KMA, McHugh AT, Crow RM et al (2005) 2004 SIVB congress symposium proceeding: biotechnological progress in dealing with dutch elm disease. In Vitro Cell Dev Plant 41:364–367
Gershenzon J, Croteau R (1991) Terpenoids. The chemical participants. Academic Press, New York
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3:408–414
Ghelardini L, Santini A (2009) Avoidance by early flushing: a new perspective on Dutch elm disease research. iForest 2:143–153
Gill RE, Hirst EL, Jones JKN (1946) Constitution of the mucilage from the bark of Ulmus Fulva (slippery elm mucilage). Part II. The sugars formed in the hydrolysis of the methylated mucilage. J Chem Soc 0:1025–1029
Glas J, Schimmel B, Alba J et al (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci 13:17077–17103
Gnonlonfin GJB, Sanni A, Brimer L (2012) Review scopoletin—a coumarin phytoalexin with medicinal properties. Crit Rev Plant Sci 31:47–56
Griebel T, Zeier J (2010) A role for beta-sitosterol to stigmasterol conversion in plant-pathogen interactions. Plant J 63:254–268
Hartmann AM, Abarzua S, Schlichting A et al (2011) Effects of elm bark extracts from Ulmus laevis on human chorion carcinoma cell lines. Arch Gynecol Obstet 284:1265–1269
Hegnauer R (1973) Chemotaxonomie der Pflanzen. Band 6. Dicotyledoneae: Rafflesiaceae - Zygophyllaceae, vol 6. Birkhäuser, Basel und Stuttgart
Hegnauer R (1989) Chemotaxonomie der Pflanzen. Band 8. Nachträge zu Band 3 und 4 (Acanthaceae-Lythraceae). Birkhäuser, Basel
Hegnauer R (1990) Chemotaxonomie der Pflanzen. Band 9. Nachträge zu Band 5 und 6 (Magnoliaceae-Zygophyllaceae). Birkhäuser, Basel
Heybroek HM (2015) The elm, tree of milk and wine. iForest 8:181
Hilker M, Fatouros NE (2015) Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515
Hilker M, Meiners T (2006) Early herbivore alert: insect eggs induce plant defense. J Chem Ecol 32:1379–1397
Hilker M, Meiners T (2010) How do plants “notice” attack by herbivorous arthropods? Biol Rev 85:267–280
Hilker M, Meiners T (2011) Plants and insect eggs: how do they affect each other? Phytochemistry 72:1612–1623
Hough L, Jones JKN, Hirst EL (1950) Chemical constitution of slippery elm mucilage: isolation of 3-methyl d-galactose from the hydrolysis products. Nature 165:34–35
Hubbes M (2004) Induced resistance for the control of Dutch elm disease. Invest Agrar Sist Recur For 13:185–196
Jeng RS, Alfenas AC, Hubbes M et al (1983) Presence and accumulation of fungitoxic substances against Ceratocystis ulmi in Ulmus americana: possible relation to induced resistance. Eur J For Pathol 13:239–244
Jia Z, Zou B, Wang X et al (2010) Quercetin-induced H2O2 mediates the pathogen resistance against Pseudomonas syringae pv. Tomato DC3000 in Arabidopsis thaliana. Biochem Biophys Res Commun 396:522–527
Jones AM, Chattopadhyay A, Shukla M et al (2012) Inhibition of phenylpropanoid biosynthesis increases cell wall digestibility, protoplast isolation, and facilitates sustained cell division in American elm (Ulmus americana). BMC Plant Biol 12:75
Jung H-J, Jeon H-J, Lim E-J et al (2007) Anti-angiogenic activity of the methanol extract and its fractions of Ulmus davidiana var. japonica. J Ethnopharmacol 112:406–409
Kappers IF, Aharoni A, van Herpen TWJM et al (2005) Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science 309:2070–2072
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Karkonen A, Murigneux A, Martinant JP et al (2005) UDP-glucose dehydrogenases of maize: a role in cell wall pentose biosynthesis. Biochem J 391:409–415
Kielbaso JG, Kennedy MK (1983) Urban forestry and entomology: a current appraisal. In: Frankie GW, Koehler CS (eds) Urban entomology: interdisciplinary perspectives. Praeger, New York, pp 423–440
Kim JP, Kim WG, Koshino H et al (1996) Sesquiterpene O-naphthoquinones from the root bark of Ulmus davidiana. Phytochemistry 43:425–430
Kim T-W, Youm S-Y, Shin S-K et al (2012) Chemopreventive effects of elm tree bark extract on Helicobacter pylori-associated mouse gastric carcinogenesis. Basic Appl Pathol 5:31–38
Klimetzek D (1993) Baumarten und ihre Schadinsekten auf der Nordhalbkugel. Mitt Dtsch Ges Allg Angew Ent 8:505–509
Kloepper JW, Tuzun S, Kuć JA (1992) Proposed definitions related to induced disease resistance. Biocontrol Sci Technol 2:349–351
Kolosova N, Bohlmann J (2012) Conifer defense against insects and fungal pathogens. In: Matyssek R, Schnyder H, Oßwald W et al (eds) Growth and defence in plants, vol 220. Springer, Berlin, pp 85–109
Kuzniak E, Urbanek H (2000) The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol Plant 22:195–203
Kwong RM, Field RP (1994) Elm leaf beetle history and distribution in southern Victoria. Plant Prot Q 9:43–47
La Camera S, Balague C, Gobel C et al (2009) The Arabidopsis patatin-like protein 2 (PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. Mol Plant Microbe Interact 22:469–481
Laitinen ML, Julkunen-Tiitto R, Yamaji K et al (2004) Variation in birch bark secondary chemistry between and within clones: implications for herbivory by hares. Oikos 104:316–326
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Ann Rev Plant Phys 48:251–275
Loureiro J, Rodriguez E, Gomes  et al (2007) Genome size estimations on Ulmus minor Mill., Ulmus glabra Huds., and Celtis australis L. using flow cytometry. Plant Biol 9:541–544
Lucas PW, Turner IM, Dominy NJ et al (2000) Mechanical defences to herbivory. Ann Bot 86:913–920
Maag D, Erb M, Köllner TG, Gershenzon J (2015) Defensive weapons and defense signals in plants: Some metabolites serve both roles. BioEssays 37:167–174
Malviya R, Srivastava P, Kulkarni GT (2011) Applications of mucilages in drug delivery—a review. Adv Biol Res 5:1–07
Mandal S, Mitra A (2007) Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol Mol Plant Pathol 71:201–209
Martemyanov V, Dubovskiy I, Belousova I et al (2012) Rapid induced resistance of silver birch affects both innate immunity and performance of gypsy moths: the role of plant chemical defenses. Arthropod Plant Interact 6:507–518
Martin D, Garcia-Vallejo MC, Lopez D et al (2004) Elm bark components and their potential influence on bark beetle feeding. Invest Agrar Sist Recur For 13:227–235
Martin JA, Solla A, Woodward S et al (2005) Fourier transform-infrared spectroscopy as a new method for evaluating host resistance in the Dutch elm disease complex. Tree Physiol 25:1331–1338
Martin JA, Solla A, Woodward S, Gil L (2007) Detection of differential changes in lignin composition of elm xylem tissues inoculated with Ophiostoma novo-ulmi using Fourier transform-infrared spectroscopy. For Pathol 37:187–191
Martin JA et al (2008) Metabolic fingerprinting allows discrimination between Ulmus pumila and U. minor, and between U. minor clones of different susceptibility to Dutch elm disease. For Pathol 38:244–256
Martin JA, Solla A, Esteban LG, de Palacios P, Gil L (2009) Bordered pit and ray morphology involvement in elm resistance to Ophiostoma novo-ulmi. Can J For Res 39:420–429
Martin JA, Solla A, Ruiz-Villar M, Gil L (2013) Vessel length and conductivity of Ulmus branches: ontogenetic changes and relation to resistance to Dutch elm disease. Trees 27:1239–1248
Martin JA, Solla A, Venturas M et al (2015) Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain. iForest 8:172
Martín JA, Solla A, García-Vallejo MC, Gil L (2012) Chemical changes in Ulmus minor xylem tissue after salicylic acid or carvacrol treatments are associated with enhanced resistance to Ophiostoma novo-ulmi. Phytochemistry 83:104–109
Martin-Benito D, Garcia-Vallejo MC, Pajares JA et al (2005) Triterpenes in elms in Spain. Can J For Res 35:199–205
McLeod G (2005) The pathogen causing Dutch elm disease makes host trees attract insect vectors. Proc R Soc 272:2499–2503
Meiners T, Hilker M (1997) Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae). Oecologia 112:87–93
Meiners T, Hilker M (2000) Induction of plant synomones by oviposition of a phytophagous insect. J Chem Ecol 26:221–232
Meiners T, Westerhaus C, Hilker M (2000) Specificity of chemical cues used by a specialist egg parasitoid during host location. Entomol Exp Appl 95:151–159
Metodiewa D, Jaiswal AK, Cenas N et al (1999) Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med 26:107–116
Miller F (2000) Insect resistance of elm genotypes. In: Dunn C (ed) The elms: breeding, conservation, and disease management. Kluwer, Boston
Miller F, Ware G (1994) Preference for and suitability of selected elms, Ulmus spp. and their hybrids for the elm leaf beetle, Pyrrhalta luteola (Coleoptera: Chrysomelidae). J Environ Hortic 12:231–235
Miller F, Ware G (1999) Resistance of elms of the Ulmus davidiana complex to defoliation by the adult elm leaf beetle (Coleoptera: Chrysomelidae). J Econ Entomol 92:1147–1150
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Mittempergher L (2000) Elm Yellows in Europe. In: Dunn C (ed) The elms: breeding, conservation, and disease management. Kluwer, Boston, pp 103–119
Mittempergher L, Santini A (2004) The history of elm breeding. Invest Agrar Sist Recur For 13:161–177
Muchero W, Labbé J, Ranjan P et al (2014) Genome resequencing in Populus: Revealing large-scale genome variation and implications on specialized-trait genomics. In: Fenning T (ed) Challenges and Opportunities for the World’s Forests in the 21st Century, vol 81. Springer, Netherlands
Myburg A, Grattapaglia D, Tuskan G et al (2011) The Eucalyptus grandis genome project: Genome and transcriptome resources for comparative analysis of woody plant biology. BMC Proc 5:I20
Myers DF, Strobel GA (1983) Pseudomonas syringae as a microbial antagonist of Ceratocystis ulmi in the apoplast of American elm. Trans Brit Mycol Soc 80:389–394
Naoumkina MA, Zhao QA, Gallego-Giraldo L et al (2010) Genome-wide analysis of phenylpropanoid defence pathways. Mol Plant Pathol 11:829–846
Nasmith C, Jeng R, Hubbes M (2008a) A comparison of in vivo targeted gene expression during fungal colonization of DED-susceptible Ulmus americana. Forest Pathol 38:104–112
Nasmith C, Jeng R, Hubbes M (2008b) Targeted gene analysis in Ulmus americana and U. pumila tissues. Forest Pathol 38:90–103
Newhouse A, Schrodt F, Liang H et al (2007) Transgenic American elm shows reduced Dutch elm disease symptoms and normal mycorrhizal colonization. In: Fladung M, Ewald D (eds) Plant Cell Reports, vol 26. Springer, Berlin, pp 977–987
Novriyanti E, Aoyama C, Watanabe M et al (2010) Plant defense characteristics and hypotheses in birch species. Eurasian J Forest Res 13:77–85
Oliveira H, Sousa A, Alves A et al (2012) Inoculation with Ophiostoma novo-ulmi subsp americana affects photosynthesis, nutrition and oxidative stress in in vitro Ulmus minor plants. Environ Exp Bot 77:146–155
Osier T, Lindroth R (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Ouellette GB, Rioux D, Simard M et al (2004) Ultrastructure of the alveolar network and its relation to coating on vessel walls in elms infected by Ophiostoma novo-ulmi and in other plants affected with similar wilt diseases. Investig Agrar: Sist Recur Forest 13:147–160
Ouellette GB, Charest PM, Chamberland H (2011) A review of ultrastructural and ultracytochemical studies of infection processes in some plant wilt diseases: the opaque matter extensively involved, its links with pathogen elements, insights into its nature. Microsc Microanal 17:137–155
Overeem JC, Elgersma DM (1970) Accumulation of mansonones E and F in Ulmus hollandica infected with Ceratocystis ulmi. Phytochemistry 9:1949–1952
Pajares JA (2004) Elm breeding for resistance against bark beetles. Forest Syst 13:207–215
Paluch G, Miller F, Zhu J et al (2006) Influence of elm foliar chemistry for the host suitability of the Japanese beetle, Popilla japonica, and the gypsy moth, Lymantria dispar. J Agri Urban Entomol 23:209–223
Pearce RB (1996) Antimicrobial defences in the wood of living trees. New Phytol 132:203–233
Perdiguero P, Venturas M, Cervera MT et al (2015) Massive sequencing of Ulmus minor’s transcriptome provides new molecular tools for a genus under the constant threat of Dutch elm disease. Front Plant Sci 6:541
Pérez-de-Luque A, Lozano MD, Cubero JI et al (2006) Mucilage production during the incompatible interaction between Orobanche crenata and Vicia sativa. J Exp Bot 57:931–942
Rajput KS, Sanghvi GV, Koyani RD et al (2009) Anatomical changes in the stems of Azadirachta indica (meliaceae) infected by pathogenic fungi. IAWA J 30:27–36
Ralph SG (2009) Studying Populus defenses against insect herbivores in the post-genomic era. Crit Rev Plant Sci 28:335–345
Rauscher KJ, Lester DT, Smallcy EB (1974) Response of elm species and clones to inoculation with Verticillium albo-atrum. Phytopathology 64:702–705
Richens RH (1983) Elm. Cambridge University Press, Cambridge
Rioux D, Ouellette GB (1991) Barrier zone formation in host and nonhost trees inoculated with Ophiostoma ulmi. I. Anatomy and histochemistry. Can J Bot 69:2055–2073
Rioux D, Chamberland H, Simard M et al (1995) Suberized tyloses in trees: An ultrastructural and cytochemical study. Planta 196:125–140
Rioux D, Nicole M, Simard M et al (1998) Immunocytochemical evidence that secretion of pectin occurs during gel (gum) and tylosis formation in trees. Phytopathology 88:494–505
Rowe JW, Seikel MK, Roy DN et al (1972) Chemotaxonomy of Ulmus. Phytochemistry 11:2513–2517
Sacchetti P, Tiberi R, Mittempergher L (1990) Preference of Scolytus multistriatus (Marsham) during the gonad maturation phase between two species of elm. Redia 73:347–354
Salminen J-P, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338
Santamour FS Jr (1972) Flavonoid Distribution in Ulmus. B Torrey Bot Club 99:127–131
Santini A, Faccoli M (2015) Dutch elm disease and elm bark beetles: a century of association. iForest 8:126
Schreiber LR (1967) A soft rot of elm root cuttings caused by Fusarium solani. Phytopathology 57:920–921
Schroeder R, Forstreuter M, Hilker M (2005) A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiol 138:470–477
Schütt P, Weisgerber H, Schuck HJ et al (1995). Enzyklopädie der Holzgewächse, Ulmus III-2. 37. Erg Lfg, 9/04
Schwachtje J, Baldwin IT (2008) Why does herbivore attack reconfigure primary metabolism? Plant Physiol 146:845–851
Seikel MK, Hostettler FD, Johnson DB (1968) Lignans of Ulmus thomasii heartwood—I: Thomasic acid. Tetrahedron 24:1475–1488
Sharan K, Mishra JS, Swarnkar G et al (2011) A novel quercetin analogue from a medicinal plant promotes peak bone mass achievement and bone healing after injury and exerts an anabolic effect on osteoporotic bone: the role of aryl hydrocarbon receptor as a mediator of osteogenic action. J Bone Miner Res 26:2096–2111
Sherman SL, Giannasi DE (1988) Foliar flavonoids of Ulmus in eastern North America. Biochem Syst Ecol 16:51–56
Shigo AL (1984) Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annu Rev Phytopathol 22:189–214
Shigo AL et al (1986) Patterns of starch reserves in healthy and diseased American elms. Can J Forest Res 16:204–210
Sinclair WA, Zahand JP, Melching JB (1975) Localization of infection in American elms resistant to Ceratocystis ulmi. Phytopathology 65:129–133
Sinclair WA, Townsend AM, Griffiths HM et al (2000) Responses of six Eurasian Ulmus cultivars to a North American elm yellows phytoplasma. Plant Dis 84:1266–1270
Slama K (1979) Insect hormone and antihormones in plants pages. Academic Press, New York
Smith DR, Altenhofer E (2011) A new elm leafmining sawfly (Hymenoptera: Tenthredinidae) from Russia. Proc Entomol Soc Wash 113:50–56
Smith CM, Clement SL (2012) Molecular bases of plant resistance to arthropods. Annu Rev Entomol 57:309–328
Soetens P, Rowellrahier M, Pasteels JM (1991) Influence of phenolglucosides and trichome density on the distribution of insects herbivores on willows. Entomol Exp Appl 59:175–187
Solla A, Gil L (2003) Evaluating Verticillium dahliae for biological control of Ophiostoma novo-ulmi in Ulmus minor. Plant Pathol 52:579–585
Stam JM, Kroes A, Li Y, Gols R, van Loon JJ, Poelman EH, Dicke M (2014) Plant interactions with multiple insect herbivores: from community to genes. Plant Biol 65:689
Sticklen MB, Sherald J (1993) Dutch elm disease research: cellular and molecular approaches. Springer-Verlag, New York
Sticklen M, Bolyard M, Hajela R et al (1991) Molecular and cellular aspects of Dutch elm disease. Phytoprotection 72:1–13
Stipes RJ, Campana RJ (1981) Compendium of elm diseases. American Phytopathological Society, Minnesota
Strunz GM, C-m Yu, Salonius A (1989) Formation of the ortho-quinone mansonone C from 7-hydroxycadalene on silica gel. Phytochemistry 28:2861–2863
Sutherland ML, Mittempergher L, Brasier CM (1995) Control of Dutch elm disease by induced host resistance. Eur J Forest Path 25:307–315
Tian D, Tooker J, Peiffer M, Chung S et al (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236:1053–1066
Tippett JT, Shigo AL (1981) Barrier zone formation-a mechanism of tree defense against vascular pathogens. IAWA Bull 2:163–168
Tuskan GA, DiFazio S, Jansson S et al (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604
Tuzun S, Bent E (2006) Multigenic and induced systemic resistance in plants. Springer, New York
Valle T, López JL, Hernández JM et al (1997) Antifungal activity of scopoletin and its differential accumulation in Ulmus pumila and Ulmus campestris cell suspension cultures infected with Ophiostoma ulmi spores. Plant Sci 125:97–101
van Dam NM, Heil M (2011) Multitrophic interactions below and above ground: en route to the next level. J Ecol 99:77–88
Vander Mijnsbrugge K, Beeckman H, De Rycke R et al (2000) Phenylcoumaran benzylic ether reductase, a prominent poplar xylem protein, is strongly associated with phenylpropanoid biosynthesis in lignifying cells. Planta 211:502–509
Velasco R, Zharkikh A, Affourtit J et al (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet 42:833–839
Velikova V, Salerno G, Frati F et al (2010) Influence of feeding and oviposition of phytophagous pentatomids on photosynthesis of herbaceous plants. J Chem Ecol 36:629–641
Veluthakkal R, Dasgupta MG (2010) Pathogenesis-related genes and proteins in forest tree species. Trees-Struct Funct 24:993–1006
Vet LEM, Dicke M (1992) Ecology of Infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wang D, Xia M, Cui Z (2006) New triterpenoids isolated from the root bark of Ulmus pumila L. Chem Pharm Bull 54:775–778
Watts CR, Rousseau B (2012) Slippery elm, its biochemistry, and use as a complementary and alternative treatment for laryngeal Irritation. J Inve Biochem 1:17–23
Webber JF, Kirby SG (1983) Host feeding preference by Scolytus scolytus. In: Research on Dutch Elm Disease in Europe. Forestry Commission Bull 60:47-49
Wegener R (2002) Identifizierung und Synthese von Inhaltsstoffen aus Blattkäfern und Pflanzen mit biologischer Aktivität in tritrophischen Systemen. TENEA, Berlin, Germany
Wegener R, Schulz S, Meiners T et al (2001) Analysis of volatiles induced by oviposition of elm leaf beetle Xanthogaleruca luteola on Ulmus minor. J Chem Ecol 27:499–515
Weidhaas JAJ (1979) Spider mites and other Acarina on trees and shrubs. J Arbor 5:9–15
Wiegrefe SJ, Sytsma KJ, Guries RP (1994) Phylogeny of elm (Ulmus, Ulmaceae): molecular evidence for a sectional classification. Syst Bot 19:590–612
Witzell J, Martin JA (2008) Phenolic metabolites in the resistance of northern forest trees to pathogens—past experiences and future prospects. Can J Forest Res 38:2711–2727
Wu WD, Jeng RS, Hubbes M (1989) Toxic effects of elm phytoalexin mansonones on Ophiostoma ulmi, the causal agent of dutch elm disease. Eur J Forest Pathol 19:343–357
Xiao Y, Wang Q, Erb M et al (2012) Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol Lett 15:1130–1139
Xu Q, Chen LL, Ruan XA et al (2013) The draft genome of sweet orange (Citrus sinensis). Nat Genet 45:59–92
Yang X, Baskin JM, Baskin CC et al (2012) More than just a coating: ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect Plant Ecol Evol Syst 14:434–442
Young CE, Hall RW (1986) Factors influencing suitability of elms for elm leaf beetle, Xanthogaleruca luteola (Coleoptera: Chrysomelidae). Environ Entomol 15:843–849
Acknowledgments
We are grateful for the support of the German Research Foundation (= Deutsche Forschungsgemeinschaft DFG (Me 1810/4-1.2 and Fe 778/1-1.2, CRC 973) and the Max Planck Society. We thank five anonymous reviewers for providing valuable comments on earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Büchel, K., Fenning, T., Gershenzon, J. et al. Elm defence against herbivores and pathogens: morphological, chemical and molecular regulation aspects. Phytochem Rev 15, 961–983 (2016). https://doi.org/10.1007/s11101-015-9442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-015-9442-0