In recent years, there has been growing exploration of organometallic transition metal complexes as promising options for developing anticancer agents that offer the potential of reduced toxicity compared with the commonly utilized cisplatin analogs. In this respect, iridium complexes containing 2-phenylimidazo- [4,5-f][1,10]-phenanthroline derivatives with different substituents were investigated in different cell lines as potential anticancer agents. All the compounds exhibited potent cytotoxic activity against Caco-2, HeLa, A549, and MDA-MB-231 cell lines. EC50 values of compound 1 were found to be 6.44 μM for Caco-2, 24.78 μM for HeLa, 9.14 μM for A549, and 4.45 μM for the MDA-MB-231 cell line. Similarly, EC50 of 3 was found at 15.07, 14.15, 10.33, and 4.48 μM respectively. The EC50 value of 2 was found to be 12.71 μM for Caco-2, 24.31 μM for HeLa, and 7.44 μM for MDA-MB-231 cells. EC50 values of compound 4 were found to be 28.20 μM for Caco-2, 12.79 μM for HeLa, 8.33 μM for A549, and 3.76 μM for the MDA-MB-231 cell line. The results of gene expression and flow-cytometry analysis showed that the compounds caused the induction of apoptosis in all cancer cell lines by changing caspase 3, Bcl-2, and Bax proteins. The obtained results demonstrate that compounds could be introduced as potent agents to prevent the progression of certain types of cancer. However, preclinical and clinical trials will be needed to evaluate these complexes to obtain safe, effective, and optimal therapeutic drugs for cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cisplatin is a platinum coordination complex classified as an antineoplastic agent used for several forms of cancer such as ovarian and bladder cancer [1, 2]. Although it has many drawbacks such as strong resistance and weak selectivity, it is one of the most widely used drugs and is considered the most active chemotherapeutic agent [3]. Many researchers have been studying new metal complexes to solve these limitations [4,5,6]. Therefore, organometallic transition metal complexes have attracted considerable attention owing to their wide and diverse structures with potent anticancer activity toward various tumor cell types [7,8,9,10,11,12]. Recently, iridium complexes have gained great interest because of their fewer side effects, selectivity, and high antiproliferative activity in the field of anticancer research [13, 14]. In addition, iridium complexes can allow the improvement of apoptosis and cytotoxic activity with the modification of ancillary ligands [15]. 2-Phenylimidazo[4,5-f][1, 10]-phenanthroline derivatives as ancillary ligands are promising for iridium complexes owing to their stability, hydrophobicity, and solubility [16]. The extended conjugation and hydrophobicity of the 2-phenylimidazo[4,5-f][1, 10]-phenanthroline ligand can increase DNA interaction with the iridium compounds [17]. Iridium complexes show multiple modes of action including mitochondria-mediated apoptosis, protein–protein interaction inhibition, and redox balance destruction [18, 19]. Enhancing our comprehension of how these complexes behave within cancerous cells would continue to be advantageous for their design and enhancement. In this paper, the apoptosis and cytotoxic activity of iridium compounds bearing arylimidazophenanthroline ligands with different substituents (methyl, isopropyl, methoxy, phenyl) were evaluated against different human cancer cells (Caco-2, HeLa, A549, MDA-MB-231) and noncancerous cell lines (HEK293). In addition, gene expression analysis has been studied. The obtained results suggest that the iridium compounds might possess anticancer agent potential.
EXPERIMENTAL
Synthesis
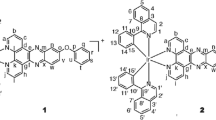
The corresponding compounds [Ir(2-(3-fluorophenyl)-4-methylpyridine)2(2-(4-methylphenyl)-1H-imidazo-[4,5-f][1, 10]-phenanthroline)][Cl] (1), [Ir(2-(3-fluorophenyl)-4-me-thylpyridine)2(2-(4-isopropylphenyl)-1H-imidazo-[4,5-f][1, 10]-phenanthroline)][Cl] (2), [Ir(2-(3-fluorophenyl)-4-me-thylpyridine)2(2-(4-methoxyphenyl)-1H-imidazo-[4,5-f][1, 10]-phenanthroline)][Cl] (3), and [Ir(2-(3-fluorophenyl)-4-methylpyridine)2(2-([1,1′-biphenyl]-4-yl)-1H-imidazo-[4,5-f][1, 10]-phenanthroline)][Cl] (4) were prepared according to the procedure described in the literature [20].
Cell culture
Human colon (Caco-2), cervical (HeLa), lung (A549), and breast (MDA-MB-23) cancer cell lines and a normal human embryonic (HEK293) cell line obtained from the European Collection of Authenticated Cell Cultures. Cells were cultured in RPMI 1640 or DMEM (Sigma-Aldrich, Germany), as previously described [21].
MTT assay
Two times 103 cells/well were seeded in 96-well plates (Costar, Corning, USA) and maintained at 37°C in a humidified incubator filled with 5% CO2 for 24 h. Then, cells were treated with different concentrations of iridium compounds, to a final concentration ranging 1.95 to 250 μM, for 24 h. All compounds were dissolved in DMSO (not exceeding 1%; Carlo Erba, Germany). The viability was evaluated through a MTT assay (Merck, Germany), as described previously [22]. A microplate reader (Epoch, BioTek, USA) was used to measure the absorbance at 590 nm. The obtained data were used to estimate the half maximal effective concentration (EC50) values and calculations were evaluated using GraphPad Prism® software v.9 (San Diego, CA USA). All experiments were performed in triplicate.
Flow cytometry analysis
The apoptosis was evaluated using the Annexin V-FITC/PI Apoptosis Kit Detection (K101, BioVision), as described previously [23]. Cells were plated in six-well plates with a density of 3 × 104 cells/well. Then, cells were treated with the EC50 values of iridium compounds for 24 h. Hydrogen peroxide (0.2 mM, Sigma-Aldrich) was used a positive control. After incubation, cells were collected following centrifugation, washed with phosphate buffered saline twice and resuspended in cold Annexin binding buffer. Cell aliquots (100 μL) were stained with 5 μL propidium iodide (PI) and 5 μL Annexin V-FITC. After, cell suspensions were incubated for 20 min in the dark. Finally, 400 μL of Annexin V binding buffer was added to each tube and analyzed using a CytoFlex flow cytometer (Beckman Coulter) with in-built CytExpert Software.
Quantitative real-time PCR
Cells (about 3 × 104 cells) were treated with the compounds at EC50 concentration for 24 h and total RNA was extracted using the innuPREP RNA Mini Kit 2.0 (Analytik, Jena, Germany). A total of 2.5 μg of RNA was reverse transcribed to cDNA using a OneScript® Plus cDNA Synthesis Kit (ABM, USA) according to the manufacturer’s instructions. Bcl-2, BAX, and CASP3 gene levels were quantified using SYBR green quantitative PCR assay following the 2-∆∆Ct method, as described previously [24]. β-Actin was used as an internal control. The sequence of the primers used in this study was presented in Table 1.
All data are presented as mean ± SD. The statistical differences between two or multiple groups were analyzed using Student′s t test or one-way analysis of variance using GraphPad Prism 9.0 software. p < 0.05 were statistically significant.
RESULTS AND DISCUSSION
Study of cytotoxic effects
The cytotoxicity of iridium compounds were evaluated against five human cell line (Caco-2, HeLa, A549, MDA-MB-231, and HEK293) using the MTT assay. In the current study, different concentrations of the compounds (1.95–250 μM) were assessed for cytotoxicity investigations for 24 h. After exposure, the cytotoxicity of the compounds to these cell lines (expressed as EC50 values) are presented in Table 2.
Our results suggested that iridium complexes induced the inhibition of cell viability in all tested cancer cell lines, although each cell showed different sensitivity, in accordance with determined EC50 values, except for compound 2 on A549 and HEK293 cells. The strongest cytotoxic activity of compounds was observed against the MDA-MB-231 cell line (4.45, 7.44, 4.48, and 3.76 μM respectively) followed by the A549 cell line (9.14, 10.33, and 8.33 μM for 1, 3 and 4 respectively). All compounds showed good cytotoxic activity against cancer cell lines, compared with weak cytotoxic activity against the HEK293 cell line. In addition, paclitaxel (20 μM) was used as a positive control in this experiment. Paclitaxel-treated Caco-2, HeLa, MDA-MB-231, and A549 cells had 21.23%, 48.16%, 50.20%, and 41.13% viability respectively.
Assessment of apoptosis via Annexin V/PI staining
To determine the type of cell death, Annexin-V/FITC and PI dual staining were utilized. As shown in Figs. 1, 2, 3, and 4, all of the tested compounds increased apoptosis in Caco-2 and A549 cells. In contrast, apoptotic cell rates decreased in HeLa treated with compound 3 and MDA-MB-231 treated with compound 4. However, other compounds were shown to induce apoptosis in both cell lines.
Gene expression analysis
Apoptosis was evaluated using the qPCR method to find out changes in the expression levels of Bax, Bcl-2, and Caspase 3 genes. Our results showed that significant increases in mRNA levels of the Bax and Caspase 3 (proapoptotic genes) were observed in cells exposed to compounds 1, 2, and 3 for the MDA-MB-231 cell line (Fig. 5). On the contrary, Bcl-2 (anti-apoptotic) mRNA levels decreased 1.09-, 1.34-, and 1.05-fold respectively. Twenty-four hours of treatment with compounds 1, 3, and 4 increased Bax and Caspase 3 mRNA levels in the A549 cell line. Similarly, Bax and Caspase 3 mRNA levels were increased as a result of compounds 1, 2, and 4 treatments in the HeLa cell line. Compound 2 has decreased 10.02-fold the Bcl-2 mRNA level in the HeLa cell line. Moreover, Caspase 3 and Bax mRNA levels were increased in the Caco-2 cell line owing to all the compounds′ treatment with respect to control cells respectively (p < 0.05). Treatment of 1, 2, 3, and 4 caused decreases at the level of Bcl-2 mRNA (1.72-, 1.85-, 1.42-, and 1.84-fold in Caco-2 cells respectively). All these results showed that our iridium complexes caused cell death by inducing apoptosis for Caco-2, HeLa, MDA-MB-231, and A549 cancer cell lines.
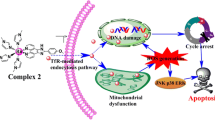
Several studies focused on developing potent anticancer and chemopreventive approaches have examined the use of iridium complexes and have shown promising results in inhibiting tumor growth, inducing apoptosis in cancer cells, and preventing metastasis. Four of the tested compounds, namely 1, 2, 3, and 4, showed cytotoxic activity on different cancer cells (Caco-2, HeLa, A549, and MDA-MB-231). In particular, all compounds showed a higher inhibitory effect on cancer cells than on HEK293 cell growth. The cytotoxicity of paclitaxel was also used as a reference. Moreover, 1, 2, 3, and 4 are more cytotoxic than paclitaxel in the tested cancer cells, which confers a biological relevance to these iridium complexes. The cytotoxic effects of iridium complexes were reported in several distinct cancer types such as human epidermoid carcinoma (A431), colorectal carcinoma (HT29) [25], liver (HepG2), gastric (BEL-7402), inhibition of cell proliferation, and inducing the apoptosis of SGC-7901 cells [26], inhibited colony-forming ability and reduced the migration of cervical carcinoma cells (HeLa) [27]. In one study, it was demonstrated that four synthesized iridium complexes showed antitumor activity against human lung carcinoma cells A549 using the MTT assay [28]. The synthesized iridium complexes contain 2-phenylimidazo[4,5-f][1, 10]-phenanthroline with different substituents (methyl, isopropyl, methoxy, phenyl) in the ancillary ligand. These ligands possess effective antitumor activity on cancer cells owing to the DNA binding ability and hydrophobicity of the 2-phenylimidazo[4,5-f][1, 10]-phenanthroline ligand [29, 30]. The important influence of side groups (methyl, isopropyl, methoxy, phenyl) in the compounds on cytotoxicity was observed. Compound 1 with the methyl group shows better anticancer activity than other compounds for Caco-2 cells at a lower concentration. For HeLa cells, compounds 3 and 4 have higher cytotoxicity in the presence of the methoxy and phenyl groups. By comparison, the phenyl group in compound 4 reveals lower EC50 values for A549 and MDA-MB-231 cells. These results may indicate that there is a relationship between chemical structure and cancer cells. Apoptosis (programmed cell death) is regulated by various genes that participate in two main pathways: extrinsic and intrinsic. Apoptosis encompasses numerous mechanisms, including the presence of antiapoptotic proteins such as the Bcl-2 family, as well as proapoptotic proteins such as Bax and caspases [31]. It was previously reported that the iridium(III) complex (Ir-1) increased caspase 3 and Bax expression and arrested the cell cycle at the G0/G1 phase in HepG2 cells [32]. In one study, it was shown that iridium complex treatment inhibited proliferation of human ovarian cancer cells (A2780) and caused the induction of apoptosis [33]. In another study, it was shown that iridium complexes induced apoptosis in lung cancer cells (A549) [34]. In a further study, it was reported that iridium(III) polypyridyl complexes induced apoptosis via decreases in mitochondrial membrane potential and inhibited the expression of PI3K and AKT in colorectal HCT116 cells [35]. These results are consistent with those of our study, and showed that iridium complexes are an inhibitory of proliferation in various cancer cell lines and induce apoptosis.
In conclusion, our findings indicate that 2-phenylimidazo[4,5-f][1, 10]-phenanthroline derivatives with different substituents containing iridium complexes have the potential as a therapeutic drug to inhibit cancer. Their unique chemical properties and tunability could offer opportunities for targeted anticancer treatments and improved cancer diagnostics. Continued exploration and development of these complexes may pave the way for novel and effective strategies to fight against cancer.
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to thank the Department of Biology, Faculty of Science, Pamukkale University, for providing us with the laboratory facility.
Authors′ Contributions
The authors declare that they have contributed equally to the article.
References
C. W. Helm and J. C. States, J. Ovarian Res., 2, 2 (2009).
B. A. Teply and J. J. Kim, J. Solid Tumors, 4(2), 25 – 35 (2014).
A. Brown, S. Kumar, and P. B. Tchounwou, J. Cancer Sci. Ther., 11(4), 97 (2019).
R. G. Deghadi, G. G. Mohamed, and N. F. Mahmoud, Appl. Organomet. Chem., 36(6), e6675 (2022).
C. M. S. Almeida, P. H. S. Marcon, É. C. M. Nascimento, et al., Appl. Organomet. Chem., 36(8), e6761 (2022).
R. Irfandi, S. Santi, S., I. Raya, et al., J. Mol. Struct., 1252, 132101 (2022).
I. Ritacco, N. Russo, and E. Sicilia, Inorg. Chem., 54(22), 10801 – 10810 (2015).
F. Shaheen, F. M. Sirajuddin, A. Saqib, et al., J. Organomet. Chem., 856, 13 – 22 (2018).
B. Askari, H. A. Rudbari, N. Micale, et al., J. Organomet. Chem., 900, 120918, (2019).
Z. Zhang, J. Zhang J, T. Yang, et al., J. Med. Chem., 66(8), 5669 – 5684 (2023).
D. Kong, M. Tian, L. Guo, et al., J. Biol. Inorg. Chem., 23, 819 – 832 (2018).
E. Sajadiyeh, M. Tabatabaee, S. M. Seifati, et al., Pharm. Chem. J., 54, 145 – 147 (2020).
T. Yang, M. Zhu, M. Jiang, et al., Front. Pharmacol., 13, 1025544 (2022).
D-L. Ma, C. Wu, K-J. Wu, et al., Molecules, 24(15), 2739 (2019).
P. Garg, U. De, N. Dehury, et al., J. Chem. Sci., 130, 76 (2018).
A. Mondal, S. Shanavas, U. Sen, et al., RSC Adv., 12, 11953 (2022).
W. J. Wang, Y. Y. Ling, Y. M. Zhong, et al., Angew. Chem. Int. Ed. Engl., 61(16), e202115247 (2022).
J. Chen, H. Liu, Y. Chen, et al., J. Inorg. Biochem., 241, 112145 (2023).
T. M. Golding, R. O. Omondi, S. Biswas, et al., Chembiochem., 16, e202300271 (2023).
C. Sahin, M. Sahin, J. Mol. Struct., 1226, 129415 (2021).
A. Kurt-Kýzýldoðan, N. Akarsu, C. Otur, et al., Anti-Cancer Agents in Med. Chem., 22(2), 362 – 370 (2022).
C. Yýlmaz, S. Arslan, D. Mutlu, et al., Anti-Cancer Agents in Med. Chem., 21(10), 1292 – 1300 (2021).
D. Mutlu, C. Cakir, M. Ozturk, and S. Arslan, Arch. Biol. Sci., 74(4), 315 – 324 (2022).
E. Kavak, D. Mutlu, O. Ozok, et al., Nat. Prod. Res., 36, 14, 3511 – 3519 (2022).
B. F. Hohlfeld, B. Gitter, C. J. Kingsbury, et al., Chemistry, 27(21), 6440 – 6459 (2021).
H. Zhang, L. Tian, R. Xiao R, et al., Bioorg. Chem., 115, 105290 (2021).
J. Hao, H. Liu, J. Wang, et al., J. Inorg. Biochem., 235, 111946 (2022).
H. Hao, X. Liu, X. Ge, et al., J. Inorg. Biochem., 192, 52 – 61. (2019).
S. Hu, W. Ma, J. Wang, et al., Eur. J. Pharmacol., 928, 175120. (2022).
N. Zhen, Q. Yang, Q. Wu, et al., Cancer Chemother. Pharmacol., 77, 169 – 180 (2016).
S. Elmore, Toxicol. Pathol., 35(4), 495 – 516 (2007).
Z. H. Liang, D. Wan, Q. Y. Yi, et al., Transit. Met. Chem., 43, 243 – 257 (2018).
J. M. Hearn, I. Romero-Canelón, B. Qamar, et al., ACS Chem. Biol., 8(6), 1335 – 1343 (2013).
J. J. Cao, C. P. Tan, M. H. Chen, et al., Chem Sci., 8(1), 631 – 640 (2017).
B. Xie, Y. Wang, D. Wang, et al., Molecules, 27(17), 5434 (2022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mutlu, D., İpek, C., Şahin, Ç. et al. Cytotoxic and Apoptosis-Inducing Activities of Iridium Complexes Bearing 2-Phenylimidazo[4,5-f][1,10]-Phenanthroline Derivatives in Human Cancer Cells. Pharm Chem J 58, 238–244 (2024). https://doi.org/10.1007/s11094-024-03139-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03139-5