A stability indicating method was developed and validated for the quantitative simultaneous estimation of hyoscine butylbromide (HBB) and paracetamol (PAR) in a combined dosage form by means of isocratic reverse-phase high-performance liquid chromatography (RP-HPLC). Separation was performed on a YMC C18 (250 × 4.6 mm, 5 μm) column using phosphate buffer–Methanol (60:40, v/v) in the isocratic elution regime at a flow rate of 1 mL/min. Quantification was achieved with UV detection at 210 nm. The retention time obtained for HBB was 9.509 min and that for PAR was 4.198 min in a continuous run up to 20 min. The detector response was found to be linear in a concentration range of 2.5 – 7.5 μg/mL for HBB and 125 – 375 μg/mL for PAR. The proposed method was validated as per ICH guidelines and shown to be specific, precise, linear, accurate, and robust. The samples were subjected to various accelerated stress conditions. The degradation products were well resolved at significantly different retention times. Thus, the proposed method can be applied successfully for the simultaneous estimation of PAR and HBB in combined pharmaceutical formulations and for their stability studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
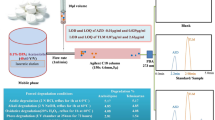
Quality and safety of commercially available formulations are ascertained by the analytical tests. These analyses become more important when the drug contains one or more ingredients in it because of its potential abuse. The present study describes validation of an easily applicable method developed for the oral combination tablet (finispa) containing 500 mg of paracetamol (PAR) and 10 mg of hyoscine butylbromide (HBB) as ingredients (Fig. 1). Hyoscine butylbromide and paracetamol are co-formulated in this oral dosage for symptomatic relief of the signs and symptoms of abdominal cramps. Hyoscine butylbromide (HBB) is chemically (1R, 2R, 4S, 5S, 7s, 9R)-9-butyl-7-(((2S)-3-hydroxy-2-phenylpropanoyl)oxy)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0]-nonane bromide [1]. HBB is a quaternary ammonium derivative which blocks the action of acetylcholine at parasympathetic sites (both muscarinic and nicotinic receptors) in smooth muscle of the gastro-intestinal, biliary, and urinary tracts and female genital organs [2, 3]. Paracetamol (PAR) is chemically N-(4-hydroxyphenyl) acetamide (Fig. 1a) [4]. The analgesic properties of PAR provide a logical addition to this action by increasing the pain threshold and increasing the blood flow across the skin, heat loss, and sweating. The combined antispasmodic and analgesic action makes PAR suitable for the treatment of paroxysmal pain in hollow organs of the abdomen [5, 6].
In the strategy of analytical method development, the stability and stress testing (forced degradation studies) are considered as key components because they are indicators of the mechanism of drug decomposition. In addition, these studies support obtaining information on the physical and chemical factors leading to instability. This in turn helps stabilizing the drug composition with increased shelf-life and improved efficacy. Thus, stress testing is defined as the stability testing of drug products under specified conditions, exceeding those used for accelerated testing and elucidating the inherent stability of drug products. The ICH Q1A(R2) guidelines describe various stress conditions including hydrolysis, oxidation, photolysis, and thermolysis to validate the stability- indicating analytical methods used for probing samples in development of these methods.
In view of the above facts, extensive literature survey has been performed for the drug combination studied in the present work. This analysis revealed few methods available for the estimation of HBB and PAR by UV [7], spectrophotometry [8,9,10,11,12], electrochemical methods [13,14,15,16], capillary electrophoresis techniques [17, 18], titrimetric methods [19,20,21] and high-performance liquid chromatography (HPLC) [22,23,24] methods. In particular, Erk [11] analyzed HBB and PAR binary mixture after precipitating HBB with acetone and ammonium reineckate and reading the absorbance of the precipitate solution in acetone for HBB and the remaining solution for PAR at specific wavelengths. Similarly, Parissi-Poulou and Panderi [7] used solid phase extraction of HBB, PAR, and lidocaine in combined injections upon separation in cation cartridges with C-18 column using acetonitrile – ammonium acetate 0.2 M (30:70, v/v) as a mobile phase, followed by reversed-phase HPLC assay. According to literature [25,26,27,28,29,30,31,32,33] it was also planned to perform stress studies for the drug combination in tablets.
Some stability indicating methods were used for the stress studies of PAR and HBB [34,35,36]. Although the binary mixture analysis was reported for this combination, still it suffered of disadvantages such as the use of complicated and lengthy procedure, expensive techniques, and in total is not suitable for the routine analysis of HBB and PAR. Moreover, these methods revealed two unknown peaks and showed poor resolution. Thus, in view of the quality control analysis, it is a topical task to develop a simple HPLC method for the routine analysis of PAR and HBB in combined tablets. Upon considering the aforementioned drawbacks, the present work was aimed at developing a simple, sensitive, selective and reproducible RP-HPLC method for simultaneous analysis of HBB and PAR in routine quality control of combined tablets.
The formation of unknown additional peaks, asymmetric shape of the main peaks, and unsatisfactory resolution of the two main peaks of PAR and HBB were the major shortcomings of reported methods. To overcome these shortcomings, all parameters in the present study were modified so as to obtain symmetric peaks and better resolution. In order to ensure complete separation of the unknown peaks from significant main peaks, the retention times of both analytes were maintained by selecting the flow rate and adjusting pH of the mobile phase. The developed method was validated as per ICH guidelines for all parameters.
2. Experimental Part
2.1. Reagents and Chemicals
Methanol and acetonitrile (Finar, HPLC grade), potassium dihydrogen phosphate and hydrochloric acid (Rankem, AR grade), trifluoroacetic acid, orthophosphoric acid, potassium hydroxide, sodium hydroxide and hydrogen peroxide (Fisher Scientific, AR grade). De-ionized water (Milli Q Plus) purification system and Membrane filters 0.45μm (Millipore Ltd.) were used throughout the study. All the glassware used was made of Class A borosil.
2.2. Instrumentation and Chromatographic Conditions
Analytical Balance (AUW220D) Shimadzu, Ultrasonicator (LMUC -12) Spectrum Tek, Digital pH meter (PH12-5P-920) Spectrum Tek, High Precision Water Bath (SS6HWB) Elchem, Hot Air Oven (7990415) Inlab equipment pvt ltd, UV Cabinet, Bio techno lab, Vaccum Pump(TID-75-S) Technics incorporation, High Performance Liquid Chromatography (HPLC), (LC-2010AHT) Shimadzu, comprising a quaternary gradient pump, solvent mixing module and Rheodyne injector with 20μL fixed loop, equipped with UV detector and LC Solution software for data acquisition and data storage.
2.3. Reference Standards and Formulation
Paracetamol BP/IP (purity: 100.14%) was purchased from Sri Krishna Pharmaceuticals, Chennai; hyoscine butylbromide EP/BP (purity: 99.36%) was purchased from Vital Laboratories Pvt. Ltd., Gujarat; film-coated tablets formulated in-house had a label claim of paracetamol (500 mg) and hyoscine butylbromide (10 mg) with an average weight of 675 mg.
2.4. Preparation of Solutions
Preparation of buffer . Potassium dihydrogen phosphate (0.01 M) buffer of pH 5.0 was prepared by dissolving 6.8 g of potassium dihydrogen phosphate by diluting with water to produce 1000 ml and adjusted to pH 5.0 by potassium hydroxide.
Preparation of mobile phase. Mobile phase was prepared by mixing 0.01 M potassium dihydrogen phosphate buffer (pH 5.0) and methanol in 60:40 (v/v) proportion, shaken vigorously and sonicated for 30 min prior to use.
Preparation of standard solution. Aqueous solutions (250 and 5 μg/mL of PAR and HBB, respectively) were prepared by taking accurately weighed 50 mg of Hyoscine Butylbromide WRS dissolved in 50 mL of mobile phase and diluted to 100 mL. Then, 1 mL of this solution was diluted to 100 mL, 25 mg of PAR was added and diluted with the mobile phase.
Preparation of sample solutions. Sample solution was prepared from 20 pulverized tablets and average tablet weight was calculated. To accurately weighed (about 675 mg) powder dissolved in 100 mL was added about 50 mL of the mobile phase and the mixture was sonicated for 30 min, cooled and diluted to mark with the mobile phase. The prepared solution was passed through 0.45 μm membrane filter and the first few milliliters were discarded. Then, 5 mL of this solution was diluted to 100 mL with the mobile phase (250 μg/mL PAR and 5 μg/mL HBB sample).
Optimized chromatographic conditions:
Mobile phase: phosphate buffer–methanol (60:40, v/v)
Column: YMC C18 (25 cm × 4.6 mm, 5 μm)
Detector wavelength: 210 nm
Flow rate: 1 mL/min
Injection volume: 20 μL
Column temperature: ambient
Run time: 20 min
2.5. Method Validation
System suitability . System suitability test was carried out on a freshly prepared standard solution of PA R and HBB to analyze various optimized parameters such as resolution, tailing factor and theoretical plates.
Linearity and range. The linearity was checked by least squares regression method. Calibration curves were generated using appropriate volumes of standard solution in a range of 125 – 375 μg/ml for PAR and 2.5 – 7.5 μg/mL for HBB. Then, chromatograms were recorded and the peak areas were computed and plotted versus concentration.
Accuracy. Accuracy was gauged by calculating percentage recovery. Recovery studies were carried out by standard addition method, using the reference standards of PAR and HBB added at three concentration levels (50, 100, and 150%) of assay concentration, each prepared sample solution was injected triplicate into the HPLC system, and the mean percentage recovery was calculated.
Precision . Precision of the method was checked in terms of the system precision, method precision, and intermediate precision. For the system precision under prescribed conditions, the standard solution of PAR and HBB (250 μg/mL and 5 μg/mL] was prepared and injected in six replicates. For the method precision, six replicates of sample solution were prepared and the content of PAR and HBB per tablet (500 and 10 mg, respectively) was determined and percentage recovery with %RSD for the area response in obtained chromatograms was calculated. For the intermediate precision characterizing the analyst, instrument and day variability of the method, the assay was performed in six replicates by different analysts using a different HPLC system on a different day.
Specificity (forced degradation study). The specificity of a method is the extent to which it can be used for the analysis of particular analytes in a mixture without interference from other components. Intentional degradation was carried out under various stress conditions using acid (0.5 M HCl heated at 60°C for 2 h), base (0.1 M NaOH heated at 60°C for 2 h), an oxidizing agent (3% H2O2 heated on a water bath at 60°C for 2 h), UV light (exposure in UV cabinet for 24 h), and thermal degradation (heating in an oven at 60°C for 24 h). These samples were analyzed by HPLC under optimized chromatographic conditions and percentage degradation was calculated.
Robustness . Robustness was established by deliberately introducing small changes in the developed method procedure such as changes in the mobile phase ratio, changes in buffer pH (±0.2), changes in the flow rate (±0.1 mL/min), and changes in the detector wavelength (±2 nm), with calculation of the corresponding %RSD values.
Solution stability. Solution stability was verified by evaluating the percentage deviation in area response from the initial value. Prepared sample solutions were stored under preset conditions and the stability of these solutions was monitored on an hour basis, depending on the nature of samples. In this study, the sample solutions were injected after 5, 10, 17, 25, 37, 49, and 73 h.
Filter study. Filter study was carried out to determine the compatibility of sample solutions with various filters (Nylon 0.45 μm, PVDF 0.45 μm, PTFE 0.45 μm) using centrifuged sample as control. The percentage deviation in area response from the centrifuged sample was calculated. The deviation in area of the filtered samples from the centrifuged sample was NMT 2.0%.
3. Results and Discussion
3.1. Method Development
The purpose of stability testing is to provide evidence on how the quality of a drug substance varies with time under the influence of a variety of environmental factors, such as temperature, humidity, and light, and to establish a retest period for the drug substance and recommended storage conditions. The safety as well as purity of a drug or API is determined by the stability studies hence the presence of trace amount of unwanted chemicals may influence the quality of pharmaceutical products. In recent years it is also a vital parameter for marketing approval. In view of this, a simple, selective and accurate isocratic RP-HPLC method for simultaneous estimation of HBB and PAR in tablets was designed and stability studies have been carried out as a part of the drug development process.
3.2. Chromatographic Separation
The developed method was validated according to the ICH guidelines. According to the literature reports, a new HPLC method was developed in which two unknown peaks were observed. To overcome this disadvantage, various parameters were modified so as to obtain an acceptable resolution between the two components. The chromatographic separation was started with C18 column as stationary phase and phosphate buffer–ACN (70:30, v/v) as a mobile phase, with the flow rate maintained at 1 mL/min, for which the system suitability failed and one unknown peak was observed. Further replacement of ACN with methanol in the mobile phase allowed us to obtain a better symmetric peak shape, while a change in the mobile phase ratio reduced the retention times. The detection was tried at different wavelengths (210, 220, 254 nm) in order to achieve considerable sensitivity of the method at 210 nm. Various pH values were tested, and pH 5.0 gave the best resolution between the components.
The optimized chromatographic separation was achieved with isocratic elution on YMC C18 (250 × 4.6 mm, 5 μm) column using phosphate buffer–methanol (60:40, v/v) mobile phase at a flow rate of 1 mL/min. Quantification was achieved with UV detection at 210 nm. The retention time obtained for HBB was at 9.509 min and that for PAR was at 4.198 min (Fig. 2) in continuous run up to 20 min.
The system suitability parameters were assessed and % RSD was found to be 0.03% for PAR and 0.18% for HBB at a resolution factor of 15.449, which was found to be within acceptable limits. In order to ensure that the method is suitable for intended use, the method validation procedure was performed according to the ICH guidelines. Linear calibration plots depicting the linearity and range for the proposed method were obtained in concentration ranges of 125 – 375 μg/mL (125, 200, 250, 300 and 375 μg/mL) for PAR and 2.5 – 7.5 μg/mL (2.5, 4, 5, 6, 7.5 μg/mL) for HBB (Tables 1a and 1b). The linear regression equation for PAR was found to be Y = 54315X + 13666.3 with correlation coefficient 0.9966 and that for HBB was Y = 19087X – 478.04 with correlation coefficient 0.9998.
Accuracy of the method was found from recovery studies ranging between 50 and 150%. At 50% level, the mean percentage recoveries of PAR and HBB were found to be 99.94 and 99.74% respectively. At 100% level, it was found to be 99.82 and 99.59%, and at 150% level it was 100.34 and 99.44% respectively. Thus, the results of recovery studies were between 98 and 102%, which indicated that the method was accurate (Table 2).
For system precision, the RSD was 0.03% for PAR and 0.18% for HBB, and the resolution factor was found to be 15.449. For method precision, the RSD was found to be 0.27 and 0.82%, respectively. For the intermediate precision, %RSD between the two sets was found to be 0.46 and 0.74%, respectively, which indicated that the method was precise (Table 3).
The method robustness was studied by deliberately changing the mobile phase composition, flow rate, buffer pH, and detection wavelength. The %RSD and resolution factor for each parameter were found within the acceptable limits and there was no significant changes in the retention time and peak area (Table 4).
Stress (sample degradation) testing was carried out to demonstrate specificity of the developed method (Table 5). The chromatogram of samples degraded under acid, base, hydrogen peroxide, thermal and photolytic conditions showed well separated main peaks, as well as some additional peaks at different retention times. The order of degradation degree for these factors was as follows: oxidative > acidic > alkaline > thermal > photolytic > control.
Two common degradation products were obtained in all hydrolyzed samples. The maximum degradation was observed in peroxide (oxidative condition) (Table 6). Stress studies revealed that there was no interference of any other peaks at the retention times of PAR and HBB. Hence, the proposed method is specific and stability indicating.
Filter study was carried out to determine the compatibility of sample solutions with various filters using centrifuged sample as control. For PVDF 0.45 μm filter, percentage deviation from the control sample for Para and HBB was 0.06 and 0.29%, respectively; for Nylon 0.45 μm filter it was 0.29 and 0.16%, respectively; for PTFE 0.45 μm filter it was 0.10 and 0.39%, respectively. The deviation in the peak area for filtered samples from the centrifuged sample was within the limit of NMT 2.0% (Tables 7a and 7b). Hence all se filters are suitable for filtering the standard and sample solutions.
Solution stability testing was performed by storing samples under preset condition for various periods of time (5, 10, 17, 25, 37, 49, and 73 h) depending on the nature of a sample. From the results, the all sample solutions were found stable upon 73 h storage (Tables 8a and 8b).
In concluding, the developed method is reliable, as the data obtained for all validation parameters show satisfactory results. Hence, the method can be employed for routine quality control of PAR and HBB in pharmaceutical industry and drug testing laboratories. Since the stress studies established the presence of some impurities, some future studies are needed for their detection, isolation and characterization using various chromatographic and spectroscopic techniques. Complete analytical profiling may help to identify and quantify detected impurities.
References
https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+7642
G. N. Tytgat, Drugs, 69(9), 1343 – 1357 (2007).
C. V. Sharma and V. Mehta, Crit. Care Pain, 14(4), 153 – 158 (2013).
M. Parissi-Poulou and I. Panderi, J. Liq. Chromatogr. Relat. Technol., 22(7) 1055 – 1068 (1999).
Nouruddin, M. Gamal, and M. Abdelkawy, Ann. Chem. Forsch., 2(1), 9 – 19 (2009).
M. S. Yonis, K. H. Al-Saidi, and Al-Nahrain, J. Sci., 19(1), 56 – 68, (2016).
N. W. Ali, G. Mohammed, and M. Abdelkawy, Brit. J. Pharm. Res., 3(3), 472 (2013).
N. Erk, Scientia Pharm., 64(2), 173 – 183 (1996).
Y. El-Saharty, F. Metwaly, M. Refaat, and S. El-Khateeb, Talanta, 72(2), 675–681 (2007).
K. Farhadi and A. Karimpour, J. Chin. Chem. Soc. (Taipei), 54(1), 165 (2007).
M. R. Ganjali, Z. Memari, B. Larijani, et al., Sensor Lett., 8(4), 545–553 (2010).
A. A. Wassel and N. Abu-Talib, J. Sensing Electroanal., 5, 229 – 242 (2010).
Y. S. Chang, Y. R. Ku, K. C. Wen, and L. K. Ho, J. Liq. Chromatogr. Relat. Technol., 23(13), 2009–2019 (2000).
S. Cherkaoui, L. Mateus, P. Christen, and J. L. Veuthey, J. Pharm. Biomed. Anal, 21, 165 – 174 (1999).
British Pharmacopoeia 2009, British Pharmacopoeial Commission, London (2009).
K. G. Kumar, R. Letha, J. Pharm. Biomed. Anal., 15, 1725–1728 (1997).
P. Vyas and R. Kharat, Indian J. Pharm. Sci., 50, 279 (1988).
R. A. Shaalan, R. S. Haggag, S. F. Belal, M. Agami, J. Appl. Pharm. Sci., 3(7), 38 (2013).
V. S. Patel, B. R. Patel, J. G. Patel, R. B. Patel, N. A. Mewada, World J, Pharm. Pharm. Sci., 6(5), 1407 – 1416 (2017).
N. W. Ali, M. Gamal, and M. Abdelkawy, Arab. J. Chem., 10, S1868-S1874 (2013).
N. W. Ali, M. Gamal, and M. Abdelkawy, Pharm. Anal. Acta, S7 – 006, 1 – 5 (2013).
Ranjna C. Dutta and Aroop K. Dutta, Pharm. Anal. Acta, 7(5), 478 (2016).
T. Bhagyasree, N. Injeti, A. Azhakesan, and U. M. Rao, Int. J. Pharm. Res. Anal., 4(8), 444 – 448 (2014).
P. Ravisankar, C. N. Navya, D. Pravallika, and D. N. Sri, IOSR J. Pharm., 5(10), 7 – 19 (2015).
G. P. Carr and J. C. Wahlich, J. Pharm. Biomed. Anal., 8(8), 613 – 618 (1990).
G. Geetha, Karanam Naga Ganika Raju, B. Vignesh Kumar, and M. Gnana Raja, Int. J. Pharm. Biol. Sci., 1(1), 64 – 71 (2012).
ICH Guidelines, Validation of Analytical Procedures: Text and Methodology Q2 (R1), International Conference on Harmonization, Geneva, Switzerland (2005), pp. 11 – 12.
R. P. Sneha Lakshmi, J. Pharm. Anal., 4(1), 1 – 15 (2015).
Suman Sood, Ranjani Bala, J. Drug Discov. Therap., 2(19), 23 – 29 (2014).
R. Singh, J. Pharm. Educ. Res., 3(1) 54 – 63 (2012).
S. Singh and M. Bakshi, Pharm. Technol. Asia, 24, 1 – 14 (2000).
F. Qiu, D. L. Norwood, J. Liq. Chromatogr. Relat. Technol., 30(5), 877 – 935 (2007).
ICH Guidelines, Stability Testing: Photostability Testing of New Drug Substances and Products, Q1B, Step 4 (1996).
Acknowledgements
The authors are thankful to Sai Mirra Innopharm Pvt. Ltd. (Chennai) for providing standards and all facilities throughout the research work. The authors sincerely show gratitude to Department of Pharmaceutical Analysis, C. L. Baid Metha College of Pharmacy (Thoraipakkam, Chennai) for providing lab facilities and for constant encouragement during the research work carried out.
Conflict of Interest
The authors declare no conflict of interest for this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagarajan, N.C., Sivaperumanan, A. & Priya Stability Indicating RP-HPLC Simultaneous Estimation of Hyoscine Butylbromide and Paracetamol in Tablets. Pharm Chem J 55, 410–415 (2021). https://doi.org/10.1007/s11094-021-02435-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02435-8