Abstract
Purpose
To explain the effects of cationic amino acids and other co-solutes on the viscosity, stability and protein-protein interactions (PPI) of highly concentrated (≥200 mg/ml) monoclonal antibody (mAb) solutions to advance subcutaneous injection.
Methods
The viscosities of ≥200 mg/ml mAb1 solutions with various co-solutes and pH were measured by capillary rheometry in some cases up to 70,000 s−1. The viscosities are analyzed in terms of dilute PPI characterized by diffusion interaction parameters (k D ) from dynamic light scattering (DLS). MAb stability was measured by turbidity and size exclusion chromatography (SEC) after 4 weeks of 40°C storage.
Results
Viscosity reductions were achieved by reducing the pH, or adding histidine, arginine, imidazole or camphorsulfonic acid, each of which contains a hydrophobic moiety. The addition of inorganic electrolytes or neutral osmolytes only weakly affected viscosity. Systems with reduced viscosities also tended to be Newtonian, while more viscous systems were shear thinning.
Conclusions
Viscosity reduction down to 20 cP at 220 mg/ml mAb1 was achieved with co-solutes that are both charged and contain a hydrophobic interaction domain for sufficient binding to the protein surface. These reductions are related to the DLS diffusion interaction parameter, k D , only after normalization to remove the effect of charge screening. Shear rate profiles demonstrate that select co-solutes reduce protein network formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hundreds of monoclonal antibodies (mAbs) are under development for treating various diseases including cancer, autoimmune diseases, and asthma (1). To avoid the need for intravenous delivery of dilute mAb solutions in the clinic, it would be desirable to deliver a high (often ≥100 mg) dose in a 1 to 2 ml injection subcutaneously (2). At such high concentrations, the interactions between closely spaced mAbs often result in high viscosities, i.e. above the ~20 cP limit for subcutaneous injection, (2–4) which would require more than 30 N of force to inject a 1.5 ml solution within one minute (5). Furthermore, concentrated mAbs are prone to reversible and irreversible aggregation, which degrades their efficacy and has the potential to produce immunogenic responses (2,6,7). Although the viscosity and stability may be improved by modifying the mAb sequence via genetic mutation, (8–10) this approach is time consuming and may have unpredictable effects on the therapeutic efficacy (4).
The viscosities of protein solutions increase exponentially with concentration due to increased protein-protein interactions (PPI) as surface separation decreases (11,12). At short surface-to-surface distances, short-ranged attractive interactions from both electrostatic (opposite local charges) and hydrophobic forces become strong. Short-ranged local anisotropic electrostatic attractive interactions are produced by the alignment of oppositely charged regions on the mAb surface and by charge-dipole interactions (3,13,14). Hydrophobic interactions are also short-ranged as they form between patches of hydrophobic residues and thus also require protein alignment to occur (15). Studies have shown that mAb solution viscosity increases with the strength of these attractive interactions, (8,16) particularly when they are strong enough to cause protein self-association, and oligomerization (3,11,16–21). Recent studies have shown that in some cases the viscosity and self-association of mAbs can be dominated by a few specific attractive interaction sites (8–10,22). These specific interaction sites most often occur between fAb regions particularly between the complementary determining regions, (9,10,12) though they have been shown to also form between negatively charged fAb and positively charged fc regions, particularly for mAbs of class IgG1 (3,13,17).
The challenge of measuring PPI at high concentrations is a major hurdle in understanding the stability and viscosity of concentrated protein solutions. In some cases advanced techniques have been used, including static light scattering, (21,23) small angle x-ray and neutron scattering, (16,19,20) neutron spin echo, (19,20) osmometry, (24) and rheology (18,20,25). However, for many of these measurements, it is difficult to model the strength and structure of the PPI. Therefore, PPI are much more often measured at dilute protein concentrations, often characterized by the second virial coefficient or the diffusion interaction parameter (k D ). In some cases, these properties are correlated with the high concentration viscosity for different mAbs at a given pH and co-solute composition (8). However, these correlations have received very limited attention for a given mAb with different co-solutes or concentrations of co-solutes and are not well understood (8).
An increasingly common strategy for manipulating PPI is to add co-solutes that indirectly or directly interact with residues on the mAb surface. Inorganic electrolytes such as NaCl, NaSCN and Na2SO4 screen charges on the protein surface, which may potentially weaken both long-range global electrostatic repulsion and short-ranged local electrostatic attraction between mAbs (14,26). However, it has been found that, depending on the nature of the PPI for a given mAb, salt can either increase or decrease net attraction at high concentration and consequently decrease, (11,12,19,27) not influence, (18,19) or increase mAb viscosities (20,21,24). In addition to simple screening of charge, salt effects on mAbs have been shown to sometimes be specific ion dependent, particularly for anions, (28) loosely following the Hofmeister series, generally with more chaotropic salts resulting in larger viscosity reductions, (11,12) presumably due to the breaking of water structure by chaotropic salts (29).
In addition to salts, organic co-solutes, which function as osmolytes or depletants, have been added to mAb solutions to influence the stability and viscosity. Neutral saccharides and amino acids are excluded from the mAb surface resulting in osmotic compression or depletion attraction that raises the accessible free volume and thus the entropy of the depletant to minimize the protein surface area (30–32). Given that folded protein is typically more resistant to irreversible aggregation than unfolded protein, osmolytes are often added as protein stabilizers (30,33,34). Additionally, the smaller occupied volume for more compact protein theoretically may lead to lower solution viscosities (12,31). However, several saccharide osmolytes have been shown to increase the viscosity of various mAbs (35).
A third class of co-solutes, organic hydrophobic electrolytes can produce drastic viscosity reductions (15). In addition to screening electrostatic PPI, these organic electrolytes can weaken hydrophobic PPI by binding to hydrophobic sites. Several studies have used arginine (Arg) to achieve large viscosity reductions (8,16,24,27,36–38) as it binds to proteins and modifies PPI (39–41). In particular, it interacts with aromatic hydrophobic residues much more strongly than other cations such as lysine (Lys) (39,40). Whereas histidine (His) is also known to interact with aromatic residues, (42,43) it has not been shown to reduce viscosity as generally as Arg. In some cases, these three amino acids yield similar viscosity reductions (27), or Arg and Lys may yield greater reductions than His, (37) or Arg greater reductions than Lys (36) depending upon the particular protein. Furthermore, the effect of pH, which influences the co-solute charge, has received very little attention, which is particularly important for co-solutes such as His with pKa values in the physiological pH range. It would be desirable to investigate the effects of co-solutes on viscosity and storage stability simultaneously. Finally, little is known about the effect of co-solutes on PPI at high concentrations, versus measurements of PPI at dilute conditions by static or dynamic light scattering.
Herein, we compare the effects of three cationic amino acids, His, Arg, and Lys on the viscosity and storage stability of concentrated mAb solutions up to 277 mg/ml over a range of pH from 5.0 to 7.6. To attempt to understand how these co-solutes modify PPI we systematically examine the effects of simpler classes of co-solutes starting with several inorganic electrolytes along the Hofmeister series to determine the effects of charge screening. We additionally test neutral osmolytes including the disaccharide trehalose (Tre) and the amino acid alanine (Ala) which provide osmotic compression that may increase protein folding and lower the effective protein volume fraction to perturb the PPI. Additionally, to further improve understanding of the three cationic amino acids, additional organic electrolytes are studied including the free side chain of His, imidazole (Im), and the hydrophobic anion, camphorsulfonic acid (CSA). These co-solutes help demonstrate which specific co-solute qualities cause viscosity reductions in mAb solutions, such as the sign of the electrolyte’s charge (cationic or anionic), the magnitude of the charge based on pKa, and the strength of hydrophobic interactions. The co-solutes are studied from pH 5 to 7.3 to vary the charge of His and Im, which change significantly with pH unlike the stronger bases Arg and Lys which remain fully protonated in this range. We hypothesize that the lowest viscosities may be achieved when the co-solute is charged to modify the PPI. Key systems are studied by shear rate dependent rheology, DLS diffusion interaction parameter (k D ), and storage stability by size exclusion chromatography, allowing us to compare the effects of co-solutes on viscosity, self-association, PPI and storage stability.
Materials and Methods
The monoclonal antibody used in this study (mAb1) is an IgG1 obtained from AbbVie at ~120 mg/ml in a proprietary buffer. The isoelectric point (pI) of mAb1 as provided by AbbVie is 9.3. Alanine, arginine, arginine hydrochloride, camphorsulfonic acid, histidine, histidine hydrochloride monohydrate, hydrochloric acid, imidazole, lysine, lysine hydrochloride, sodium chloride, sodium sulfate and sodium thiocyanate were purchased from Fisher Scientific, Fairlawn, NJ. Sodium camphorsulfonate was purchased from TCI America, Portland, OR. Trehalose dihydrate was obtained from Ferro Pfanstiehl Laboratories Inc., Waukegan, IL.
Centrifugal Filtration
For each sample the mAb1 solution was diluted in a buffer containing the desired concentrations of co-solutes. The resulting solution was then centrifuged in an Eppendorf Centrifuge 5810R (Hamburg, Germany) at 4500 rcf in a centrifugal filter with a molecular weight cutoff of 30 kDa for 15 min. The retained protein solution was then re-diluted using the same buffer and then centrifuged again. This process was repeated until the volumetric fraction of original buffer was less than 1% assuming ideal mixing. After diafiltration the solution was concentrated by centrifugal ultrafiltration until the desired concentration was reached. The large volume samples used for viscosity versus shear rate measurements were made by combining and mixing the retentate from six separate filters. The co-solute concentrations selected for diafiltration are explained in the supplemental section.
Samples with multiple reported concentrations were concentrated to the highest reported concentration then diluted for lower concentration measurements. The pH reported is that at the highest concentration, but the pH change upon dilution was always <0.1 pH units.
Lyophilization Dilution (LD)
Some mAb1 samples were made by lyophilization dilution (LD), in which lyophilized protein powder was mixed into an aqueous buffer to achieve 100–150 μl of a ~ 250 mg/ml mAb1 solution. The lyophilized powder contained a 0.2:1 mass ratio of trehalose to mAb1 without any other solids. Therefore, all LD solutions contain ~130 mM trehalose and all additional co-solutes are added via the solvent. The lyophilized powder was made by centrifugal diafiltration of mAb1 into DI water and then adding a solution of 300 mg/ml trehalose to achieve the 0.2:1 mass ratio. The solution was then frozen at −40°C in a freezer (Industrial Freezer Sales), and lyophilized in a VirTis AdVantage Plus tray lyophilizer (SP Scientific, Warminster, PA) at −40°C for 1250 min and then −25°C for 4250 min before being ramped to 25°C over the course of 300 min.
Viscosity Measurements
Rheosense m-VROC
The viscosities of the samples made by CF were measured using a microfluidic Viscometer/Rheometer-on-Chip (m-VROC, Rheosense Inc. San Ramon, CA) with a C05 chip for samples viscosities >10 cP and a B05 chip for samples with viscosities <10 cP. Viscosity measurements were taken at shear rates of 500 s−1 and 1000 s−1, until the measurement stabilized (at least two consecutive runs with measured viscosities within 5%). The viscosity and standard deviation reported is from the average and standard deviation of the measurements at 1000 s−1 after the measurement had stabilized, though Newtonian behavior was observed for all samples in this shear range.
The viscosities were measured at 25 ° C as a function of shear rate using both a C05 chip and an E04 chip, and consistency was observed in the overlap region. The shear rate was increased and decreased at least twice to check for reversibility. This process was first performed with Newtonian standards N35 and N10, (Cannon Instrument Company, State College, PA), shown in Fig. S1, to demonstrate the reliability of the measurement and the consistency between chips.
Due to their small volume, the viscosity of LD samples was measured in triplicate at 25°C with a capillary syringe viscometer calibrated using viscosity standards as described previously (38). The shear range measured by this viscometer is shown in Fig. S2.
Protein Concentration
The concentration of the mAb1 solutions were measured in duplicate, where 2 μl of solution was mixed into 998 μl of 50 mM pH 6.4 phosphate buffer. The background-corrected absorbance of the resulting solution was measured over a spectrum of 400 to 250 nm using a Cary 3E UV-visible spectrophotometer. The concentration was determined from the corrected absorbance using Beer’s law (\( A{280}_{corr}=\varepsilon *b*c \)) where A280 corr is the absorbance at 280 nm after correcting for scattering. The method for removing the scattering contribution from the absorbance was adopted from prior work by Englander and Epstein and is described in the supplemental material (44), ε is the extinction coefficient (1.42 ml mg−1 cm−1 for mAb1 as provided by AbbVie) and b is the cell path length (1 cm). Representative absorbance spectra can be seen in Fig. S3.
Protein Turbidity
The protein turbidity, τ, was measured using a Cary 3E UV-visible spectrophotometer over a spectrum of 1100 to 200 nm. The turbidity was measured in triplicate on both the full concentration sample without any dilution and the protein-free solvent. Representative turbidity spectra can be seen in Fig. S4. The reported values of τ are the average measured A350 of the sample minus that of the solvent divided by the path length (0.2 cm). The normalized turbidity, τ/C p, is equal to the value of τ divided by the protein concentration, C p , in g/ml during the measurement.
Dynamic Light Scattering (DLS) for Diffusion Interaction Parameter (k D ) Measurement
The diffusion coefficients of mAb1 solutions were measured by DLS at a scattering angle of 90° with a Brookhaven ZetaPlus using the diffusion coefficient calculated from the quadratic cumulant algorithm. Each sample was run four times for 2 min. The diffusion interaction parameter (k D ) was obtained by fitting the diffusion coefficients at 2, 5, 10, 15 and 20 mg/ml with the equation \( D={D}_0\left(1+{k}_d{C}_p\right) \) where D is the measured diffusion coefficient, D 0 is the fit diffusion coefficient at infinite dilution and C p is the protein concentration. The data plots to determine k D are available in Fig. S5.
Size Exclusion High Performance Liquid Chromatography (SE-HPLC)
The measurement of irreversible aggregation was measured by SE-HPLC as described previously, (38) with a Tosoh Biosciences TSKgel3000SWXL column (Tosoh Corporation, Tokyo, Japan).
Accelerated Storage
Small aliquots (50 μL) of the final mAb1 solutions were stored at 40°C in a convection oven (model number 107905; Boekel Industries, Feasterville, PA) for four weeks in 300 μL HPLC vial inserts inside 1 mL HPLC vials sealed with caps as well as two layers of Parafilm surrounding a layer of aluminum foil. The mAb1 concentration of each sample stored can be found in Table SI under the column title “C p,2 (mg/ml)”. For the samples that do not have value in this column, the mAb1 concentration was diluted to ~200 mg/ml for storage.
Results and Discussion
Effect of pH on Viscosity With Low Co-Solute
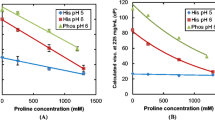
To determine the effect of charge on the behavior of mAb1, highly concentrated solutions were made at pH 5, 6 and 7 by CF with minimal co-solute, 30 mM His-HCl buffer to control the pH. However, due to the low ionic strength of the solution at pH 7, the final pH drifted up to 7.6 (45); therefore, another sample was made at a slightly lower pH of 6.8, which only drifted to 7.2. As shown in Fig. 1, the mAb1 solution viscosity increases exponentially with concentration for all pH values tested, as previously seen for other mAbs (11,12). The viscosity for a given concentration decreases markedly with pH from 7.6 to 5.1 at each mAb concentration. Given that the pI is 9.3, part of this reduction in viscosity was driven by the change in the charges on the protein (26).
Effect of pH on solution viscosity for concentrated mAb1 solutions containing 30 mM His-HCl made by CF. Lines represent fits to the Ross-Minton equation. Error bars are standard deviations of viscosity and concentration measurements, some error bars are too small to be seen. The inset shows the inherent viscosity of these systems at the measured concentration closest to 200 mg/ml mAb1.
Upon reducing the pH below the pI, the electrostatic and charge dipole interactions are influenced in two ways. First, the increase in the net positive charge of the mAb increases the global long-ranged charge repulsion. Additionally, lowering the pH will neutralize some negative charges on the mAb surface weakening the anisotropic local electrostatic attraction between opposite charges and dipoles. Several studies have demonstrated the importance of anisotropic attractive electrostatic interactions near physiological pH for IgG1 antibodies, in which the fc region is positively charged and the fAb region is often negatively charged (3,13,17). In this case, a decrease in pH reduces the number of negative charges in the fAb region, weakening local anisotropic attractive fAb-fc electrostatic and charge-dipole interactions. Therefore, as the pH is lowered starting at the pI of 9.3, the local attractive protein-protein interactions (PPI) become weaker and the repulsive PPI become stronger, both of which would be expected to decrease net attraction and therefore reduce the viscosity, (8,13,16) as is seen in Fig. 1.
A widely used equation to fit viscosity versus concentration data for protein solutions is the Ross-Minton equation (Eq. 1), (11,12)
which relates the relative viscosity, η rel , which is the ratio of the solution to solvent viscosity, η/η 0 , to the protein concentration, C p , the intrinsic viscosity, [η], the crowding factor, k, and the Simha (shape-determining) parameter, ν. The curves shown in Fig. 1 are fits to Eq. 1 using [η] and k/ν as adjustable parameters and 1 cP for η 0 . Because of the limited number of data points used for some of these fits (given the availability of the mAb and large quantities needed for viscosity measurement), the fits are only meant to be semi-quantitative. The inherent viscosities, η inh , at protein concentrations of ~200 mg/ml are reported in the inset of Fig. 1, and are shown to decrease from 29.1 to 14.6 ml g−1 at pH values from 7.6 to 5.1. All reported η inh are measured at ~200 mg/ml unless otherwise stated. The η inh is more appropriate for comparing formulations than η in order to mitigate the large dependence of η on protein concentration (46). Since η increases exponentially with C p , (11) a small difference in C p has a large influence on η. On the other hand, the change in η inh with concentration is much more gradual, as shown by Eq. 2 determined by simultaneously solving the definition of inherent viscosity, η inh ≡ ln(η/η 0 )/C p , with the Ross-Minton equation (Eq. 1).
Thus, η inh is useful in comparing formulations with variations or error in C p on the order of 200 ± 10 mg/ml; however, it becomes less precise for larger variations. For example a change in C p on the order of 20 mg/ml typically produces a change in η inh of <1 ml g−1. A summary of the η inh at ~200 mg/ml of these systems can be found in Fig. 2a.
The PPI of these solutions were also measured under dilute conditions by the diffusion interaction parameter (k D ). The k D is a common measurement of PPI as strong correlations have been shown between the k D measured under dilute conditions and the viscosity at high concentration for many mAbs (8). PPI are measured by k D based on how increased interactions, through increased concentration, affect the rate of diffusion. When increased concentration slows down diffusion the resulting k D will be negative corresponding to attractive interactions. Alternatively when diffusion speeds up with concentration the k D will be positive corresponding to repulsive interactions. The measured k D of the 30 mM His-HCl solutions are displayed in Fig. 2b, showing a small decrease in k D from pH 5 to pH 6 and a large drop between pH 6 and 7. The high concentration viscosities of these systems qualitatively match the k D as the systems with more repulsive interactions by k D have lower viscosities.
Effect of Inorganic Electrolytes on Viscosity
Since charge was shown to have a major effect on mAb1 viscosities through pH, the next step was to test how the manipulation of charge through ionic strength affects mAb1 viscosity. As stated earlier, decreasing the pH simultaneously strengthens global repulsion and weakens local attraction between mAbs, decreasing net attraction. Increasing ionic strength screens all electrostatic interactions and therefore weakens both attractive and repulsive electrostatic interactions, and may thus produce various net effects on mAb interactions, (26) which explains why the effects of salt on viscosity is mAb dependent (19). For systems in which salts reduce net attraction and therefore, reduce mAb solution viscosities the viscosity minimum tends to occur around an ionic strength of 150 to 500 mM, (11,12,27,36,37) and thus 250 mM was chosen for the current study. As seen in Fig. 3 and Tables I and SI, mAb1 solutions were made with 220 mM NaCl and 30 mM His-HCl at pH values of 5.1, 6.0 and 6.9. At pH ~5, the NaCl weakly increased the solution viscosity with η inh increasing from 14.6 to 14.9 ml g−1, while at pH 6 it caused a minor decrease in η inh from 19 to 17.5 ml g−1, and at pH 7 it caused a major decrease in η inh , 29.1 to 17.7 ml g−1. This behavior likely occurs because NaCl is screening all electrostatic interactions which are more repulsive at low pH and more attractive at high pH, so at pH 7 the NaCl is screening significant attractive interactions and is thus causing a large reduction in viscosity, while at pH 5 it screens more repulsive than attractive interactions. In addition to NaCl the inorganic electrolytes NaSCN and Na2SO4 were added to 30 mM His-HCl systems, to achieve a total ionic strength of ~250 mM, and these electrolytes resulted in similar viscosities as NaCl as seen in Fig. S6, indicating at most a weak Hofmeister effect implying that the effects of NaCl are likely general for inorganic electrolytes. Therefore, the screening of local electrostatic attractive interactions from an ionic strength of 250 mM is not sufficient to significantly alter mAb1 PPI to reduce viscosities at low pH.
Effect of mAb concentration and pH on viscosity of mAb1 solutions containing 30 mM His-HCl and 220 mM NaCl made by CF. Lines represent fits to the Ross-Minton equation. Error bars are standard deviations of measurements, some error bars are too small to be seen. The inset shows the inherent viscosity of these systems at the measured concentration closest to 200 mg/ml mAb1 and compares them to the inherent viscosities at 200 mg/ml for samples containing only 30 mM His-HCl.
The k D was measured for the NaCl solutions, shown in Fig. 2b. Although the solutions with 30 mM His-HCl at pH 5 and 6 with and without 220 mM NaCl had similar viscosities at high concentration, the k D measurement shows far stronger attractive interactions with NaCl, as the addition of 220 mM NaCl reduces the k D from 12.0 to −10.0 ml g−1 at pH 5 and 8.0 to −14.7 ml g−1 at pH 6. The k D is measured under dilute conditions where the average protein-protein separation is much larger than under the high concentrations during the viscosity measurements. Therefore, the length scales relevant to PPI are different for measurements of k D and viscosity with greater emphasis on longer ranged forces for k D measurements. Since inorganic electrolytes screen both long-ranged global electrostatic repulsion and short-ranged local electrostatic attraction, the relative significance of repulsion will be overemphasized under dilute conditions, such as a k D measurement. Therefore, even though at high concentration ionic screening causes similar reductions in attractive and repulsive electrostatic PPI, under dilute conditions the reduction in repulsion from screening is far greater than that of attraction, leading to a net increase in measured attraction.
Effect of Neutral Organic Osmolytes on Viscosity
A second method to lower viscosity is to lower the protein volume fraction by forming more compact folded protein structures, as shown in Eq. 1, where compact structures will have lower [η] (12,31). Additionally, the folding generally internalizes more hydrophobic residues, reducing attractive hydrophobic PPI. More compact structures may be formed by osmotic compression with neutral saccharides or amino acids, which are preferentially excluded from the protein surface (30). Fig. S7 and Tables I and SI show that the addition of either of two common osmolytes, the disaccharide trehalose (Tre), or the amino acid alanine (Ala) at 220 mM to concentrated mAb1 solutions at pH 6 causes an increase in viscosity. Although osmolytes promote compact protein conformations, which could theoretically lead to lower viscosity, (12,31) they also may promote protein attraction through depletion attraction. This attraction likely causes the increase in viscosity for mAb1 as with other mAbs previously tested (35).
Effect of Organic Electrolytes on Viscosity
Lysine (Lys)
Several studies have shown that organic electrolytes, for example the cationic protonated amino acids Lys, His and Arg, can cause significant viscosity reductions for certain mAbs, (15) possibly due to their ability to hydrogen bond with the protein to modulate surface charge. Adding 250 mM Lys-HCl to mAb1 resulted in a η inh of 17.2 ml g−1 with a viscosity profile similar to the samples containing inorganic electrolytes, as seen in Fig. S6. This suggests that the effects of Lys-HCl on viscosity occur mainly through ionic screening, similar to the inorganic electrolytes. In comparison, much larger viscosity reductions were observed for the other cationic amino acids as will now be described.
Histidine (His)
Unlike Lys, His is known to interact with aromatic residues on proteins via its imidazole ring (42,43). The η inh at pH 6 with 250 mM His-HCl dropped down to 14.8 ml g−1 (Fig. 4), significantly lower than the value of 17.2 ml g−1 achieved with Lys-HCl or 19.0 for 30 mM His-HCl. In fact, it was essentially as low as the sample with 30 mM His-HCl at pH 5. Thus, the addition of His is an interesting alternative to pH for weakening PPI to lower viscosity.
Effect of mAb concentration and pH on viscosity of mAb1 solutions containing 250 mM His-HCl made by CF. Lines represent fits to the Ross-Minton equation. Error bars are standard deviations of measurements, some error bars are too small to be seen. The inset shows the inherent viscosity of these systems at the measured concentration closest to 200 mg/ml mAb1 and compares them to the inherent viscosities at 200 mg/ml for samples containing only 30 mM His-HCl.
Another difference between His and the cationic amino acids Lys and Arg is its weaker basicity. The side-chain of His has a pKa of ~6.0, relative to ~10.5 and ~12.5 for the stronger bases, Lys and Arg, respectively. Therefore, at pH 7, the fraction of protonated His is ~10% and at pH 6 it is ~50% as shown in Fig. S8. To test the effects of His charge on mAb1 viscosity, 250 mM His-HCl was added to concentrated mAb1 solutions. As with 30 mM His-HCl, the pH of the pH 7 sample drifted upward during ultrafiltration to 7.3, so a new sample was made at lower pH to achieve a final pH closer to 7. As seen in Fig. 4 and Tables I and SI, the viscosity of mAb1 with 250 mM His-HCl decreased with pH, as seen for previous systems. However, unlike at 30 mM His-HCl, the viscosities were very similar at pH values of 5 and 6 with η inh values of 13.3 and 14.8 ml g−1, respectively, well below the value of 19.0 ml g−1 at pH 7. At each pH value tested, increasing the His-HCl concentration from 30 to 250 mM decreased the viscosity. However, this reduction was much smaller at pH 5, where mAb1 is already highly repulsive and the viscosity was already quite low.
To complement these results, mAb1 solutions with similar formulations were made by lyophilization dilution (LD). An advantage of LD is that the final concentration of the co-solute is completely known, because all of the components are measured gravimetrically and mixed into the sample. In contrast for CF, partitioning of co-solutes across the filter leads to uncertainty in the final co-solute concentration (45).
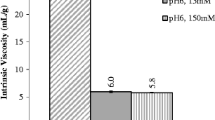
For these LD samples, the mAb1 was concentrated to ~250 mg/ml with either 30 mM or 250 mM His-HCl over the same 5–7.5 pH range. Due to the strong buffer capacity of the lyophilized protein powder, the pH of the 30 mM His samples only ranged from 5.5 to 7. Unlike the CF samples, the LD samples all contained 130 mM Tre in addition to the His-HCl, as Tre was used as lyo-protectant during formation of the lyophilized powder. This amount of Tre did not affect the viscosity, as seen for CF samples in Fig. S9 and Table SII.
Although the LD samples were more turbid, as discussed in the supporting information, the final viscosities for CF and LD systems were similar for a given formulation and mAb1 concentration, as shown in Fig. 5 (data in Tables SI and SIII), in terms of the ~250 mg/ml η inh . For 250 mM His, η inh decreases sharply with pH from 7.5 to 6, then remains relatively steady between pH 6 and 5. As shown in Fig. 6, the η inh continually decreases with His-HCl concentration over the full range tested of 30 to 750 mM for LD samples at pH 5.5. This trend was also seen for CF samples at pH 6 as increasing the His-HCl concentration from 250 to 500 mM further reduced the viscosity (Fig. 6 and Table SII). The rate of reduction of η inh decreases steadily with co-solute concentration, suggesting that His-HCl may begin to saturate protein interaction sites, or that changes in PPI become progressively smaller. Figure 7 combines the effects of His-HCl concentration and pH into one plot for these LD samples, showing that increasing His-HCl concentrations and reducing pH both reduce the η inh across the spectrum tested. The upper right-hand region of Fig. 7 (corresponding to high concentration His-HCl and high pH) is not filled in because His is not sufficiently soluble in this pH region.
Effect of pH and His-HCl concentration on inherent viscosity of ~250 mg/ml mAb1 solutions made by CF and LD. Hollow symbols represent samples made by centrifugation filtration (CF), while filled symbols represent samples made by lyophilization dilution (LD). Blue diamonds contain 30 mM His-HCl, while red squares contain 250 mM His-HCl. Error bars were determined by propagation of errors in measurement of concentration and viscosity.
The large viscosity reduction for His-HCl at pH 6 versus the minor reductions for the other electrolytes, Lys-HCl and inorganic electrolytes, may be used to infer indirectly how the co-solutes are modulating PPI. The screening of the charges on the protein surface by ions in the diffuse layer could potentially weaken both global repulsive and local anisotropic attractive electrostatic interactions. Given very limited viscosity reduction for Lys-HCl and the inorganic electrolytes, other factors must be present for His-HCl. Similarly to Lys, the carboxylate and amino groups of His may interact with the charged sites on the protein via hydrogen bonding, and charge-dipole interactions and therefore bind to the surface, increasing the effective charge of the mAb (47–49). However, these interactions only caused minor viscosity reductions for Lys. Unlike Lys, the aromatic imidazole group of His is known to bind to hydrophobic aromatic residues through cation-π, and π-π stacking interactions, (42,43,50) which have interaction strengths on the same order as hydrogen bonds (50,51). Furthermore, it may even interact with positively charged sites as imidazole rings have been shown to bind to other imidazole and guanidyl groups, even when both are positively charged (50). In contrast, Lys may bind more weakly to the mAb as it does not have an aromatic functionality. Thus bound HisH+ can neutralize negatively charged residues, add positive charge to neutral aromatic residues, and strengthen the positive charge of surface Arg and His residues. These changes may strengthen the global electrostatic repulsion by increasing net charge and simultaneously weaken the local anisotropic electrostatic attraction, by neutralizing negatively charged surface sites. Eventually, for His-HCl concentrations >250 mM, the degree of co-solute binding and/or these changes in charge appear to get saturated and the viscosity reduction with added co-solute slows down. Furthermore the effect will be limited at pH 7 where only ~10% of the His is protonated, which explains why fully ionic NaCl causes a greater viscosity reduction than His at pH 7. Finally, the smaller viscosity reductions from His at pH 5 may be attributed to a large number of positively charged sites even without added co-solute.
In addition to modulating the electrostatic PPI, His can block attractive hydrophobic interactions that have been hypothesized to play a large role in self-association, which is known to raise protein solution viscosities (10,15,23,46). The replacement of a hydrophobic aromatic site with a positive charge could have a large influence on PPI, as removing hydrophobic residues via mutations has had dramatic effects on PPI (9,10,22). Even bound neutrally charged, zwitterionic His could reduce hydrophobic interactions, as proteins interact weakly with zwitterions (52). This behavior may partially explain why His can cause a large viscosity reduction at pH 7, even when ~90% of it is net neutral.
Arginine (Arg)
The third and final naturally occurring cationic amino acid, Arg, like His is known to interact with aromatic residues via its side-chain, in this case a guanidyl group (39,40). Arg is a strong base with a pKa of ~12.1 and therefore like Lys is essentially 100% positively charged from pH 5 to 7 (Fig. S8). As seen in Fig. 8 and Tables I and SI, the η inh values for mAb solutions containing 250 mM Arg-HCl are 13.1, 13.7 and 14.4 at pH values of 5.3, 5.9 and 6.9 respectively. Not only are these values much more pH independent than previous systems, but they are exceptionally low. Direct comparisons of systems with low His-HCl, high His-HCl, NaCl, and Arg-HCl at pH values of 5, 6 and 7 (Figs. S10–S12) show that 250 mM Arg-HCl causes larger viscosity reductions than the other systems at all pH values tested, with larger differences at higher pH. The effect of Arg-HCl on the mAb1 PPI viscosity-concentration profile (and consequently PPI) relative to that of 30 mM His-HCl sample, both at pH 6, is shown in Fig. 9a and b with the Ross-Minton equation (Eq. 1) and its linearized version Eq. 3 (53)
Effect of mAb concentration and pH on viscosity of mAb1 solutions containing 250 mM Arg-HCl made by CF. Lines represent fits to the Ross-Minton equation. Error bars are standard deviations of measurements, some error bars are too small to be seen. The inset shows the inherent viscosity of these systems at the measured concentration closest to 200 mg/ml mAb1 and compares them to the inherent viscosities at 200 mg/ml for samples containing only 30 mM His-HCl.
Effect of concentration and co-solute on viscosity of mAb1 solutions at pH 6 made by CF. The numbers in the legend are the co-solute concentration in mM. Lines are fits to the data by the Ross-Minton equation. Error bars are standard deviations of measurements, some error bars are too small to be seen. The fit values of [η] are 10.3 and 8.7 ml g−1 for 30 mM His-HCl and 250 mM Arg-HCl, respectively, while the values for k/ν are 0.2176 and 0.2041.
As seen in Fig. 9b, the linearized representation of the low co-solute system has significant curvature indicating attractive interactions beyond hard quasi-spherical interactions, while the Arg-HCl system displays linear behavior. Thus Arg-HCl weakens the non-ideal PPI exhibiting behavior closer to hard particles (21).
The dilute PPI for both the 250 mM Arg-HCl and His-HCl solutions yielded very similar results having k D values of approximately −5 ml g−1 at pH 5 and 6 and approximately −8 ml g−1 at pH 7, as seen in Fig. 2b. Since the viscosities of Arg-HCl and His-HCl systems at pH 5 and 6 are similar, and the two co-solutes have similar ionic strengths under these conditions, it is unsurprising that the k D values were similar. Unexpectedly the k D values were more attractive for these high co-solute systems than their 30 mM His-HCl counterparts, at both pH 5 and 6, despite the lower viscosities. This behavior may be explained by the large effect of screening of electrostatic repulsion with added electrolyte at dilute conditions, as was seen with the NaCl solutions. Screening of this repulsion had little influence on viscosity at high concentrations, where the local attractive anisotropic electrostatic interactions are strong. Note that the k D values of the His-HCl and Arg-HCl solutions were less attractive than the ones with concentrated NaCl at similar ionic strength. Since Arg-HCl, His-HCl and NaCl screen long-ranged global electrostatic repulsion similarly, the increase in k D for His-HCl and Arg-HCl relative to NaCl must correspond to reductions in other attractive interactions such as hydrophobic PPI. At pH 7 on the other hand, the viscosities were much lower for Arg-HCl than His-HCl, despite similar k D measurements, because most of the His is uncharged at pH 7, resulting in a lower ionic strength and therefore less screening of electrostatic repulsion. Figure 10 shows a heat map suggesting that the η inh is correlated to a combination of the measured interactions under dilute conditions through k D , and the strength of electrostatic screening, represented by the solvent Debye length. The plot reveals that for a given Debye length the η inh increases with decreasing k D , as has been reported previously (8). However, due to the large effect of screening of long-ranged electrostatic repulsion under dilute conditions, low viscosities at high protein concentrations can be achieved for solutions with relatively low k D when strong ionic screening is present. This analysis shows some generality amongst mAbs, as it qualitatively agrees with prior results from Connolly et al., in which the k D and viscosity were tested for 16 unique mAbs each in two formulations, one with and one without 200 mM Arg-HCl (8). Connolly’s data and the data from the current study were simultaneously fit to calculate a predicted η inh from both the measured k D and the solvent Debye length. This predicted η inh is correlated to the measured η inh in Fig. S13 (described in supplemental).
Plot of inherent viscosity at 200 mg/ml mAb1 as a function of solvent Debye length and k D . Systems included in plot are in Fig. 2. The heat map is a linear interpolation of the data points, marked by black triangles.
To further test PPI modulation by co-solutes, the shear rate dependence of viscosities of concentrated mAb1 (~220 - 230 mg/ml) solutions was investigated for both 30 mM His-HCl and 250 mM Arg-HCl at pH 6, as well as 30 mM His-HCl at pH 5 (with fewer data points to conserve mAb). As seen in Fig. 11, a shear thinning viscosity profile is seen for the 30 mM His-HCl solution, while the Arg-HCl and low pH solution exhibit mostly Newtonian behavior. Shear thinning has been seen for concentrated solutions of other mAbs due to shear-induced deformation of mAb networks (18,25). Therefore, the high viscosity for the 30 mM His-HCl solution at pH 6, as well as the shear thinning behavior suggests formation of reversible aggregates and networks for this mAb. In contrast, the low viscosity and Newtonian behavior for the Arg-HCl and pH 5 systems may be attributed to the weakening of the attractive PPI, disrupting network formation. Due to limitations on the pressure sensors of the instrument, the viscosity could not be measured at the highest shear rates for the 30 mM His-HCl solution; therefore it was diluted from 220 to 205 mg/ml, where it still displayed shear thinning behavior (Fig. 11). As has been seen previously, the shear thinning is more pronounced at the higher mAb concentration, due to stronger network formation (25). Previous work has shown that shear thinning of another mAb could be reduced by the addition of NaCl; (18) however, the NaCl also resulted in an overall viscosity reduction, likely because screening electrostatic attraction was sufficient to disrupt networks of the mAb studied. The addition of NaCl only weakly reduced the viscosity of the pH 6 mAb1 solutions tested (Fig. 3), and as seen in Fig. 11, did not remove the shear thinning behavior, indicating the presence of protein networks at 200 mg/ml. The low shear viscosities were similar for the ~230 mg/ml pH 5 system and the ~200 mg/ml NaCl system. However strong shear forces only reduced the viscosity of the latter system, as only it had networks to break. The shear thinning was quantified with the Carreau model, (25) (described in supplemental) with parameters shown in Table SIV.
Effect of shear rate on viscosity of concentrated mAb1 solutions at pH 6 made by CF. Filled symbols with solid lines are 220–230 mg/ml mAb1, while hollow symbols with dashed lines are 200–205 mg/ml. Legend shows concentration of co-solute in mM followed by mAb1 concentration in mg/ml. Lines are fits to the data by the Carreau model. Error bars are standard deviations of measurements, some error bars are too small to be seen.
In addition to mAb1, Arg has been shown to reduce the viscosity of various proteins, (8,16,24,27,36,37) by preferentially interacting with the protein surface (39–41,47). Like His, these surface interactions can include double-layer screening, hydrogen bonding through the amino, carboxylate and the side chain (guanidyl group). Also like the imidazole group of histidine, the guanidyl group preferentially interacts with aromatic residues as well as other neutral and positively charged guanidyl and imidazole groups, allowing it to bind with Arg and His residues on the mAb surface (50,51). Therefore the guanidyl group of Arg functions analogously to the imidazole group of His, allowing Arg to screen both electrostatic and hydrophobic interactions. Static light scattering measurements have shown that Arg directly binds to mAb surfaces, (23) which would increase the effective charge of the mAb. Arg reduces the viscosity much more significantly than His at high pH, but only slightly more at low pH where the weaker base His becomes protonated. Further evidence that the higher charge of Arg is largely responsible for the greater viscosity reduction relative to His is that 500 mM His-HCl at pH 6, with ~250 mM in the protonated HisH+ state, produces approximately the same viscosity as 250 mM fully protonated Arg-HCl at pH 6, as seen in Fig. S14. However, in addition to charge other more complex factors may also influence the interactions. For example, Arg is a larger and more flexible molecule than His, and simulations have shown that Arg forms stacks in solution increasing its interactions with protein surfaces (40,41).
It is instructive to compare co-solute effects on various types of proteins with a range of types of PPI. For instance, viscosity reductions were similar for human serum albumin (HSA) (37) and mAb JM1 (27) for all electrolytes tested including several inorganic electrolytes, Arg-HCl and Lys-HCl. It is likely that the electrostatic interactions were dominant for these proteins, such that the additional hydrophobic screening of Arg-HCl had minimal effect. At pH 7.4 where His-HCl is <5% charged (Fig. S8), it did not reduce the viscosity of HSA as in the uncharged state it does not screen these electrostatic PPI. Alternatively, another study from Inoue et al. showed that Arg-HCl caused greater viscosity reductions than Lys-HCl or NaCl with polyclonal gamma globulin (36). Therefore, polyclonal gamma globulin likely has significant hydrophobic PPI as seen for mAb1. For a second mAb, JM2, studied by Wang et al. (27) viscosity reductions were greater for the cationic amino acids, Arg-HCl, His-HCl, and Lys-HCl over other salts tested. The fact that Lys caused a similar viscosity reduction as His and Arg for JM2 was consistent with the weak aromatic hydrophobic PPI reported by three dimensional excitation-emission-intensity (27).
Imidazole (Im)
Given the large viscosity reduction for His, it is instructive to investigate the behavior of its side chain the basic co-solute imidazole (Im). As shown in Fig. S15 and Tables I and SI, at pH 6, 250 mM Im-HCl and His-HCl yield similar η inh values of 13.9 ml g−1 and 14.8 ml g−1, respectively. At pH 7 however, the η inh was 15.5 ml g−1 for Im-HCl much smaller than His-HCl at 19.0 ml g−1. This result is consistent with those above for cationic bases, as seen in Fig. S8, approximately half of the stronger base Im is positively charged at pH 7, much higher than the ~8% for His, allowing Im to screen more interactions and produce a larger increase in the number of positive charges on the mAb surface and thus cause a greater reduction in viscosity. His-HCl is approximately half charged at pH 6 and lowers the viscosity more than half-charged Im-HCl at pH 7. This difference likely occurs for two reasons: one, the protein itself is inherently more positively charged at lower pH regardless of the co-solute; and two, the uncharged His still contains a zwitterionic moiety and therefore may still screen hydrophobic interactions, unlike neutral Im.
Camphorsulfonic Acid (CSA)
Recent studies have shown viscosity reductions of mAb solutions from anionic organic co-solutes, for example camphorsulfonic acid (CSA) which contains both a negative sulfonate group and a bulky aliphatic hydrophobic moiety (15). Adding 220 mM Na-CSA to a concentrated mAb1 solution containing 30 mM His-HCl at pH 6, caused a large viscosity reduction similar to that of cationic 250 mM His-HCl as shown in Fig. S16 and Tables I and SI. Likewise, at pH 7 the viscosity reduction from Na-CSA was similar to that obtained with Im-HCl. Since direct binding of the anionic CSA to the mAb surface would add negative charges to a positively charged protein, below its pI, it would be expected to increase the viscosity by increasing net attraction. Thus, the addition of positive charges is not a necessary condition for viscosity reduction. Instead, screening of both electrostatic and hydrophobic PPI is likely how CSA reduces the viscosity.
Since ArgH+ yielded the lowest viscosities for all of the cations tested and CSA− for the anions, these two ions were used together to test for synergistic behavior. However, as seen in Fig. S17 and Tables I and SI Arg-CSA yielded similar to slightly higher viscosities than Arg-HCl or Na-CSA. Without measurements of co-solute binding for the various cases, the interpretation of this result is unclear, but it is likely that the binding sites for these co-solutes became saturated such that the increased concentration in total binding organic electrolyte had a minimal effect.
Effects of Co-Solutes and pH on Turbidity and Storage Stability
For pharmaceutical applications, it is essential to maintain high mAb stability during processing and storage. Therefore, the initial (pre-storage) stabilities of several of the CF samples, reported in Tables I and SI, were measured at full concentration (~200 mg/ml) via turbidity, τ, at 350 nm and were reported as both a τ and a normalized τ/C p . As all of the samples were visually transparent, the measured τ were all relatively low, less than 0.5 cm−1, except for 250 mM His-HCl at pH 7.3 where it was 0.79 cm−1. The τ/C p values reported in Table I, generally increase with pH for a given co-solute type and concentration. This trend is expected as the higher pH is closer to the pI of the mAb where stronger attractive PPI (26) promote aggregation resulting in higher turbidity, and viscosity (3,11,12,17,19–21). Consequently, Fig. 12a shows a correlation is present between τ/C p and η inh . Interestingly, η inh increases much faster with turbidity with 30 mM co-solute than with 250 mM co-solute.
Correlation between normalized turbidity at ~200 mg/ml and (a) inherent viscosity at ~200 mg/ml (b) percent aggregates by SEC after 4 weeks of 40°C accelerated storage at ~200 mg/ml for mAb1 solutions containing 30 mM and 250 mM ionic co-solute. Data for plots can be found in Table I. The lines are just guides to the eye.
To characterize storage stability, the irreversible aggregate formation for CF samples was measured by SE-HPLC after accelerated storage at 40°C for four weeks. The percent of aggregates is reported instead of the percent of monomer, as we were unable to de-convolute the monomer peak from the first degradate peak in some samples; the full SE-HPLC chromatograms can be found in Fig. S18. The largest degradate peaks are seen for the samples at pH ≥7 and the sample containing 220 mM Tre.
As shown in Tables I and SI, the stability of the 30 mM His-HCl samples steadily decreased with an increase in pH. The stability also decreased with pH for all high co-solute samples tested at multiple pH values, except for Arg-HCl which had a maximum at pH 6 and Na-CSA which had the lowest storage stability of any co-solute system tested. For a given pH, the addition of any co-solute improved the storage stability above the low co-solute 30 mM His-HCl case as has been seen with other mAbs stored at high concentration (27). Particularly low aggregate formation was seen for the organic electrolytes His-HCl, Arg-HCl and Arg-CSA, the inorganic electrolyte NaSCN and the osmolyte Tre, as seen in Tables I and SI. Figure 12b shows a correlation between the aggregate formation during storage and the initial value of τ/C p . As was also seen in Fig. 12a, in Fig 12b the rate of aggregates formed during storage increases with turbidity much more rapidly for the low co-solute (30 mM) systems than for the 250 mM systems, indicating the important role of co-solute for improving stability.
Conclusions
For a mAb solution with a viscosity highly sensitive to pH, the addition of various classes of co-solutes caused very different effects on the viscosity and stability. In particular, large viscosity reductions were achieved with the addition of the basic amino acids arginine (Arg), and histidine (His) as well as imidazole (Im) when protonated in the cationic state, and anionic camphorsulfonic acid (CSA). At pH 7 where His is mostly uncharged, it lowered the viscosity significantly less than the other stronger bases that were protonated and cationic. Furthermore, co-solutes without hydrophobic sites that only screened electrostatic interactions, including inorganic electrolytes and Lys-HCl, also had little effect on the viscosity at pH 6. Thus, it is likely that weakening of both local anisotropic electrostatic and hydrophobic attractions was necessary for achieving large viscosity reductions by breaking protein networks. This analysis was supported by the lack of shear-thinning behavior for the Arg-HCl system. At high concentrations where the average protein-to-protein surface separation is small, short-ranged interactions are very important, and thus viscosities were not correlated with k D measured at dilute conditions. However, the viscosity reduction at high concentration was correlated with k D for systems with similar electrostatic screening. After accounting for this contribution of screened electrostatic repulsion, the k D was found to characterize the effect of the remaining attractive interactions which play an important role for the highly concentrated solutions. The effects of the co-solutes on viscosity were similar when the mAb solutions were made by either centrifugation filtration or lyophilization dilution, despite the opposite pathways, indicating the protein likely did not get trapped in different metastable states. In addition to reducing viscosities, co-solutes increased the accelerated storage stability of mAb1 during 4 weeks at 40°C, as measured by SE-HPLC. In particular, protein solutions formed with the basic co-solutes that led to the lowest viscosities also had high storage stabilities, as both properties are improved by weakening PPI.
Abbreviations
- CF:
-
Centrifugal filtration
- CSA:
-
Camphorsulfonic acid
- DLS:
-
Dynamic light scattering
- Gdm:
-
Guanidinium
- Im:
-
Imidazole
- k D :
-
Diffusion interaction parameter
- LD:
-
Lyophilization dilution
- mAb:
-
Monoclonal antibody
- pI:
-
Isoelectric point
- PPI:
-
Protein-protein interactions
- Tre:
-
Trehalose
- η inh :
-
Inherent viscosity
References
Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–57.
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93:1390–402.
Buck PM, Chaudhri A, Kumar S, Singh SK. Highly viscous antibody solutions are a consequence of network formation caused by domain-domain electrostatic complementarities: insights from coarse-grained simulations. Mol Pharm. 2015;12:127–39.
Wang W. Advanced protein formulations. Protein Sci. 2015;24:1031–9.
Burckbuchler V, Mekhloufi G, Paillard Giteau A, Grossiord JL, Huille S, Agnely F. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm. 2010;76:351–6.
Roberts CJ. Protein aggregation and its impact on product quality. Curr Opin Biotechnol. 2014;30:211–7.
Carpenter JF, Randolph TW, Jiskoot W, et al. Overlooking subvisible particles in therapeutic protein products: gaps that May compromise product quality. J Pharm Sci. 2009;98:1201–5.
Connolly BD, Petry C, Yadav S, et al. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78.
Chaudhri A, Zarraga IE, Yadav S, Patapoff TW, Shire SJ, Voth GA. The role of amino acid sequence in the self-association of therapeutic monoclonal antibodies: insights from coarse-grained modeling. J Phys Chem B. 2013;117:1269–79.
Pindrus M, Shire SJ, Kelley RF, et al. Solubility challenges in high concentration monoclonal antibody formulations: relationship with amino acid sequence and intermolecular interactions. Mol Pharm. 2015;12(11):3896–907.
Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94:1928–40.
Kanai S, Liu J, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97:4219–27.
Li L, Kumar S, Buck PM, et al. Concentration dependent viscosity of monoclonal antibody solutions: explaining experimental behavior in terms of molecular properties. Pharm Res. 2014;31:3161–78.
Robertsand CJ, Blanco MA. Role of anisotropic interactions for proteins and patchy nanoparticles. J Phys Chem B. 2014;118:12599–611.
Guo Z, Chen A, Nassar RA, et al. Structure-activity relationship for hydrophobic salts as viscosity-lowering excipients for concentrated solutions of monoclonal antibodies. Pharm Res. 2012;29:3102–9.
Fukuda M, Moriyama C, Yamazaki T, Imaeda Y, Koga A. Quantitative correlation between viscosity of concentrated MAb solutions and particle size parameters obtained from small-angle x-ray scattering. Pharm Res. 2015;32(12):3803–12.
Chaudhri A, Zarraga IE, Kamerzell TJ, et al. Coarse-grained modeling of the self-association of therapeutic monoclonal antibodies. J Phys Chem B. 2012;116:8045–57.
Zarraga IE, Taing R, Zarzar J, et al. High shear rheology and anisotropy in concentrated solutions of monoclonal antibodies. J Pharm Sci. 2013;102:2538–49.
Yearley EJ, Godfrin PD, Perevozchikova T, et al. Observation of small cluster formation in concentrated monoclonal antibody solutions and its implications to solution viscosity. Biophys J. 2014;106:1763–70.
Godfrin PD, Zarzar J, Zarraga IE, Porcar L, Falus P, Wagner NJ, et al. The effect of hierarchical cluster formation on the viscosity of concentrated monoclonal antibody formulations studied by neutron scattering. J Phys Chem B. 2015;120:278–91.
Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B. 2013;117:6373–84.
Quang LJ, Sandler SI, Lenhoff AM. Anisotropic contributions to protein-protein interactions. J Chem Theory Comput. 2014;10:835–45.
Scherer TM. The role of cosolute-protein interactions in the dissociation of monoclonal antibody clusters. J Phys Chem B. 2015;119(41):13027–38.
Binabaji E, Ma J, Zydney AL. Intermolecular interactions and the viscosity of highly concentrated monoclonal antibody solutions. Pharm Res. 2015;32:3102–9.
Allmendinger A, Fischer S, Huwyler J, et al. Rheological characterization and injection forces of concentrated protein formulations: an alternative predictive model for non-newtonian solutions. Eur J Pharm Biopharm. 2014;87:318–28.
Chari R, Jerath K, Badkar AV, Kalonia DS. Long- and short-range electrostatic interactions affect the rheology of highly concentrated antibody solutions. Pharm Res. 2009;26:2607–18.
Wang S, Zhang N, Hu T, et al. Viscosity-lowering effect of amino acids and salts on highly concentrated solutions of two IgG1 monoclonal antibodies. Mol Pharm. 2015;12:4478–87.
Saluja A, Crampton S, Kras E, et al. Anion binding mediated precipitation of a peptibody. Pharm Res. 2009;26:152–60.
Melanderand W, Horvath C. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins - interpretation of lyotropic series. Arch Biochem Biophys. 1977;183:200–15.
Arakawaand T, Timasheff SN. The stabilization of proteins by osmolytes. Biophys J. 1985;47:411–4.
Johnston KP, Maynard JA, Truskett TM, et al. Concentrated dispersions of equilibrium protein nanoclusters that reversibly dissociate into active monomers. ACS Nano. 2012;6(2):1357–69.
Borwankar AU, Dinin AK, Laber JR, et al. Tunable equilibrium nanocluster dispersions at high protein concentrations. Soft Matter. 2013;9:1766–71.
Cheungand JK, Truskett TM. Coarse-grained strategy for modeling protein stability in concentrated solutions. Biophys J. 2005;89:2372–84.
Shen VK, Cheung JK, Errington JR, Truskett TM. Coarse-grained strategy for modeling protein stability in concentrated solutions. II: phase behavior. Biophys J. 2006;90:1949–60.
He F, Woods C, Litowski J, et al. Effect of sugar molecules on the viscosity of high concentration monoclonal antibody solutions. Pharm Res. 2011;28:1552–60.
Inoue N, Takai E, Arakawa T, Shiraki K. Specific decrease in solution viscosity of antibodies by arginine for therapeutic formulations. Mol Pharm. 2014;11:1889–96.
Inoue N, Takai E, Arakawa T, Shiraki K. Arginine and lysine reduce the high viscosity of serum albumin solutions for pharmaceutical injection. J Biosci Bioeng. 2014;117:539–43.
Borwankar AU, Dear BJ, Twu A, Hung JJ, Dinin AK, Wilson BK, et al. Viscosity reduction of a concentrated monoclonal antibody with arginine·HCl and arginine·glutamate. Ind Eng Chem Res. 2016;55:11225–34.
Arakawa T, Ejima D, Tsumoto K, et al. Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. Biophys Chem. 2007;127:1–8.
Shuklaand D, Trout BL. Interaction of arginine with proteins and the mechanism by which It inhibits aggregation. J Phys Chem B. 2010;114:13426–38.
Vagenende V, Han AX, Mueller M, Trout BL. Protein-associated cation clusters in aqueous arginine solutions and their effects on protein stability and size. ACS Chem Biol. 2013;8:416–22.
Loewenthal R, Sancho J, Fersht AR. Histidine aromatic interactions in barnase - elevation of histidine PK(A) and contribution to protein stability. J Mol Biol. 1992;224:759–70.
Du QS, Meng JZ, Liao SM, Huang RB. Energies and physicochemical properties of cation-pi interactions in biological structures. J Mol Graph Model. 2012;34:38–45.
Englanderand SW, Epstein HT. Optical methods for measuring nucleoprotein and nucleic acid concentrations. Arch Biochem Biophys. 1957;68:144–9.
Teeters M, Bezila D, Benner T, Alfonso P, Alred P. Predicting diafiltration solution compositions for final ultrafiltration/diafiltration steps of monoclonal antibodies. Biotechnol Bioeng. 2011;108:1338–46.
Hung JJ, Borwankar AU, Dear BJ, Truskett TM, Johnston KP. High concentration tangential flow ultrafiltration of stable monoclonal antibody solutions with low viscosities. J Membr Sci. 2016;508:113–26.
Houand Y, Cramer SM. Evaluation of selectivity in multimodal anion exchange xystems: a priori prediction of protein retention and examination of mobile phase modifier effects. J Chromatogr A. 2011;1218:7813–20.
Holstein MA, Parimal S, McCallum SA, Cramer SM. Mobile phase modifier effects in multimodal cation exchange chromatography. Biotechnol Bioeng. 2012;109:176–86.
Chen B, Bautista R, Yu K, Zapata GA, Mulkerrin MG, Chamow SM. Influence of histidine on the stability and physical properties of a fully human antibody in aqueous and solid forms. Pharm Res. 2003;20:1952–60.
Heyda J, Mason PE, Jungwirth P. Attractive interactions between side chains of histidine-histidine and histidine-arginine-based cationic dipeptides in water. J Phys Chem B. 2010;114:8744–9.
Vondrasek J, Mason PE, Heyda J, Collins KD, Jungwirth P. The molecular origin of like-charge arginine-arginine pairing in water. J Phys Chem B. 2009;113:9041–5.
Jiangand S, Cao Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–32.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99:4812–29.
Acknowledgments and Disclosures
We acknowledge support from AbbVie, the National Science Foundation (12474795) and the Welch Foundation (KPJ F-1319 and TMT F-1696). We thank Christian Reid for useful comments during the course of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1787 kb)
Rights and permissions
About this article
Cite this article
Dear, B.J., Hung, J.J., Truskett, T.M. et al. Contrasting the Influence of Cationic Amino Acids on the Viscosity and Stability of a Highly Concentrated Monoclonal Antibody. Pharm Res 34, 193–207 (2017). https://doi.org/10.1007/s11095-016-2055-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2055-5