Abstract
Purpose
Non-viral gene delivery vehicles such as polyethylenimine and polyamidoamine dendrimer effectively condense plasmid DNA, facilitate endocytosis, and deliver nucleic acid cargo to the nucleus in vitro. Better understanding of intracellular trafficking mechanisms involved in polymeric gene delivery is a prerequisite to clinical application. This study investigates the role of clathrin and caveolin endocytic pathways in cellular uptake and subsequent vector processing.
Methods
We formed 25-kD polyethylenimine (PEI) and generation 4 (G4) polyamidoamine (PAMAM) polyplexes at N/P 10 and evaluated internalization pathways and gene delivery in HeLa cells. Clathrin- and caveolin-dependent endocytosis inhibitors were used at varying concentrations to elucidate the roles of these important pathways.
Results
PEI and PAMAM polyplexes were internalized by both pathways. However, the amount of polyplex internalized poorly correlated with transgene expression. While the caveolin-dependent pathway generally led to effective gene delivery with both polymers, complete inhibition of the clathrin-dependent pathway was also deleterious to transfection with PEI polyplexes. Inhibition of one endocytic pathway may lead to an overall increase in uptake via unaffected pathways, suggesting the existence of compensatory endocytic mechanisms.
Conclusions
The well-studied clathrin- and caveolin-dependent endocytosis pathways are not necessarily independent, and perturbing one mechanism of trafficking influences the larger trafficking network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Branched 25-kDa polyethylenimine (PEI) has been a benchmark for non-viral gene delivery since its use was first reported in 1995 (1). Dendrimers such as polyamidoamine (PAMAM) have also been explored as gene delivery agents (2,3) due to their potential for modification and flexible chemistry. Both polymers complex effectively with negatively charged nucleic acids (4) and exhibit relatively efficient in vitro transfection (5). In addition, PEI and PAMAM are prototypical “proton-sponge” polymers that are hypothesized to facilitate endosome escape via their strong buffering capacity, which leads to osmotic swelling and rupture of acidified endocytic vesicles (6,7). Thus, PEI and PAMAM serve as excellent models for investigating polymer-mediated gene delivery mechanisms, providing a basis for design of safer and more efficient vectors.
Cellular internalization and subsequent intracellular processing of polyplexes are key barriers to successful in vitro gene delivery. In particular, two endocytic pathways, clathrin- and caveolin-dependent endocytosis, have been extensively studied in the past decade (8,9). Clathrin-dependent endocytosis results in acidified vesicles (pH 5–6) that fuse with lysosomes (pH ~4.5). Caveolin-dependent uptake is less well-characterized but is associated with formation of caveosomes that are less acidified and are believed to avoid trafficking to lysosomes (10,11). The differences in endocytic vesicles and subsequent processing may significantly impact the intracellular destination of associated cargo. Indeed, recent literature has demonstrated the importance of uptake pathway in the efficacy of gene delivery with polyplexes. We and others have shown that, surprisingly, the caveolar uptake pathway is primarily responsible for PEI/DNA polyplex transfection (12–15). Several investigators have also reported the importance of uptake pathway on PAMAM dendrimers. In particular, Qi et al. suggested that, in contrast to PEI-mediated gene delivery, caveolin-dependent uptake does not play a role in PAMAM/DNA polyplex internalization (16).
To better understand the roles of clathrin- and caveolin-dependent pathways in polymer-mediated gene delivery, and to investigate the generality of the previous findings regarding the importance of the caveolar pathway in PEI-mediated gene delivery (12–15), we have directly compared the role of uptake pathway on PAMAM/DNA and PEI/DNA polyplex uptake and transfection. Importantly, we employed endocytosis inhibiting drugs (Table I) over a range of concentrations to discern specific effects on uptake and reduce the impact of cytotoxicity. Confocal fluorescence micrographs also demonstrate colocalization of polymer-DNA polyplexes with clathrin- or caveolin-tagged endocytic vesicles, and provide further evidence that these vectors utilize both clathrin- and caveolin-dependent pathways.
Materials and Methods
Materials
YOYO-1 dye, NHS-ester functionalized Alexa Fluor 488, Alexa Fluor 647 cholera toxin subunit B (CTxB) conjugate, and Alexa Fluor 647 transferrin (Tf) conjugate were obtained from Invitrogen (Carlsbad, CA). Methyl-β-cyclodextrin was obtained from Roquette America, Inc. (Keokuk, IA). Generation 4 PAMAM dendrimer, 25-kDa branched polyethylenimine, genistein, chlorpromazine, and amantadine were obtained from Sigma-Aldrich (St. Louis, MO).
Cells and Plasmids
The HeLa human cervical carcinoma cell line used in this study was a gift from Dr. Sandra McMasters (University of Illinois, Urbana, IL). The cells were cultured according to their ATCC protocols at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Luciferase plasmid pGL3 (Promega Corp., Madison, WI) carried the reporter gene used to assess gene delivery vehicle efficiency. The plasmid encodes the firefly luciferase protein, driven by the SV40 promoter and enhancer domains. Plasmid production and purification were conducted by Elim Biopharmaceuticals (San Diego, CA).
Polyplex Sizing
Polyplexes were prepared using 3.0 μg DNA diluted in 20 mM PIPES, 150 mM NaCl, and polymer was added to bring the total polyplex volume to 250 μL for a given polymer-DNA (N/P) ratio. Following 20 min incubation at room temperature, polyplexes were transferred to a transparent cuvette and diluted with deionized water to a final volume of 1.8 mL. Each sample was subjected to size measurement on a Brookhaven Instruments Corporation 90 Plus Particle Size Analyzer (Holtsville, NY) immediately after dilution, and again after 2, 4, 6, and 24 h at room temperature.
Polymer Buffering Capacity
One milligram of each polymer was dissolved in 1 mL of deionized water and adjusted to pH 12.2 with 1 M NaOH. The polymer solution was titrated with 5 μL aliquots of 2 M HCl, and the pH was read (Accumet AB15, Hudson, MA) after each aliquot to generate a polymer pH buffering curve.
Transfections
HeLa cells were seeded at 70,000 cells/well in 24 well plates 24 h prior to transfection. Polymer/pGL3 polyplexes were prepared at room temperature by mixing 1 mg/mL polymer in 150 mM NaCl, 20 mM PIPES, pH 7.2, with 1 mg/mL pGL3 in endotoxin free water, to achieve the desired N/P ratio. Polyplexes were then incubated at room temperature for 10 min. Prior to transfection, the growth media was replaced with serum-free media and 50 μl of polyplexes was added to each well. In experiments using endocytosis inhibiting drugs, drugs were added to serum free media 30 min before transfection. The transfection medium was removed and replaced with serum-supplemented media 4 h post-transfection. Luciferase expression was quantified 48 h post-transfection using the Promega luciferase assay system (Promega, Madison, WI). Luciferase activity was measured in relative light units (RLU) using a Lumat LB 9507 luminometer (Berthold, GmbH, Germany), and was subsequently normalized by total cell protein using the Pierce BCA protein assay kit (Pierce, Rockford, IL) to yield RLU/mg protein. All results were normalized to gene delivery activity of G4 PAMAM or PEI at N/P 10 in the absence of drugs.
Polyplex Uptake
pGL3 plasmid was tagged with intercalating dye YOYO-1 at 1 dye/100 base pairs. Polyplexes were formed and cells were transfected as described above. Two hours post-transfection, the cells were rinsed twice with 0.001% SDS in PBS and PBS, respectively, to remove surface-bound polyplexes. Next, 100 μl of 0.25% trypsin in PBS was added to each well. The cells and trypsin were allowed to incubate for 5 min, and 50 μl of fetal bovine serum was added to each well. The cells were then collected and stored on ice. FACS analyses were performed on a Coulter EPICS XL-MCL flow cytometer (Beckman-Coulter, Fullerton, CA). Uptake was measured using the following formula
where Fblank is autofluorescence of untreated cells, x = N:P ratio of sample, and y = drug concentration. Normalization was done by comparing the corrected fluorescence to N:P = 10 and in the absence of drugs.
Time-Lapse Live Confocal Fluorescence Microscopy
Primary amines on both polymers were reacted with NHS-ester functionalized Alexa Fluor 488 in 0.1 M sodium bicarbonate buffer (pH 8.3) for 1 h. Labeled polymer was purified from unreacted dye by gel filtration chromatography. HeLa cells were seeded at 50,000 cells/well and grown for 2 days prior to transfection in 60 mm Petri dishes (Corning®, Manassas, VA). Alexa Fluor 647-conjugated CTxB and Tf were added to the plates, followed by Alexa Fluor 488 labeled polyplexes (N:P = 10). Cells were visualized with the Multiphoton Confocal Microscope Zeiss 710 for a period of 1 h at 37°C with a 100× oil immersion lens to investigate colocalization with clathrin and caveolin vesicles. The videos were then analyzed via Imaris© software (Bitplane, Belfast, UK).
Cytotoxicity
Cytotoxicity of endocytosis inhibiting drugs was characterized in HeLa cells using the CellTiter-Blue cell viability assay (Promega, Madison, WI). Cells were seeded in 96-well microplates at 2 × 104 cells/well and grown in medium containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C, 5% CO2. Growth medium was replaced with serum free DMEM 24 h after seeding. Endocytosis inhibiting drugs were added at the following concentrations: genistein, 0–62.5 μg/mL; methyl-β-cyclodextrin, 0–12.5 mg/mL; chlorpromazine, 0–6.25 μg/mL; amantadine, 0–12.5 mM. DMEM along with inhibiting drugs were aspirated after 4 h incubation and replaced with growth medium. The cells were incubated for a further 20 h. Subsequently, 20 μl of the CellTiter-Blue reagent was added to each well and incubated for an additional 4 h. Absorbance was read at 570 nm. The background absorbance of cells killed with ethanol was subtracted from the viable cell absorbance.
Results
PEI/DNA and PAMAM/DNA Polyplex Formation and Physicochemical Characterization
The proton-sponge hypothesis suggests that buffering plays a key role in polyplex escape from the endosome by inducing endosomal rupture (1,7,17). To compare the buffering capacities of PAMAM and PEI, aqueous polymer solutions were titrated with HCl between pH 12–2 (Fig. 1). Buffering capacity of the two polymers is defined here as the reciprocal slope of the titration plots over pH 7.5–4.5 (units of μL of 1 M HCl/pH unit, or simply μL). PEI is a strong buffer, exhibiting a slope of 5.78 μL. PAMAM buffering capacity (4.84 μL) was slightly less than PEI but appears to be sufficient to act as a “proton sponge.”
Particle size may influence the mechanism of endocytosis and subsequent intracellular trafficking (18). Polyplexes were prepared at N/P 10–50, and sizes measured within 15 min. Sizes ranged from 90 to 150 nm for PAMAM, and from 105 to 133 nm for PEI (Table II). Polyplexes measured after 2 h incubation at room temperature showed variable increases in size, most likely due to aggregation (not shown). Subsequent experiments were conducted with vectors across the range of N/P ratios, with only N/P 10 results shown here as the optimal vector for PEI and PAMAM.
Cytotoxicity of Endocytosis Inhibitors
We transfected HeLa cells in the presence of endocytosis inhibitors to understand the interplay of trafficking mechanisms and pathways at different degrees of inhibition. Cytotoxicity of inhibitors was evaluated over a range of concentrations. Although the drugs exhibited cytotoxicity increasing with concentration, cell viability was not significantly affected at the highest drug concentrations (red arrow) used in our experiments (14,19,20) (Fig. 2) (p > 0.05).
Cytotoxicity of caveolin- (genistein, methyl-β-cyclodextrin) and clathrin-dependent pathway (chlorpromazine, amantadine) inhibiting drugs was determined as the normalized metabolic activity of HeLa cell line in the presence of varying amounts of the drugs. Metabolic activity was normalized to cells grown in the absence of drugs. Red arrows indicate the highest concentration at which drugs were administered during transfection and uptake experiments. The standard deviation is shown for each data point (N = 6, mean ± SD).
Gene Delivery Activity and Uptake of PEI and PAMAM Polyplexes
Recent work has demonstrated the importance of polymer-DNA binding (21) and endolysosomal escape (22) in polymeric gene delivery. However, we have also reported that PEI-mediated gene delivery does not correlate with buffering capacity (19,23). We thus hypothesize that uptake pathway and intracellular trafficking are critical parameters for design of gene delivery vehicles. The goal of these transfection experiments is to determine the interplay of clathrin- and caveolin-dependent endocytosis in gene delivery with proton sponge polymers.
Investigation of pathway-specific polyplex internalization and transfection is often accomplished by treatment of cells with well-known biochemical inhibitors of specific endocytosis pathways (Table I). As we have previously validated that siRNA knockdown of clathrin and caveolin expression and chemical inhibition of clathrin- and caveolin-dependent pathways are comparable (14), we did not pursue further siRNA inhibition in this study. Amantadine prevents clathrin-mediated uptake by stabilizing clathrin-coated vesicles and preventing clathrin-lattice recycling to the cell surface, thus blocking the budding of clathrin-coated vesicles (24). Chlorpromazine similarly prevents coated pit formation on the plasma membrane by promoting clathrin lattice assembly on endosomal membranes (25,26) and has a probable inhibitory effect on the formation of large intracellular vesicles (27). Methyl-β-cyclodextrin (MβCD) sequesters membrane cholesterol and disrupts the lipid-rich domains on the cell surface that form caveolae. Acute plasma membrane cholesterol depletion at high MβCD concentrations also affects clathrin-coated pit formation (28–30). Genistein prevents tyrosine phosphorylation of caveolin (31) and microtubule polymerization (32), thereby inhibiting caveosome formation and subsequent trafficking of the caveolin-dependent pathway. Thus, lower uptake or transgene expression in the presence of amantadine and chlorpromazine indicate that clathrin-dependent endocytosis and subsequent trafficking play an important role, while decreases in the presence of MβCD and genistein point to the importance of the caveolar pathway.
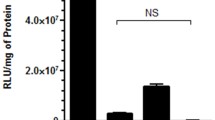
In vitro transfection efficiency and uptake of PEI/DNA and PAMAM/DNA polyplexes was quantified in HeLa cells at N/P 10, which was found to be optimal for transfection of both polyplexes, in the presence of each endocytic inhibitor (Fig. 3). Genistein resulted in greater than five-fold reduction (p < 0.001) in transfection by both PEI/DNA and PAMAM/DNA at the highest drug concentration (Fig. 3a, e). In contrast, cellular internalization of PEI/DNA increased more than two-fold, while that of PAMAM/DNA increased by 30% before decreasing over the same range of genistein concentrations. MβCD exhibited similar yet less dramatic behavior (Fig. 3b, f). PEI/DNA and PAMAM/DNA transfection decreased greater than two-fold (p < 0.01) at the highest MβCD concentration. As with genistein, PEI/DNA uptake in the presence of MβCD doubled at the highest concentration, while PAMAM/DNA uptake increased before decreasing over the same concentration range. The observed increase followed by decrease in our PAMAM/DNA uptake data with both caveolar inhibitors suggests that compensatory uptake via clathrin-mediated endocytosis and other alternative routes may occur when the caveolar pathway is progressively inhibited.
Normalized in vitro transfection efficiency (RLU/μg Protein)) and median uptake fluorescence (YOYO-1 Fluorescence) of PAMAM (a–d) and PEI (e–h) polyplexes in HeLa cells in the presence of increasing concentrations of genistein (a, e), MβCD (b, f), amantadine (c, g) or chlorpromazine (d, h). All results were normalized to polyplexes at N/P 10, under inhibitor-free conditions. (N = 6; error bars represent standard deviation; *, +p < 0.05; **, ++p < 0.01; ***, +++p < 0.001 compared to the same polyplex in the absence of drug).
In the presence of chlorpromazine and amantadine, PEI/DNA gene delivery decreased by greater than ten-fold (p < 0.001) at the highest drug concentrations while uptake was minimally affected by either inhibitor (Fig. 3g, h). In contrast, internalization of PAMAM/DNA decreased to less than 30% of the control at maximum chlorpromazine and amantadine concentration (p < 0.001) (Fig. 3c, d). Additionally, both inhibitors had interesting effects on PAMAM/DNA transfection. PAMAM/DNA-mediated transgene expression increased 2.5-fold at intermediate chlorpromazine concentrations (p < 0.01) and 3.5-fold at intermediate amantadine concentrations (p < 0.001) but, at the highest inhibitor concentrations tested, returned to levels similar to those in the absence of inhibitors (Fig. 3c, d).
Confocal Microscopy
To further understanding of the uptake and trafficking pathways, we performed confocal microscopy for visual corroboration via colocalization of fluorescently tagged polyplexes and pathway-specific ligands. Tf and CTxB are well-documented as cargo of clathrin-coated and caveolar vesicles, respectively (33). PEI colocalized with both Tf and CTxB, while PAMAM colocalized more strongly with Tf than with CTxB (Fig. 4). Images were taken within 1 h of transfection and are representative of early endocytic behavior.
Confocal fluorescence micrographs of HeLa cells transfected with PEI or PAMAM (Alexa-Fluor 488, green), and endocytic vesicle marker transferrin or cholera toxin (Alexa-Fluor 647, red) at 30 min post transfection. Polyplexes were formed at N/P ratio 10. (a) PAMAM-Tf, (b) PEI-Tf, (c) PAMAM-CTxB, (d) PEI-CTxB. Left panel, combined cross-sectional view; right panel, corresponding brightfield views.
Discussion
Formation of well-condensed, cationic polyplexes is important for effective polymeric gene delivery. Particle size may influence polyplex transport to the cell surface and subsequent internalization (34,35). In our study, both PEI and PAMAM exhibited comparable sizes and buffering capacity (Fig. 1). Cell viability in the presence of endocytic inhibitors also was not a confounding factor based lack of significant cytotoxicity (Fig. 2) and previously published research (14,19,20).
As summarized in Table I, the role of the various endocytic inhibitors is well documented in literature. Non-specific effects of these inhibitors have been reported (27). However, we have previously demonstrated that the effects of the inhibitors, at the concentrations used here, on polyplex uptake and transfection are the same as when clathrin or caveolin expression was knocked down using siRNA (14). The relationship between degree of inhibition and gene delivery has not been made clear, largely because most inhibition experiments are conducted at only one drug concentration. Our results broadly show that interfering with caveolin-dependent trafficking significantly decreases gene delivery activity of both PEI and PAMAM polyplexes (Fig. 3a, b, e, f). In contrast, the role of clathrin-dependent trafficking in gene delivery is less easily generalized. Both polyplex uptake and gene delivery may increase or decrease in response to endocytic inhibition, and not necessarily concomitantly (Fig. 3e, f, g, h).
Depletion of plasma membrane cholesterol with MβCD resulted in moderate increases in PEI and PAMAM polyplex uptake, but poor gene delivery at high MβCD concentrations (Fig. 3b, f). This supports previous findings demonstrating the importance of lipid raft and caveolar trafficking in polymeric gene delivery (14,36). Increases in uptake despite decreased gene delivery may be explained by upregulation of endocytic mechanisms that do not involve cholesterol and do not lead to effective transfection. This compensatory uptake is a phenomenon repeatedly seen in our data. In addition to the well-characterized clathrin- and caveolin-dependent pathways that are dynamin-dependent, less well-characterized, dynamin-independent mechanisms have also been identified that involve small GTPase ARF6 and flotillin (37). Several studies have shown that endocytosis via caveolin-dependent and dynamin-independent mechanisms is often complicated by the possibility that the same cargo may be internalized by multiple routes or switch routes in a given cell line depending on environmental conditions (38–41). In contrast to clathrin-dependent endocytosis, defined by cargo association with clathrin-coated pits, adaptor molecules for caveolin-dependent and dynamin-independent mechanisms have not been identified. Our results point to this condition-dependent interchangeability among clathrin- and caveolin-dependent, dynamin independent mechanisms as a likely explanation for “compensatory uptake”.
As with MβCD, caveolar inhibition by genistein resulted in similar uptake and gene delivery patterns for both polymers (Fig. 3a, e). The caveolar pathway is hence crucial for gene delivery via PAMAM/DNA and PEI/DNA polyplexes. Further, any compensatory uptake that occurs during caveolar inhibition appears to result in trafficking along an inefficient pathway that leads to poor gene delivery. In contrast to the dissociation between uptake and gene delivery seen in our caveolar inhibition experiments, Gabrielson et al. reported decreased uptake and decreased gene delivery with genistein, albeit using PEI/DNA polyplexes at N/P 3.7 (14). In our hands PEI gene delivery activity peaked between N/P ratios 5–10, and we thus focused on this range of polyplex formulations in our analysis of polyplex trafficking. In concordance with studies from other groups showing that internalization of PAMAM polyplexes is mainly caveolin-independent in HeLa cells (16), our uptake data demonstrated minimal change with genistein or MβCD. Minimal colocalization of PAMAM with CTxB, a caveosome marker, was also evident in our confocal fluorescence micrographs (Fig. 4).
Inhibition of clathrin-dependent uptake with amantadine and chlorpromazine yielded more disparate results. Uptake decreased for PAMAM but not PEI polyplexes. In contrast, gene delivery dramatically decreased for PEI but not PAMAM polyplexes (Fig. 3c, d, g, h). Thus, inhibition of either caveolin- or clathrin-dependent pathways was deleterious for PEI gene delivery. Decreased PEI gene delivery upon inhibition of clathrin-dependent uptake has also been reported in several other cell lines (42). Uptake was minimally affected upon either clathrin or caveolar pathway inhibition but is a poor indicator of successful PEI transfection (Fig. 3e–h), possibly because multiple routes of PEI internalization are possible in HeLa cells (13).
Upon inhibition of clathrin-dependent uptake, PAMAM polyplex gene delivery increased to varying degrees despite decreased uptake (Fig. 3c, d). We further observed that PAMAM gene delivery increased before decreasing at high levels of inhibition of clathrin-dependent uptake (Fig. 3c, d). An explanation for this dissociation of uptake and transfection results is that partial inhibition of clathrin-dependent internalization resulted in PAMAM polyplex uptake along a non-clathrin-dependent pathway that led to high gene delivery activity. However, increased gene delivery via this alternate route was only maintained while clathrin-dependent uptake mechanisms were only moderately inhibited, suggesting clathrin-dependent trafficking is required at some level for successful PAMAM gene delivery.
We further observed a high degree of colocalization between PAMAM-containing polyplexes and Tf during the first 60 min of transfection. The Tf receptor is located in clathrin-coated pits and is a common marker for clathrin-coated vesicles. In contrast, minimal PAMAM colocalization was observed with CTxB. Our confocal microscopy colocalization studies with PEI polyplexes and both CTxB and Tf (Fig. 4b, d) indicated that while some association between the polyplex and each endocytic marker was noted, colocalization with neither marker was dramatic, consistent with PEI polyplex uptake data showing that internalization was not significantly decreased when either mechanism of endocytosis was inhibited. Interestingly, Rejman et al. reported 50–70% decreases in PEI polyplex uptake in the same cell line at a chlorpromazine concentration twice the maximum concentration used here (6.25 μg/mL) (13).
Because of the different effects of clathrin inhibition on the PEI and PAMAM gene delivery processes, we suggest that the importance of clathrin-dependent trafficking in gene delivery is polymer-specific, while that of the caveolar pathway is shared in common between PEI and PAMAM. In other words, successful gene delivery with both PAMAM and PEI is caveosome-dependent despite caveosomes playing a minimal role in initial internalization of the polyplexes (Figs. 3 and 4). Other groups have reported similar findings of caveosome dependence for dendrimer-based gene delivery but did not compare initial mechanism of uptake to gene delivery activity, a process that may take as long as 4 h (43).
While caveolar trafficking is important to both PEI and PAMAM, the precise role of the clathrin-dependent pathway is still unclear (36,44). These pathways may not be entirely independent of each other. In the case of PEI polyplexes, activity of only clathrin- or caveolin-dependent pathways is insufficient for successful gene delivery. It is known that caveolae do not undergo the same degree of endosomal acidification and targeting to endolysosomes as clathrin-coated vesicles (11). However, lysosomal trafficking does not necessarily preclude efficient gene delivery. It is also known that both clathrin-dependent and caveolar pathways can lead to effective gene delivery activity for lipoplexes and polyplexes (13,18,36). The dependence of PEI on both pathways might indeed be evidence of trans-pathway sorting. Such a phenomenon would also explain the poor correlation between polyplex uptake and gene delivery in this study and previous publications (36). Recent studies have pointed to Golgi complex trafficking as a contributor to this clathrin-caveolin communication network (11,45), showing that, like clathrin-coated vesicles, caveolae can localize to intracellular structures such as endosomes, endoplasmic reticulum or the Golgi complex (10,15). Previous drug inhibition studies utilizing only one drug concentration were unable to demonstrate such interplay between clathrin-coated vesicles and caveolae.
Conclusion
The interaction between clathrin- and caveolin-dependent endocytosis is poorly understood. Polyplexes may be internalized via several mechanisms simultaneously. We suggest that intracellular trafficking, rather than initial endocytosis at the cell surface, regulates gene delivery efficiency. Early association of polyplexes with clathrin-coated vesicles or caveolae is of limited importance in predicting gene delivery effectiveness because these pathways may not be mutually exclusive. Further, inhibition of one pathway may lead to compensatory increases in another, resulting in unpredictable changes in gene delivery. 25-kD PEI and G4 PAMAM share similar physical properties but exhibit different mechanisms of uptake and gene delivery. Successful PEI-mediated gene delivery is dependent on both clathrin- and caveolin-dependent pathways. In contrast, uptake of PAMAM polyplexes is heavily clathrin dependent, while gene delivery is caveolin dependent. Thus, rather than relying on a single pathway for successful gene delivery, PEI and PAMAM polyplexes depend on the interplay between clathrin-dependent and caveolin-dependent pathways in HeLa cells.
Abbreviations
- PEI:
-
Polyethylenimine
- PAMAM:
-
Polyamidoamine
- CTxB:
-
Cholera toxin subunit B
- Tf:
-
Transferrin
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- RLU:
-
Relative light units
- MβCD:
-
Methyl-β-cyclodextrin
References
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci. 1995;92:7297–301.
Eichman JD, Bielinska AU, Kukowska-Latallo JF, Baker Jr JR. The use of PAMAM dendrimers in the efficient transfer of genetic material into cells. Pharm Sci Technol Today. 2000;3:232–45.
Braun CS, Vetro JA, Tomalia DA, Koe GS, Koe JG, Middaugh CR. Structure/Function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J Pharm Sci. 2005;94:423–36.
Svenson S. Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm. 2009;71:445–62.
Son S, Kim WJ. Biodegradable nanoparticles modified by branched polyethylenimine for plasmid DNA delivery. Biomaterials. 2010;31:133–43.
Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–31.
Behr J-P. The Proton Sponge: a trick to enter cells the viruses did not exploit. Chimia (Aarau). 1997;2:34–6.
Doherty GJ, McMahon HT. Mechanisms of endocytosis. Biochemistry. 2009;78:857–902.
Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–95.
Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20.
Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94.
Van der Aa MAEM, Huth US, Häfele SY, Schubert R, Oosting RS, Mastrobattista E, et al. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm Res. 2007;24:1590–8.
Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–74.
Gabrielson NP, Pack DW. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J Control Release. 2009;136:54–61. Elsevier B.V.
Reilly M, Larsen J, Sullivan M. Polyplexes traffic through caveolae to the Golgi and endoplasmic reticulum en route to the nucleus. Mol Pharm. 2012;9:1280–90.
Qi R, Mullen DG, Baker JR, Holl MMB, Dendrimers DI, Polymers OD. The mechanism of polyplex internalization into cells: testing the GM1/caveolin-1 lipid raft mediated endocytosis pathway. Mol Pharm. 2010;1517–26
Pack D, Putnam D, Langer R. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol Bioeng. 2000;67:217–23.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69.
Gabrielson NP, Pack DW. Acetylation of Polyethyleminimine enhances gene delivery via weakened polymer/DNA Interactions. Biomacromolecules. 2006;7:2427–35.
Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC, et al. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther Nat Publ Group. 2009;18:561–9.
Hartmann L, Hafele S, Peschka-Suss R, Antonietti M, Borner HG. Tailor-made poly(amidoamine)s for controlled complexation and condensation of DNA. Chemistry (Easton). 2008;14:2025–33.
Forrest ML, Pack DW. On the kinetics of polyplex endocytic trafficking: implications for gene delivery vector design. Mol Ther. 2002;6:57–66.
Forrest ML, Meister GE, Koerber JT, Pack DW. Partial Acetylation of Polyethylenimine enhances in vitro gene delivery. Pharm Res. 2004;21:365–71.
Phonphok Y, Rosenthal KS. Stabilization of clathrin coated vesicles by amantadine, tromantadine and other hydrophobic amines. FEBS. 1991;281:188–90.
Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–72.
Sofer A, Futerman A. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the golgi apparatus and the subsequent elevation of cyclic AMP. J Biol Chem. 1995;270:12117–22.
Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15–33.
Shogomori H, Futerman AH. Cholesterol depletion by methyl-beta-cyclodextrin blocks cholera toxin transport from endosomes to the Golgi apparatus in hippocampal neurons. J Neurochem. 2001;78:991–9.
Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE, et al. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci. 1999;96:6775–80.
Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–74.
Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253:629–36.
Mukherjee S, Acharya BR, Bhattacharyya B, Chakrabarti G. Genistein arrests cell cycle progression of A549 cells at the G2/M Phase and depolymerizes interphase microtubules through binding to a unique site of tubulin. Biochemistry. 2010;49:1702–12.
Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44.
Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS Pharm Sci. 2001;3:E21.
Wagner E. Polymers for siRNA delivery: inspired by viruses to be targeted, dynamic and precise. Acc Chem Res. 2012;45:1005–13.
McLendon PM, Fichter KM, Reineke TM. Poly(glycoamidoamine) vehicles Promote pDNA uptake through multiple routes and efficient gene expression via caveolae-mediated endocytosis. Mol Pharm. 2010;7:738–50.
Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12.
Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1 – dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14:3254–65.
Damm E-M, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–88.
Massol RH, Larsen JE, Fujinaga Y, Lencer WI, Kirchhausen T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol Biol Cell. 2004;15:3631–41.
Torgersen ML, Skretting G, Van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–42.
Gonçalves C, Mennesson E, Fuchs R, Gorvel J-P, Midoux P, Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol Ther. 2004;10:373–85.
Manunta M, Tan PH, Sagoo P, Kashefi K, George AJT. Gene delivery by dendrimers operates via a cholesterol dependent pathway. Nucleic Acids Res. 2004;32:2730–9.
Gersdorff V, K S, Nn V, R DS, Sc W, E, et al. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Gene Ther. 2006;14:745–53
Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745:273–86.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Institutes of Health grants GM085222 and DK083875 (to M.E.H.). We thank Sandy McMasters at the Cell Media Facility and Barbara Pilas at the Flow Cytometry facility at the University of Illinois. The authors have no competing financial interests relating to the work reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, M.E., Keswani, R.K. & Pack, D.W. Dependence of PEI and PAMAM Gene Delivery on Clathrin- and Caveolin-Dependent Trafficking Pathways. Pharm Res 32, 2051–2059 (2015). https://doi.org/10.1007/s11095-014-1598-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1598-6