Ritter reactions of 3,4-(R1)2-benzylcyanides with 1,1-(R2)2-2-[3,4-(R3)2-phenyl]ethanols synthesized 1-[3,4-(R1)2-benzyl]-3,3-(R2)2-6,7-(R3)2-3,4-dihydroisoquinolines (R1 = H, MeO, EtO; R2 = Alk; R3 = MeO, EtO). The obtained isoquinolines can be considered 3,3-dialkyl-substituted analogs of drotaverine. Aseries of benzo[f]isoquinolines were similarly prepared. Hydrochlorides of all isoquinolines were tested for analgesic activity. Ten of thirteen compounds were active in the hot-plate test. Four of them exhibited an analgesic effect in the acetic-acid-induced writhing test. The most active isoquinoline had R1 = MeO, R2 = Et, and R3 = EtO and inhibited acetic-acid-induced cramps by 71.01%. An analysis of the structure—activity relationship study showed that the analgesic effect was not related to variation of the R1 radical but was typical of structures with an ethoxy group on the isoquinoline ring. The effect increased upon substitution of ethyl for methyl groups (R2 radical) in the isoquinoline 3-position.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
3-Alkyl- and 3,3-dialkyl-3,4-dihydroisoquinolines with a variety of structures have been reported to exhibit analgesic (antinociceptive) activity [1,2,3,4,5,6,7,8, – 9]. The studied compounds include only isolated examples of 1-benzylisoquinolines despite benzylisoquinolines being lead compounds in series of isoquinoline alkaloids. Natural isoquinolines with a 1-benzyl fragment have various structures and are often biogenetic precursors of other isoquinoline alkaloids [10]. Benzylisoquinoline alkaloids and their synthetic analogs are included in several drug compositions [11, 12]. It is also noteworthy that little was known until now about ethoxy-substituted 1-benzylisoquinolines, which can be considered drotaverine (No-Spa) analogs.
The goal of the present work was to synthesize derivatives of 1-benzyl-3,3-dialkyl-3,4-dihydroisoquinoline containing ethoxy groups on the isoquinoline ring and benzyl moiety and to study the structure—activity (analgesic) relationship.
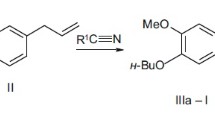
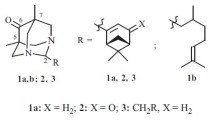
Compounds IIIa-k were synthesized by the known method [13] using Ritter reactions of nitriles Ia-c and carbinols IIa-e in benzene–H2SO4 with added HOAc at 60 – 70°C. Benzo[f]-analogs of isoquinolines IIIa-k (compounds Va and Vb) were prepared to study the structure—activity (analgesic) relationship [14]. These compounds were similarly synthesized by cyclocondensation of carbinols IVa and -b with nitrile Ia in benzene–H2SO4. Biological tests used the hydrochlorides (IIIa-k)·HCl and (Va,b)·HCl, which were prepared by passing dry HCl through a solution of the corresponding base in Et2O or EtOAc.
Table 1 lists the characteristics of the synthesized compounds. The obtained hydrochlorides were crystalline yellow substances. Hydrochlorides (IIId,j,h)·HCl and Va,b)·HCl were poorly soluble in H2O. The other compounds were water-soluble.

The structures of the obtained compounds were proven by PMR spectra (Table 2). Spectra of hydrochlorides (IIIa-k)·HCl contained a singlet for the benzyl CH2 protons at 4.59 – 4.77 ppm. This resonance in spectra of the benzo-[f]isoquinolines appeared at 4.83 and 4.82 ppm. The aromatic region of spectra of (IIIa-k)·HCl exhibited singlets in the range 6.65 – 6.83 ppm that could correspond to the isoquinoline ring 5-H or the benzyl ring 2-H. The NH+ group gave a singlet at 14.57 – 14.99 ppm. The spectra also contained resonances for the R1, R2, and R3 protons.
IR spectra of the synthesized bases contained C=N absorption bands in the range 1630 – 1640 cm–1.
EXPERIMENTAL CHEMICAL PART
IR spectra were taken on a Specord M-80 spectrometer. PMR spectra were recorded in DMSO-d6 solution with HMDS internal standard (0.05 ppm vs. TMS) on a Bruker Avance III HD 400 instrument (400 MHz).
Compounds were recrystallized from i-PrOH. Elemental analyses (C, H, N, and Cl) agreed with those calculated. The purity of products was monitored using TLC and CHCl3–Me2CO (9:1) with detection by I2 vapor.
1-[3,4-(R 1 ) 2 -Benzyl]-3,3-(R 2 ) 2 -6,7-(R 3 ) 2 -3,4-dihydroisoquinolinium chlorides (IIIa-k)·HCl. A mixture of the appropriate nitrile (Ia-e, 0.03 mol) and carbinol IIa (0.03 mol) in benzene (150 mL) at <5°C was treated successively with glacial HOAc (6 mL) and dropwise with conc. H2SO4 (10 mL). The mixture was stirred vigorously at 60 – 70°C for 30 min, cooled, and poured into ice water (150 mL). The benzene layer was separated. The aqueous phase was neutralized with ice-cold NH4OH solution. The resulting oil was extracted with Et2O, dried by NaOH, and distilled (30 – 50 mL) to remove traces of NH3. Passage of dry HCl through the solution precipitated the hydrochloride, which was filtered off, dried, and recrystallized.
4-(3,4-Diethoxybenzyl)-2,2-(R 2 ) 2 -1,2-dihydrobenzo[ f ]-isoquinolinium chlorides (Va, b)·HCl. A mixture of nitrile Ia (0.03 mol) and the appropriate carbinol (IVa, b, 0.03 mol) in benzene (100 mL) at <10°C was treated dropwise with conc. H2SO4 (12 mL). The mixture was stirred vigorously at 60 – 70°C for 30 min, cooled, and poured into ice water (150 ml). The benzene layer was separated. The aqueous phase was neutralized with aqueous NH4OH (25%) and cooled to 20°C to precipitate the base, which was filtered off, dried, and dissolved in EtOAc (100 mL). Passage of dry HCl produced the corresponding hydrochloride, which was filtered off, dried, and recrystallized.
EXPERIMENTAL PHARMACOLOGICAL PART
Tests used inbred white mice of both sexes (18 – 24 g) that were obtained from the Andreevka nursery, Moscow Region. Animals were kept in a typical vivarium with natural 12-hour light cycles at air temperatures of 20 ± 2°C and were fed according to feed standards for experimental animals. The animals had free access to water using special feeders for rodents. The water was analyzed beforehand for chemical and bacteriological impurities. The animals were kept according to good laboratory practice (GLP) rules and MH RF Order No. 199n of Apr. 1, 2016 “Good laboratory practice rules.”
Analgesic activity was studied using the hot-plate test [15, 16]. The reference drug was metamizole sodium tablet powder (Medisorb, Russia) that met pharmacopoeial requirements. The dose of the tested compounds was set at 50 mg/kg so that the results could be compared correctly with previous data [1,2,3,4,5,6,7,8, – 9]. However, the tests showed that the compounds at this dose possessed pronounced irritating effects with signs of intoxication. This was probably related to their good solubility in water and; correspondingly, rapid uptake in the blood. Therefore, the dose was halved (25 mg/kg). The tested compounds were injected intraperitoneally (i.p.) 30 min before testing as suspensions in starch solution (1%). The reference standard was metamizole sodium at a dose of 50 mg/kg, which was used in previous studies [1,2,3,4,5,6,7,8, – 9]. Control and test groups included eight mice each.
Animals were placed on a metal surface heated to 54°C and surrounded by a cylinder. The time from the moment of placement on the hot surface until the manifestation of a behavioral response to the pain (licking hind paws, jumping, lifting hind paws) was recorded. The criterion for an analgesic effect was considered a statistically significant increase of the latent period for the response after injection of the compound. Test results were statistically processed using the Student coefficient. Differences were considered statistically significant for p < 0.05 [17].
Test results for analgesic (antinociceptive) activity (Table 3) showed that all compounds at a dose of 25 mg/kg except for IIIc·HCl, IIIj·HCl, and Vb·HCl caused statistically significant increases of the time for a defensive reflex as compared to the control. The increases of the latent period of the pain reaction for IIId·HCl, IIIe·HCl, IIIh·HCl, and Va·HCl were statistically significantly greater than that of metamizole sodium at a dose of 50 mg/kg. The activities of the other compounds were comparable to that of the reference drug. It is noteworthy that administration of the compounds to several experimental groups caused suppression of respiratory activity and signs of irritation that manifested as contractions of abdominal muscles after i.p. administration of the compounds.
Compounds IIId·HCl, IIIe·HCl, IIIh·HCl, and Va·HCl were tested in the acetic-acid-induced writhing model [16]. Acetic acid was injected (i.p., 0.2 mL) as a 0.75% solution. The cramps occurring in 15 min were counted. The tested compounds were injected i.p. at a dose of 25 mg/kg 30 min before injecting the HOAc. An effect was assessed from the decrease in the number of cramps as compared to control animals. The reference standard was metamizole sodium at a dose of 25 mg/kg. Control and test groups included 10 mice each.
The studies showed that all four compounds at a dose of 25 mg/kg except for IIId·HCl possessed analgesic activity for the acetic-acid-induced writhing model (Table 4). The reference drug metamizole sodium at a dose of 25 mg/kg in this model was inactive [18]. All four compounds had higher analgesic activity than metamizole sodium. The most active compound was IIIe·HCl, which contained methoxyls on the benzyl moiety, ethyl groups in the 3-position, and ethoxyls on the isoquinoline ring. The number of cramps after injection of this compound was 8.87 ± 1.70 while the number was 30.6 ± 2.2 for the control. Thus, the percent of cramps decreased by 71.01% as compared to the control. Also, benzo[f]isoquinoline Va·HCl exhibited high activity and decreased the number of cramps by 65.06%.
All four compounds showed irritating effects that manifested as chest muscle contractions for 15 – 20 min after i.p. administration. Furthermore, suppression of respiratory activity was observed after injection of IIIe·HCl, which could be a manifestation of toxic activity
An analysis of the structure—activity relationship showed that the analgesic effect was not related to variation of the R1 radical but was characteristic of structures with ethoxy groups on the isoquinoline ring (IIId·HCl, IIIe·HCl, IIIb·HCl). The effect increased if methyls in the isoquinoline 3-position (R2 radical) were replaced by ethyls.
A comparison of the analgesic activities of the tested compounds with previous results [1,2,3,4,5,6,7,8, – 9] showed that benzo[f]-annelation enhanced the antinociceptive activity. The pharmacological target of the analgesic (antinociceptive) activity could be assumed to be nerve endings because the observed effect had a pronounced peripheral character.
It could be concluded based on the results that an analgesic effect in the series of 3,3-dialkyl-substituted drotaverine analogs was most probable for compounds containing ethoxyls in the 6- and 7-positions of the isoquinoline ring.
References
A. G. Mikhailovskii, E. V. Vikhareva, N. G. Ismailova, et al., Khim.-farm. Zh., 41(10), 19 – 21 (2007); Pharm. Chem. J., 41(10), 529 – 531 (2007).
L. V. Anikina, Yu. B. Vikharev, V. A. Safin, et al., Khim.-farm. Zh., 36(2), 19 – 23 (2002); Pharm. Chem. J., 36(2), 18 – 22 (2002).
V. A. Glushkov, L. V. Anikina, Yu. B. Vikharev, et al., Khim.-farm. Zh., 39(10), 27 – 29 (2005); Pharm. Chem. J., 39(10), 533 – 536 (2005).
Yu. B. Vikharev, Yu. V. Shklyaev, L. V. Anikina, et al., Khim.-farm. Zh., 39(8), 13 – 15 (2005); Pharm. Chem. J., 39(8), 405 – 408 (2005).
L. V. Anikina, Yu. B. Vikharev, A. A. Gorbunov, et al., Khim.-farm. Zh., 47(8), 23 – 25 (2013); Pharm. Chem. J., 47(8), 419 – 421 (2013).
O. V. Surikova, E. S. Limanskii, G. A. Aleksandrova, et al., Khim.-farm. Zh., 47(4), 20 – 23 (2013); Pharm. Chem. J., 47(4), 198 – 201 (2013).
O. V. Surikova, A. S. Yusov, R. R. Makhmudov, et al., Khim.-farm. Zh., 51(1), 20 – 22 (2017); Pharm. Chem. J., 51(1), 22 – 25 (2017).
A. G. Mikhailovskii, A. S. Yusov, R. R. Makhmudov, et al., Khim.-farm. Zh., 52(8), 36 – 40 (2018); Pharm. Chem. J., 52(8), 716 – 720 (2018).
A. S. Yusov, S. V. Chashchina, A. G. Mikhailovskii, et al., Khim.-farm. Zh., 53(1), 36 – 40 (2019); Pharm. Chem. J., 53(1), 35 – 39 (2019).
V. G. Kartsev (ed.), Selected Methods for Synthesis and Modification of Heterocycles. Natural Isoquinolines: Chemistry and Biological Activity [in Russian], Part 8, ICSPF, Moscow (2011).
M. D. Mashkovskii, Drugs [in Russian], Novaya Volna, Moscow (2017).
E. A. Tolmachev (ed.), Vidal Handbook [in Russian], Vidal’ Rus, Moscow (2018).
A. G. Mikhailovskii, O. V. Gashkova, I. P. Rudakova, et al., Khim.-farm. Zh., 51(7), 25 – 27 (2017); Pharm. Chem. J., 51(7), 546 – 549 (2017).
A. G. Mikhailovskii, O. V. Gashkova, I. P. Rudakova, et al., Khim.-farm. Zh., 53(11), 17 – 20 (2019); Pharm. Chem. J., 53(11), 1005 – 1008 (2019).
N. B. Eddy and D. Leimbach, J. Pharmacol. Exp. Ther., 107(3), 385 – 393 (1953).
R. U. Khabriev (ed.), Handbook for Experimental (Preclinical) Studies of New Drugs [in Russian], Moscow (2012).
L. M. Belen’kii, Elements of Quantitative Assessment of Pharmacological Effects [in Russian], Medgiz, Leningrad (1963).
Ya. A. Sigidin, G. Ya. Shvarts, A. P. Arzamastsev, and S. S. Liberman, Medicinal Therapy of the Inflammatory Process: Experimental and Clinical Pharmacology of Anti-inflammatory Preparations [in Russian], Meditsina, Moscow (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 1, pp. 19 – 24, January, 2021.
Rights and permissions
About this article
Cite this article
Mikhailovskii, A.G., Pogorelova, E.S., Chashchina, S.V. et al. Synthesis and Analgesic Activity of 3,3-Dialkyl-Substituted Drotaverine Analogs. Pharm Chem J 55, 17–22 (2021). https://doi.org/10.1007/s11094-021-02365-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02365-5