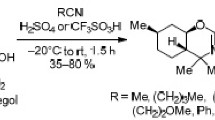
Cyclocondensation of O-butylated eugenol with various nitriles was used to synthesize 1-substituted derivatives of 3-methyl-6-methoxy-7-(n-butoxy)-3,4-dihydroisoquinoline. Hydrochlorides of the study compounds were tested for analgesic activity using the “hot plate” test. The experiments showed that the study compounds had analgesic effects greater than those of metamizole sodium and similar to those of ibuprofen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We have previously prepared ureide derivatives of isoquinoline ethanoic acid and studied their analgesic properties [1]. Pharmacological studies of these compounds showed that the further search for analgesics among the isoquinolines has potential.
The aim of the present work was to synthesize 1-substituted 3-methyl-6-methoxy-(n-butoxy)-3,4-dihydroisoquinolines and study their analgesic activity.
Isoquinoline derivatives IIIa-l were synthesized by the known cyclocondensation reaction between O-alkylated eugenol and the corresponding nitriles [2, 3].
The phenolic hydroxyl group in eugenol I was quantitatively alkylated with iodobutane by interphase catalysis in an 18-crown-6/KOH system. The resulting ester II was used without purification for subsequent Ritter cyclocondensation. The nitrile constituents were hydrogen cyanide for the synthesis of IIIa, acetonitrile for IIIb, butyronitrile for IIIc, p-bromobenzonitrile for IIId, benzylcyanide for IIIe, 3-methoxypropionitrile for IIIf, 3-phenoxyproprionitrile for IIIg, chloracetonitrile for IIIh, and cyanoacetic acid amides for IIIi-l. Pharmacological studies used hydrochlorides formed by passing dry HCl through solutions of the corresponding bases. The resulting hydrochlorides (IIIa-l).HCl were yellow crystalline substances soluble in water. The properties of the newly synthesized compounds are presented in Table 1.
Compound structures were confirmed by PMR spectroscopy. The spectra of the bases of compounds IIIa-h, which exist in the azomethine form, differed from those of enamines IIIi-l. The spectra of the latter contained singlet signals from the vinyl proton (5.22 – 5.27 ppm) and from the ring NH group proton (7.85 – 8.15 ppm), which corresponds to the H-chelate form of the enaminoamide.
The spectra of the hydrochlorides (Table 2) contained a singlet from the isoquinoline ring NH+ group at 11.15 – 14.38 ppm, a set of signals characteristic of the CHAHB system at 3.05 – 3.18 ppm, as well as proton signals from the substituents in position 1 of the isoquinoline ring. All spectra also contained a doublet from the position 3 CH3 group at 1.22 – 1.37 ppm, a quadruplet form the position 3 proton at 2.72 – 3.05 ppm, and signals from methoxy and n-butoxy group protons in positions 6 and 7 of the isoquinoline ring.

The IR spectra of the bases of compounds IIIa-h contained absorption bands from the azomethine group at 1620 – 1640 cm-1, while the spectra of the bases of compounds IIIi-l had broad absorption bands from the chelated ring NH group (3100 – 3150 cm-1). This supports the fact that the base of the amide molecule has the Z configuration, stabilized by an intramolecular hydrogen bond [4]. The spectra of amides IIIj and IIIk had an absorption band from the carbonyl group of the tertiary amide group (1660 – 1670 cm-1). The spectrum of amide IIIl contained an absorption band from the carbonyl group of the secondary amide group (1680 – 1685 cm-1) and the amide NH group at 3280 – 3300 cm-1. The spectrum of the base of amide IIIi contained an absorption band from the free NH2 group at 3350 – 3400 cm-1.
Experimental Chemical Section
IR spectra were taken on a Specord M-80 in Vaseline; PMR spectra were recorded on a Bruker AMX 300 (300 MHz) in DMSO-d6 solution with HMDS as the internal standard (0.05 ppm relative to TMS).
Substances were recrystallized from acetonitrile. Elemental analysis data (C, H, N, and Cl) were consistent with calculated values. The purity of reaction products was monitored by TLC in a solvent system consisting of chloroform and acetone (9:1) with detection using iodine vapor. Bases for recording PMR and IR spectra were prepared by treating the corresponding hydrochloride with 25% ammonia solution.
1-Substituted 3-methyl-6-methoxy-7-butoxy-3,4-dihydroisoquinoline hydrochlorides (IIIa-l).HCl. A mixture of 21.6 ml (0.12 mol) of eugenol (I), 17.2 ml (0.15 mol) of n-butyl iodide (n-iodobutane), and 0.5 g (0.0019 mol) of 18-crown-6 in the presence of 20 g (0.36 mol) of KOH in 150 ml of benzene was mixed intensely at 40 – 50°C for 2 h and then cooled to 20°C. Cooled solution was filtered, washing with two aliquots of alkaline benzene. Filtrate was evaporated to a volume of about 70 ml. The resulting benzene solution of compound II was supplemented with 0.1 mol of the corresponding nitrile, 15 ml of glacial acetic acid, and then, dropwise, with 30 ml of concentrated sulfuric acid. The reaction was mixed intensely at 50 – 60°C for 30 min and then poured into 300 ml of glacial acetic acid, after which the benzene layer was evaporated. The aqueous phase was neutralized with ammonia solution. The crystalline precipitate of the base precipitating on cooling to 5 – 7°C was collected by filtration, dried, and dissolved in 250 ml of ethyl acetate; dry HCl was passed through the solution to prepare the hydrochloride, which was collected by filtration, dried, and recrystallized.
Experimental Pharmacological Section
Analgesic activity was studied in mongrel white mice weighing 18 – 22 g by thermal stimulation by the “hot plate” method [5]. Test compounds were given i.p. as suspensions in 2% starch paste at a dose of 50 mg/kg. The indicator of pain sensitivity was the time spent by the animal on the hot plate in response to thermal stimulation - with licking of the hindpaws, shaking them, or trying to jump away. Experiments used animals with initial defensive reflex durations of no more than 15 sec. The standard reference agent was metamizole sodium at a dose of 93 mg/kg, which corresponded to the ED50 for i.p. administration, and ibuprofen at a dose of 50 mg/kg [6, 7]. Statistical analysis was run using results from 54 experiments. Effects were regarded as significant at p ≤ 0.05 compared with controls and the reference drug.
Data from pharmacological studies (Table 3) showed that all 12 compounds had analgesic activity at a dose of 50 mg/kg which was greater than that of metamizole sodium and comparable with that of ibuprofen. The most active compounds were 1-(n-bromopheny)-3-methyl-6-methoxy-7-(n-butoxy)-3,4-dihydroisoquinoline hydrochloride (IIId. HCl) and 2-(3-methyl-6-methoxy-7-(n-butoxy)-3,4-dihydroisoquinolyl-1-ethanolic acid N-[2(3,4-dimethoxyphenyl)-ethyl]amide hydrochloride (IIIl. HCl), for which defensive reflex durations were 22.70 and 22.30 sec respectively.
These data lead to the conclusion that studies seeking analgesics in the 3-alkyl-3,4-dihydroisoquinoline series have potential.
References
O. V. Surikova, E. S. Limanskii, G. A. Aleksandrova, et al., Khim-Farm. Zh., 47(4), 20 – 22 (2013); Pharm. Chem. J., 47(4), 198 – 201 (2013).
A. G. Mikhailovskii, O. V. Surikova, E. S. Limanskii, and M. I. Vakhrin, Khim. Prirod. Soedin., 2, 254 – 256 (2012).
O. V. Surikova, A. G. Mikhailovskii, B. Ya. Syropyatov, and M. I. Vakhrin, Khim-Farm. Zh., 48(10), 33 – 36 (2014); Pharm. Chem. J., 48(10), 665 – 668 (2015).
É. Prech, F. Byul’man, and K. Affol’ter, Identification of the Structures of Organic Compounds [in Russian], Mir, BINOM, Laboratoriya Znanii, Moscow (2006), p. 301.
N. B. Eddy and D. Leimbach, J. Pharmacol. Exp. Ther., 107(3), 385 – 393 (1953).
V. É. Kolla and B. Ya. Syropyatov, Doses of Drugs and Chemical Compounds for Laboratory Animals [in Russian], Meditsina, Moscow, (1998), p. 14.
Ya. A. Sigidin, G. Ya. Shvarts, A. P. Arzamastsev, and S. S. Liberman, Drug Treatment of It Inflammatory Process: Experimental and Clinical Pharmacology of Anti-inflammatory Drugs [in Russian], Meditsina, Moscow (1988).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 1, pp. 20 – 22, January, 2017.
Rights and permissions
About this article
Cite this article
Surikova, O.V., Yusov, A.S., Makhmudov, R.R. et al. Synthesis and Analgesic Activity of 1-substituted 3-methyl-6-methoxy-7(n-butoxy)-3,4-dihydroisoquinolines. Pharm Chem J 51, 18–21 (2017). https://doi.org/10.1007/s11094-017-1549-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1549-8