Abstract
Background Patient reporting of adverse drug reactions (ADRs) could supplement the existing reporting system and contribute to early detection of ADRs. The confidence in ADR identification and their attribution of ADRs were limited to outpatients. Objective To determine the type and frequency of ADRs reported by outpatients, to evaluate confidence and accuracy in ADR identification as well as contributing factors. Setting University hospital in northeastern Thailand Method Cross-sectional study using questionnaires distributed to 500 outpatients who claimed to have experienced an ADR. Confidence in identifying ADRs was measured by visual analogue score (VAS), while accuracy of reported ADRs was determined using Naranjo algorithm and WHO criteria. Main outcome measure Number and type of ADRs, confidence rating and accuracy category. Results In total, 390 outpatients completed the questionnaire (response rate = 78.0%). Rash (19.0%), nausea/vomiting (7.4%), and dizziness (5.8%) were the top three reported ADRs. Sixty-one percent of respondents rated their level of confidence in identifying ADRs as high (VAS 9.2 ± 0.95), which was associated with having underlying diseases (OR 1.93), low number of reported symptoms (OR 0.38) and severe ADRs (OR 1.33). Causality assessment was classified as true ADRs in 90.0% and 88.9% of cases, using Naranjo algorithm and WHO criteria, respectively. Respondents with low number of reported symptoms (OR 0.27) and high level of confidence had greater accuracy in ADR identification (OR 1.11). Conclusion The outpatients reported a high proportion of potential ADRs with high confidence and accuracy. Patient reporting of ADRs has potential to support the pharmacovigilance system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on practice
-
Thai outpatients are confident of identified ADRs, particularly those experiencing more severe ADRs, or those with a lower number of reported symptoms, or those with underlying diseases.
-
Outpatients with high confidence in identifying ADRs and with a low number of symptoms contribute to a greater accuracy in the reporting of ADRs.
-
Healthcare professionals should educate and encourage outpatients to report ADRs, and the reports should be integrated into the formal pharmacovigilance system at national level.
Introduction
Adverse drug reactions (ADRs) have become a common clinical problem, and are one of the leading causes of morbidity and mortality [1, 2]. ADRs now cause up to 6.5% of hospital admissions. A meta-analysis comprising 39 prospective studies in hospitals revealed that the incidence of serious ADRs is about 6.7% [3]. Early detection of ADRs is thus the primary step in prevention of harm [2]. The main pharmacovigilance method is a worldwide ADR reporting system used by health professionals. Under-reporting, however, is a drawback of this voluntary approach [3]. A direct reporting system by patients is a feasible method to augment the existing system. Its benefits include enhanced ADR reporting rates, new information regarding unrecognized reactions from marketed drugs, and identification of ADRs from new drugs [3,4,5]. Direct reporting by patients is an effective tool for early detection of serious ADRs and ADRs affecting daily life [6]. Many recent studies revealed that ADR reporting, whether by patients or healthcare professionals, is similar in quality and evaluation of the degree of seriousness of reactions [4, 7, 8]. Furthermore, patients often report easily noticeable and preventable ADRs [9, 10]. Previous studies of patient accuracy in ADR reporting showed that patients were able to report true ADRs (i.e. related to the specific suspected drug group) in the range of 60–76% [11,12,13,14]. In addition, patients consulted with health professionals about their suspected symptoms, leading to a decrease in false ADR reports [15, 16]. However, patients with chronic diseases receiving a high number of medicines had an increased risk of ADRs, and they had difficulty distinguishing ADR symptoms caused by drugs from other causes [17]. Patients’ accuracy in ADR reporting was reduced by high number of reported symptoms, concomitant drugs and concurrent diseases [12, 13, 18, 19].
In one study, approximately half of patients in community had relatively high confidence in their ADR reporting [18]. Nevertheless, patient confidence regarding ADR identification remains unclear in hospital setting and it depends on knowledge about ADRs [6]. Little is known regarding confidence and accuracy of ADRs attributed by outpatients with respect to a wide variety of chronic diseases and prescribed drugs. Therefore, the current study was conducted to explore outpatient ADR experiences, which had been initially identified by the patients themselves, and evaluate their confidence and accuracy in identifying ADRs.
Aims of the study
The objectives of study were to (a) determine the type and frequency of experienced ADRs reported by outpatients, (b) evaluate their confidence in identifying ADRs and the accuracy of their reported ADRs, and (c) determine the factors associated with these aspects.
Ethics approval
The study protocol was approved by the Khon Kaen University Ethics Committee for Human Research (Number HE591086).
Methods
Study design and setting
This was a cross-sectional study conducted among 500 outpatients at a tertiary care teaching hospital in northeastern Thailand between July 2016 and February 2017.
Participants
Study participants were aged over 18 and had experienced symptoms suspected to be medicine related within the previous year. The study excluded patients who had a previously known drug allergy in order not to confound our analysis with information given by other health professionals. Based on sample size calculation, number of patients was at least 445 patients needed, therefore this study required 500 enrolled patients.
Questionnaire development and testing
The 4-section questionnaire was developed from our previous study [20]. It was assessed by two pharmacists with proficiency in ADR identification. The index of consistency (IOC) was calculated from each expert in order to (a) assess the consistency between each question, (b) guarantee the questions met the study objectives, and (c) check the suitability of wording (IOC = 0.90). The IOC value of > 0.5 is considered acceptable in consistency [21]. The questionnaire was thereafter adjusted and piloted in 20 patients at outpatient clinics in the study hospital to evaluate patient’s ability to complete the questionnaire and to gain any suggestions for improving the questionnaire. The piloted outpatients were excluded from the main study and final analysis.
Section 1—Overall confidence about suspected symptoms. The systemic body organ checklist comprised 87 general adverse symptoms characterized by 12 organ systems and extra space for other symptoms that allowed patients to report other symptoms not listed. All symptoms in the checklist were modified to be in user-friendly language, according to the developed questionnaire from our previous study. Patients could check-off any experienced symptoms that they suspected were caused by drugs. Each symptom also had a checkbox regarding their level of confidence in assigning the suspected symptom as an ADR. Patients were subsequently asked about their overall confidence in the suspected symptoms checklist using a visual analogue score (VAS: range 0–10 cm); ‘Less confident’ (score = 0), ‘Most confident’ (score = 10). Patients marked “X” on the VAS line according to their own assessment.
Section 2—Specific confidence in selected symptoms. Patients were asked to select one suspected symptom that they had the greatest confidence in identifying as an ADR. Their confidence in identifying this symptom was rated using the visual analogue score (VAS: range 0–10 cm); ‘Less confident’ (score = 0), ‘Most confident’ (score = 10). The specific symptom was also rated for severity, anxiousness and disturbance on daily activity using the visual analogue score (VAS: range 0–10 cm); ‘highest’ (score = 0), ‘lowest’ (score = 10).
Other collected data included onset of symptoms, frequency and outcome.
Section 3—Suspected drug. Information about the drug that patients suspected to cause the ADR symptoms included its name, dose, frequency, indication, route of administration, and start and stop date. If patients were unable to specify the suspected drug name, there was a space for them to explain the details of the drug shape, color and features in order that the research pharmacist could retrieve further information related to the drug name from pharmacy database. Other information that patients did not provide would be obtained from the outpatient records (OPD cards and database). Moreover, the accuracy of the data provided by patients was repeatedly checked and confirmed by the research pharmacist from the medical records.
Section 4—Patient characteristics. Demographic data included gender, age, education, concomitant diseases, and history over the previous year taking medication(s) and/or herb(s).
Data collection
The self-administered questionnaires were directly distributed to 500 outpatients by purposive sampling that aimed to include patients having experienced ADRs. The participants were over 18 and had experienced symptoms related to drugs sometime in the previous year. The outpatients were recruited while they were waiting to see doctors at outpatient clinics at Srinagarind Hospital, Khon Kaen. The research pharmacist asked them about any experience of ADRs during the previous year. Consent forms were given to ask patients’ permission to participate in the study. Data were collected between July 2016 and February 2017. In addition, the outpatient records of all responders were assessed by a pharmacist for information about all concomitant drug treatments, start and stop dates, comorbidities, and potential recorded symptoms related to ADRs. This information would be necessary for assessment of the causal relationship between implicated drugs and the reported adverse reactions.
Assessment of symptoms
The Naranjo algorithm and WHO criteria were used to evaluate the accuracy of symptoms that patients were most confident in identifying as an ADR, indicating a causal relationship between reported symptoms and suspected drug. The Naranjo algorithm has four categories including: definite (score > 9), probable (score 5–8), possible (score 1–4), and doubtful (score < 0) [22]. The WHO criteria also has four categories: certain, probably/likely, possible, and unlikely [23]. The accuracy of reported symptoms were considered as true if the ADRs were classified as definite, probable or possible using the Naranjo algorithm or certain, probably/likely or possible using the WHO criteria. In contrast, inaccurate symptom attribution (or false ADRs) were doubtful using the Naranjo algorithm or unlikely using the WHO criteria.
Data analysis
The valid data questionnaires were entered into SPSS version 19 for analysis. Demographic data, frequency, and type of ADR were analyzed using descriptive statistics. Patient reported confidence in identifying symptoms as an ADR were classified as low confidence with the lowest score being ≤ 4, medium confidence score being > 4 and ≤ 7, and high confidence score being > 7, and are presented with mean ± SD. The levels of confidence were divided into binary variables as follows; low/medium and high. Univariate analysis of the factors related to the variables were analyzed using the Chi square test. Logistic regression of factors related to patient confidence in identifying symptoms as an ADR were used for the multivariate analysis. The results with p values less than 0.05 were considered statistically significant.
Results
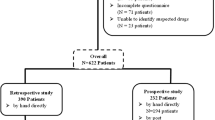
A total 500 patients were invited to participate by research pharmacist and agreed to complete the questionnaire. Of the 484 returned questionnaires, 94 were invalid due to incomplete data in required parts of Sections 1 and 2 (n = 71), or unable to identify or describe the suspected drugs in Section 3 (n = 23). There were 390 participants who completed the questionnaire and reported at least one ADR experience (response rate = 78.0% of 500 invited patients). The majority of respondents were female with an average age of 50.46 ± 0.75 years (range 18–94). The majority of patients completed education levels of diploma or lower (n = 249, 51.4%) or a bachelor’s degree or higher (n = 235, 48.6%). Two-thirds (329; 68.0%) of respondents (226 females (68.7%) and 103 males (31.3%)) reported their confidence in the overall symptoms check list to be ≥ 50%. Age, number of underlying diseases, and number of concomitant drugs were all significantly associated with patient confidence in the overall symptoms check list ≥ 50% (p = 0.043, p = 0.032, p < 0.001, respectively) (Table 1).
Of the 390 respondents, the top three reported ADRs classified by systemic organ class (SOC) were (a) skin tissue disorders (N = 127, 25.4%) including rash (N = 74, 19.0%) caused by amoxicillin (N = 9), ibuprofen (N = 5); (b) gastrointestinal disorders (N = 98, 19.6%) including nausea/vomiting (N = 29, 7.4%) caused by tramadol (N = 8), gabapentin (N = 2); and (c) nervous system disorders (N = 59, 11.8%) including dizziness (N = 23, 5.8%) caused by efavirenz (N = 5), valproic acid (N = 2). Furthermore, the most common suspected drugs with their reported ADRs classified by anatomical therapeutic chemical (ATC) were: amlodipine (N = 23) induced peripheral edema (N = 21), peg-interferon (N = 18) induced fever (N = 8), amoxicillin (N = 14) induced rash (N = 9), respectively (Tables 2, 3).
Approximately two-thirds of respondents rated their level of confidence in identifying ADRs from the overall list of selected symptoms as high (N = 238, 61%) with a mean ± SD of VAS of 9.2 ± 0.95, followed by moderate (N = 85, 21.8%) with a mean ± SD of VAS of 5.5 ± 0.79 and low (N = 67, 17.2%) with a mean ± SD of VAS of 2.0 ± 1.31, respectively. Additionally, their level of confidence in the symptom most confidently identified as an ADR was classified as high (N = 231, 59.2%) with a mean ± SD of VAS of 9.3 ± 0.95, followed by moderate (N = 103, 26.4%) with a mean ± SD of VAS of 5.6 ± 0.80, and low (N = 56, 14.4%) with mean ± SD of VAS of 2.1 ± 1.22, respectively (Table 4).
The univariate analysis regarding factors related to respondents’ confidence showed that respondents with underlying diseases (p = 0.016), concomitant drugs (p = 0.037), increased severity of ADRs (p < 0.001), anxiousness about ADRs (p < 0.001), and bothersome ADRs (p < 0.001) were significantly associated with a high confidence in reported ADRs. The multiple logistic regression showed the factors independently associated with patient confidence were having underlying diseases (OR 1.931; 95% CI 1.067, 3.493; p = 0.030), lower number of reported symptoms (OR 0.379; 95% CI 0.172, 0.836; p = 0.016) and increased severity of ADRs (OR 1.332; 95% CI 1.236, 1.436; p < 0.001) (Table 5).
The 390 symptoms that patients were most confident in identifying as an ADR were further assessed by the pharmacist. Data on diseases and concomitant drugs were obtained from outpatient records and computerized database. According to the Naranjo algorithm, the number of definite ADRs was 2 (0.5%), probable 198 (50.8%), possible 151 (38.7%), for a total of 351 (90.0%) symptoms being true ADRs, while the number of unlikely or false ADRs was 39 (10.0%). According to the WHO criteria, the percentage of certain ADRs classified was 2.3% (N = 9), probable 60.0% (N = 234) and possible 26.6% (N = 104), for a total of 88.9% (N = 347) of symptoms being true ADRs. Forty-three symptoms were classified as doubtful or false ADRs (11.1%). The multiple logistic regression showed that factors independently associated with accuracy included number of reported symptoms (OR 0.267; 95% CI 0.119, 0.602; p < 0.001) and confidence in overall reported symptoms (OR 1.105; 95% CI 0.999, 1.223; p = 0.052) (Tables 6, 7).
Discussion
The current study was a retrospective cross-sectional design aimed to assess patient experience evaluating ADRs; notably their confidence in identifying ADR symptoms and their accuracy using a systemic symptom checklist questionnaire. The overall response rate was 78.0%, which was comparable to previous studies in Thailand (76.0%) and the Netherlands (76.5%) [24, 25]. The most common ADRs reported by patients in this study were rash, nausea/vomiting, dizziness, myalgia, and peripheral edema. These symptoms were recognized as known ADRs and were easily noticeable by patients. This finding is in line with other studies which found that patients frequently reported noticeable ADRs including rash, itching, and edema [9, 26]. The current study also confirmed the finding of the previous studies, which indicated that the common organ systems involved in reported ADRs were cutaneous, gastrointestinal, and nervous [26,27,28]. The most common suspected drug group was anti-infective agents, which are in agreement with reports from healthcare professionals in Thailand [29]. Pharmacists and doctors should thus encourage patients to report any potential adverse symptoms related to their medications, particularly easily noticeable and common ADRs.
The results from the current study showed that 61% of outpatients had a high level of confidence in identifying ADRs. Previous surveys showed that around one-half of the general public and out-patients had a high level of confidence regarding the identification of ADRs (52%, 56% respectively) [18, 26]. Patients are likely to report high confidence in identifying ADRs because they have had direct experience with ADRs, or because their understanding of ADRs has been confirmed by their physicians or other source of information before completing the questionnaire. Nevertheless, level of confidence in recognizing ADRs in this study setting does not directly translate to high patient reporting rates in Thailand, where patient reporting system is inadequately promoted by the health authority and rarely recognized by Thai patients [18]. Unlike the European Countries, patient reporting could complement the reporting system by healthcare professionals although the patient reporting rates were relatively low, compared to healthcare professional reporting [30]. In this study, the factors that were related to patient confidence in identifying ADRs included underlying diseases, number of symptoms reported and severity of ADRs. The respondents having underlying diseases seemed to be more confident in their reporting of symptoms perhaps because they had taken a number of medicines leading to more awareness of their adverse effects. However, a previous qualitative study suggested that ADR symptoms similar to disease states might make it difficult for patients to identify true ADRs [6]. Moreover, patients who reported greater number of symptoms were more likely to be less confident in their reporting of ADRs than those who reported lower number of symptoms, which may relate to some symptoms caused by their diseases. Similarly, this finding confirmed a previous study that patients tended to have high confidence in ADR reporting when they had experienced more severe ADRs [18]. This is also in line with previous studies from the Netherlands and Portugal in which patient reporting of ADRs was related to both the severity of reactions and concern about reactions [25, 31].
When considering the accuracy of attributed ADRs by patients, it was found that the majority of reported ADRs were classified as being a ‘probable’ rather than a ‘possible’ causal relationship according to both the WHO criteria and the Naranjo algorithm. Notwithstanding, the findings do not support two previous studies [12, 20] which found a higher proportion of ‘possible’ than ‘probable’ ADRs. This difference might be explained by the fact that our analysis of the accuracy of the reported ADRs in the current study focused on the symptom that the patients were most confident in identifying as an ADR, while the other reports covered all suspected ADRs. In addition, patient accuracy in our study was reduced by increased numbers of symptoms reported, which confirms previous studies in Thailand [6, 13]. In this study, patients who had higher confidence in identifying overall symptoms were more likely to report ADRs with greater accuracy.
An explanation for the high level of confidence reported in the current study might be due to the fact that most symptoms were noticeable and directly affected patient health. Patients were, thus, able to report true ADRs with reasonable confidence by using the checklist questionnaires. While the patients had a high level of confidence, these reports were confirmed by the causality assessment performed by the pharmacist using the WHO criteria and Naranjo’s algorithm. The accuracy of ADRs in the current study, however, depended on the assessment of healthcare professionals, which required additional information from the patient records. If patients were themselves able to contribute to the assessment of ADRs from the suspected drugs, it would greatly increase the immediacy and relevance of drug safety monitoring.
Strengths and limitations of study
The study targeted outpatients who had experienced suspected ADRs. A self-administered questionnaire directly distributed to the patients achieved a high response rate. The study setting was the largest tertiary care hospital in northeastern Thailand, where a wide variety of drug groups are used. The research we conducted had high levels of patient confidence in identifying ADRs and high levels of accuracy in the symptoms patients attributed to potential ADRs. These measures of confidence and accuracy in the targeted hospital outpatient community are likely to be higher than those in the wider population as outpatients attending a hospital are likely to have previously discussed the issue of ADR symptoms with their healthcare provider before completing the questionnaire, which would have affected their level of confidence. In addition, the data were collected in patients who had experienced potential ADRs sometime during the year, so recall bias might be an issue.
Conclusion
A high proportion of Thai outpatients reported ADRs with a high level of confidence. Accuracy of the symptoms most confidently attributed by patients was also acceptable. The higher the confidence in experienced ADRs, the greater the accuracy of attributed ADRs reported by patients. The findings of the current study confirm that patient reporting could be a beneficial method aiding in the screening and reporting of ADRs.
References
Langerova P, Vrtal J, Urbanek K. Adverse drug reactions causing hospital admissions in childhood: a prospective, observational, single-centre study. Basic Clin Pharmacol Toxicol. 2014;115:560–4.
Khan LM, Al-Harthi SE, Osman AM, Sattar MA, Ali AS. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm J. 2015;24:485–93.
Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63:148–56.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–96.
Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30:1073–82.
Chaipichit N, Krska J, Pratiparnwat T, Uchaipichat V, Jarernsiripornkul N. A qualitative study to explore how patients identify and assess symptoms as adverse drug reactions. Eur J Clin Pharmacol. 2014;70:607–15.
McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L. Yellow Card Collaboration. Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf. 2010;33:775–88.
Agbabiaka TB, Savovic J, Ernst E. Methodos for causality assessment of adverse drug reactions. Drug Saf. 2008;31:21–37.
Gäwert L, Hierse F, Zink A, Strangfeld A. How well do patient reports reflect adverse drug reactions reported by rheumatologists? Agreement of physician- and patient-reported adverse events in patients with rheumatoid arthritis observed in the German biologics register. Rheumatology (Oxford). 2011;50:152–60.
Ramakrishnaiah H, Naidu S, Jyothsnya S. A comparative study of adverse drug reactionsnreported by healthcare professional and patients in tertiary care teaching hospital. Int J Basic Clin Pharmacol. 2017;6:1078.
Jarernsiripornkul N, Senacom P, Uchaipichat V, Chaipichit N, Krska J. Patient reporting of suspected adverse drug reaction to antiepileptic drugs: factors affecting attribution accuracy. Epilepsy Behav. 2012;24:102–6.
Jarernsiripornkul N, Chaisrisawadsuk S, Chaiyakum A, Krska J. Patient self-reporting of potential adverse drug reactions to non-steroidal anti-inflammatory drugs in Thailand. Pharm World Sci. 2009;31:559–64.
Jarernsiripornkul N, Kakaew W, Loalukkana W, Krska J. Adverse drug reaction monitoring: comparing doctor and patient reporting for new drugs. Pharmacoepidemiol Drug Saf. 2009;18:240–5.
Jarernsiripornkul N, Krska J, Richards ME, Capps PAG. Patient reporting of adverse drug reactions: useful information for pain management? Eur J Pain. 2003;7:219–24.
Van Den Bemt PM, Egberts AC, Lenderink AW, Verzijl JM, Simons KA, Van Der Pol WS, et al. Adverse drug events in hospitalized patients. A comparison of doctors, nurses and patients as sources of reports. Eur J Clin Pharmacol. 1999;55:155–8.
Fisher S, Bryant SG. Postmarketing surveillance: accuracy of patient drug attribution judgments. Clin Pharmacol Ther. 1990;48:102–7.
Tangiisuran B, Wright J, Van der Cammen T, Rajkumari C. Adverse drug reactions in elderly: challenges in identification and improving preventative strategies. Age Ageing. 2009;38:358–9.
Jarernsiripornkul N, Patsuree A, Krska J. Public confidence in ADR identification and their views on ADR reporting: mixed method study. Eur J Clin Pharmacol. 2017;73:223–31.
Jarernsiripornkul N, Krska J, Capps PAG, Richards ME, Lee A. Patient reporting of potential adverse drug reaction: a methodological study. Br J Clin Pharmacol. 2002;53:318–25.
Jarernsiripornkul N, Chaipichit N, Pratipanawatr T, Uchaipichat V. Initial development and testing of an instrument for patient self-assessment of adverse drug reactions. Pharmacoepidemiol Drug Saf. 2016;25:54–63.
Turner RC, Carlson L. Indexes of item-objective congruence for multidimensional items. Int J Test. 2009;3:163–71.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardizes case causality assessment. https://www.who-umc.org/media/2768/standardised-case-causality-assessment.pdf. Accessed 20 Mar 2018.
Chaipichit N, Jarernsiripornkul N, Uchaipichit V, Pratiparnwat T, Krska J. Patients’ attitude towards self-reporting of adverse drug reactions. Srinagarind Med J. 2014;29:461–8.
van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, Grootheest K. Motives for reporting adverse drug reactions by patient-reporters in the Netherlands. Eur J Clin Pharmacol. 2010;66:1143–50.
Jarernsiripornkul N, Arunrot P, Krska J. Survey of patients’ experiences and their certainty of suspected adverse drug reactions. Int J Clin Pharm. 2015;37:168–74.
Isacson D, Johansson L, Bingefors K. Nationwide survey of subjectively reported adverse drug reactions in Sweden. Ann Pharmacother. 2008;42:347–53.
Parretta E, Rafaniello C, Magro L, Coggiola Pittoni A, Sportiello L, Ferrajolo C, et al. Improvement of patient adverse drug reaction reporting through a community pharmacist-based intervention in the Campania region of Italy. Expert Opin Drug Saf. 2014;13:21–9.
Center Health Product Vigilance. Adverse drug reactions reporting 2015. Thai Food and Drug Administration, Ministry of Public Health. 2015. http://thaihpvc.fda.moph.go.th/thaihvc/Public/News/uploads/hpvc_1_3_4_100718.pdf. Accessed 20 Mar 2018.
Banovac M, Candore G, Slattery J, Houyez F, Haerry D, Genov G, et al. Patient reporting in the EU: analysis of EudraVigilance data. Drug Saf. 2017;40:629–45.
Matos C, van Hunsel F, Joaquin J. Are consumers ready to take part in the pharmacovigilance system? A Portuguese preliminary study concerning ADR reporting. Eur J Clin Pharmacol. 2015;71:883–90.
Acknowledgements
The authors thank the patients for their participation and the staff at Srinagarind Hospital for their assistance.
Funding
This study received financial support from Graduate School, Khon Kaen University (Grant Number 59121109). The funding organization had no role in the design or conducting of the study.
Conflicts of interest
None of authors declared any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kampichit, S., Pratipanawatr, T. & Jarernsiripornkul, N. Confidence and accuracy in identification of adverse drug reactions reported by outpatients. Int J Clin Pharm 40, 1559–1567 (2018). https://doi.org/10.1007/s11096-018-0732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0732-7