The effect of thioctic acid (TA), 2-ethyl-6-methyl-3-hydroxypyridine hydrochloride (emoxypine), and their ester derivative (2-ethyl-6-methylpyridinol-3-yl-thiooctanoate, thioxypine) on the behavior of rats in an elevated plus maze (EPM) was studied. Intraperitoneal administration (three times) of TA, emoxypine, and their equimolar mixture in single doses of 36.25, 72.5, and 145 μmol/kg enhanced individual manifestations of anxious behavior in the EPM but did not cause full-blown anxiogenic effects. Similar pro-anxiogenic effects were observed when TA was administered in a dose of 36.25 μmol/kg and emoxypine in doses of 72.5 and 145 μmol/kg. The equimolar mixture of the non-esterified components of thioxypine had a pro-anxiogenic effect in doses of 36.25 and 72.5 μmol/kg. Combination of TA and 2-ethyl-6-methyl-3-hydroxypyridine into the ester potentiated their pro-anxiogenic activity, which manifested as an extensive anxiogenic effect of thioxypine when administered three times in a dose of 145 μmol/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Thioctic (α-lipoic) acid possesses potent redox-modulating activity that is closely related to its antihypoxic and psychotropic potential [1,2,3,4]. 2-Ethyl-6-methyl-3-hydroxypyridine hydrochloride (emoxypine) displays an analogous spectrum that causes a nootropic effect and substantially affects the experimental and clinical affective status, and includes antioxidant and antihypoxic activity [2, 4,5,6]. Thioctic acid (TA) and emoxypine administered to mice at doses equivalent to the human therapeutic doses cause comparable thymoanaleptic effects and exhibit equivalent antihypoxic activity with experimental asphyxia [2, 4]. Combination of TA and the cationic part of emoxypine (2-ethyl-6-methyl-3-hydroxypyridine) into the ester thioxypine (2-ethyl-6-methylpyridinol-3-yl-thiooctanoate) potentiates the antihypoxic activity of its free components with complete loss of their antidepressant activity [1, 7]. Administration of thioxypine at a subantihypoxic dose causes an effect like sedation due to the acyl (thiooctanoyl) component of the ester [7]. This raises the question of potential anxiolytic activity of thioxypine. Also, the above effect may also indicate not sedation but a freezing response due to the probable anxiogenic activity of thioxypine. The present article focused on a check of this hypothesis according to criteria for the effects of thioxypine and its free components on the behavior of rats in an elevated plus maze (EPM), the use of which is a basic approach to screening potential tranquilizers [8].
Experimental Chemical Part
Thioxypine (2-ethyl-6-methylpyridinol-3-yl-thiooctanoate) was synthesized at the Central Research Laboratory of South Ural State Medical University, Ministry of Health of Russia, according to the previously published [1] and RF patent-protected technology (No. 2,797,949; Appl. No. 2023106552; Jun. 13, 2023). The starting reagents were (R,S)-thioctic acid (NuSci, USA; CAS No. 1077-28-7) and 2-ethyl-6-methyl-3-hydroxypyridine, the base that was produced from powdered methylethylpyridinol hydrochloride drug substance (ZAO Obninsk Chemical and Pharmaceutical Co.) by treating it with NaOH by the previously described method [1].
Experimental Biological Part
The test plan complied with ethical standards given in domestic and international regulatory documents [8, 9]. The study used 130 Wistar rats (180 – 250 g). Each experimental group of animals included an equal number of males and females. Thioxypine, its free components (TA and emoxypine), and their equimolar mixture (reference substance) were administered as emulsions prepared in a medium consisting of aqueous NaCl solution (9 g/L) and Tween-80 (0.84 g/L). The pH of the administered emulsions in all instances was in the range 7.2 – 7.6.
The tested compounds were administered three times intraperitoneally in a volume of 25 mL/kg 24 and 4 h and 30 min before evaluating their anxiotropic activity. Thioxypine and the reference substance were administered at the same doses (36.25, 72.5, and 145 μmol/kg), which were previously used to study the thymoanaleptic potential of the corresponding drugs [7]. Control animals received equal volumes (25 mL/kg) of the injection medium [Tween-80 (0.84 g/L) in aqueous NaCl solution (9 g/L)] that was used for emulsification of the tested compounds.
The effects of the tested compounds on anxiety reactions of the rats were evaluated from their behavior in an EPM. The work used a standard EPM for rats (NPK Otkrytaya Nauka, Russia) with four arms (50 × 14 cm) and a central area (14 × 14 cm). Two opposite arms of the EPM had side walls of height 30 cm and were considered closed arms. The other two arms did not have barriers, were considered open arms, and were situated at a right angle relative to the closed arms. The EPM was placed on a stand so that the device was raised over the floor by 50 cm. Rats were placed into the central area at the start of the test so that their noses were facing one of the open arms. The number of entries of a test animal into open and closed arms and the number of entries into the central area were recorded for 5 min. Simultaneously, the total residence time of the rats in these EPM sections was recorded. The resulting parameters were evaluated as both absolute values and percents of the total number of entries into the EPM sections and the times spent in them (of the total test time). Entry into EPM sections were measured from the time all four paws were located on the floor of any arms or the central area. The manifestation of anxiety was judged from the number of entries into the EPM closed arms and the residence time in them according to generally accepted recommendations [8]. The number of entries into open arms and the EPM central area and the total time spent in them were considered criteria of anxiolytic activity of the tested compounds. Substantial shifts in visits to EPM sections and residence times in them were judged only for unidirectional changes of the absolute and relative values of the corresponding parameters. The overall locomotor activity of the animals, which was judged from the total number of entries into the various EPM sections, was recorded separately. The anxiety index (AI) was the integral characteristic of the animal behavior in the EPM and was calculated using the formula [10]:
The nonconventional (ethological) parameters of risk assessment behavior (RAB) [11] were calculated in addition to the above commonly accepted (conventional) behavior parameters. The RAB was judged from the number of head-dippings from the open arms (when the animal dropped its head below the EPM floor) and from the number of end-arm explorations (within 10 cm from the edge). Vegetative manifestations of anxiety were assessed from the number of fecal boluses. Locomotor activity (the number of crossed squares on the EPM arms) and orientational reactions (number of vertical rearings) of the rats were recorded besides the conventional and nonconventional parameters.
Statistical analysis used the SPSS-17.0 applied computer program suite. The results were processed by descriptive statistics and given as mean (Me) and the range between the lower (LQ, 25 percentile) and upper (UQ, 75 percentile) quartiles. The significance of intergroup differences was judged using a two-tailed version of the Mann(Whitney U-criterion. Statistical hypotheses were checked using significance level P = 0.05. The statistical significance of found effects was evaluated according to the intersection-union principle excluding corrections for multiple comparisons [12]. The existence of an effect of each tested compound on the manifestation of anxiety in the EPM was judged from the same statistically significant changes of at least three parameters, including the AI and a minimum of one conventional and nonconventional parameter. Pro-anxiolytic or pro-anxiogenic effects were considered if these criteria were not completely met.
Results and Discussion
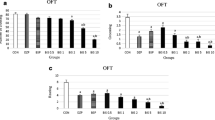
The research established that the ester derivative of 2-ethyl-6-methyl-3-hydroxypyridine and TA, i.e., thioxypine, affected the behavior of rats in the EPM much more than each of its free components (Tables 1 and 2). This appeared most clearly after three-fold administration of the tested compounds at the maximum one-time dose (145 μmol/kg). This administration regime of thioxypine more than halved the absolute number of entries of the animals into EPM closed arms and completely prevented visits of its open arms. This led to a decrease in the total number of transitions between EPM sections by 2.6 times as compared to the control animals. The results indicated that the locomotor activity of the rats was suppressed by thioxypine. This conclusion was confirmed by the practically halving of the number of crossed squares after administration of a course of thioxypine at the maximum dose (Table 2).
The decreased locomotor activity of rats that received thioxypine at the maximum dose was nonuniform in nature and was mainly associated with fewer visits of the EPM open arms. This parameter, in contrast to visitation of the closed arms, was reduced not only in the absolute but also the relative values (Table 1). This result was interpreted in terms of concepts about an anxiogenic effect that manifested in avoidance of the EPM open arms [8]. Avoidance of the open arms under the influence of the maximum dose of thioxypine was also confirmed by the pronounced drop in the absolute and relative parameters of time spent by the rats in these EPM sections (Table 1). Integral analysis of conventional manifestations of anxiety using AI data also confirmed an anxiogenic effect for thioxypine. Thioxypine used at the maximum dose was observed to increase the AI by 24% as compared with the control (Table 1). It is noteworthy that thioxypine intensified the nonconventional signatures of anxiety in addition to the conventional manifestations. This produced statistically significant decreases of RAB parameters such as the number of head-dippings from EPM open arms and the number of end-arm explorations (Table 2).
The effect of thioxypine on the AI and both nonconventional anxiety manifestations disappeared if the one-time dose was reduced by two and three times (Table 1). This result showed that thioxypine lacked significant anxiogenic activity with three administrations at doses of 36.25 and 72.5 μmol/kg. It is noteworthy that thioxypine, even in this range of relatively low doses, caused separate proanxiogenic effects, the integral sum of which nevertheless did not lead to a conclusion about a substantial increase in anxiety. Only the absolute parameters of the number of entries into closed and open arms and the associated number of transitions between EPM sections decreased if thioxypine was used at the minimum dose (36.25 μmol/kg) (Tables 1 and 2). A reduction of locomotor activity of the animals according to the criterion of the number of crossed squares was simultaneously observed (Table 2). Three administrations of thioxypine at the medium dose (72.5 μmol/kg) led to a decrease only in the absolute number of entries into the EPM closed arms (Table 1) and a decrease in the number of vertical rearings (Table 2).
The isolated components of the thioxypine ester were significantly inferior to it with respect to the influence on behavior of the rats in the EPM. This concerned primarily the acyl (thiooctanoyl) component, which exhibited a distinct psychotropic effect only after administration at the minimum dose. Three-fold administration of TA at a dose of 36.25 μmol/kg reduced the absolute number of entries into closed arms, decreased the number of transitions between EPM sections (Tables 1 and 2), and practically completely prevented anxiogenic defecation (Table 2). Nevertheless, the obvious anxiolytic tendency of these effects did not lead to a conclusion about genuine tranquilizing activity. This was related to the lack of its significant influence on the relative parameters of visitation of EPM closed arms, the studied RAB parameters, and the AI value. The hydrochloride salt of the thioxypine alcohol component (2-ethyl-6-methyl-3-hydroxypyridine hydrochloride, emoxypine), in contrast to TA, exerted its activity at relatively high doses. Administration of emoxypine at the maximum dose increased the absolute and relative parameters of time spent in closed arms but did not affect other parameters of behavior in the EPM. Use of emoxypine at the medium dose had a significant effect only on the absolute number of entries into open arms and the number of end-arm explorations. In both instances, these parameters decreased significantly. Administration of emoxypine at the minimum dose had no effect on the behavior of the animals in the EPM. The results did not suggest that emoxypine had a significant influence on the manifestation of anxiety because it did not change the AI parameter at any studied dose.
The change in the behavior of rats in the EPM under the influence of an equimolar mixture of the non-esterified components of thioxypine deserves separate attention. Administration of this mixture three times at the minimum one-time dose (36.25 μmol/kg for each component) produced effects like those of thioxypine. An equimolar mixture of emoxypine and TA used at the minimum dose practically completely prevented visitation of the EPM open arms (Table 1). Simultaneously, the absolute and relative values of the time spent in the EPM closed arms were observed to increase. The proanxiogenic tendency of these effects was confirmed by a substantial increase in the AI. Also, the minimum dose of the mixture of the non-esterified components of thioxypine did not affect any of the studied RAB parameters and completely suppressed anxiogenic defecation (Table 2). This did not lead to a conclusion about a genuine anxiogenic effect of the equimolar mixture of emoxypine and TA. It is noteworthy that the strength of the influence of the mixture of thioxypine free components on the behavior in the EPM progressively decreased as the dose was increased. Only the absolute parameters of entry of rats into closed and open arms with an associated decrease in the number of transitions between EPM sections decreased if the equimolar combination of emoxypine and TA was used at a one-time dose of 72.5 μmol/kg (Tables 1 and 2). Use of this mixture at the maximum one-time dose (145 μmol/kg) led only to suppression of anxiogenic defecation (Table 2).
A similar dose-dependent effect was observed in previous research demonstrating that TA, its equimolar mixture with emoxypine, and their ester derivative (thioxypine) suppressed exploratory activity of mice in an open field only after administration at the minimum dose [7]. TA-containing drugs lost their influence on exploratory activity of animals if the dose was increased. This similarity in the dose-dependences of TA-containing drugs on the behavior of rats in the EPM (Table 1) and the exploratory activity of mice in an open field [7] suggested that these effects had a single basis. This basis could be the proanxiogenic activity of TA at the minimum dose (Table 1), which provoked neophobia and suppressed exploratory activity of mice in an open field.
A similar mechanism could underly the suppressive influence of thioxypine on locomotor and orienting activity of rats in the EPM (Table 2). Apparently, combination of 2-ethyl-6-methyl-3-hydroxypyridine and TA into the ester thioxypine potentiated their proanxiogenic effects, the strength of which increased to the level of genuine anxiogenic activity. The observed reduction in the number of locomotor movements and vertical rearings should be considered the result of a freezing response [13] and not sedation as previously proposed [7]. This effect of thioxypine could also be related to potentiation of the proanxiogenic activity of its non-esterified components due to forming the ester derivative. This possibility was illustrated by the lack of any influence of emoxypine and TA on the number of crossed squares and the number of vertical rearings in the EPM (Table 2).
It is entirely possible that the reduction in the locomotor activity and orientational reaction of rats under the influence of thioxypine could be directly related to its antihypoxic activity. The fact that the resistance of stressed rats to hypoxia is known to increase as their spontaneous locomotor activity decreases is indicative of this [14].
Conflict of Interest
We declare no explicit and potential conflict of interest related to publication of the present article.
Contributions of authors
All authors participated equally in preparation for publication and development of the concept of the article, production and analysis of experimental data, writing and editing of the article text, and checking and confirming the article text.
Financing
We declare no external financing for the research and publication of the article.
Acknowledgments
We thank Prof. I. I. Dolgushin, President of South Ural State University and Russian Academy of Science member, for interest in the research and support at all stages.
Compliance with ethics principles
The research protocol was approved by the local ethics committee of SUSMU (protocol No. 15 of Dec. 16, 2022).
References
I. A. Volchegorskii, S. I. Grobovoi, A. I. Sinitskii, et al., Khim.-farm. Zh., 57(3), 14 – 19 (2023).
I. A. Volchegorskii, I. Yu. Miroshnichenko, L. M. Rassokhina, and R. M. Faizullin, Eksp. Klin. Farmakol., 76(7), 6 – 10 (2013).
G. P. Biewenga, G. R. M. M. Haenen, and A. Bast, Gen. Pharmacol.: Vasc. Syst., 29(3), 315 – 331 (1997).
I. A. Volchegorskii, I. Yu. Miroshnichenko, L. M. Rassokhina, and M. P. Malkin, Ross. Fiziol. Zh. im. I. M. Sechenova, 105(3), 363 – 374 (2019).
I. A. Volchegorskii and N. V. Mester, Klin. Med., 85(2), 40 – 45 (2007).
I. A. Volchegorskii, L. M. Rassokhina, I. Y. Miroshnichenko, et al., Bull. Exp. Biol. Med., 150(3), 327 – 332 (2011).
I. A. Volchegorskii, I. Yu. Miroshnichenko, A. I. Sinitskii, et al., Khim.-farm. Zh., 57(10), 9 – 15 (2023).
A. N. Mironov (ed.), handbook for Preclinical Drug Studies [in Russian], Grif i K, Moscow (2012).
S. Louhimies, Altern. Lab. Anim., 30, 2 Suppl. (2002).
H. Cohen, T. Liu, N. Kozlovsky, et al., Neuropsychopharmacology, 37(2), 350 – 363 (2012).
A. M. B. Garcia, R. Martinez, M. L. Brandao, and S. Morato, Physiol. Behav., 85(4), 440 – 447 (2005).
Points to consider on multiplicity issues in clinical trials, Committee for Proprietary Medicinal Products, CPMP, London (2002).
M. L. Brandao, J. M. Zanoveli, R. C. Ruiz-Martinez, et al., Behav. Brain Res., 188(1), 1 – 13 (2008).
I. A. Volchegorskii, V. E. Tseilikman, S. A. Ship, et al., Probl. Endokrinol., 48(6), 41 – 44 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 58, No. 4, pp. 11 – 16, April, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Volchegorskii, I.A., Grobovoi, S.I., Sinitskii, A.I. et al. Effect of 2-ethyl-6-methylpyridinol-3-yl-thiooctanoate and its Non-Esterified Components on the Behavior of Rats in an Elevated Plus Maze. Pharm Chem J 58, 554–559 (2024). https://doi.org/10.1007/s11094-024-03178-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03178-y