Abstract
Background Community pharmacists have a role in identifying drug–drug interactions (DDIs) when processing prescription orders and dispensing medications to patients. The harmful effects of DDIs can be prevented or minimized by using an electronic DDI checker to screen for potential DDIs (pDDIs). However, different DDI checkers have variable rates of detecting pDDIs. Aim To estimate the prevalence of pDDIs in prescriptions dispensed in a community pharmacy setting using two electronic DDI databases and to evaluate the association between the pDDIs and contributory factors. Method Eligible prescription orders dispensed by a community pharmacy chain in Qatar from January to July 2020 were included in this retrospective observational study. For each prescription, Micromedex® and Lexicomp® were simultaneously used to identify pDDIs, and the interactions categorized based on severity and risk rating. Results Seven hundred-twenty prescriptions met the inclusion criteria, of which Micromedex® and Lexicomp® respectively identified 125 prescriptions (17.4%) and 230 prescriptions (31.9%) as having at least one pDDI. Moderate strength of agreement was found between Lexicomp® and Micromedex® in identifying pDDIs (Cohen’s Kappa = 0.546). Micromedex® classified 61.6% of DDIs as major severity, while Lexicomp® classified 30.8% as major severity. The number of concurrent medications per prescription was significantly and positively associated with pDDI. Conclusion This study demonstrates a high prevalence of pDDIs among prescriptions dispensed in a community pharmacy setting. It is advisable that community pharmacists in Qatar, who typically do not have access to computerized patient profiles, use these DDI checkers to ensure all pDDIs are communicated to respective prescribers for appropriate action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on practice
-
Community pharmacists rely on their knowledge, drug information books, drug interaction software provided by employers, and accessible electronic resources to screen for potential drug–drug interactions (pDDIs).
-
Electronic DDI resources have varying rates of detecting pDDIs, leading to differences in their severity classification.
-
Both Lexicomp® and Micromedex® identified a high rate of pDDIs; however, a wide variability of the pDDIs classified as moderate or major severity were different between the two electronic resources.
-
Community pharmacies should provide at least two drug interactions software and encourage pharmacists to utilize both to screen for pDDIs when processing prescription orders.
-
Utilizing more than one software to screen for pDDIs will optimize medication therapy by preventing significant and harmful DDIs.
Introduction
Multiple medications are commonly prescribed concomitantly to treat single or multiple medical conditions. However, concomitant medications may result in undesired drug–drug interactions (DDIs). DDIs are defined as “a clinical response to the administration of drug combinations that is different from the expected effects of the individual agents when administered alone” [1]. DDIs have been reported to increase the risk of treatment failure, drug-related morbidity and mortality [2,3,4,5,6,7]. They are responsible for more than 30% of all adverse drug reactions (ADRs) and are associated with 44% of drug-related deaths [5, 8, 9]. A Swedish study reported that out of 35.5 million prescriptions dispensed within a 4-month period, 7% were associated with potential DDIs (pDDIs) [10]. Several predictors such as the number of concomitant medications, medical conditions, gender, and age have been reported to increase the risk of pDDIs [11, 12].
Healthcare professionals have the responsibility to prevent harmful DDIs through several strategies, including the avoidance of interacting combinations, spacing co-administered medications, monitoring for early detection, implementing computerized screening or decision support systems, and providing patient education with regards to prescription and non-prescription medications [13]. Kheshti and colleagues reported that one of the most important tools that is trusted by clinicians for detecting pDDIs is the use of well-established computerized DDI screening databases such as Lexi-Interact®, Micromedex® Drug Interactions, iFacts®, Medscape®, Drugs.com®, and Epocrates® [14]. While some of these databases are available free online, others require subscriptions or memberships. Lexi-Interact® (Lexicomp® Drug Interactions) and Micromedex® were shown to have the best performance in detecting pDDIs among all other DDI screening programs with the highest detection sensitivity of 0.77 and 0.78, respectively [14, 15]. Thus, pharmacists and clinicians should be aware of the features, functionality, advantages, and disadvantages of different DDI databases. Consequently, evaluation of the systems that expose patients to potential medication misadventures in a systematic fashion would enable clinicians to address the severity and contributing factors of misadventures appropriately [16]. Databases would help identify the pDDIs risk-categorization with severity indicators such as none, minor, major or contraindication, and allow clinicians to act accordingly.
Community pharmacies commonly receive a large volume of prescriptions to dispense and many of these may contain actual DDIs or pDDIs. Therefore, this setting may serve as a source of potential patient safety risk if prescriptions are not scrutinized for pDDIs or actual DDIs [17, 18]. A majority of pDDIs studies was conducted in hospital settings and only few studies have addressed pDDIs in ambulatory settings [19]. Several studies in community pharmacies from developed countries and a few from developing countries have reported the incidence and prevalence of DDIs or pDDIs; 13.9% of prescriptions in community pharmacies from Greece and 41.6% of community and hospital prescriptions from Iran had at least one pDDIs [20,21,22,23,24,25]. However, the prevalence and severity of DDIs in Qatar’s healthcare sector have not been previously investigated.
Aim
This study aimed to: (1) estimate the prevalence of pDDIs in prescriptions dispensed in a community pharmacy and categorize them based on severity and risk rating using Micromedex® and Lexicomp® databases and; (2) identify the relationship between pDDI and factors contributing to the DDIs such as age, gender, and number of concomitantly prescribed medications.
Ethics approval
The study was approved for exemption from review by the Institutional Review Board of Qatar University on 9 October 2017.
Method
Study design and setting
A retrospective observational study was conducted to identify pDDIs among prescriptions in a community pharmacy setting using two electronic drug information databases equipped with drug interaction tools (IBM Micromedex® and Lexicomp®). The study was conducted between January and July 2020, using prescriptions dispensed at Wellcare Pharmacy in Qatar. Wellcare Pharmacy is one of the leading networks of retail pharmacy chains in Qatar with over 50 branches nationwide [26]. Prescriptions commonly come from private clinics and hospitals, public hospitals, and primary healthcare centers within the country, with the majority generated from the private sector.
Prescriptions selection criteria
Prescriptions were eligible for inclusion if they fulfil all of the following criteria: (1) an adult patient aged 18–65 years; (2) contained two or more oral and/or parenteral medications (3) considered legible and legal (stamped, signed and dated). Incomplete prescriptions (i.e. missed any of the criteria above) or those prescribed only topical medications such as creams, eye drops, ointment and sprays were excluded. Prescriptions containing a combination of topical and oral medications were included in the study, but only the oral medications were evaluated for pDDIs. Prescriptions were sequentially collected within each participating Wellcare Pharmacy branch, blinded for identifying information, and photocopied for further analyses. Prescription orders were considered as the unit of analysis instead of patients. All prescriptions obtained from the community pharmacy were anonymized; consequently, we were unable to determine if some patients (not prescriptions) were included more than once.
Sample size
Sample size was calculated using the following criteria—confidence interval of 95%, a Z statistic value of 1.96, precision of 5% [27] and expected pDDI prevalence range of 19.3–48.0% as analyzed by Lexicomp® and Micromedex® [25, 28,29,30]. Therefore, the minimum sample size for the number of prescriptions required to analyze the prevalence of pDDI within a confidence of 95% was 384 [25, 27,28,29,30].
Drug interaction databases
As indicated before, Lexi-Interact® (the drug interaction component of the Lexicomp® clinical modules) and Micromedex® were selected because they have demonstrated the best performance in detecting pDDIs when compared to all other DDI screening databases [14]. We utilized Qatar University’s Library E-Resources to access Lexicomp® Drug Interactions via UpToDate® [31]. This is a copyrighted drug–drug, drug-herb, and herb-herb analysis tool, provided by UpToDate® (UpToDate, Inc. and/or its affiliates) utilizing Lexicomp® clinical content. The Lexi-Interact Online® combines literature and scientific understanding of DDIs with a state-of-the-art electronic platform. A patient specific drug regimen was entered to evaluate for pDDIs. Lexicomp® classifies pDDIs based on risk rating or severity. Severity is classified as minor, moderate and major. Table 1 shows the detailed definition of each risk and severity rating according to Lexicomp®.
IBM Micromedex® was accessed via IBM Micromedex® Web Application Access (IBM Corp., USA) for academic purposes under the license of Qatar University Library E-Resources [32]. The drug interaction tool screens patients’ drug regimen, if an interaction exists; the database provides information by reporting severity, documentation, and summary for each DDI. Micromedex® classifies pDDI based on severity. Table 1 shows the detailed definition of each severity rating according to Micromedex®. The severity levels of contraindicated and major seem to be serious concerns in drug dispensing in community pharmacy settings.
Data collection and potential drug–drug interaction identification
Data collection was achieved and analyzed using each eligible prescription dispensed at the pharmacy. After dispensing a particular prescription that appeared to fulfill the eligibility criteria, a community pharmacist would conceal the patient’s identification to ensure confidentiality. There was no interaction or direct contact made with any patient in this study. Since eligibility was based on the prescription presented to the community pharmacy, it is possible for a patient who frequently uses the participating pharmacies to have more than one prescription included in the study. The following information were collected from each eligible medical prescription: age, gender, name, number and date of concurrent medications, and source of written prescription sheet.
For each included prescription, the pDDIs was determined for the set of medications by inserting the non-proprietary (i.e. generic) names of all the drugs contained in the prescription into each electronic DDI software. To avoid any errors during the transferring and evaluation procedure, two researchers (AA1 and SA) independently undertook the data collection and drug interaction analysis for each medical prescription. Discrepancies between the two assessors were resolved using the method of consensus or adjudication by a third assessor (SS or AA2). All the evaluated medical prescriptions with or without pDDIs were coded and imported to the IBM SPSS® statistical software version 26.0.
Primary outcome measures
The primary outcome was the prevalence of pDDI using Micromedex® and Lexicomp® databases and was determined by computing the number of prescriptions that detected at least one pDDIs over the total number of prescriptions analyzed.
Statistical analyses
Categorical and continuous data were analyzed using descriptive statistics. Chi-square test was used to determine the association between pDDIs and other variables such as age, gender, source and number of concurrent medications. Cohen’s kappa test was performed to determine the degree of agreement between the pDDIs identified by the two databases used (IBM Micromedex® and Lexicomp®). A Cohens kappa of 0–0.2 indicates a slight agreement; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial and 0.81–1.0, perfect agreement. A p value of less than 0.05 was considered statistically significant for all inferential statistics. Data analyses were performed using the IBM SPSS® statistical software version 26.0.
Results
Characteristics of prescriptions dispensed
From January to July 2020, 840 prescriptions that meet the study eligibility criteria were collected by the pharmacists and upon review by the research team, 720 prescriptions that met the study’s inclusion criteria were included for further DDI analysis. The mean age (± SD) of patients was 37.06 ± 10.1 years (range 18–65 years) where 66.8% of the patients were young adults < 40 years of age. The number of concurrent medications in the evaluated prescriptions ranged between two and eight with a mean of 3.07 ± 1.08. Table 2 shows characteristics of the prescriptions.
Prevalence of potential drug–drug interactions detected by Lexicomp® and Micromedex®
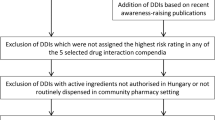
A total of 720 dispensed prescriptions were analyzed for pDDIs using two databases namely Lexicomp® and Micromedex®. Lexicomp® and Micromedex® identified 230 and 125 prescriptions as having at least one pDDI, with a prevalence of 31.9% and 17.4%, respectively (Fig. 1). These pDDIs, were categorized based on Lexicomp® risk rating as follows: category C (69.83%), category D (12.64%), category B (10.92%) and category X (6.61%) (Fig. 2a). The severity of these interactions as categorized by Lexicomp® were identified as follows: moderate (57.47%), major (30.75%), and minor (11.78%) (Fig. 2b). The interactions detected by Micromedex® were rated with the following severity categories of major (61.63%), moderate (33.14%), minor (4.65%), and 0.58% contraindicated (Fig. 2b).
The agreement rate of pDDI between Lexicomp® and Micromedex® as assessed by Cohen’s Kappa is 0.546 with a p value < 0.001. Upon identifying the agreement rate, it was estimated that only 50% of the pDDIs detected by Lexicomp® were also detected by Micromedex®. On the other hand, 90% of the pDDIs detected by Micromedex® were also detected by Lexicomp®.
Factors associated with potential drug–drug interactions
The association of age, gender, number of concurrent medications and source of prescriptions with the risk of pDDI is reported in Table 2. Age and gender were not significantly associated with pDDIs. On the other hand, the prevalence of pDDI rises from a prevalence of 14.3% (Micromedex®) and 24.1% (Lexicomp®) in prescriptions containing 2–3 drugs to 58.1% (Micromedex®) and 80.6% (Lexicomp®) in prescriptions containing 6–8 drugs (p < 0.001). Moreover, prescriptions obtained from hospitals had a significantly higher rate of pDDIs of 28.6% by Micromedex® and 50% by Lexicomp® in comparison to those identified from clinics/medical centers with 16.3% (Micromedex®) and 29.9% (Lexicomp®). Table 2 shows the effect of the different factors on the rate of pDDIs.
Potential clinical consequences of the potential drug–drug interactions
The number of pDDIs per prescriptions ranged from 1 to 7 with a mean of 1.54 ± 1.13. A total of 240 prescriptions (115 detected by both Micromedex® and Lexicomp®, 115 detected only by Lexicomp®, 10 detected only by Micromedex®) have at least one pDDI. Whereas 66 (27.5%) had more than one case of pDDI and up to 7 interactions per prescription. The potential clinical consequences with related mechanisms of interaction for the top 20 pDDIs are listed in Table 3. The most frequently detected pDDI pair was between NSAIDs and quinolone drug classes observed in 41 prescriptions. The clinical consequence of this interaction was identified as C (monitor therapy) with a severity of major by Lexicomp® and severity of moderate by Micromedex®. Moreover, the most recurrently reported contraindicated pDDI with major severity was between atypical antipsychotics and selective serotonin reuptake inhibitors (SSRI) drug classes.
Discussion
Statement of key findings
There are only a few studies conducted from the context of developing countries to determine the prevalence of pDDIs in prescription orders dispensed in community pharmacy settings and to compare the rates based on commonly available drug interaction databases. The present study revealed that the overall prevalence of at least one pDDI in prescriptions dispensed in community pharmacies was 17.4% and 31.9% as detected by Micromedex® and Lexicomp®, respectively. This finding is in agreement with a few other studies, which reported a rate of 30–40% of DDIs in prescriptions regardless of where they were dispensed [25, 33, 34]. Based on our results, among prescriptions dispensed in community pharmacies in Qatar, prescriptions issued in hospital settings had the most number of pDDIs compared to those issued in clinics and medical centers, which is consistent with a previous study in Iran [25]. This high rate of pDDIs in prescriptions from the hospital is most likely due to multiple medications per prescription written for patients that are more complex from hospitals. This is also consistent with our finding that patients receiving more medications per prescriptions tend to have higher rates of pDDIs per prescription.
Consistent with several studies, the majority (69.8%) of pDDIs detected by Lexicomp® were of risk rating C, while pDDI with higher risk ratings of D and X accounted for 12.6% and 10.9%, respectively [25, 35, 36]. The risk rating for pDDIs provided by Lexicomp®, but not Micromedex®, offers clinicians an additional perspective on the interpretation and clinical significance of the interaction, as well as the action to take to avert any potential harms. Even though the majority of the pDDIs only required monitoring, clinical judgement is necessary because of high-risk patients who are more prone to experiencing the effect of the interaction because of their age, polypharmacy, concomitant diseases and organ dysfunction. Discrepancies were noted concerning the severity of pDDI between the two databases. For example, Lexicomp® identified 57% and 30.7% of pDDI as moderate and major severity respectively, while Micromedex® identified 33.1% and 61.2% of pDDIs as moderate and major severity, implying Micromedex® over interprets severity of pDDIs. This was consistent with a study that noted a difference in DDI severity ratings using the same databases [37]. However, the differences in the moderate and major severity ratings of pDDIs for Lexicomp® and Micromedex® may be mitigated in practice if all pharmacists review the pDDIs flagged as moderate or major severity and inform prescribers to assess the clinical significance of the interactions taking into account patient-related factors. Nevertheless, the discrepancy in the severity of pDDIs between Lexicomp® and Micromedex® underscores the need for community pharmacists to have access to more than one drug–drug interaction software in their practices.
Based on the risk rating of the pDDIs, most of the interactions required monitoring. The risk of a clinically significant interaction occurring is very much influenced by patient factors such as age, concomitant diseases and number of chronic medications. Since most community pharmacists do not have access to comprehensive patient profiles, they should educate patients or their caregivers about these potential interactions and instruct them on when to seek medical care. We found that the five most common pDDIs were among the following drug class pairs beginning with the most frequent: quinolone with NSAIDs, 2nd generation antihistamine with macrolide, atypical antipsychotics with selective serotonin reuptake inhibitor (SSRI), 1st generation antihistamine with 2nd generation antihistamine, and glucocorticoids with NSAIDs. Ismail et al. reported that the common drug pair in outpatient department in Pakistan also included quinolone and NSAIDs [38]. Furthermore, Dirin et al. found that one of their most common drug pairs were corticosteroids and NSAIDs [25]. While other studies had discrepancies among most common drug pair reported with pDDI, this could be attributed to the differences in drug prescribing pattern, number of prescriptions analyzed and sources of prescriptions.
We observed that Lexicomp® is more likely to detect drug interactions as compared to Micromedex® and provide risk ratings for clinicians to intervene as needed. This variation in databases could be due to the following: Micromedex® screens published data on evidence-based concepts, using peer-reviewed scientific journals and provides an assessment of the quality of documentation [39]. On the other hand, Lexicomp® focuses on depth and provides both risk rating and severity of interactions based on duplicated information, hence serves as a better tool for decision-making [40]. However, there was a moderate agreement between the two databases regarding the detection of pDDI with weighted kappa of 0.546.
In general, we identified that increasing age and polypharmacy increased the risk of pDDIs which was consistent with other studies [11, 25, 34, 36]. This is probably because elderly patients took a higher number of medications potentially due to higher number of comorbidities. Consistent with other studies, there were no statistically significant differences between men and women regarding the prevalence of pDDIs [36, 41]. We observed interesting data in relation to pDDIs based on source of prescriptions. Prescriptions originating from hospitals tend to have a higher prevalence of pDDIs compared to those from clinics.
Strengths and weaknesses
Our study has a few limitations. Firstly, our study focused on prescriptions brought to community pharmacies. We therefore are not aware of pDDIs in prescriptions provided to the same patient and dispensed in hospitals as most hospital prescriptions are dispensed within the respective hospital pharmacies. Secondly, the community pharmacy chain did not have EHR and does not have access to medications prescribed elsewhere. Therefore, we may underestimate the prevalence of pDDI. Finally, Micromedex® did not have data on interactions for some medications and therefore the results could have been underreported.
Interpretation
The current study has shown that there is a high rate of at least one pDDI in prescription orders with multiple medications presented to the community pharmacy. In addition, Lexicomp® identifies pDDIs at a higher rate (1.8 times) than Micromedex®, but both software programs have similar rates of detection of the composite of pDDIs with severity ratings of moderate and major respectively. There was however, a wide discrepancy between the software programs in their respective categorization of moderate and major pDDIs with Micromedex® categorizing pDDIs as major severity almost double the rate by Lexicomp®.
Further research
Future research studies should assess the clinical significance of identified pDDIs and investigate the impact of pharmacist-led interventions to prevent the potential harm associated with these.
Conclusion
Prescriptions dispensed in community pharmacy settings contained relatively high rates of pDDIs as determined by Lexicomp® (31.9%) and Micromedex® (17.4%) having at least one pDDIs. The simultaneous use of Lexicomp® and Micromedex® to screen for pDDIs in the community pharmacy setting would increase the rates of detection of pDDIs and provide community pharmacists with a higher composite rate of pDDIs of major severity to evaluate and use clinical judgement to take the appropriate action. Educating Qatar pharmacists about the most common drug pairs with potential for DDIs can assist them to be more vigilant and recognize at an earlier stage for harmful prevention. This study will raise awareness of the importance of implementing a computerized warning system in community pharmacies and help alert pharmacists about pDDIs in optimizing medication therapy and promoting patient safety. It is recommended that community pharmacists in Qatar, who typically do not have access to computerized patients' profiles or EHR, use these electronic DDI resources to ensure all pDDIs are detected and communicated to respective prescribers for appropriate actions. The study has potential implication on improving patient safety in community pharmacy setting.
References
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ. 2004;329(7456):15–9.
Becker ML, Kallewaard M, Caspers PW. Hospitalisations and emergency department visits due to drug–drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641–51.
Brvar M, Fokter N, Bunc M, et al. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol. 2009;9:8. https://doi.org/10.1186/1472-6904-9-8.
Juurlink DN, Mamdani M, Kopp A, et al. Drug–drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289(13):1652–8.
Montane E, Arellano AL, Sanz Y, et al. Drug-related deaths in hospital inpatients: a retrospective cohort study. Br J Clin Pharmacol. 2018;84(3):542–52.
Peyriere H, Cassan S, Floutard E, et al. Adverse drug events associated with hospital admission. Ann Pharmacother. 2003;37(1):5–11.
Suriyapakorn B, Chairat P, Boonyoprakarn S, et al. Comparison of potential drug–drug interactions with metabolic syndrome medications detected by two databases. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0225239.
Iyer SV, Harpaz R, LePendu P, et al. Mining clinical text for signals of adverse drug–drug interactions. J Am Med Inform Assoc. 2014;21(2):353–62.
Strandell J, Bate A, Lindquist M, et al. Drug–drug interactions—a preventable patient safety issue? Br J Clin Pharmacol. 2008;65(1):144–6.
Holm J, Eiermann B, Eliasson E, et al. A limited number of prescribed drugs account for the great majority of drug–drug interactions. Eur J Clin Pharmacol. 2014;70(11):1375–83.
Gagne JJ, Maio V, Rabinowitz C. Prevalence and predictors of potential drug–drug interactions in Regione Emilia-Romagna, Italy. J Clin Pharm Ther. 2008;33(2):141–51.
Nikolic B, Jankovic S, Stojanov O, et al. Prevalence and predictors of potential drug–drug interactions. Cent Eur J Med. 2014;9(2):348–56.
Ansari J. Drug interaction and pharmacist. J Young Pharm. 2010;2(3):326–31.
Kheshti R, Aalipour M, Namazi S. A comparison of five common drug–drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract. 2016;5(4):257–63.
Clauson KA, Marsh WA, Polen HH, et al. Clinical decision support tools: analysis of online drug information databases. BMC Med Inform Decis Mak. 2007. https://doi.org/10.1186/1472-6947-7-7.
Weant KA, Bailey AM, Baker SN. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med. 2014;6:45–55.
Becker ML, Caspers PW, Kallewaard M, et al. Determinants of potential drug–drug interaction associated dispensing in community pharmacies in the Netherlands. Pharm World Sci. 2007;29(2):51–7.
Becker ML, Kallewaard M, Caspers PW, et al. Potential determinants of drug–drug interaction associated dispensing in community pharmacies. Drug Saf. 2005;28(5):371–8.
Tache SV, Sonnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–89.
Andersson ML, Bottiger Y, Kockum H, et al. High prevalence of drug–drug interactions in primary health care is caused by prescriptions from other healthcare units. Basic Clin Pharmacol Toxicol. 2018;122:512–6.
Letinier L, Cossin S, Mansiaux Y, et al. Risk of drug–drug interactions in out-hospital drug dispensings in france: results from the drug–drug interaction prevalence study. Front Pharmacol. 2019. https://doi.org/10.3389/fphar.2019.00265.
Reis AM, Cassiani SH. Prevalence of potential drug interactions in patients in an intensive care unit of a university hospital in Brazil. Clinics (Sao Paulo). 2011;66(1):9–15.
Tragni E, Casula M, Pieri V, et al. Prevalence of the prescription of potentially interacting drugs. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0078827.
Chatsisvili A, Sapounidis I, Pavlidou G, et al. Potential drug–drug interactions in prescriptions dispensed in community pharmacies in Greece. Pharm World Sci. 2010;32(2):187–93.
Dirin MM, Mousavi S, Afshari AR, et al. Potential drug–drug interactions in prescriptions dispensed in community and hospital pharmacies in East of Iran. J Res Pharm Pract. 2014;3(3):104–7.
Wellcare Pharmacy. The care you can trust. https://wellcarepharmacies.com. Accessed 7 July 2020.
Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–7.
Ahmad A, Umair Khan M, Haque I, et al. Evaluation of potential drug–drug interactions in general medicine ward of teaching hospital in Southern India. J Clin Diag Res. 2015;9(2):66.
Dookeeram D, Bidaisee S, Paul JF, et al. Polypharmacy and potential drug–drug interactions in emergency department patients in the Caribbean. Int J Clin Pharm. 2017;39(5):1119–27.
Sancar M, Kasik A, Okuyan B, et al. Determination of potential drug–drug interactions using various software programs in a community pharmacy setting. Turk J Pharm Sci. 2019;16(1):14–9.
UpToDate®. Lexicomp drug interactions. https://0-www.uptodate.com.mylibrary.qu.edu.qa/drug-interactions/?source=responsive_home#di-druglist. Accessed 7 July 2020.
IBM Micromedex Solutions®. Drug interactions. https://0-www.micromedexsolutions.com.mylibrary.qu.edu.qa/micromedex2/librarian/CS/4C7995/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/4CD5EB/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.FindDrugInteractions?navitem=topInteractions&isToolPage=true. Accessed 7 July 2020.
Mousavi S, Norouz M, Ashouri A, et al. Study of potential drug–drug interactions in prescriptions of university-based pharmacies. J Pharm Care. 2014;2(2):60–5.
Ren W, Liu Y, Zhang J, et al. Prevalence of potential drug–drug interactions in outpatients of a general hospital in China: a retrospective investigation. Int J Clin Pharm. 2020;42(4):1190–6.
Aljadani R, Aseeri M. Prevalence of drug–drug interactions in geriatric patients at an ambulatory care pharmacy in a tertiary care teaching hospital. BMC Res Notes. 2018. https://doi.org/10.1186/s13104-018-3342-5.
Doubova SV, Reyes-Morales H, Torres-Arreola LP, et al. Potential drug–drug and drug–disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007. https://doi.org/10.1186/1472-6963-7-147.
Armahizer MJ, Kane-Gill SL, Smithburger PL, et al. Comparing drug–drug interaction severity ratings between bedside clinicians and proprietary databases. ISRN Critical Care. 2012. https://doi.org/10.5402/2013/347346.
Ismail M, Noor S, Harram U, et al. Potential drug–drug interactions in outpatient department of a tertiary care hospital in Pakistan: a cross-sectional study. BMC Health Serv Res. 2018. https://doi.org/10.1186/s12913-018-3579-7.
IBM Micromedex® Solutions. Drug interactions policy. https://www.ibm.com/downloads/cas/ZVLXDL7X. Accessed 30 Aug 2020.
Chatfield AJ. Lexicomp online and Micromedex 2.0. J Med Libr Assoc. 2015;103(2):112–3.
Teixeira JJ, Crozatti MT, dos Santos CA, et al. Potential drug–drug interactions in prescriptions to patients over 45 years of age in primary care, southern Brazil. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0047062.
Acknowledgements
The authors would like to thank the pharmacists of all the Wellcare Pharmacy outlets in Qatar where data for the study were collected from prescriptions.
Funding
This study was funded by Qatar University under the Student Grant number QUST-1-CPH-2018-16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbas, A., Al-Shaibi, S., Sankaralingam, S. et al. Determination of potential drug–drug interactions in prescription orders dispensed in a community pharmacy setting using Micromedex® and Lexicomp®: a retrospective observational study. Int J Clin Pharm 44, 348–356 (2022). https://doi.org/10.1007/s11096-021-01346-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-021-01346-8