Abstract
In this study we tested the effects of rapid induced resistance of the silver birch, Betula pendula, on the performance and immune defense of the gypsy moth, Lymantria dispar. We also measured the effects of defoliation on the concentrations of plant secondary metabolites, particularly on phenolics and terpenoids. It was found that severe natural defoliation (by moth larvae) of silver birch led to an increase in lipophilic flavonoids on the leaf surface. The concentration of some simple phenolics and monoterpenes (linalool and geraniol) also increased, while that of several glycosides of quercetin decreased. The female pupal weights and survival rates of moths decreased, and larval development time increased, when the insects fed on defoliated trees. However, the feeding of caterpillars with the leaves of defoliated trees led to an increase in lysozyme-like activity in their hemolymph, with an increase in their ability to encapsulate potential parasites. Our data show that the silver birch deploys a rapid chemical defense against gypsy moth larvae. We suggest that lipophilic flavonoids are important compounds in the direct silver birch defense against L. dispar caterpillars. The increased strength of immune defense of insects exposed to trees that had deployed a rapid induced resistance may be an adaptation of the herbivores to resist the rising density of parasites when host population density is high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbivores often trigger host plant defenses, which can be seen as changes in leaf chemistry (as primary and secondary metabolites), physical factors (e.g., toughness of the leaves), or phenology (Larsson 2002). It is because of high levels of variation of ontogenetic and physiological origin that primary metabolites are usually not considered as part of a plant’s resistance mechanisms, although this may be erroneous (Berenbaum 1995). Secondary compounds such as terpenoids, phenolics, and alkaloids are commonly involved in both constitutive and induced types of plant defense against insect herbivores, which can lead to a negative feedback relationship with the insects’ performance (Rosenthal and Berenbaum 1992). There are two types of herbivore-induced defense in perennial plants, and these can be separated temporally: (1) A rapidly induced resistance (RIR) appearing during the season of a plant’s defoliation that begins in the first hours after initial tissue damage and (2) a delayed induced resistance (DIR) that occurs during the following seasons after the plant is damaged (Haukioja 1991; Neuvonen and Haukioja 1991). However, some researchers have identified a third type of temporal defense, intermediate-delayed induced resistance (IDIR), which occurs up to 2 months after any initial damage within the same defoliation season (Stevens and Lindroth 2005).
A major forest defoliator in North America and Eurasia is the gypsy moth, Lymantria dispar L. (Lepidoptera: Lymantriidae) (Doane and McManus 1981). Gypsy moth populations are typically eruptive, and when densities reach very high levels, trees are often completely defoliated. The main host plant of L. dispar in Western Siberia is the silver birch, Betula pendula Roth. Many authors have demonstrated that herbivores are able to induce both rapid and delayed resistance in different birch species including silver birch (Kaitaniemi et al. 1998; Keinänen et al. 1999; Mutikainen et al. 2000, Haukioja 2005; Roden and Mattson 2008). However, studies of the B. pendula—L. dispar interaction are lacking.
The main top-down forces involved in regulating Western Siberian L. dispar are parasitic insects (e.g., dipterans, hymenopterans) and some pathogens (Martemyanov and Bakhvalov 2007). The successful development of parasites and pathogens does not only depend on host density but also on the status of the host such as its immune function. It has been shown that insect innate immunity, measured as hemolymphal phenoloxidase activity, encapsulation response, or phagocytosis activity, is associated with their overall resistance against parasitoids (Smilanich et al. 2009), fungi (Wilson et al. 2001), and viruses (Trudeau et al. 2001). The host’s immune defense has been found to be phenotypically plastic and dependent on many biotic factors, such as food quality and quantity (e.g., Siva-Jothy and Thompson 2002; Ojala et al. 2005; Lee et al. 2006; Yang et al. 2007). Changes in biotic factors may also affect the likelihood of parasitoids and pathogens successfully attacking hosts, which could change the insect–pathogen interaction through a change in host immune status. Since food quality for insect herbivores is influenced by previous and/or current host plant defoliation, we suggest that their immune traits may be affected by the status of the tree they have fed on. However, we are unaware of any previous studies on the effects of RIR on the immunity of insect herbivores.
The aim of this study was to measure any RIR effect(s) of B. pendula on gypsy moth performance and immune defense. We also tested whether the RIR of silver birch Betula pendula Roth. was associated with general (systemic and local) changes in the content and composition of secondary compounds (phenolics and terpenoids).
Materials and methods
Experimental plot
The field experiments were conducted in suburban stands of early-successional birch at Novosibirsk (54055′N 83012′E), Western Siberia, Russia, during 2007. All trees were of vegetative origin and thereby had low genetic variation. During the study period, and in the previous 4 years, the natural densities of gypsy moths and other insect defoliators in the study area were low with no visible defoliation apparent. The insects for the experiment were therefore collected during a natural outbreak from birch stands in the Novosibirsk region, 200 km west of the experimental plot in the autumn of 2006. This was done by collecting clutches of L. dispar eggs, then keeping them in a refrigerator at +4 °C during the subsequent winter for use as stock for the experiments the following spring (Lazarević et al. 2002).
Experimental design and field experiments
In our study we simulated the severe defoliation of host plants that can occur at high levels of gypsy moth population density. Our experimental design was modified from that of Parry et al. (2003). Ten 9–11-year-old B. pendula trees (height ~ 5 m) were haphazardly chosen for defoliation, with another ten used as control trees. The defoliation treatment was conducted by enclosing larvae in a big mesh bag that covered the whole crown of each tree. The laboratory-reared moth larvae were each allocated to one of three groups: “A”—a group to induce severe defoliation, “B”—a group to estimate insect performance, and “C”—a group to estimate the immune parameters of the insects (Fig. 1). To produce insects for the group A treatment, we first placed 25 clutches of gypsy moth eggs (6,000–8,000 eggs in total) from the refrigerated stock into the laboratory at room temperature until they hatched. This process was timed so the eggs hatched in the middle of May 2007 were synchronous with the birch budbreak. The first-instar caterpillars were reared in plastic hatcheries on cut tips of birches until they molted to their second instar. This was done to prevent small larvae from escaping from the mesh bags placed over each experimental tree in the field. The second-instar larvae were then released into the bags of each of the ten treatment trees in the field, with 250–300 larvae per tree depending on the size of the tree. According to our preliminary experiments, this density was enough to cause severe defoliation to the crowns of young trees. The crowns of the control trees were covered with bags, but no larvae were introduced. This allowed us to control the effect of shadow as well as changing microclimate conditions inside the bag on chemical compositions of tree foliage.
Insects for groups “B” and “C” were reared identically to those for group A. Before hatching we mixed the eggs of 50 females after which we allowed them to hatch as described above. We haphazardly selected 2000 first-instar caterpillars and reared them in large 19-l plastic containers with 50 larvae per container. In twenty containers we reared insects for group “B.” The larvae in the remaining twenty containers were allocated to group “C.” Each container for each group was randomly allocated to one of twenty trees chosen for the experiment. All first-instar caterpillars for groups B and C were reared on foliage cut from the tree to which they would subsequently be introduced as a second-instar caterpillar. To maintain leaf turgor, freshly cut tips were placed in 10-ml glass vials filled with water and sealed around the stem with parafilm. When they reached the second instar, group B and C larvae were released into the mesh sleeve bags (30 cm × 70 cm) placed on the branches of their appropriate tree (control or treated) (Fig. 1). Finally, each of twenty studied trees contained insects of both groups B and C. Ten trees destined for defoliation contained additional larvae from group A. The releasing of larvae from all three groups was carried out on the same day. First, insects of groups “B” and “C” were released into small mesh bags located in the middle canopy of the trees. We then covered the whole crowns of the young plants with a single big bag and housed the larvae of group “A” into a big bag on ten treated trees as described above. Thus, all groups of larvae were isolated from each other. When the larvae in each small bag had consumed all of the foliage inside their bag, they were moved to another branch after first removing (by shaking) the “defoliating” caterpillars. After the treated plants had been defoliated by 60–70 % (approximately 4 weeks after the larvae were released), we removed the big bag together with the “defoliating” insects by shaking the tree. The severe defoliation in our experiment was synchronized with a natural outbreak of L. dispar in western Novosibirsk.
Gypsy moth performance
Three parameters of group “B” insects were measured: (1) larval development time, (2) female pupal mass, and (3) survival to adulthood. Larval development time was measured as the mean duration in days between hatching and pupal molt (Lazarević et al. 2002). Larval pupation was checked daily, with all new pupae being collected. The pupae were returned to the laboratory, where they were weighed to the nearest 0.01 g and then kept separately until metamorphosis into adult moths. The sex of each insect was determined using morphological parameters of the imago, particularly the structure of the antenna. The viability of individuals was measured as egg to adult survival.
Immune assays
After the larvae in group “C” had reached the fourth instar, we took them to the laboratory for immune assays. To collect hemolymph, each caterpillar was pierced with a thin needle. Then, about 25–30 μl of hemolymph was withdrawn, with each sample being split equally by being placed into two Eppendorf tubes that had been previously cooled to 4 °C. Part of the hemolymph from one tube (4.5 μl) was mixed with 20 μl of cooled anticoagulant with phenylthiourea for a total hemocyte count (THC). THC was immediately measured with a hemocytometer as the number of hemocytes per 1 ml of hemolymph. The other Eppendorf tube with hemolymph was centrifuged at 500g at 4 °C for 10 min. The supernatant was then used to estimate PO activity, lysozyme-like activity, and protein concentration. PO activity was measured using l-dopa as a substrate. To do it, 10 μl of supernatant was mixed with 500 μl of l-dopa (dioxyphenylalanine, concentration of stock solution 2 mg/ml) in a phosphate buffer (pH 7.2). After incubation for 1 h at 28 °C, PO activity was measured with an Agilent 8453 UV–visible spectroscopy system at 490 nm. Hemolymph protein levels were measured by the method described by Bradford (1976), using the standard curve created from a bovine serum albumin standard. The final PO activity was recalculated as the difference in the absorbencies between control and hemolymph present in samples per 1 min per 1 mg of protein. To estimate lysozyme-like activity against gram-positive bacteria, the bacterium Micrococcus luteus was used (Lee et al. 2006). The remaining hemolymph samples were mixed with 1 μl of saturated solution of phenylthiourea to prevent the following melanization, and then 2 μl of each sample was used. Each sample was placed into holes in agar medium that contained the M. luteus. All samples were incubated for 24 h at 28 °C. Data were recorded as the area of the lytic zone, which was photographed using a digital camera and then measured with Image Pro-Plus software (Media Cybernetics, Silver Spring, MD, USA). Then, each datum was recalculated using a lysozyme standard.
The strength of encapsulation response was quantified by measuring the degree of melanization of a nylon monofilament that was inserted into the hemocoel of each animal. This is a commonly used technique to measure the strength of immunity in lepidopterans and in other insects (e.g., Lee et al. 2006; Rantala and Roff 2007; Dubovskiy et al. 2008). The encapsulation rate against a nylon monofilament has been shown to correlate with the resistance of the autumnal moth against an entomopathogenic fungus (Rantala and Roff 2007), suggesting that the method is biologically valid. Likewise, Smilanich et al. (2009) found that the encapsulation response against artificial implants was associated with resistance to parasitoids. To measure the encapsulation response, we used insects of the same group immediately after hemolymph collection. A piece of nylon monofilament (2 mm long) was inserted into the caterpillar’s body cavity through the hole made for hemolymph collection. After 3 h, the nylon implant was carefully removed and then photographed from three different angles using a digital camera. Then, the degree of melanization was calculated as the difference between the mean of the three measurements and a control nylon filament that was not inserted into any larva. All measurements were taken using Image Pro-Plus software. The final result is presented as the difference between melanized and control implants.
Foliage phenolics analysis
We estimated the response of the birch trees to herbivory as the sum of both local and systemic responses. To control for any effects of feeding by larvae of groups “B” and “C” on control trees, we used ten additional native trees in the same plot. The foliage from all trees was collected twice: directly before insect release and after the removal of the big bags. Approximately 50 leaves were haphazardly collected from the canopy of each tree. The freshly collected leaves were then taken to the laboratory and were air-dried at room temperature in the shade for 10 days. According to Keinänen and Julkunen-Tiitto (1996), this method of drying B. pendula leaves before the extraction of phenolic compounds is acceptable “for comparative purposes.” The dried leaves were then ground to a fine powder for chemical extraction. We detected three groups of phenolics in the samples: (1) simple phenolics, (2) water-soluble flavonoid glycosides, and (3) lipophilic flavonoid aglycones. We analyzed hydrolysable tannins in the samples as well but none was found. Other groups of tannins, that is, proanthocyanidins (condensed tannins), were not measured, since earlier studies have suggested that they do not significantly affect gypsy moth performance (Osier and Lindroth 2001; Martemyanov et al. 2006; Barbehenn et al. 2009). To extract simple phenolics and flavonoid glycosides, 20 mg of the powdered leaves was added to 0.5 ml of acetone/water (7/3, V/V, containing 0.1 % ascorbic acid, m/V) and then shaken for 45 min with a mechanical shaker. After being centrifuged, residues of samples were re-extracted twice by the same methods. Thus, finally, we had 1.5 ml of supernatant of each sample that contained extracted phenolics. Then, acetone was allowed to evaporate from the supernatant by placing it into an Eppendorf concentrator 5301 (Eppendorf AG, Hamburg, Germany). For each sample, the remaining water was frozen and the remaining extract freeze-dried. The dried extract was dissolved in 1.5 ml of distilled water, filtered, and analyzed with the HPLC system (Merck-Hitachi, Tokyo, Japan). This consisted of a pump L-7100, a diode array detector L-7455, a programmable autosampler L-7250, and an interface D-7000. For separation, we used a Superspher 100 RP-18 column (75 mm × 4 mm i.d., 4 μm, Merck, Germany). Lipophilic leaf surface flavonoids were extracted from 10 mg of leaf powder with 1 ml of 95 % ethanol for 1 min and subsequently filtered and analyzed with the same HPLC system and columns as previously described using the methods of Lahtinen et al. (2006). Simple phenolics were detected at 280 nm, with flavonoid glycosides and lipophilic flavonoid aglycones being detected at 349 nm. Also, several samples that presented maximal amounts of peaks were analyzed by HPLC–MS according to the methods outlined by Salminen et al. (1999). Compounds were identified on the basis of their UV and mass spectra and retention times reported in the literature (Ossipov et al. 1996; Keinänen and Julkunen-Tiitto 1998; Valkama et al. 2003). Simple phenolics were quantified as gallic acid equivalents, and water-soluble flavonoid glycosides and lipophilic flavonoid aglycones as quercetin equivalents. Results of phenolic content in leaves are presented as the average amount of the compound per gram of dried foliage with standard errors (Table 1). Peaks of unknown phenolic # 2 and chlorogenic acid; two glycosides of myricetin; two unidentified flavonoid glycosides; as well as acacetin, tetrahydroxy-flavone dimethyl ether, and pentahydroxyflavone are presented as their summarized concentrations (Table 1) since the resolution of the separation did not allow us to calculate the concentrations of chemicals separately. Compounds 1–6 are simple phenolics, compounds 7–14 are water-soluble flavonoid glycosides, and compounds 15–18 are lipophilic flavonoid aglycones.
Foliage terpenoid analysis
The fresh leaves for this analysis (70–100 g per sample) were collected at the same time as the samples for phenolics analysis. Afterward, the collected samples were weighed and treated with ethanol (250 ml) at room temperature for 72 h. The alcoholic extract was concentrated to a volume of 5–10 ml by rotary evaporation in a vacuum at room temperature to prevent loss of volatiles. Then, it was subjected to simultaneous hydrodistillation–extraction (Likens and Nickerson 1964) with hexane for 3 h. The hexane extract was dried over anhydrous Na2SO4 and filtered. The solvent was removed by rotary evaporation at room temperature under reduced pressure.
GC–MS analyses were made on a HP 5890 gas chromatograph coupled to a HP 5972A quadrupole mass selective detector [30 m × 0.25 mm HP-5 ms; film thickness, 0.25 μm; temperature programming, 50 °C (2 min)–240 °C (4 °C/min)–280 °C (20 °C/min)–280 °C (5 min)]. Helium was used as the carrier gas, at a flow rate of 1 ml/min, and the injector temperature was set at 280 °C. The volume injected of each sample was 1 μl of ~1 % solution, with a split ratio of 1:100, ionization 70 eV, an ion source temperature of 280 °C, and a mass range from 29 to 500. The component compounds were identified by their retention indexes (relative to C8–C9–…–C24 n-alkanes used as the internal standards) and full mass spectra (Tkachev 2008). Percentage of each compound was calculated from GC peak areas by internal normalization without correction coefficients. The absence of chiral column meant that enantioselective analysis of studied volatiles was not carried out.
Statistical analysis
All analyses were performed with SPSS 11.5.1 statistical software (SPSS inc.). The concentrations of foliage chemicals were tested for normality with a series of Kolmogorov–Smirnov tests. All were significantly different to a normal distribution with an equal mean and variance so we ln-transformed our data before analysis. We revealed a lot of terpenoids in trace amounts that did not replicate in every tree. Therefore, we have included in the model only the six terpenoids that occurred in most trees of same group with a concentration above 0.001 mg g−1 of damp leaves. These were geraniol, linalool, eugenol, hexadecanol, eudesmadienol, and germacratrienol. The phenolics that we identified were present in most individuals so all were included in the analytical model. First, we compared the effects of slight current defoliation by larvae of both groups “B” and “C” (without group “A”) on the secondary compounds of control trees with additional native trees (see “Foliage phenolics analysis” section). We used a repeated measures ANOVA with treatment as the fixed factor and time (the beginning and finishing of defoliation) as the within-subject factor. All identified chemicals did not statistically differ between these groups. This may be explained by a permanent low level of herbivory and/or phytopathogen presence in native trees under natural conditions. On the basis of this result, we combined the leaf chemical data from native and control trees to one large control group to increase the power of further statistical tests. After combining these data, the tests were repeated using the defoliated and control groups in the treatment category. The result of statistical analysis of treatment x time interaction is presented in Tables 1 and 2.
The distributions of PO, hemocyte counts, and lysozyme-like activity were not normal. Therefore, we log-transformed the data for all of these measurements to conform to the assumptions that underlie parametric tests. Nested ANOVA with individual trees as a random factor (nested under treatment) was used to test for the effects of treatment on larval immune traits. It is because we have found correlations between larvae body mass and lysozyme-like activity, as well as between PO and larvae body mass, we include body mass as a covariate in our models. We used one-way ANOVAs to test for the effects of treatment on life history traits.
Results
The effect of same-season tree defoliation on foliage chemicals content
We found that severe defoliation by gypsy moth larvae led to significant increases (more than two times) in all studied flavonoid aglycones (Table 1). Such simple phenolics as 1-(4″-hydroxyphenyl)-3′-oxopropyl-β-d-glucopyranose and the unknown phenolic # 1 were also increased after severe defoliation by gypsy moth larvae (Table 1). In spite of the seasonal reduction in the concentrations of both derivatives of coumaroyl quinic acid, the decrease in those chemicals in the foliage of treated birches occurred to less of a degree compared with control trees (Table 1; for derivative # 2 only the trend was revealed). The concentrations of all found glycosides of quercetin (except glycoside # 1) were significantly decreased by moth defoliation. All of the phenolics we found changed their concentrations between two measurements (for all phenolics, presented in Table 1 p ≤ 0.05 for time effect). Additionally, we found that defoliation increased the foliar content of monoterpenes such as linalool and geraniol (Table 2). The concentration of the volatile phenolic eugenol also was increased, while that of hexadecanol was decreased by the presence of defoliators. We did not identify any novel phenolics in the leaves of the defoliated birches.
The effect of birch response after defoliation on gypsy moth performance
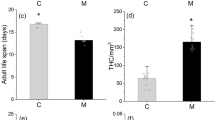
We found that the different effects of RIR on the performance of different sexes of the moths led to the increasing of female development time (MS = 81.67; df = 1; F = 10.27; p = 0.005) and a decreasing of female pupae mass (MS = 0.182; df = 1; F = 9.839; p = 0.007) (Fig. 2). However, the larval development time (MS = 1.042; df = 1; F = 0.261; p = 0.613) and pupae mass (MS = 0.005; df = 1; F = 0.588; p = 0.449) of males were unaffected by tree defoliation (Fig. 2). Egg to adult survival also decreased as RIR increased (MS = 174.8; df = 1; F = 7,875; p = 0.012) (Fig. 2).
The effect of birch after defoliation on larvae immune traits
RIR of birch enhanced the lysozyme-like activity in the hemolymph of the moth larvae. However, the lysozyme-like activity of the hemolymph did not differ between larvae reared on different trees within the same treatment (Fig. 3, Table 3). There was a significant difference in encapsulation response between larvae reared on different individuals of trees (Table 3). Defoliation treatment increased the encapsulation response of the larvae. Instead, no RIR or tree individual had any effect on THC or PO activity in the hemolymph of the moth larvae.
The effect of birch defoliation on the following gypsy moth immune parameters: lysozyme-like activity (a), encapsulation rate of a nylon monofilament inserted into the hemocoel (b), PO activity (c), and total hemocyte counts (d). Original data were in log units (a), gray value (b), log ∆A490/min/mg protein (c), and log cells/ml (d). The bars marked with asterisks are statistically differed from control (** for p ≤ 0.01)
Discussion
The effect of same-season tree defoliation on the content of foliage chemicals
We found that severe current-season defoliation by gypsy moth larvae leads to changes in plant chemistry. Most previous studies carried out on other birch species, and other tree species, investigated mainly the content of water-soluble flavonoid glycosides, proanthocyanidins, hydrolyzable tannins, and simple phenolics (Ossipov et al. 2001; Lempa et al. 2004; Stevens and Lindroth 2005). Other phytochemical studies begin to pay attention to the surface-bound lipophilic aglycones of flavonoids that accumulate in the glandular trichomes (Lahtinen et al. 2004; Valkama et al. 2005). However, these studies did not measure the effect of current-season tree defoliation on the flavonoid aglycone content in the leaves. Keinänen et al. (1999) mention that artificial defoliation of birch (B. pendula) clones in the current-season leads to a significant increase in flavonoid aglycones, but the strength of the effect was weaker than in our study. There are at least two explanations for this difference: (1) differences in the nature of defoliation (artificial vs. natural) and (2) differences in the duration after the beginning of the defoliation (3 days in the study with clones vs. 4 weeks for our study). Thus, we show for the first time that natural defoliation of B. pendula trees by gypsy moth larvae strongly increases the content of lipophilic flavonoids in their leaves. One possible reason contributing to the strength of this effect is natural herbivory, particularly the effect of larvae regurgitant that was shown for other species (e.g., Walling 2000 and works cited within there).
Our result concerning flavonoid glycosides does not concur with the data presented by Lempa et al. (2004), who did not find any effect of current-year defoliation of mountain birch (B. pubescens ssp. czerepanovii (Orlova)) by Autumn Moth (Epirrita autumnata Borkh) larvae on the content of flavonoid glycosides. However, Lempa and colleagues did not report the exact level of tree defoliation, only mentioning that a moderate level of tree damage was induced. It is possible that the level of defoliation in their study was not enough to induce the change in flavonoid glycoside concentrations, as found in our study. The differences in the effect found between the two studies may also be explained by differences between birch species (see Ossipov et al. 1996). Likewise, the trends shown for glycosidic flavonoids were the opposite to those found by Keinänen et al. (1999), who found that defoliation by scissors did not affect the concentration of quercetin glycosides. This discrepancy may have been influenced by the manner of defoliation, which may demonstrate the importance of actual larvae in inducing a plant response. Since we found the opposite effect of defoliation on aglycones and glycosides of flavonoids, we were interested to investigate any trade-off between these chemicals. Using Pearson correlation (for ln-transformed data), we found a negative correlation between the sum of quercetin glycosides (decreased by defoliation treatment) and the sum of all lipophilic aglycones in the leaves of defoliated trees (r = −0,74; p = 0,013). A similar pattern was found in B. pendula by Keinänen et al. (1999), which may be explained by the efficient transportation of flavonoid building blocks from foliar vacuoles (the site for flavonoid glycoside biosynthesis) to glandular trichomes (the site for flavonoid aglycone biosynthesis). Alternatively, defoliation may have simply increased the metabolic activity of the glandular trichomes and thus the production of flavonoid aglycones. In general, the concentration of flavonoid aglycones is known to decrease, not increase, during leaf growth and development (e.g., Valkama et al. 2004).
Osier and Lindroth (2001) showed that in another species of woody tree (Populus tremuloides), phenolic glycosides (particularly salicortin and tremulacin) present the constitutive defense against the gypsy moth. These did not change by defoliation, whereas in our case different phenolic glycosides (1-(4″-hydroxyphenyl)-3′-oxopropyl-β-d-glucopyranose) of a closely related biosynthetic pathway were increased, reflecting birch-induced defense. This example suggests that the same class of phenolic compounds are involved, or possibly the same biosynthetic pathway in constitutive or induced defense is species specific in the trees.
The increase in linalool in silver birch leaves when the tree was damaged by E. autumnata was also shown in recent work that compared the tree response against both herbivores and phytopathogens (Vuorinen et al. 2007). Moreover, the increase in some volatiles in that work was a consumer-specific response, that is, the emission of linalool was increased by herbivore defoliation but was unaffected by phytopathogen infestation. Our result has partially replicated that study (in our case the contents of linalool and some other volatiles were increased by gypsy moth defoliation) and thus concurs with the suggestion that the involvement of some volatile organic compound is a specific response of birch against different types of consumers. However, Vuorinen and co-authors did not measure any life history traits of the insects, unlike in our study.
The effects of birch response after defoliation on gypsy moth performance
Interestingly, we found that the effect of defoliation on the insects was sex dependent, that is, affected both female pupal mass and larval development time but not males (Fig. 2). Similar effects have been shown in gypsy moths (L. dispar ssp. dispar) that have been feeding on other birch species (B. papyrifera) as well as other tree genera (Quercus rubra, Populus tremuloides) (Roden and Mattson 2008). Only female pupal mass in that study was affected by artificial tree defoliation, although the larval development time of both sexes was unaffected. The different effect on larval growth in that study, in comparison with our results, may be caused by at least two reasons: (1) the manner of defoliation and (2) the plant species studied. This could be tested by comparing different studies on the effects of the rapid response of trees on herbivores. For example, Osier and Lindroth (2001) did not find any effect of same-season artificial defoliation of Populus tremuloides on gypsy moth performance, with the exception of a decrease in relative growth rates of L. dispar larvae. In another herbivorous insect, Epirrita autumnata, using Betula pubescens ssp. czerepanovii as a host plant, those individuals reared on leaves from defoliated trees developed 16 % faster than those on leaves from control trees (Lempa et al. 2004). However, a similar study on E. autumnata fed on B. pendula showed that larval growth rate was unaffected by same-season artificial defoliation (Mutikainen et al. 2000). Thus, rapid induced response of plants may lead to totally opposite effects on their herbivores. In our study it was possible that females were more susceptible to plant defenses than males because of their additional instar and longer feeding time. One more possible explanation for the sex-dependent effect of treatment on pupal mass may be a difference between the sexes in the accumulation of nutrients during the last larvae stage. More specifically, adult males are better fliers than females (in a population of L. dispar ssp. asiatica), and they need to accumulate more energy reserves as larvae before the pupation. Females, however, need to accumulate more protein to produce eggs (see Stockhoff 1993). Possibly because of the increase in phenolic compounds in defoliated trees (Table 1), the availability of proteins in the diet may be reduced owing to an increase in binding protein–phenols.
The increase in the concentration of flavonoid aglycones, as well as that of some simple phenolics, may be the reason for the suppressive effect of the treated plants against caterpillars. Earlier, a defensive effect of flavonoid aglycones was shown for E. autumnata feeding on B. pubescens ssp. czerepanovii (Lahtinen et al. 2004; Valkama et al. 2005), although for B. pendula only trend of negative correlation between the concentrations of aglycones and the performance of the insects was found (Valkama et al. 2005).
It is possible that monoterpenes (linalool and geraniol) may also be involved in the rapid defense of B. pendula against gypsy moths, because the concentration of these chemicals in their leaves increased after defoliation. Previous experiments using gypsy moth larvae reared on artificial diet showed the deterrent ability of geraniol in a closed concentration (3.75 mg/ml) (Doskotch et al. 1980). It is also known that monoterpenes inhibit the activity of acetylcholine esterase by means of hydrophobic binding with the enzyme that will lead to accumulation of acetylcholine in cholinergic sites (Ryan and Byrne 1988; Keane and Ryan 1999). Thus, volatile monoterpenes may be involved in both types of defenses of birch trees (direct and indirect through the attraction of parasitoids) and may contribute to the decline in performance of the insects fed on defoliated birches.
It is noteworthy that the low level of survival in the control group (Fig. 2) may be caused by at least four factors other than those associated with induced defense: (1) the effects of high-density stress of the maternal generation (see methods); (2) unfavorable climatic conditions (there was high rainfall in June 2007); (3) the effects of some predators (mainly bugs and scorpion flies) that are able to attack the larvae near to the mesh bag; and (4) the effects of unknown pathogens. However, we also suggest that the induced response of silver birch may contribute to regulation of the density of present and following generations of gypsy moths.
The effect of birch response after defoliation on larvae immune traits
In this study we found a strong effect of tree defoliation on the lysozyme-like activity in the hemolymph of the moths; feeding on the damaged trees increased this immune parameter. We got similar results in previous studies in which we tested the effect of delayed induced resistance of birch on gypsy moth immunocompetence (unpublished data). However, in that study we found no significant changes in the content of the same phytochemicals. Thus, there is likely to be another factor that increases the lysozyme-like activity which is related to both the effects of same-season and previous-year defoliation. Interestingly, we found a strong tendency of increase in the encapsulation response against the nylon monofilament when the larvae were fed on the leaves of the treated birches. Ruuhola et al. (2010) found no association between encapsulation and the amount of aromatic amino acids in the food of E. autumnata. Likewise, we did not find any correlation between birch phenolics and larval encapsulation in our previous data (Martemyanov et al. 2012). Thus, it seems that after severe defoliation, plants induce some unidentified changes in leaf quality, which may increase the immune response of some of their insect herbivores. The adaptive significance of this finding may be that it is a mechanism of herbivores that an increase in some immune parameters prepares them to be ready to resist against a rise in population density of both generalized pathogens and parasitoids, which inevitably follows after a rise in moth population density. In other words, gypsy moth larvae may use some chemical signals from damaged plants, which give information about a rise in herbivore population density. These signals can trigger the insect immune response. Taking into account the findings of Kapari et al. (2006), where the same result was shown for delayed induced response in B. pubescens after geometrid moth defoliation, it is possible that this plant–Lepidopteran phenomenon is widespread in nature.
In conclusion, our study provides evidence that silver birch produces a chemical defense after current-year defoliation that includes a change in the balance between glycoside phenolics and its aglycones, as well as an increase in terpenoids. In other words, we have demonstrated a direct and potentially an indirect rapid induced defense in birch. We have shown that the crucial rise in flavonoid aglycones after larvae defoliation is more significant to compare with the artificial treatment of silver birch presented by Keinänen et al. (1999). This highlights the importance of the nature of defoliation in interpretation of subsequent data. In spite of the significant effect of tree defoliation on gypsy moth population parameters, we suggest that tree-induced defenses play only a small role in the density regulation of later herbivore generations. This is because low survival rates in control treatments were caused by factors other than those associated with plant defoliation. It is possible that high density induced stress in the maternal generation or pathogens are more important. One more finding that needs to be emphasized is the increased immune function in larvae reared on damaged trees. This may be an adaptation in herbivores that increases individual survival during outbreaks.
References
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen J-P (2009) Hydrolyzable tannins as “quantitative defenses”: limited impact against Lymantria dispar caterpillars on hybrid poplar. J Insect Physiol 55:297–304
Berenbaum MR (1995) Turnabout is fair play: secondary roles for primary compounds. J Chem Ecol 21:925–940
Bradford JM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Doane CC, McManus ML (1981) The gypsy moth: research toward integrated pest management. Department of Agriculture, Forest Service, Science and Education Agency. Animal and Plant Health Inspection Service, Washington, USA
Doskotch RW, Cheng H-Y, Odell TM, Girard L (1980) Nerolidol: an antifeeding sesquiterpene alcohol for gypsy moth larvae from Melaleuca leucadendron. J Chem Ecol 6:845–851
Dubovskiy IM, Krukova NA, Glupov VV (2008) Phagocytic activity and encapsulation rate of Galleria mellonella larvae hemocytes during bacterial infection by Bacillus thuringiensis. J Invert Pathol 98:360–362
Haukioja E (1991) Induction of defenses in trees. Ann Rev Entomol 36:25–42
Haukioja E (2005) Plant defenses and population fluctuations of forest defoliators: mechanism-based scenarios. Ann Zool Fenn 42:313–325
Kaitaniemi P, Ruohomäki K, Ossipov V, Haukioja E, Pihlaja K (1998) Delayed induced changes in the biochemical composition of host plant leaves during an insect outbreak. Oecologia 116:182–190
Kapari L, Haukioja E, Rantala MJ, Ruuhola T (2006) Defoliating insect immune defense interacts with induced plant defense during a population outbreak. Ecology 87:291–296
Keane S, Ryan MF (1999) Purification, characterization, and inhibition by monoterpenes of acetylcholinesterase from the wax moth, Galleria mellonella (L.). Insect Biochem Mol Biol 29:1097–1104
Keinänen M, Julkunen-Tiitto R, Mutikainen P, Walls M, Ovaska J, Vapaavuori E (1999) Trade-offs in phenolic metabolism of silver birch: Effects of fertilization, defoliation, and genotype. Ecology 80:1970–1986
Keinänen M, Julkunen-Tiitto R (1996) Effect of sample preparation method on birch (Betula pendula Roth) leaf phenolics. J Agric Food Chem 44:2724–2727
Keinänen M, Julkunen-Tiitto R (1998) High-performance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. J Chromatogr A 793:370–377
Lahtinen M, Salminen J-P, Kapari L, Lempa K, Ossipov V, Sinkkonen J, Valkama E, Haukioja E, Pihlaja K (2004) Defensive effect of surface flavonoid aglycones of Betula pubescens leaves against first-instar Epirrita autumnata larvae. J Chem Ecol 30:2257–2268
Lahtinen M, Lempa K, Salminen J-P, Pihlaja K (2006) HPLC analysis of leaf surface flavonoids for the preliminary classification of birch species Phytochem Anal 17:197–203
Larsson S (2002) Resistance in trees to insects—an overview of mechanisms and interactions. In: Wagner MR et al (eds) Mechanisms and deployment of resistance in trees to insects. Kluwer Academic Publishers, Netherlands, pp 1–29
Lazarević J, Perić-Mataruga V, Stojković B, Tucić N (2002) Adaptation of the gypsy moth to an unsuitable host plant. Entomol Exp App 102:75–86
Lee KP, Cory JS, Wilson K, Raubenheimerand D, Simpson SJ (2006) Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc R Soc London Ser B 273:823–829
Lempa K, Agrawal AA, Salminen J-P, Turunen T, Ossipov V, Ossipova S, Haukioja E, Pihlaja K (2004) Rapid herbivore-induced changes in mountain birch phenolics and nutritive compounds and their effects on performance of the major defoliator, Epirrita autumnata. J Chem Ecol 30:303–321
Likens ST, Nickerson GB (1964) Detection of certain hop oil constituents in brewing products. Proc Am Brewing Chem 5:5–13
Martemyanov VV, Bakhvalov SA (2007) Interrelationships of plant–insect–parasite systems and their influence on the development and population dynamics of forest defoliators. Eurasian Entomol J 6:205–221
Martemyanov VV, Bakhvalov SA, Dubovskiy IM, Glupov VV, Salakhutdinov NF, Tolstikov GA (2006) Effect of tannic acid on the development and resistance of the gypsy moth Lymantria dispar L. to viral infection. Dokl Biochem Biophys 409:219–222
Martemyanov VV, Dubovskiy IM, Rantala, MJ, Salminen JP, Belousova IA, Pavlushin SV, Bakhvalov SA, Glupov VV (2012) The effects of defoliation-induced delayed changes in silver birch foliar chemistry on gypsy moth fitness, immune response, and resistance to baculovirus infection. J Chem Ecol 38:295–305
Mutikainen P, Walls M, Ovaska J, Keinanen M, Julkunen-Tiitto R, Vapaavuori E (2000) Herbivore resistance in Betula pendula: effect of fertilization, defoliation, and plant genotype. Ecology 81:49–65
Neuvonen S, Haukioja E (1991) The effects of inducible resistance in host foliage on birch-feeding herbivores. In: Tallamy DW, Raupp MJ (eds) Phytochemical induction by herbivores. Wiley, New York, pp 277–291
Ojala K, Julkunen-Tiitto R, Lindström L, Mappes J (2005) Diet affects the immune defence and life-history traits of an Arctiid moth Parasemia plantaginis. Evol Ecol Res 7:1153–1170
Osier TL, Lindroth RL (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Ossipov V, Nurmi K, Loponen J, Haukioja E, Pihlaja K (1996) HPLC separation and identification of phenolic compounds from leaves of Betula pubescens and Betula pendula. J Chromatogr A 721:59–68
Ossipov V, Haukioja E, Ossipova S, Hanhimäki S, Pihlaja K (2001) Phenolic and phenolic-related factors as determinants of suitability of mountain birch leaves to an herbivorous insect. Biochem Syst Ecol 29:223–240
Parry D, Herms DA, Mattson WJ (2003) Responses of an insect folivore and its parasitoids to multiyear experimental defoliation of aspen. Ecology 84:1768–1783
Rantala MJ, Roff DA (2007) Inbreeding and extreme outbreeding causes sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 98:329–336
Roden DB, Mattson WJ (2008) Rapid induced resistance and host species effects on gypsy moth, Lymantria dispar (L.): implications for outbreaks on three tree species in the boreal forest. For Ecol Manag 255:1868–1873
Rosenthal GA, Berenbaum MR (1992) Herbivores, their interactions with secondary plant metabolites. The chemical participants, vol I. Academic Press, New York
Ruuhola T, Yang S, Rantala MJ (2010) Increase in substrate availability down-regulates PO activity in Epirrita autumnata. Chemoecology 20:11–18
Ryan MF, Byrne O (1988) Plant-insect coevolution and inhibition of acetylcholinesterase. J Chem Ecol 14:1965–1975
Salminen J-P, Ossipov V, Loponen J, Haukioja E, Pihlaja K (1999) Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography–mass spectrometry. J Chromatogr A 864:283–291
Siva-Jothy MT, Thompson JW (2002) Short-term nutrient deprivation affects immune function. Physiol Entomol 27:206–212
Smilanich AM, Dyer LE, Gentry GL (2009) The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 90:1434–1440
Stevens MT, Lindroth RL (2005) Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145:298–306
Stockhoff BA (1993) Ontogenetic change in dietary selection for protein and lipid by gypsy moth larvae. J Insect Physiol 39:677–686
Tkachev AV (2008) The investigation of plant volatiles. Izdatelsko-poligraficheskoe predprijatie “Ofset”. Novosibirsk, Russia
Trudeau D, Washburn JO, Volkman LE (2001) Central role of hemocytes in Autographa californica M nucleopolyhedrovirus pathogenesis in Heliothis virescens and Helicoverpa zea. J Virol 75:996–1003
Valkama E, Salminen J-P, Koricheva J, Pihlaja K (2003) Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann Bot 91:643–655
Valkama E, Salminen JP, Koricheva J, Pihlaja K (2004) Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann Bot 94:233–242
Valkama E, Koricheva J, Salminen J-P, Helander M, Saloniemi I, Saikkonen K, Pihlaja K (2005) Leaf surface traits: overlooked determinants of birch resistance to herbivores and foliar microfungi? Trees 19:191–197
Vuorinen T, Nerg A-M, Syrjälä L, Peltonen P, Holopainen JK (2007) Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Interact 1:159–165
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wilson K, Cotter ShC, Reeson AF, Pell JuK (2001) Melanism and disease resistance in insects. Ecol Lett 4:637–649
Yang S, Ruuhola T, Rantala MJ (2007) Impacts of starvation on immune defense and other life history traits of an outbreaking geometrid, Epirrita autumnata: a possible ultimate trigger of the crash phase of population cycle. Ann Zool Fenn 44:89–96
Acknowledgments
We thank Alexey Tkachev for help in phytochemical assay and for his comments on the earlier version of the manuscript and thank Derek Roff and Will Sillitoe for the language correction. We also thank Ekaterina Chertkova for her assistance in the study of insect immunecometens, and Elena Shatalova, Elena Bojarischeva, and Ekatirina Grizanova for their help in field work. The study was financially supported by a Kone Foundation grant for V. Martemyanov, by a grant from the Russian Foundation for Basic Research (grant Nr 07-04-00870), and by a grant of the President of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martemyanov, V.V., Dubovskiy, I.M., Belousova, I.A. et al. Rapid induced resistance of silver birch affects both innate immunity and performance of gypsy moths: the role of plant chemical defenses. Arthropod-Plant Interactions 6, 507–518 (2012). https://doi.org/10.1007/s11829-012-9202-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9202-7