Abstract
Objectives To validate and pilot in Thailand a questionnaire to enable patients to identify and report symptoms perceived as potential ADRs from NSAIDs. To determine the questionnaire’s usefulness in enabling Thai out-patients to report potential ADRs. To determine the frequency with which symptoms patients reported were recorded by health professionals and the frequency of ADRs to these drugs reported to the APRM Centre. To assess whether patients reported symptoms from non COX-selective inhibitors and COX-2 selective NSAIDs with different frequencies. Setting Out-patient departments (OPD) of a University teaching hospital in North-East Thailand. Method A questionnaire which incorporated an extensive symptoms checklist, developed and validated in English, was translated, piloted and validated in Thai. This was distributed to patients receiving one of five NSAIDs. Causality assessment of the symptoms reported was undertaken by a pharmacist, using data on concomitant medicines and disease states from OPD records. Outcome measures Frequency and type of symptoms reported by patients, recording of these in OPD records, reports sent to APRM Centre. Results Piloting found that patients were able to understand the questionnaire, but were unaware of drug names. A response rate of 42% was obtained: 694 usable questionnaires were returned out of 1,654 distributed. Overall 73% of respondents reported at least one symptom perceived to be an ADR. Sixty percent of symptoms reported were classed as probably or possibly an ADR. Fewer symptoms per patient were reported by those taking COX-2 selective inhibitors (3.5) than those taking non-selective NSAIDs (5.5), although there were no differences in the frequency of GI symptoms reported between these two sub-classes, which may relate to other factors, such as age, previous GI problems and prescription of protective ulcer-healing therapy. Only 5% of symptoms were recorded in OPD records and reporting of ADRs to these drugs to the APRM Centre of the Thai FDA during the study was very limited. Conclusion Thai out-patients were willing and able to complete questionnaires regarding potential ADRs. The questionnaire could form part of routine out-patient monitoring, aiding identification of ADRs, and may help to increase ADR reporting in Thailand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact of research on practice
-
The described checklist can be used as a routine tool in an outpatient setting for detecting ADRs from NSAIDs.
-
The described tool can be used by health professionals to increase the awareness of possible drug-related ADRs, and to increase ADR reporting in Thailand.
Introduction
Spontaneous reporting systems play an important role in identifying adverse drug reactions (ADRs) to drugs. In Thailand, spontaneous reporting by health professionals is managed by the Adverse Product Reaction Monitoring Center (APRM) of the Thai Food and Drug Administration (FDA), but suffers from under-reporting [1] as occurs elsewhere [2]. Thai hospital pharmacists are widely regarded as having expertise in ADRs, encouraging others to submit reports and submitting reports themselves. They also play an important role in educating patients about ADRs and the reporting of these to doctors [3].
The national ADR registers in several countries, including the USA and UK, accept reports directly from patients, although this is not the case in Thailand. Involving Thai patients in self-reporting, particularly when initiated by pharmacists, might be feasible and could improve health professional reporting rates [4], but has not been investigated. Elsewhere direct patient reporting may improve early detection of ADRs, particularly symptomatic reactions [5]. A generic self-completed questionnaire incorporating an extensive symptoms checklist, previously used in the UK, may be suitable for encouraging Thai patients to report symptoms which they perceive to be ADRs [6].
NSAIDs constitute one of the largest groups of drugs taken around the world and are a mainstay of therapy in musculoskeletal conditions [7], but frequently cause ADRs. Each year, NSAIDs account for an estimated 7,600 deaths and 76,000 hospitalization in the United States [8], while in the UK, they are among the drugs most likely to cause hospital admission [9]. The most common ADRs to NSAID affect the gastrointestinal (GI) system, many of which are detectable by patients. This group of drugs therefore was considered appropriate for a study into patient reporting of ADRs. Furthermore, it presents an opportunity to assess whether this tool can detect differences in patient-reported GI symptoms between non-selective NSAIDs and COX-2 selective inhibitors [10] in a clinical setting.
The objectives of this study were therefore:
-
(i)
to validate and pilot in Thailand a questionnaire designed to enable patients to identify and report symptoms perceived as potential ADRs from NSAIDs
-
(ii)
to determine the usefulness of this questionnaire in enabling Thai out-patients to report potential ADRs by assessing causality of symptoms reported
-
(iii)
to determine the frequency with which symptoms patients reported were recorded by health professionals and the frequency of ADRs reported to the APRM Centre to the drugs studied
-
(iv)
to assess whether patients reported symptoms from non COX-selective inhibitors and COX-2 selective NSAIDs with differing frequencies
Method
Ethics approval was obtained from Khon Kaen University Ethics Committee for Human Research.
Questionnaire development
The questionnaire, developed in English, was designed to enable patients to report symptoms they perceive to be ADRs and has been described in detail elsewhere [6, 11]. It consists of an extensive series of symptoms, each with tick boxes, divided into sections for body systems/regions. The option of ‘none’ and space for addition of other symptoms within each section means the questionnaire covers all possible symptoms and that patients can report no symptoms. Respondents are requested to consider whether they have experienced any of the symptoms listed or others they wish to add, which they believe were due to the specific drug named on the questionnaire (index drug) during the last 12 months. Additional questions request demographic data, concurrent therapy and disease states, whether patients have reported symptoms to doctors and any reasons for stopping the index drug.
Questionnaire validation and piloting
The questionnaire was translated into Thai by one bilingual researcher and checked by another, then tested for validity by obtaining the views of five Thai health professionals (two doctors, three pharmacists) on clarity, meaningfulness and symptoms included. An index of consistency between reviewers was calculated for each question/section, scored between −1 (do not agree) and +1 (fully agree). This exercise resulted in small changes being made to five questions for which the average score was below +0.5, namely: all strengths of the index drug were listed, the text of a question relating to administration was changed and the lists of symptoms included for mouth, cardiovascular system and sexual function were modified.
The resultant questionnaire was piloted in a sample of out-patients from the Department of Medicine, a similar population to the main study. Patients were asked to complete both the Thai questionnaire translated from the English version and modified as above, plus a shorter version, not using body systems, since it was recognized that questionnaire length may be of concern. Respondents were then interviewed to determine their views on all aspects of both questionnaires, including understanding of the wording as a further check on the translation and to facilitate format selection. The pilot confirmed that the longer questionnaire incorporating body systems was preferred, therefore the shorter version was discarded. Several small modifications were made to the original questionnaire from comments received. These were: removal of the question about other medicines being taken and the inclusion of descriptions and pictures of the products to facilitate recognition.
Data collection
A total of 1,654 questionnaires were posted to all patients receiving a prescription for one of five NSAIDs (diclofenac, naproxen, nabumetone, meloxicam or celecoxib) over a 1-month-period, identified from computer databases at the Out-patient Departments (OPD) of Orthopaedics and Physical Rehabilitation at Srinagarind Hospital, an 800-bed teaching hospital in North-East Thailand. Questionnaires were accompanied by a prepaid envelope for return to the Faculty of Pharmacy, Khon Kaen University. A second mailing was sent to non-responders after 1 month.
For all patients who returned a completed questionnaire, the OPD record cards were reviewed by a pharmacist to collect information about concomitant medicines, disease states and symptoms recorded by doctors. Information on the number of ADR reports from the whole of Thailand for the study period on these NSAIDS was obtained from the Thai APRM Center.
Causality assessment of potential ADRs reported by patients
For each symptom reported, the ADR literature was searched and, together with the information obtained from OPD cards about concomitant drugs and diseases, used to assess causality of each symptom based on the criteria developed by Jarernsiripornkul et al. [6]. The method was developed to enable classification in the absence of temporal drug administration data. It uses data on concomitant drugs and disease states, plus previous ADR reports to index drugs and results in four levels of causality: probable, possible, unlikely and not attributable. Relationships between demographic data and causality were assessed using Chi-squared tests with P < 0.05 indicating statistical significance.
Comparison of reported symptoms between drug sub-groups
Symptom reporting frequency between patients receiving prescriptions for non COX-selective inhibitors and COX-2 selective inhibitors was also compared using Chi-squared test at a significance level of P < 0.05.
Results
Piloting
Twenty-four patients completed the pilot study, of whom most (21) agreed that the longer body system format was clearer. Nine patients forgot to tick ‘none’ when they had experienced no symptoms. Since this was considered an important feature, it was moved to the start of each section. The average time taken to complete the long questionnaire was 18.3 and 14.0 min for the shorter version. Patients had difficulty recognizing the name of the index drug, therefore descriptions and pictures were added. All patients confirmed that this addition enabled correct identification of products. Patients also did not know the names of other medicines they were taking. Therefore this question was removed and the information obtained from medial records.
Response rates
Questionnaires were distributed to 1,654 patients taking one of five NSAIDs: diclofenac and naproxen, regarded as non COX-selective inhibitors and nabumetone, meloxicam and celecoxib, regarded as COX-2 selective inhibitors (Table 1). A total of 766 (46%) questionnaires were returned, of which 72 were unusable because of lack of completion (25), not taking the prescribed drug (13) and no OPD record available (32), giving a usable response rate of 42%. The distribution of response rates between the five NSAIDs is shown in Table 1.
Demographic data
The average age of respondents was 49 years (SD 14.0, range 12–87 years) and the majority (66%) were female. The highest proportion of respondents (326, 47%) had completed only primary school education, with 16% having completed secondary and 35% tertiary education (2% data not available). Most (74%) patients had been taking one of the index NSAIDs for less than 3 months, although 9% had taken them for longer than 1 year. The NSAIDs were prescribed for non-specific pain (29%), degenerative joint diseases (25%), muscular pain (22%), nerve compression (15%) and rheumatic diseases (9%). The majority of respondents (430, 62%) had no other diseases, 236 (34%) had one or two and only 28 (4%) more than two. Most were taking one or two other drugs (391, 56%), 248 (36%) were taking three or more drugs and only 55 (8%) no other concomitant drugs. Only 29 respondents were taking drugs which may have increased risk of GI symptoms, distributed evenly between the different index drugs. The majority of the respondents 643 (93%) had already stopped taking their NSAID. Almost half (313) had discontinued these themselves, due to lack of perceived need or problems, while 185 had them stopped or changed by health professionals and no information was available for the remaining 130.
Symptoms reported/causality assessment
Of the total 694 respondents, 509 (73%) reported at least one symptom, the proportion varying between the different NSAIDs (Table 1). The highest proportion was in patients taking meloxicam and lowest in those taking celecoxib. Blurred vision was the most frequent symptom reported by patients taking diclofenac and meloxicam, while bloated feeling was the commonest among those taking naproxen, nabumetone and celecoxib. A total of 4,016 symptoms were reported. The number of different symptoms and overall symptom numbers for each drug are shown in Table 2. Overall 80% of respondents identified GI symptoms, 77% CNS symptoms, 27% dermatological symptoms and 21% symptoms associated with the kidney and urinary tract. However 19% identified bone or joint pain.
Using the classification system developed previously, 60% of the symptoms reported by patients were assessed as being probably or possibly related to the NSAID (Table 2). No demographic characteristic was found to relate consistently with respondents’ ability to identify probable or possible ADRs. Patients with no concomitant underlying diseases identified slightly more probable/possible ADRs than unlikely/not attributable symptoms (62% vs. 55%, P < 0.05), although the same was not true of patients taking no concomitant drugs compared to those who were (54% vs. 60%, NS). It was found however that the greater the number of symptoms reported, the greater the likelihood that some were classed as unlikely to be an ADR or not attributable (χ2 = 89.709, df = 3, P < 0.001).
Frequency of symptom recording and ADR reporting
Of the 694 patients reporting symptoms, 153 (22%) claimed they had informed their doctor about all the symptoms they reported on the questionnaire, while a further 122 (18%) had informed doctors about some symptoms. Examination of the medical records of all 694 patients found documented evidence of only 122 (5%) of the 2,402 symptoms reported by these patients. During the study period, a total of 507 ADR reports to these five drugs were received by the APRM Centre of the Thai FDA from the whole of Thailand.
Comparison between drug groups
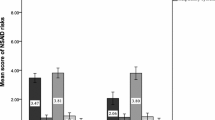
There were 32% of patients taking non-COX selective NSAIDs and 29% taking COX-2 selective NSAIDs who reported at least one symptom. Overall the number of symptoms reported per patient was 5.5 for non-selective and 3.5 for selective drugs. A higher proportion of symptoms reported by patients taking non-selective NSAIDs were classed as probably or possibly ADRs, while patients taking COX-2 selective NSAIDs were more likely to report symptoms not identified in the literature as previously reported ADRs to these drugs. No statistical difference was found in the proportion of respondents who reported symptoms involving the GI system, urinary system or dermatological system between the drug groups (Table 3). The highest proportion of patients reporting a GI symptom was found among those taking celecoxib, although this was the smallest number of patients. Only symptoms involving the central nervous system (CNS) differed between the two groups, with a slightly higher proportion of patients taking non-selective NSAIDs reporting symptoms (P < 0.001). This was mainly due to the low reporting rate of CNS symptoms reported by patients taking nabumetone.
Patients taking Cox-2 selective NSAIDs tended to be older (proportion elderly 29% vs. 19%, P = 0.006) and have had more previous GI events (19% vs. 9%, P = 0.001) than those receiving non-selective drugs, but were less likely to receive concomitant ulcer-healing therapy (17% vs. 38%, P = 0.001) than those taking non-selective drugs.
The overall frequency of drug discontinuation was slightly higher among patients who had received non COX-selective NSAIDs than those who had received COX-2 selective NSAIDs (95% vs. 93%, P = 0.008). Of these, more respondents among those taking selective NSAIDs had stopped the drug because they no longer needed it (46% vs. 36%, P = 0.01) but there were no differences in the proportion who stopped the drug because they experienced side effects (9% vs. 6%, P = 0.24).
Discussion
This study is the first to encourage patients in Thailand to report perceived side effects to prescribed medicines using a questionnaire incorporating a symptom checklist. The overall response rate was 46%, with a valid response rate of 42%, higher than that found in similar published studies from other countries. An Australian study involving two NSAIDs obtained a response rate of 39% [12], while the UK study using the English questionnaire had a response rate of 36% [6]. The study therefore shows that Thai out-patients were willing and able to complete and return questionnaires about potential ADRs.
Since the method precludes the use of standard algorithms for assessing causality, symptoms were classified as reported previously [6], using information obtained from OPD records about concomitant medicines and conditions. No method of assessing causality is universally accepted, all require a degree of judgement [13] and all can over- or under-estimate ADR frequency. This study was further limited by lack of information about purchased medicines, which may be significant since NSAIDs can be purchased in Thailand without a prescription. Study respondents reported a large number of symptoms which were classed as either unlikely to be ADRs or were unattributable to any medicine or condition, including 19% who reported bone and joint pain—symptoms for which the NSAID was prescribed. While this may indicate the index drug’s lack of efficacy, it could alternatively indicate that some respondents misinterpreted questionnaire instructions and included all symptoms they had experienced since starting the index drug. It may also relate to the lack of awareness of drug names and it is possible that patients incorrectly identified the index drug, despite the inclusion of pictures. Similar apparently inappropriate reports were found in the equivalent UK study, although to a lesser extent. The number of concomitant medicines and medical conditions and educational level may be expected to be associated with the likelihood of reporting symptoms. However the study found no consistent relationship between these factors and the ability to identify symptoms assessed as probable or possible ADRs. As with the UK study [6], patients who reported more symptoms were more likely to report symptoms assessed as unlikely to be an ADR. The usefulness of the questionnaire may therefore be greatest in patients who report few symptoms, since their ability to relate symptoms to drug therapy may be higher. Furthermore, it must be acknowledged that patients who responded may be those most likely to have experienced symptoms they perceived as ADRs. Given that 56% of those given the questionnaire did not respond, the incidence of symptoms can only be realistically estimated as a potential range, assuming that non-responders did not experience symptoms.
The risk of a significant GI complication in patients on chronic NSAID therapy is 1–4% per year, but the highest risk is in the first 90 days [10, 14]. In the present study, most patients used the drugs short-term and approximately 80% of respondents reported GI symptoms. There were no differences in reporting rates for GI symptoms between COX-selective and non-selective NSAIDs, although the study was sufficiently powered to detect a difference of 10% in reporting rates. The only difference found was in the reporting of CNS symptoms. This could have been due to the relatively low response rate, although this did not differ between the two groups. Other contributory factors may have included differences in patient characteristics, including age, previous GI events and co-prescription of ulcer-healing therapy, plus a low number of patients receiving celecoxib, the most selective index drug.
The 694 respondents in this study reported a total of 2,369 symptoms to one of five NSAIDs, although only 122 of them were recorded in medical records and there were relatively few reports submitted to the APRM Centre over the same period. This is similar to our UK study, in which primary care doctors recorded a small proportion of the symptoms which patients claim to have reported to them and even fewer resulted in Yellow Card reports to the relevant UK authority [6]. Subsequent changes in UK regulations mean patients are now encouraged to report directly, but this has not been considered in Thailand. Self-reporting can be an additional source of information to health professional reports and is increasingly accepted in many countries [4, 15–17]. Patient involvement in recognizing and reporting potential ADRs is however also key to increasing the number of health professional ADR reports, in Thailand [3] as elsewhere. The questionnaire may prove to be a useful method of encouraging patients to monitor their therapy and to report their symptoms to health professionals. While it may result in over-reporting and some inaccurate attribution to drug therapy, if the completed questionnaire is used as part of a consultation by a pharmacist or other health professional, it could enable the identification of ADRs with greater frequency and potentially increase ADR reporting to the relevant authority. The questionnaire may benefit from further study and refinement to modify instructions and improve response rates. Further work should also explore how patients identify symptoms and associate them as potential ADRs to particular drugs.
Conclusion
Thai out-patients were willing and able to complete lengthy questionnaires regarding potential ADRs experienced to prescribed NSAIDs. The questionnaire could usefully form part of routine monitoring of out-patients and may help to increase ADR reporting in Thailand. It detected few differences in reporting rates of symptoms between non-COX selective NSAIDs and COX-2 selective NSAIDs.
References
Adverse Product Reaction Monitoring Center. Adverse Drug Reactions Reporting 2004. Bangkok; 2004.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–96.
Chaikoolvatana A, Chanakit T, Juengrakpong A. The evaluation of a recurrent adverse drug reaction prevention program in the North-East region of Thailand. J Med Assoc Thai. 2006;89(5):699–705.
Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63(2):148–56. doi:10.1111/j.1365-2125.2006.02746.x.
Egberts TCG, Smulders M, Koning FHP. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. Br Med J. 1996;313:530–1.
Jarernsiripornkul N, Krska J, Capps PAG, et al. Patient reporting of potential adverse drug reactions: a methodological study. Br J Clin Pharmacol. 2002;53:318–25.
Brooks P. Use and benefits of non-steroidal anti-inflammatory drugs. Am J Med. 1998;104(3):9S–13S.
Gurwitz JH, Avorn J. The ambiguous relation between aging and adverse drug reactions. Ann Intern Med. 1991;150:841–5.
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. Br Med J. 2004;329:15–9.
Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a cochrane collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28.
Jarernsiripornkul N, et al. English version of questionnaire. http://www.blackwellpublishing.com/products/journals/suppmat/BCP/BCP1547/BCP1547_fsms1.pdf.
Mitchell AS, Henry DA, Hennrikus D, et al. Adverse drug reactions: can consumers provide early warning? Pharmacoepidemiol Drug Saf. 1994;3:257–64.
Agbabiaki TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions. Drug Saf. 2008;31:21–137.
Lewis SC, Langman MJS, Laporte J-R, et al. Dose-response relationships between individual non-aspirin non-steroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320–6.
Mitchell AS, Henry DA, Fisher S, et al. Patients as a direct source of information on adverse drug reactions. Br Med J. 1988;297:891–3.
Van den Bemt PMLA, Egberts ACG, Lenderink AW, et al. Pharmacoepidemiology and prescription: adverse drug events in hospitalized patients. A comparison of doctors, nurses and patients as sources of reports. Eur J Clin Pharmacol. 1999;55:155–8.
Cox AR. Patient reporting of adverse drug reactions. PharmacoVigilance Rev. 2009;3:18–21.
Acknowledgements
We would like to thank the pharmacists, doctors and other staff working at Srinagarind Hospital, Khon Kaen University. Special thanks are extended to all respondents and Adverse Product Reaction Monitoring Center of the Thai FDA for supplying valuable data.
Funding
This study was funded by a grant from Khon Kaen University.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jarernsiripornkul, N., Chaisrisawadsuk, S., Chaiyakum, A. et al. Patient self-reporting of potential adverse drug reactions to non-steroidal anti-inflammatory drugs in Thailand. Pharm World Sci 31, 559–564 (2009). https://doi.org/10.1007/s11096-009-9310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-009-9310-3