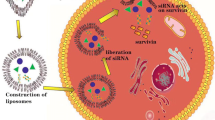
Abstract
Purpose
We have developed and evaluated novel peptide-targeted gemini surfactant-based lipoplexes designed for melanoma gene therapy.
Methods
Integrin receptor targeting peptide, cyclic-arginylglycylaspartic acid (cRGD), was either chemically coupled to a gemini surfactant backbone or physically co-formulated with lipoplexes. Several formulations and transfection techniques were developed. Transfection efficiency and cellular toxicity of the lipoplexes were evaluated in an in vitro human melanoma model. Physicochemical properties were examined using dynamic light scattering, zeta-potential, and small-angle X-ray scattering measurements.
Results
RGD-modified gemini surfactant based lipoplexes showed significant enhancement in gene transfection activity in A375 cell lines compared to the standard non-targeted formulation, especially when RGD was chemically conjugated to the gemini surfactant (RGD-G). The RGD had no effect on the cell toxicity profile of the lipoplex systems. Targeting specificity was confirmed by using an excess of free RGD and negative control peptide (RAD) and was demonstrated by using normal human epidermal keratinocytes. Physicochemical characterization showed that all nanoparticles were in the optimal size range for cellular uptake and there were no significant differences between RGD-modified and standard lipoplexes.
Conclusions
These findings indicate the potential of RGD-modified gemini surfactant-based lipoplexes for use in melanoma gene therapy as an alternative to conventional chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is a malignant tumor of melanocytes, the most progressive form of skin cancer, and its incidence is growing, causing the majority of skin cancer-related deaths. Melanoma can be cured by surgical excision if diagnosed at an early stage. However, in advanced stages, especially in metastatic forms, the response to current therapeutic options is poor with a very low survival rate of less than 5% over 5 years (1). Therefore, new treatment strategies are required. The advances in elucidating the biology of melanoma and the growing knowledge in the genetic derivation of the disease can be utilized to tailor melanoma-specific treatment.
Gene therapy is a promising therapeutic approach for melanoma management. Several gene therapy approaches have been investigated in preclinical and clinical trials to control melanoma (2,3). These include: immuno-gene, oncogene inactivation, tumor suppression and genetic pro-drug activation. In fact, gene therapy approaches are expected to revolutionize targeted melanoma therapy as the first approved gene therapy treatment in the USA (Talimogene laherparepvec, IMLYGICTM) spearheads this approach (4).
Gemini surfactants are a group of cationic lipids that have been studied extensively as non-viral gene delivery carriers for both in vitro and in vivo applications (5,6). These agents have versatile chemical structure, can be produced easily on a laboratory scale, are able to compact DNA to nano-sized lipoplexes and show relatively low toxicity compared to monomeric surfactants (7–9). Chemical structure modification of gemini surfactants has resulted in an increase in activity and improvement of cytotoxicity profile (7). In vivo, topical delivery of pDNA encoding interferon-gamma (IFN-γ) by using diquaternary ammonium gemini surfactant (16–3–16) lipoplexes, induced higher gene expression compared to untreated control group and naked pDNA delivery in an IFN-γ-deficient mice model (10). The same topical lipoplex system was evaluated in a cutaneous scleroderma mouse model (Tsk/+ mice) and showed a decrease in disease manifestation by modulation of excessive collagen synthesis (11). Modification of gemini surfactant structure by introducing amino acid(s) moieties in the spacer region enhanced the bio-compatibility of the lipoplexes and also produced a pH-sensitive system (9,12,13). One of these amino acid modified gemini surfactants, 12-7 N(GK)-12 (Fig. 1a), was evaluated in vivo to develop non-invasive topical lipoplexes for vaginal genetic vaccination (14). Muco-adhesive lipoplexes composed of a model pDNA, 12-7 N(GK)-12, poloxamer 407, and penetration enhancer diethylene glycol monoethyl ether induced gene expression in the mucosa, providing evidence for the possibility of using gemini surfactant-based lipoplexes as noninvasive mucosal gene delivery systems (14).
To improve gene delivery, and specifically tailor the lipoplexes towards melanoma therapy, the gemini surfactants can be modified with targeting moieties to deliver the gene of interest to the melanoma cells. Integrins are a group of cell adhesion proteins that form heterodimeric receptors for extracellular matrix molecules. Integrin receptors are involved in essential intracellular functions including cell adhesion, differentiation, and apoptosis (15). They consist of two-subunits, α and β, with several sub-classes and numerous dimerization combinations (16). Integrins are over-expressed in several tumors, including melanoma, playing a major role in cancer invasion and migration (17–19). In melanoma, overexpressed α3/β1 integrin is substantially involved in disease progress, invasion and metastasis (20–22). This integrin phenotype is homogeneously expressed in cutaneous malignant melanoma (20). Over-expressed integrins can be targeted with an arginine-glycine-aspartate (RGD) moiety (23–25).
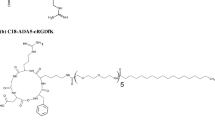
In this work, a new RGD-modified gemini surfactant (Fig. 1c) was synthesized and lipoplexes formulated and characterized for targeted melanoma gene delivery. Various formulation strategies were developed to optimize the physicochemical properties of the delivery system and to achieve efficient in vitro transfection. The in vitro targeting efficiency of the lipoplexes was evaluated in A375 human melanoma cell lines and normal human keratinocytes.
Materials and Methods
Materials
Cyclo(Arg-Gly-Asp-D-Phe-Lys) [RGD], cyclo(Arg-Ala-Asp-D-Phe-Lys) [RAD] and cyclo[Arg-Gly-Asp-D-Phe-Lys(PEG-PEG)] were purchased from Peptides International, Inc. (Louisville, KY, USA). N, N-diisopropylethylamine (DIPEA), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxidehexafluorophosphate (HATU) and dimethyl sulfoxide (DMSO spectroscopy grade) were purchased from Sigma-Aldrich (Oakville, ON, Canada). Helper lipid 1,2 dioleyl-sn-glycero-phosphatidylethanolamine (DOPE) was purchased from Avanti Polar Lipids, (Alabaster, AL, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Invitrogen Corporation (Grand Island, NY, USA). All solvents used were HPLC grade.
Synthesis of Gemini Surfactants
The synthesis and characterization of the gemini surfactant 12-7 N(GK)-12 used in this study (coded as G in lipoplexes) has been previously described (9,26).
Synthesis of RGD Modified Gemini Surfactant [RGD-G]
Under a N2 atmosphere using standard Schlenk techniques, a 25 mL Schlenk flask equipped with a magnetic stir bar was charged with DMF (5 mL), gemini-COOH (synthesis described previously) (9) (9.35 mg, 0.0134 mmol), HATU (5.6 mg, 0.0148 mmol) and DIPEA (0.005 mL, 0.0295 mmol). This mixture was stirred at 0°C for 10–15 min, at which time the color changed from yellow to orange. Cyclo[Arg-Gly-Asp-D-Phe-Lys(PEG-PEG)] (molecular weight of 893.98 with two PEG moieties attached to lysine amino acid) (10 mg, 0.021 mmol) was then added and the reaction mixture allowed to warm to room temperature. After stirring for 18 h, DMF was removed under vacuum. The resultant solid was extracted with dichloromethane saturated with NaHCO3. The combined organics were dried over sodium sulfate and the solvent removed. The product obtained was dissolved in 50:50 (v/v) H2O/methanol and subjected to 3 cycles of cold acetone precipitation. The structure and purity of the product compound, 12-7 N(RGD-PEG-PEG)-12, was confirmed by using a high resolution positive ESI API QSTAR XL MS/MS hybrid QqToF tandem mass spectrometer (Applied Biosystems Inc., CA, USA), that showed a triply charged ion, m/z 500.68 [M + 3H]3+. No peaks were observed for uncoupled gemini surfactant at m/z 284.79 nor for uncoupled cyclo[Arg-Gly-Asp-D-Phe-Lys(PEG-PEG)] (singly charged ion at m/z 894.46 and doubly charged ion at m/z 447.735).
Lipoplex Formulations
The construction of the model plasmid pGThCMV.IFN-GFP, used as a model for a robust plasmid, was described previously (7). Plasmid DNA (coded as P in lipoplexes) was isolated and purified using QIAGEN Plasmid Giga Kit (Mississauga, ON, Canada) following the manufacturer’s protocol. Aqueous solutions of 3 mM gemini surfactant/peptide were used to prepare all lipoplex formulations evaluated in this work.
Lipoplexes, Table I, were formulated using a plasmid to gemini surfactant (−/+) charge ratio of 1:10 in the presence of (DOPE) as a helper-lipid (coded as L in lipoplexes) creating plasmid/gemini surfactant/lipid lipoplexes [P.G.L], as described previously (7). Briefly, the DOPE film was dispersed in 9.25% sucrose solution (pH 9) in nuclease-free ultrapure water (Gibco, Invitrogen Corporation, Grand Island, NY, USA) at 1 mM DOPE final concentration and filtered through Acrodisc® 0.45 μm syringe filters (Pall Gelman, Ann Arbor, MI). The [P/G] lipoplexes were prepared by mixing an aliquot of 200 μg pDNA aqueous solution with an appropriate amount of 3 mM gemini surfactant solution and incubated at room temperature for 20 min. The [P.G.L] systems were prepared by mixing [P/G] lipoplexes with the DOPE vesicles at gemini surfactant to DOPE molar ratio of 1:10 and incubated at room temperature for 20 min.
Peptide-modified lipoplex formulations were prepared in two different ways: 10% of total non-modified gemini surfactant, 12-7 N(GK)-12, was replaced by either peptide-modified gemini surfactant (RGD-G) in F2 and F3, (Table I) or targeting peptide (RGD) in F4 and F5 (Table I).
Size and Zeta Potential Measurements
Formulations (800 μL of each) were transferred into a special cuvette (DTS1061, Malvern Instruments, Worcestershire, UK) for size distribution and zeta-potential measurements using a Zetasizer Nano ZS instrument (Malvern Instruments, Worcestershire, UK). Each sample was measured four times, and the results are expressed as an average ± standard deviation (SD) of three samples (n = 3) with a corresponding polydispersity index (PDI) value.
Small-Angle X-ray Scattering (SAXS) Analysis
Small-angle X-ray Scattering (SAXS) technique was performed at Stanford Synchrotron Radiation Lightsource, Menlo Park, CA, California. Lipoplexes prepared as described above were concentrated (10x) by speed vacuuming at 35°C. A wavelength of 1.1271 Å (11KeV energy) was utilized. Samples were loaded in 1.5 mm boron-rich glass capillaries (Charles Supper Company, USA). The scattered X-ray was detected on MAR225-HE detector (225 mm x 225 mm [3072 x 3072 pixels], pixel size 73.24 μm) at 20s exposure time and at a sample to detector distance of 1.1 m. The SAXS detector was calibrated with silver behenate. GSASII software was used to plot diffraction intensity versus 2θ (where θ is the diffraction angle) or q (scattering vector) by radial integration of the 2D patterns.
Cell Culture and In Vitro Transfection
Human malignant melanoma (A375) cell line (ATCC® CRL-1619™) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic and incubated at 37°C under an atmosphere of 5% CO2/95% air. Normal adult human epidermal keratinocyte cells (HEKa,C-005-5C, Cascade Biologics, Invitrogen), were grown on a T-75 Cell + tissue culture flask (Sarstedt AG & Co.) in Medium 154 (Gibco) supplemented with Human Keratinocyte Growth Supplement (HKGS, Gibco), 10% fetal bovine serum and 1% antibiotic and incubated at 37°C under an atmosphere of 5% CO2/95% air.
The day before transfection, cells were seeded in 96-well tissue culture plates (BD Mississauga, ON, Canada) at a density of 1.5 × 104 cells/well for A-375 cell line and for HEKa cell line at a density of 2 × 104 cells/well in 96-well Cell + tissue culture plate (Sarstedt AG & Co.). One hour prior to transfection, the media was replaced with non-supplemented media. Quadruplicate cells were transfected with lipoplexes containing 0.2 μg pGThCMV.IFN-GFP plasmid/well. Lipofectamine Plus reagent (Invitrogen Life Technologies) was used to monitor the transfection conditions with 0.2 μg pDNA/well. The 96-well tissue culture plates were then incubated at 37°C under 5% CO2 for 5 h. The transfection agents were removed and replaced with fresh supplemented media. Supernatants containing the secreted IFN-γ were collected at 24 and 48 h and replaced with fresh supplemented media. The collected supernatants were stored at −80°C until further evaluated. All experiments were conducted in triplicate.
Transfection Activity (ELISA)
Enzyme-linked immunosorbent assay (ELISA) was performed using Immulon 2 flat bottom 96-well plates (Greiner Labortechnik, Frickenhausen, Germany) following the BD Pharmingen protocol as previously described (13). The concentration of expressed IFNγ was calculated from a standard IFNγ curve using recombinant mouse IFN-γ standard (BD Pharmingen, BD Biosciences).
Cytotoxicity Assay
MTT assay was used to evaluate cellular toxicity of lipoplex systems in A375 and HEKa cell lines. A sterile solution of 5 mg/mL of MTT in PBS buffer was prepared. Cell lines were seeded on 96-well plates and transfected with lipoplexes (as described above). After 48 h, the cell lines were evaluated for cytotoxicity. The supplemented media was removed from the wells and replaced with 0.5 mg/mL MTT in supplemented media and incubated at 37°C in 5% CO2 for 3 h. The supernatant was removed and each well washed with PBS. The purple formazan crystal that formed was dissolved in DMSO. Plates were incubated for 10 min at 37°C. Absorbance was measured at 580 nm using BioTek microplate reader (Bio-Tek Instruments, VT, USA). The cellular toxicity is expressed as a percentage of the non-transfected control cells ± SD.
Statistical Analysis
Statistical analyses were performed using SPSS software (Version 23.0). Results expressed as the average of n = 3 ± SD. One way analysis of variance (ANOVA, Dunnett’s test) was used for statistical analyses. Significant differences were considered at p < 0.05 level.
Results
Physicochemical Characterization of RGD-Modified Gemini Surfactant Lipoplexes
Particle size and surface charge of the lipoplexes are important factors that determine cellular uptake, transfection efficiency, intracellular distribution and lipoplex stability (27,28). The mean particle diameter of standard non-modified lipoplexes [P.G.L] was 172 ± 0.6 nm and all lipoplexes assembled into particles less than 200 nm (Table I). The addition of 10% of RGD-modified gemini surfactant [RGD-G] to the lipoplex system (i.e., chemically conjugated F2:Ch[P.G.RGD-G.L] and F3: Ch[P.G.L].RGD-G) caused a slight increase in particle size (~ 10 nm). On the other hand, physical co-formulation of 10% free RGD peptide [RGD] with [P.G.L] lipoplexes (F4: Ph[P.G.RGD.L] and F5: Ph[P.G.L].RGD) caused a significant increase in particle diameter in comparison to non-modified (~ 30–130 nm,). Zeta potential measurements showed that all lipoplexes bear an overall positive charge ranging from 20–26 mV for non-modified and chemically conjugated lipoplexes. Incorporation of free RGD [RGD] in the formulations caused significant reduction in values of zeta potential (50% reduction) (Table I) (F4: Ph[P.G.RGD.L] and F5: Ph[P.G.L].RGD).
Small-angle X-ray scattering (SAXS) measurements were carried out to evaluate the influence of the targeting component on the super-molecular arrangement of the lipoplexes. The standard non-modified lipoplexes F:1[P.G.L] evaluated in this work retained an inverted hexagonal phase arrangement (HII) as the SAXS scattering profile shows distinctive Bragg peaks at q values of 0.104, 0.181 and 0.209 with corresponding relative ratios of 1, \( \sqrt{3} \) and \( \sqrt{4} \) (Fig. 2, Table II). The addition of RGD peptide into lipoplexes (chemical or physical co-formulation) had no substantial impact on the lipid phase arrangement or inter-lattice spacing.
In Vitro Evaluation
Influence of Formulation Methods on Transfection Efficiency
We evaluated the influence of the amount of the targeting ligand on the transfection and targeting efficiency of developed lipoplex systems. Increasing amounts of RGD-conjugated gemini surfactant (RGD-G) or physical co-formulation of free RGD peptide were incorporated in the lipoplexes (5%, 10%, 15%, and 20% of the total amount of cationic lipid) and the transfection efficiency was evaluated in A375 cell line (Fig. S- 1). Incorporation of 10% RGD-conjugated gemini surfactant (RGD-G) or physical co-formulation of free RGD in the lipoplex systems produced 2–3 times higher levels of INF-γ compared to standard non-targeting lipoplexes [P.G.L] (Fig. S- 1). Increasing the amount of targeting ligand in the formulation (20% and more) significantly diminished the IFN-γ levels. Based on these findings, 10% targeting ligands (RGD-conjugated gemini surfactant (RGD-G) or physical co-formulation of free RGD) was used in our further studies.
In order to optimize the formulation and to enhance the targeting efficiency, we evaluated different preparation techniques (Table I, Fig. 3). In the F2:Ch[P.G.RGD-G.L] and F4:Ph[P.G.RGD.L] systems the RGD-conjugated gemini surfactant or free RGD targeting ligands (RGD-G or free RGD) were added during the formation of lipoplexes. For the other systems, F3:Ch[P.G.L].RGD-G and F5:Ph[P.G.L].RGD, the targeting ligands (RGD-G or free RGD) were added after [P.G.L] lipoplexes were formulated with the non-modified gemini surfactant. The highest IFN-γ expression (Fig. 3A) was obtained when the formulation was prepared by adding 10% of RGD-conjugated gemini surfactant (RGD-G) during the formation of lipoplexes F2:Ch[P.G.RGD-G.L], namely 1058 ± 246 pg INF-γ/1.5 × 104 A375 cells, which is more than two-fold higher compared to the INF-γ levels achieved by standard non-modified F1:[P.G.L] lipoplexes of 385 ± 157 pg INF-γ/1.5 × 104 A375 cells. Physical co-formulation of free RGD in the lipoplexes (F4: Ph[P.G.RGD.L] and F5: Ph[P.G.L].RGD) showed no significant improvement of the transfection activity in comparison to the standard F1:[P.G.L] lipoplexes. As the formulation prepared with RGD-conjugated gemini surfactant added during lipoplex formation, F2:Ch[P.G.RGD-G.L], showed the highest level of enhancement in IFN- expression, this lipoplex formulation was used for further evaluation.
a IFN-γ expression in A375 cells at 48-h post-treatment with lipoplexes constructed at 1:10 −/+ charge ratio. P: pDNA, G:12-7 N(GK)-12, L: DOPE, RGD-G: 12-7 N(RGD)-12, RGD: RGD peptide. (Ch) indicates that the lipoplexes were built using chemically conjugated RGD-gemini and (Ph) indicates a physical co-formulation of free RGD with the non-targeted lipoplexes. IFN-γ level was determined by ELISA. Significant increase (* p < 0.01, one-way ANOVA) in IFN-γ expression was observed when cell treated with RGD chemically-conjugated lipoplexes (F2: Ch[P.G.RGD-G.L]) compared to non-modified lipoplexes (F1:[P.G.L]) b Cell viability in A375 cells after a 48-h treatment with RGD-modified lipoplex formulations as determined by MTT assay. Cell viability was calculated as % relative to non-transfected cells. Four wells of each formulation were loaded in three different experiments. The results are expressed as mean of the three experiments (n = 3). Bars represent standard deviation. * Indicates significance at p < 0.01 in comparison to standard formulation [P.G.L] (F1).
In Vitro Cellular Toxicity Assay
The MTT assay was used to assess the effect of the targeting ligands (RGD-G or free RGD) on the cellular toxicity of the developed lipoplexes (Fig. 3b). After 48 h transfection, the standard non-modified lipoplexes F:1[P.G.L] showed over 80% cell viability and the presence of RGD ligands in the lipoplexes showed very little (not significant) change from this value.
In Vitro Targeting Efficiency
In order to evaluate the specificity of the RGD-modified gemini surfactant-based lipoplexes to integrin receptors (over-expressed on the melanoma cells), we conducted two control experiments. In the first experiment, we used an excess of free RGD peptide and a false-negative control peptide [cyclo(Arg-Ala-Asp-D-Phe-Lys) (RAD)], which shows a marginal binding to integrin receptors, (Fig. 4) (29–31). A375 cell lines which over-express α3/β1 integrins, were treated with excess amount of RGD or RAD (1 μM) 1 h prior to transfection with RGD-modified lipoplexes. A significant reduction in the transfection activity was observed compared to the cells without pre-treatment with free peptides (RGD or RAD) (Fig. 4). The level of IFN-γ showed a reduction from 2357 ± 374 pg to 375 ± 23 pg when treated with F2:Ch[P.G.RGD-G.L] lipoplexes after pre-treatment with free RGD (IFN-γ 375 ± 23 pg) and to 162 ± 70 pg when pre-treated with RAD peptide.
RGD specificity study: IFN-γ expression in A375 cells at 48-h post-treatment with (1) non-modified lipoplexes, F1:[P.G.L] and (2) RGD chemically-conjugated lipoplexes, F2: Ch[P.G.RGD-G.L]. (3) A375 cells were pre-treated with 1 μM RAD peptide (a negative control for integrins) 1 h prior to transfection with F2: Ch[P.G.RGD-G.L] lipoplexes. (4) A375 cells were pre-treated with 1 μM free RGD (1 h prior to transfection with F2: Ch[P.G.RGD-G.L] lipoplexes. Significant reduction (* p < 0.01, one-way ANOVA) in IFN-γ expression was observed when cells treated by RAD and free RGD prior transfection compared to (2), indicating the specificity of RGD chemically-conjugated lipoplexes (F2: Ch[P.G.RGD-G.L]) toward integrins. Four wells of each formulation were loaded per experiment. The results are expressed as mean of the three experiments (n = 3).
In the second control experiment, we used primary human epidermal keratinocytes, HEKa which express normal levels of integrin receptors in normal growth conditions (Fig. 5) (32,33). We transfected the HEKa cells with three lipoplex formulations: non-modified F1:[P.G.L], formulation prepared with RGD-conjugated gemini surfactant added during lipoplex formation F2:Ch[P.G.RGD-G.L], and formulations prepared with physical incorporation of free RGD added during lipoplex formation F4:Ph[P.G.RGD.L]. A slight, but not significant increase (p > 0.05), was observed with the formulation prepared with the chemically conjugated RGD-gemini surfactant added during lipoplex formation, F2:Ch[P.G.RGD-G.L], compared to standard [P.G.L] lipoplexes and no difference was observed with the physical incorporation of the free RGD in the formulation process F4:Ph[P.G.RGD.L] (Fig. 5A). The addition of targeting ligands to the lipoplexes caused no change to the cellular toxicity profile of the standard formulation [P.G.L] (Fig. 5b).
a IFN-γ expression in HEKa cell line at 48-h post-treatment with lipoplexes constructed at 1:10 −/+ charge ratio non-modified lipoplexes (F1:[P.G.L]), RGD chemically-conjugated lipoplexes (F2: Ch[P.G.RGD-G.L]) and RGD peptide co-formulated with the lipoplexes F4: Ph[P.G.RGD.L]. IFN-γ level determined by ELISA. b Cell viability of HEKa cells after a 48-h treatment with RGD-modified lipoplex formulations as determined by MTT assay. Cell viability values were calculated as % relative to non-transfected cells. HEKa cells are normal adult human epidermal keratinocyte cells which express normal level of integrins. No statistically significant differences were observed between non-modified lipoplexes and RGD modified lipoplexes. Four wells of each formulation were loaded per experiment. The results are expressed as mean of the three experiments (n = 3). Bars represent standard deviation.
Discussion
Achieving the ultimate goal of effective melanoma gene therapy depends on the design of efficient, safe and melanoma-specific gene delivery systems. Tumor specific uptake is essential to avoid non-specific gene expression and possible related toxicities. Targeted gene delivery can be achieved by designing a gene delivery system that preferentially interacts with a cancer-specific bio-molecule (i.e., protein) that has an essential role in tumor growth or progression (34). Cancer-specific molecules can be: a) a receptor or enzyme that is over-expressed in tumor cells, b) a tumor specific antigen, or c) a cancer specific gene (i.e., oncogene). Integrins are a family of cell adhesion proteins that are up-regulated in different cancers, including melanoma, and promote tumor growth and metastasis (35). Thus, for this study, we synthesized a new RGD-conjugated gemini surfactant to incorporate into the [P.G.L] lipoplexes.
For non-viral gene delivery systems, optimization of the physicochemical properties of lipoplexes is essential to achieve a maximum level of gene expression and formulation stability. Several factors can influence the cellular uptake, and subsequently the transfection activity of the lipoplexes. These include the particle size and shape, overall charge, supramolecular structure and the presence of targeting motifs (attached by surface chemistry) (27,36). The particle size of the lipoplexes plays an important role in formulation stability, cellular uptake, biodistribution and clearance. Addition of new components such as the RGD-modified gemini surfactant (RGD-G) to the lipoplexes might influence their assembly, cellular uptake mechanism and ultimately their biological behaviour.
Previously, we evaluated the mechanism of cellular uptake of lipoplexes constructed with 12-7 N(GK)-12 gemini surfactant, the parent cationic lipid used in the current study (12). It was demonstrated that gemini surfactant-based lipoplexes can be internalized to the cells either by clathrin-mediated and/or caveolae-mediated endocytosis (12). Thus, in the current formulation development process, our goal was to maintain the innate physicochemical characteristics of the gemini surfactant-based lipoplexes, despite addition of the RGD peptides. Incorporation of 10% of chemically conjugated gemini surfactant (RGD-G) in the lipoplexes (F2: Ch[P.G.RGD-G.L]), with the highest transfection, showed a slight but not significant increase in particle size with a polymodal particle distribution ranging from 50 nm up to 400 nm (as indicated by the PDI value) compared to the non-targeted formulation F1: [P/G/L] (Table I). Previously, we reported that [P.G.L] lipoplexes formulated with 12-7 N(GK)-12 gemini surfactant form larger nanoparticles with cylindrical shape while lipoplexes formed using an older generation of gemini surfactant, 12-7NH-12, formed spherical-shaped lipoplexes with smaller particle size (12). The main cellular uptake pathway for 12-7 N(GK)-12 lipolexes employed both clathrin- and caveolae-mediated uptake. The larger cylindrical lipoplexes formed with the peptide-modified gemini surfactant was believed to confer the ability to these lipoplexes to engage multiple pathways and facilitate the cellular uptake (12,36). In the current work, the polymodal distribution of F2:Ch[P.G.RGD-G.L] lipoplexes can be an indication of heterogeneity of size and shape of the lipoplexes which could similarly enhance use of different endocytotic pathways mechanisms. Gao et al. showed that the modification of poly(ethyleneglycol)-poly(e-caprolactone) nanoparticles PEG-PCL NPs with interleukin 13 (IL-13) peptide to target IL-13Ra2 over-expressed receptor, caused significant changes in the cellular uptake mechanism compared to non-conjugated PEG-PCL NPs. Macropinocytosis was suggested to be an important cellular uptake for non-conjugated NPs, while the IL-modified NP showed preferable uptake by clathrin-dependent pinocytosis and receptor-mediated endocytosis.
As well, the increase of the IFN-γ levels in the case of F2:Ch[P.G.RGD-G.L] lipoplexes could be related to the RGD motif. The RGD structure is anticipated to cause specific binding to cell surface integrins leading to receptor-mediated endocytosis for the Ch[P.G.RGD-G.L] lipoplexes, hence enhancing the transfection activity (37–39).
Another important characteristic of the lipoplexes that influences the transfection efficiency is the supramolecular arrangement of the lipids in the context of the genetic material. Lipoplexes can form different lipid phases including lamellar, cubic, hexagonal and inverted hexagonal phases. All gemini surfactant lipoplexes investigated in this work adopted inverted hexagonal phase (HII) (Fig. 2 and Table II). The inverted hexagonal phase was found to be associated with the transfection activity of gemini surfactant-based lipoplexes as it can facilitate the fusion of the lipoplexes with the cell membrane and boost DNA endosomal escape as a function of helper-lipid, DOPE (7,40).
Optimization of the preparation method is a critical step when evaluating new components or formulating novel lipoplex systems. In this study, we have evaluated the influence of chemically-conjugated RGD gemini surfactant [RGD-G] and the physically co-formulated RGD (Table I) on the transfection activity of gemini-based lipoplexes. Only lipoplexes formulated with chemically conjugated RGD gemini surfactant (i.e, F2:Ch[P.G.RGD-G.L]) showed improvement in transfection activity (Fig. 3). Targeting by physical co-formulation of free RGD in the gemini surfactant lipoplexes had no positive effect on the level of gene expression. This can be related to the possibility of dissociation of the RGD from the lipoplexes prior to cellular uptake. It was found in this work that the optimal amount of RGD-targeting ligand that can achieve an increase in transfection activity is 10% of overall cationic lipid concentration. Further increase in the proportion of RGD negatively affected gene expression, more noticeably when [RGD-G] is used. It is possible that increasing the [RGD-G] content in the formulation caused a disturbance in the lipid phase arrangement as the [RGD-G] bears a long PEG chain in the spacer region which can hinder the cellular uptake of the lipoplexes and delay DNA release. In fact, the SAXS profile of lipoplexes formulated with 100% [RGD-G] showed the disappearance of the distinctive Bragg peaks associated with inverted hexagonal phase (Fig. S- 2). Consequently, lipoplexes formulated with 100% [RGD-G] gemini surfactant showed a negligible level of gene expression (Fig. 3a), underlining the importance of the inverted hexagonal arrangement. The incorporation of 10% of [RGD-G] during the formation of lipoplexes (i.e, F2:Ch[P.G.RGD-G.L] achieved a significant enhancement in gene expression (p < 0.01) with no change in cytotoxicity (Fig. 3). In contrast, the addition of [RGD-G] after the formation of [P.G.L] (formulation F3: Ch[P.G.L].RGD-G) did not improve the transfection activity compared to non-modified lipoplexes. This observation can be explained by the hypothesis that the cellular uptake of F2:Ch[P.G.RGD-G.L] lipoplexes was more efficient than the other RGD-modified formulations due to the arrangement of the RGD-conjugate on the outer surface of the lipoplexes, presenting a higher availability for interaction with the surface integrins. Thus, the capability and specificity of RGD modified gemini surfactant to target the over-expressed integrins, α3/β1, on A375 malignant melanoma cells was validated.
The incorporation of 10% of [RGD-G] in formulation F2:Ch[P.G.RGD-G.L] caused a significant enhancement in transfection activity which implies higher cellular uptake. In addition, the integrin-competition studies with free RGD peptide and negative RAD peptide controls confirmed that the modification of the lipoplexes with RGD-conjugated lipoplexes was internalized preferably by melanoma cells, involving a new mechanism of cellular uptake (receptor-mediated endocytosis) as all lipoplexes failed to achieve a significant transfection level when the A375 cells were pre-treated with the control peptides (Fig. 4). Similarly, the transfection studies in HEKa cells, which express low levels of integrins during early and normal cell growth, (33,41) showed no improvement in the cellular uptake of the targeted lipoplexes compared to the non-targeted formulation, confirming the targeting specificity of the RGD-modified lipoplexes to melanoma (Fig. 5a).
Conclusion
In this work, the specificity of a novel RGD-modified gemini surfactant-based nano-platform to target integrin receptors in melanoma was evaluated. The RGD-conjugated lipoplexes showed higher transfection activity in melanoma cell lines compared to non-modified lipoplexes. The improvement in transfection activity associated with RGD-modified lipoplexes can be returned to baseline by blocking the integrin receptors with free RGD. The specificity of RGD-conjugated lipoplexes toward melanoma cells was proved by transfection in normal keratinocytes cells (HEKa cells) which express a normal level of integrins. Lipoplex modification with the RGD-ligands had no significant influence on the physicochemical properties of the original lipoplex system or on the cellular toxicity profile. Future applications of the RGD-modified gemini surfactant-based lipoplexes will encompass delivery of tumor-suppressor genes directly into melanoma cells with high specificity, to halt uncontrolled cell proliferation and trigger programmed cell death without affecting healthy surrounding cells in the skin.
Abbreviations
- cRGD:
-
Cyclic-arginylglycylaspartic acid
- DOPE:
-
Phosphatidylethanolamine
- GFP:
-
Green fluorescent protein
- IFN-γ:
-
Interferon-gamma
- SAXS:
-
Small-angle X-ray scattering
References
Gershenwald JE, Giacco GG, Lee JE. Cutaneous Melanoma. 60 Years of Survival Outcomes at The University of Texas MD Anderson Cancer Center: Springer; 2013. p. 153–65.
Viola JR, Rafael DF, Wagner E, Besch R, Ogris M. Gene therapy for advanced melanoma: selective targeting and therapeutic nucleic acids. J Drug Deliv. 2013;2013. doi:10.1155/2013/897348.
Gene Therapy Clinical Trials Worldwide: John Wiley and Sons Ltd. The Journal of Gene Medicine Clinical Trial site. http://www.wiley.com//legacy/wileychi/genmed/clinical/ (2016). Accessed 25 April 2012.
Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology. 2015;5(1):e1115641.
Bombelli C, Giansanti L, Luciani P, Mancini G. Gemini surfactant based carriers in gene and drug delivery. Curr Med Chem. 2009;16(2):171–83.
Wettig SD, Verrall RE, Foldvari M. Gemini surfactants: a new family of building blocks for non-viral gene delivery systems. Current Gene Ther. 2008;8(1):9–23.
Badea I, Verrall R, Baca-Estrada M, Tikoo S, Rosenberg A, Kumar P, et al. In vivo cutaneous interferon-γ gene delivery using novel dicationic (gemini) surfactant–plasmid complexes. J Gene Med. 2005;7(9):1200–14.
Donkuru MD, Wettig SD, Verrall RE, Badea I, Foldvari M. Designing pH-sensitive gemini nanoparticles for non-viral gene delivery into keratinocytes. J Mater Chem. 2012;22:6232–44.
Yang P, Singh J, Wettig S, Foldvari M, Verrall RE, Badea I. Enhanced gene expression in epithelial cells transfected with amino acid-substituted gemini nanoparticles. Eur J Pharm Biopharm. 2010;75(3):311–20.
Badea I. Gemini cationic surfactant-based delivery systems for non-invasive cutaneous gene therapy: University of Saskatchewan; 2006.
Badea I, Virtanen C, Verrall R, Rosenberg A, Foldvari M. Effect of topical interferon-γ gene therapy using gemini nanoparticles on pathophysiological markers of cutaneous scleroderma in tsk/+ mice. Gene Ther. 2012;19(10):978–87.
Singh J, Michel D, Chitanda JM, Verrall RE, Badea I. Evaluation of cellular uptake and intracellular trafficking as determining factors of gene expression for amino acid-substituted gemini surfactant-based DNA nanoparticles. J Nanobiotechnol. 2012;10(1):7.
Al-Dulaymi MA, Chitanda JM, Mohammed-Saeid W, Araghi HY, Verrall RE, Grochulski P, et al. Di-peptide-modified Gemini surfactants as Gene delivery vectors: exploring the role of the alkyl tail in their physicochemical behavior and biological activity. AAPS J. 2016:1–14.
Singh J, Michel D, Getson HM, Chitanda JM, Verrall RE, Badea I. Development of amino acid substituted gemini surfactant-based mucoadhesive gene delivery systems for potential use as noninvasive vaginal genetic vaccination. Nanomedicine. 2015;10(3):405–17.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87.
Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31(1):485–516.
Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90(3):561–5.
Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8(2):96–103.
Eble JA, Haier J. Integrins in cancer treatment. Curr Cancer Drug Targets. 2006;6(2):89–105.
Natali P, Bartolazzi A, Cavaliere R, Bigotti A, Nicotra M. Integrin expression in cutaneous malignant melanoma: association of the α3/β1 heterodimer with tumor progression. Int J Cancer. 1993;54(1):68–72.
Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Kim DK, Gupta TKD. Beta1 integrin expression in malignant melanoma predicts occult lymph node metastases. Surgery. 1995;118(4):669–75.
Van Belle PA, Elenitsas R, Satyamoorthy K, Wolfe JT, Guerry D, Schuchter L, et al. Progression-related expression of β3 integrin in melanomas and nevi. Hum Pathol. 1999;30(5):562–7.
Harvie P, Dutzar B, Galbraith T, Cudmore S, O'Mahony D, Anklesaria P, et al. Targeting of lipid-protamine-DNA (LPD) lipopolyplexes using RGD motifs. J Liposome Res. 2003;13(3–4):231–47.
Shi W, Bartlett JS. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol Ther. 2003;7(4):515–25.
Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Maeda M, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med. 2005;7(5):604–12.
Mohammed-Saeid W, Buse J, Badea I, Verrall R, El-Aneed A. Mass spectrometric analysis of amino acid/di-peptide modified gemini surfactants used as gene delivery agents: establishment of a universal mass spectrometric fingerprint. Int J Mass Spectrom. 2012;309:182–91.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159.
Resina S, Prevot P, Thierry AR. Physico-chemical characteristics of lipoplexes influence cell uptake mechanisms and transfection efficacy. PLoS One. 2009;4(6):e6058.
Dubey PK, Mishra V, Jain S, Mahor S, Vyas S. Liposomes modified with cyclic RGD peptide for tumor targeting. J Drug Target. 2004;12(5):257–64.
Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van der Schaft DW, Storm G, et al. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005;19(14):2008–10.
Schiffelers RM, Koning GA, ten Hagen TL, Fens MH, Schraa AJ, Janssen AP, et al. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release. 2003;91(1):115–22.
Adams JC, Watt FM. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991;115(3):829–41.
Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, et al. Keratinocytes in human wounds express αvβ6 integrin. J Investig Dermatol. 1996;106(1):42–8.
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22.
Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci. 2008;105(33):11613–8.
Kunath K, Merdan T, Hegener O, Häberlein H, Kissel T. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J Gene Med. 2003;5(7):588–99.
Xiong XB, Huang Y, WL LU, Zhang X, Zhang H, Nagai T, et al. Intracellular delivery of doxorubicin with RGD-modified sterically stabilized liposomes for an improved antitumor efficacy: in vitro and in vivo. J Pharm Sci. 2005;94(8):1782–93.
Panetti T, McKeown-Longo P. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993;268(16):11492–5.
Foldvari M, Badea I, Wettig S, Verrall R, Bagonluri M. Structural characterization of novel gemini non-viral DNA delivery systems for cutaneous gene therapy. J Exp Nanosci. 2006;1(2):165–76.
Solomon DE. An in vitro examination of an extracellular matrix scaffold for use in wound healing. Int J Exp Pathol. 2002;83(5):209–16.
Acknowledgments and Disclosures
The authors report no conflicts of interest. The authors are grateful for financial support from NSERC and SHRF to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S-1
(DOCX 66 kb)
Figure S-2
(DOCX 72 kb)
Rights and permissions
About this article
Cite this article
Mohammed-Saeid, W., Chitanda, J., Al-Dulaymi, M. et al. Design and Evaluation of RGD-Modified Gemini Surfactant-Based Lipoplexes for Targeted Gene Therapy in Melanoma Model. Pharm Res 34, 1886–1896 (2017). https://doi.org/10.1007/s11095-017-2197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2197-0