Transdermal delivery of drugs is a current alternative route of administration for systemic actions because of a whole series of unarguable advantages, including high compliance due to its noninvasive, pain-free and simple use, as well as reductions in drug doses and the consequent reductions in side effects. However, most candidate drugs encounter the problem of low drug permeability though the skin barrier. One of the simplest means of enhancing skin permeability consists of introducing a permeability enhancer (penetrator) into the medicinal formulation. The aim of the present review was to consider different types of chemical permeability enhancers presented in the literature in recent years. A classification of permeability enhancers is provided and possible mechanisms of action are indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Transdermal drug delivery is a current alternative to the oral and parenteral routes of administration. Transdermal drug administration avoids first-pass hepatic metabolism and provides constant drug release and maintenance of therapeutic blood concentrations without significant oscillations over prolonged periods of time, which decreases the numbers of doses required and side effects. This means of drug use can provide high treatment compliance because of its painlessness and simplicity of use [1, 2].
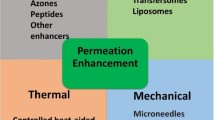
One task in the development of transdermal drug formulations is that of overcoming the natural transport barrier imposed by the skin. Recent years have seen the appearance of technological methods for solving this problem, which can be divided into physical and chemical approaches.
Physical methods consists of using mechanical, electrical, magnetic, or thermal energy sources to stimulate the transport of drug molecules by degrading skin membranes, for example, using microneedles, iontophoresis, sonopheresis, ultrasound, etc. [1,2, – 3]. These methods have many limitations.
The chemical method consists of adding permeability enhancers to the composition of formulations; in the literature these are also termed absorption activators and penetrators. About 300 chemical compounds able to increase the transport of drug molecules through the skin have been described [1, 2, 4]. Inclusion of absorption activators into the repertoire of drug formulations is currently the best studied approach to transdermal enhancement of skin permeability. Their use is quite economical and simple, and from the technological point of view they can be combined with active pharmaceutical substances in the compositions of a variety of formulations and they have different mechanisms of transcutaneous transport.
The mechanisms of penetration of drug through the skin are multifactorial processes linked with the complex morphological structure of the skin. The skin is a multifunctional membrane formation. The skin consists of three layers: the avascular epidermis, the dermis, and the hypodermis. Each layer has its own physiological characteristics and functional features. Thus, the hypodermis (the subcutaneous fat layer) consists mainly of fatty tissue, is quite thick, protects the body from cold, and has a storage function. Above this, between the subcutaneous tissue and the epidermis, is the dermis itself, which consists of connective tissue. This layer is 2 – 3 mm thick and contains large numbers of lymphatic and blood vessels, as well as nerve endings, hair follicles, and sebaceous and sweat glands. This skin layer has the largest blood supply, such that it supports the concentration gradient required for transport of drugs directly into the systemic circulation. The epidermis consists of multiple layers of flattened epithelium differentiated into the strata corneum, lucidum, granulosum, spinosum, and basale. The stratum corneum of the epidermis carries out one of the most important functions of the skin – that of a barrier. The stratum corneum of the skin consists of keratinocytes (70%) and lipids (20%), which decrease water evaporation from the dermis and block the penetration of chemical and biological substances [5,6, – 7].
The main route of penetration of drugs across the skin into the body is transepidermal (intracellular and intercellular) and transfollicular. The transepidermal route allows transport of low molecular weight compounds both across epidermis cells and between them. Substance transport occurs as a result of passive diffusion, which consists of the passage of drugs from an area of higher concentration to an area of lower concentration. Transfollicular transport makes a small contribution to maintaining a constant drug concentration in the blood because of the small area of follicles, accounting for about 1% of the total skin surface. At the same time, the transfollicular route promotes faster onset of therapeutic effects and can operate as a “gate” for the transport of large polar molecules, polymers, and colloid particles [5, 8].
Skin drug permeability is influenced by physiological factors such as age, anatomical location of the skin area to which patches are applied, the moisture content of the skin, its functional state, level of metabolism, race, sex, and body temperature [2, 4, 9].
Drug molecules able to be incorporated into the composition of transdermal formulations must meet a number of quite stringent criteria: the lipophilicity of the molecules must be no more than logP = 5, molecular weight must be no more than 500 Da, the therapeutic dose must be no more than 10 mg/day, and the molecule must be neutral [1, 4, 9]. A large proportion of drug molecules do not meet these criteria. The largest problem in transdermal drug delivery is that of achieving safe and reversible transport across the skin. The safety of enhanced skin permeability technologies must be assessed at the drug formulation development stage, along with the duration of enhanced permeability and the absence of penetration of undesired substances [1, 7, 9, 10]. From this point of view, the use of chemical permeability enhancers is more predictable and safe than physical methods of increasing skin permeability.
Permeability enhancers increase drug transport across the skin by means of a variety of mechanisms. They can have effects on skin structure indirectly, acting on intercellular lipids or keratinocytes. Penetrators are able to extract intercellular lipids from the skin, this creating diffusional pathways for drug penetration, or they can increase the fluidity of lipid bilayers in the stratum corneum, inducing pseudoliquefaction. In addition, permeability enhancers can increase skin drug transport by increasing its thermodynamic activity, for example by inducing oversaturation at the administration site. The mechanisms of a number of permeability enhancers are known, based on increases in the hydration of the stratum corneum or changes in the protein components of the skin [1, 11].
Classification of permeability enhancers. Permeability enhancers are classified on the basis of their chemical structures rather than their mechanisms of action on the skin, which are not always known. Chemical substances belonging to one group can act on the skin by different means depending on their individual physicochemical properties. Table 1 presents the classification and nomenclature of the most widely used penetrators.
Water is the most physiological permeability enhancer for both hydrophilic and lipophilic drugs. The state of hydration of the stratum corneum is important for increasing the penetration of drug molecules: as a rule, increases in the hydration of the stratum corneum increase transdermal transport. Additional quantities of water in the stratum corneum can alter the solubility of the penetrating substance, causing swelling of keratinocytes and leading to pore formation [1, 9].
Hydrocarbons generally promote drug penetration through the skin due to derangement of the ordered structure of lipid bilayers and separation of the stratum corneum. Studies using alkanes with different molecular chain lengths showed that alkanes with shorter chains have more marked penetration properties [1, 15, 16]. The most widely used permeability enhancers are alkanes, alkenes, halogenated alkanes, squalane, and squalene [1, 9, 16].
Alcohols are often used as penetrators for improving transdermal drug delivery. These include alkanols, alkenols, glycols, polyglycols, and glycerols. Alcohols can enhance skin permeability via various mechanisms, such as lipid and protein extraction, swelling of the stratum corneum, and increases in the solubility of the active pharmaceutical substance in the formulation. Alcohols function not only as penetrators, but also as solvents and cosolvents in formulations [1, 17].
Primary, secondary, tertiary, and cyclic and acyclic amines have been used with success to increase skin permeability by separating lipid bilayers and forming pores [1, 4, 17]. Like alcohols, they are solvents, i.e., they combine these functions in medicinal formulations. The most widely used members of this class are diethanolamine and triethanolamine.
Cyclic and acyclic amides form another large class of chemical substances studied as permeability enhancers, which includes azone, pyrrolidones, urea, and its derivatives. Azone (1-dodecylazacyloheptan-2-one or laurocapram) was the first synthetic permeability enhancer and its analogs, along with pyrrolidones, are the most widely used of the amides studied. Azone provokes dynamic structural derangements to the intercellular lamellar lipid structure throughout the stratum corneum, increasing the fluidity of the lipid layer [1, 9, 13, 15]. The use of pyrrolidones as permeability enhancers for many molecules, both hydrophilic (for example, mannitol and 5-fluorouracil) and lipophilic (progesterone and hydrocortisone) has been studied. Pyrrolidones distribute well in the stratum corneum of the skin and can alter the hydrophilic-lipophilic ratio in the membrane [1, 9, 15]. Urea and its analogs are generally used as permeability enhancers in soluble form, and they can have different influences on the skin depending on the solvent system selected though they generally act by degrading skin lipids and/or increasing the hydration of the stratum corneum with formation of hydrophilic channels [1, 9].
Fatty acids increase the transport of drug molecules through the skin by various mechanisms: extraction of intercellular lipids and formation of lipophilic complexes with drugs. Thus, fatty acids can operate as penetrators, delivery systems, and solvents. Oleic, linoleic, valeric, lauric, caproic, caprylic, linolenic and other acids have the ability to increase the transport of active pharmaceutical substances through the skin. A number of studies have shown that fatty acids with chains of length C9 -C12 have the greatest activity levels. In addition, there is a correlation between fatty acid molecule chain length and lipophilicity. The ability to alter the lipid organization of the skin is obtained when the permeability enhancer itself is in the liquid phase at physiological temperature. For example, long-chain unsaturated fatty acids cannot create a liquid phase in lipid domains in the skin membrane unless they form eutectic mixtures with lipids [1, 13, 16, 18, 19]. Fatty acid esters can increase transcutaneous transport of quite large quantities of active pharmaceutical substances. The best known member of this group of penetrators is transcutol. Transcutol is the monoethyl ether of diethylene glycol. It is very widely used in cosmetics and is approved for use in the pharmaceutical industry. It is used as a solvent, a cosolvent, and penetrator in transdermal, ophthalmological, and intranasal delivery systems. It has marked solubilizing and dehydrating properties and decreases the charge on drug molecules. It rapidly penetrates the stratum corneum of the skin, increasing the solubility of active pharmaceutical substances and disorganizing the lipid structure of the epidermis, forming transport channels. Use with other classes of permeability enhancers produces synergistic effects. It is not toxic, carcinogenic, or genotoxic [20,21,22, – 23].
Terpenes, terpenoids, and essential oils are widely used as permeability enhancers in transdermal drug delivery systems. Terpenes and terpenoids obtained from plant essential oils are natural isoprene derivative hydrocarbons with high penetration properties and relatively low toxicity; they are weak skin irritants at low concentrations (1 – 5%). The actions of a given terpene or terpenoid on the skin depend on its chemical structure and physicochemical properties, such as lipophilicity, size, chirality, level of saturation, and boiling temperature. They increase skin permeability via one or more mechanism; interaction with stratum corneum lipids and/or keratinocytes and increasing the solubility of active pharmaceutical substances in lipids [24, 25].
Essential oils obtained from aromatic herbs are complex aromatic volatile mixtures of compounds with low molecular weight and different chemical structures. The components of essential oils can increase the penetration of various drugs via different mechanisms of action based on the disruption of highly ordered intercellular lipid structure and interactions with intercellular proteins, inducing conformational changes and increasing drug solubility in lipids. Essential oils and their components (terpenes and terpenoids) are regarded as safe, as they are rapidly metabolized, do not accumulate, and are rapidly eliminated from the body after application to the skin [1, 13, 25, 26]. Some essential oils, for example, niaouli oil, which contains a mixture of terpene components (1,8-cineol, α-pinene, α-terpineol, and d-limonene), have been shown to have greater penetrant ability than individual terpenes, even at high concentrations, as well as lower toxicity. Analogous results have also been shown for other oils [26, 27].
Sulfoxides. Dimethylsulfoxide was first chemical substance studied in detail as a permeability enhancer. It is widely used as a universal strong bipolar aprotic solvent and is less toxic than other compounds of this group, such as dimethylformamide and dimethylacetamide. Dimethylsulfoxide has its own pharmacological effects: anti-inflammatory, local anesthetic, moderate antiseptic, and fibrinolytic actions. It penetrate the skin, mucous membranes, and bacterial cell walls (increasing their sensitivity to antibiotics), and other biological membranes, increasing their permeability for drugs. As a permeability enhancer, dimethylsulfoxide rapidly penetrates the skin; its penetrating effect depends on the concentration, which is usually quite high, about 60%. Dimethylsulfoxide has complex effects on skin: it extracts lipids, increases lipid fluidity by disrupting the ordered structure of lipid chains, making the stratum corneum more permeable, forming aqueous channels, and also denaturing intercellular protein structures in the stratum corneum. Despite quite high efficacy, use of this substance is associated with a number of side effects. Use of this compound can produce contact dermatitis, a garlic odor to exhaled air, increased skin pigmentation, allergic reactions, erythematous rash, dry skin, and mild burning. Nonetheless, dimethylsulfoxide is regarded as a low-toxicity compound (LD50 3 – 25 g/kg) [9, 13, 28, 29].
Dimethylacetamide and dimethylformamide are analogous aprotic solvents and also have marked penetrating abilities but less marked side effects. Apart from higher toxicity, dimethylformamide produces irreversible membrane damage, so the use of these substances as permeability enhancers is more limited [9, 13, 28].
Surfactants. Many surfactants have been actively studied as penetrators. These include anionic, cationic, and nonionic surfactants. Their activity depends on the hydrophilic-lipophilic balance, charge, and tail length. Surfactants affect the permeability of a number of biological membranes, including skin. Many of the properties of surfactants may be linked with their ability to concentrate at phase boundaries, leading to decreases in interphase tension. In biological systems, the actions of surfactants are complex in nature; in particular, they affect not only cell and other membranes, but can also lead to changes in permeability characteristics. The ability of surfactants to adsorb to phase separation surfaces and bind via hydrophobic or polar interactions may lead to desired and undesired actions on the skin depending on the concentration of surfactant, the type of action, the duration of contact, and individual reactions. The undesirable effects of surfactants on the skin have been extensively studied in vivo using volunteers, as well as using in vitro screening tests, with the aim of predicting the clinical effects of individual surfactants and medicinal formulations. Selection of a surfactant as permeability enhancer must be based not only on its efficacy in increasing skin permeability, but also its dermal toxicity and physicochemical and biological compatibility with other components of the system [1, 4, 9, 13].
The mechanism of the penetrating action of surfactants is based on their binding to and denaturation of skin proteins, solubilization, disorganization of intercellular skin lipids, and extraction of lipids. Surfactant molecules can diffuse through the lipid area of the skin, bind to proteins, and induce protein denaturation, leading to swelling of the stratum corneum of the skin [1, 13, 30,31, – 32]. Surfactants integrate into lipid bilayers to disrupt them, increase their fluidity, and alter the barrier function of skin. Surfactant monomers easily reach intercellular lipids, making the penetrating effect dependent on the relative proportion of monomer in solution [1, 13, 30,31, – 32].
Anionic surfactants such as sodium lauryl sulfate and sodium dodecyl sulfate impair the barrier function of the skin, damaging lipids and proteins in the stratum corneum. They are more effective than cationic and nonionic surfactants in increasing the skin penetration of target molecules. After penetration into the stratum corneum, anionic surfactants bind with proteins and denature them, and also extract intercellular lipids. Repeat exposure leads to skin dryness and irritation, impairing the barrier function and health of the skin. Anionic surfactants rapidly increase the electrical conductivity of the skin and its water penetrability, leading to nonlinear increases in the permeability of the skin to surfactants with time. The zeta potential of surfactants has been found to correlate with skin conductivity and the penetration of water and the surfactants themselves across the skin, especially on prolonged exposure [33, 34].
Cationic surfactants also interact with skin proteins via bipolar interactions and hydrophobic binding. Hydrophobic interactions between surfactant chains and proteins lead to the formation of ionic groups and subsequent swelling of the stratum corneum. Cationic molecules are more disruptive to skin tissues than anionic surfactants. Examples of permeability enhancers of this group are: benzalkonium chloride, cetyltrimethylammonium bromide, cetylpyridinium chloride, and dodecylamine [1, 9, 30, 31, 32, 35].
Nonionic surfactants form a relatively safe class of permeability enhancers as compared with other surfactants. They penetrate the intercellular space of the stratum corneum, increase the fluidity of the lipid layer and solubilize and extract lipid components. They can bind with proteins via hydrophobic interactions, but without disrupting them. Polysorbates, Spans, and poloxamers are the most widely used [1, 4, 9, 13, 30, 31, 32].
Cyclodextrins. Many studies have described the actions of cyclic oligosaccharides, particularly β-cyclodextrins, on increasing skin permeability. Cyclodextrins form inclusion complexes with various drugs, which improves drug stability by preventing degradation, oxidation, or hydrolysis and increases drug solubility. In addition, cyclodextrins support dissociation and penetration of drugs across membranes [36, 37]. The efficacy of cyclodextrins for estradiol, tixocortol, avobenzone, various non-steroidal anti-inflammatories, etc. has been demonstrated [1, 38, 39, 40]. Apart from providing a stabilizing effect for drugs, cyclodextrins can operate synergistically with penetration enhancers to improve their absorption through the skin. For example, the inclusion complex of prostaglandin E1 with O-carboxymethyl-Oethyl- β-cyclodextrin in a fatty alcohol – propylene glycol ointment base – supplemented with permeability enhancer 1-[2-(decyl)ethyl]azacyclopentan-2-one improved the transdermal transport of prostaglandin through the skin by a factor of approximately 100. The use of cyclodextrin with quaternary ammonium salts decreased the toxic side effects of the latter on the skin, preserving their ability to permeabilize the skin, thus demonstrating synergy for safety and efficacy [1, 9, 13].
Having considered the main classes of permeability enhancers, we will now address the limitations and problems associated with use of the chemical method of enhancing skin permeability. An important limitation of chemical permeability enhancers is that many of them display weak penetration, such that their activity is limited to a few upper layers of the stratum corneum of the skin. As their concentrations through the stratum corneum decrease, their activities also decrease. As a result, these chemical substances provide insufficient transdermal drug delivery. The physicochemical properties determining the activity of permeability enhancers, such as charge, ability to bind hydrogen, polarity, lipophilicity, solubility, etc. can be used to develop quantitative measures linking the chemical structure of a permeability enhancer with its penetration activity. These data can be used to develop novel permeability enhancers with significantly greater efficacy than standard chemical agents. One of the first examples of such “synthetic” permeability enhancers developed using molecular properties relevant to skin permeabilization [1, 35, 41,42, – 43] is azone.
A further problem in using chemical substances to enhance transdermal transport is their ability to induce skin irritation. This relates to any side effects induced by the interactions of chemical substances with components of skin and can include local inflammation, erythema, edema, dermatitis, etc. Highly effective permeability enhancers disrupt the stratum corneum of the skin or highly ordered lipid bilayers in the stratum corneum. The stratum corneum is the main transport barrier for diffusion of drug molecules, though at the physiological level it consists of dead cells. However, penetrators enter the stratum corneum more deeply and can interact with living cells in the epidermis and have cytotoxic effects on them. It is difficult to design permeability enhancers acting exclusively in the stratum corneum of the skin. As a result, it is difficult to find the optimum balance between the safety and efficacy of chemical enhancers [1, 35, 41,42, – 43].
Mixtures of permeability enhancers can be used to overcome these problems. The individual components of mixtures can affect the skin by similar or mutually supplementary mechanisms, leading to adaptive or synergistic actions as permeability enhancers. For example, the combination of two chemical enhancers, one of which acts on lipids and the other on keratinocytes, can open intercellular hydrophobic, as well as intracellular hydrophilic, pathways for penetration of drug molecules. Another example in which enhancers can show additive or synergistic properties is the situation where one enhancer stabilizes the drug or prevents its metabolism in the skin and the other creates a diffusion pathway for drug penetration. Apart from displaying synergistic effects in improving drug transport, mixtures of chemical substances can also demonstrate synergy between efficacy and safety. High efficacy requires penetrators to have disrupting properties in relation to the stratum corneum of the skin, while safety requires them not to interact with the viable epidermis. It is extremely difficult to achieve this separation of mechanisms for diffusion of individual chemical substances through the skin, though use of combinations of penetrators can separate their actions in the stratum corneum and epidermis [1, 41, 42].
Mixtures of chemical penetrators can be divided into mixtures of solvents, mixtures of permeability enhancers of different classes, eutectic mixtures, and microemulsions.
Solvent mixtures. Most transdermal drugs contain a solvent such as water, fatty acids, alcohols, glycols, and fatty esters, used in pure form. Mixing of two or more solvents is one of the most widely studied strategies for increasing drug transport through skin. The mechanisms by which such systems increase transdermal transport may include changes in the thermodynamic activity of drugs, specific interaction with the stratum corneum of the skin, and increases in drug solubility in the stratum corneum [1, 4, 13]. For example, propylene glycol is widely used as a cosolvent in many studies of propylene glycol with fatty acids, alcohols, and fatty acid esters [1, 13, 45]. For example, skin permeability for clebopride from a binary mixture of the monoethyl ester of diethylene glycol and isopropyl myristate (40:60) was 80 times higher than for isopropyl myristate alone [13]. Transdermal transport of lipophilic drugs such as estrogens can be increased by using a mixture of propylene glycol and lauric acid (90:10) [1]. Increases in skin permeability from binary solvent mixtures can additionally be improved by including a third component in the mixture. For example, increases in permeability to estradiol and acyclovir using a ternary mixture of oleic acid, laurocholine, and propylene glycol were significantly greater than with binary mixtures [1].
Mixtures of permeability enhancers from different classes. Studies have demonstrated positive results with the combined use of penetrators belonging to different classes, for example, carboxylic acids (octanoic acid or isostearic acid) and aliphatic amines (diisopropanolamine or triisopropanolamine). These substances interact with each other to form salts in the form of a so-called ionic liquid. This liquid forms a molecular complex including a hydrophilic drug and promotes transdermal transport [44]. Another example is the use of natural terpenes (citronellol, geraniol, nerol, farnesol, linalool, perillyl alcohol, menthol, borneol, and carveol) or cinnamyl alcohol with 6-(dimethylamino)hexanoic acid. The ability of these mixtures to increase theophylline and hydrocortisone transport in human skin was studied ex vivo. Citronellyl, bornyl, and cinnamyl mixtures showed properties increasing permeability (increasing the coefficient of enhancement to 82) but low cellular toxicity [25]. Simultaneous use of β-cyclodextrin with 10% urea increased the permeability of epalrestat by a factor of 300 as compared with pure substance [46].
Another type of mixture demonstrating efficacy is the eutectic mixture. Mixing of solid drugs with certain solid permeability enhancers can form eutectic mixtures which are liquid at environmental temperature and display significant penetrating ability due to high thermodynamic activity. The melting temperature of a formulation is inversely proportional to its lipophilicity and solubility in skin lipids. As a result, decreases in melting temperature lead to increases in transdermal permeability [1, 47]. In addition, the penetrator acts directly on the skin, increasing drug permeation [1]. A eutectic mixture of meloxicam and thymol (6:4) had penetration from cream more than six times higher than from a simple mixture [48]. Use of a eutectic mixture of menthol and camphor (1:1) and glabridine in microemulsions increased the transport of this hydrophobic compound more than seven-fold [49].
One effective means of increasing transdermal penetration consists of using microemulsions. Microemulsions are thermodynamically or kinetically stable transparent dispersions of two liquid phases in which one is hydrophilic and the other is lipophilic and the mixture is stabilized by an interphase film of surfactant and cosurfactant substances. Microemulsions have a number of advantages: increases in the bioavailability of poorly soluble drugs, high absorption and penetration, as well as simplicity of preparation. Drop size in microemulsions is less than 0.1 _m; drops are invisible as they are small than the wavelength of visible light (400 – 800 nm), they are unable to reflect light, and cannot be seen through an optical microscope, which makes microemulsion systems transparent [50,51,52, – 53]. The mechanism of permeability enhancement by microemulsions is linked with decreases in interphase tension, drug solubilization, and disorganization of the lipid layer of the skin with pore formation, such that transport can occur both across cells and between cells and through follicles [50,51,52, – 53].
We will present a number of examples of the composition of microemulsions. A microemulsion of minoxidil consisting of oleic acid as the oil phase, polysorbate 80 as surfactant substance, and polyethylene glycol as cosurfactant demonstrated release of more than 90% over 24 h [50]. A microemulsion using Capryol-90 as the oil phase, Cremophor EL as surfactant, and Transcutol P as additional surfactant for repaglinide demonstrated high efficacy [50]. Use of cyclodextrin and a microemulsion system consisting of oleic acid glyceride Macrogol-6 as oil phase, Solutol HS 15 (a mixture of polyglycolic mono- and diesters of hydroxystearic acid and polyethylene glycol) as surfactant, Transcutol as penetrator, and aqueous cyclodextrin solution for ketoconazole significantly increased transdermal transport [54].
In addition, a significant number of studies addressing the simultaneous use of chemical penetrators and physical methods of increasing skin permeability have been reported [7, 9, 12, 13, 55].
Overall, interest in transdermal drugs has increased in recent years and there has been an enormous number of developments in this area. Data from Scopus and Pubmed for 2019 – 2020 indicate that more than 250 reports have been published on this theme. Transdermal permeability enhancers play the decisive role in the therapeutic efficacy of these medicinal formulations. The use of chemical permeability enhancers, which have been well studied and have predictable efficacy and safety, is very relevant.
References
P. Karande and S. Mitragotri, Biomembranes, 1788(11), 2362 – 2373 (2009).
V. Mathur, Y. Satrawala1, and M. S. Rajput, Asian J. Pharmaceutics, 4(3), 173 – 183 (2010).
H. X. Nguyen and A. K. Banga, Pharmaceutics, 10(3), 1 – 18 (2018).
K. S. Paudel, M. Milewski, C. L. Swadley, et al., Ther Deliv., 1(1), 109 – 113 (2010).
P. C. Pandey, S. Shukla, S. A. Skoog, et al., Sensors (Basel), 9(5), 1028 (2019).
Y. M. Fuh, D. C. Pham, and C. F. Weng, Medicina (Kaunas), 55(5), 121 (2019); https://doi.org/10.3390/medicina55050121.
B. J. Aungst, Am. Assoc. Pharm. Sci. J., 14(1), 10 – 18 (2012); https://doi.org/10.1208/s12248-011-9307-4.
H. A. E. Benson, Drug Deliv., 3(6), 727 – 737 (2006).
H. J. Patel, D. G. Trivedi, A. K. Bhandari, D. A. Shah, IJPI J. Pharm. Cosmetol., 1(2), 67 – 80 (2011).
Z. Sidat, T. Marimuthu, P. Kumar, et al., Pharmaceutics., 11(2), 96 (2019); https://doi.org/10.3390/pharmaceutics11020096.
D. Prasanthi and P. K. Lakshmi, ISRN Pharm., 2012, 1 – 8 (2012).
A. Kováèik, M. Kopeèná, K. Vávrová, Expert Opin. Drug Deliv., 17(2), 145 – 155 (2020).
M. E. Lane, Int. J. Pharm., 15, 12 – 21 (2013).
G. N. Sharma, S. Jyotsana, K. Avinash, and D. Abha, Int. Res. J. Pharmacy, 3(5), 82 – 88 (2012).
B. Y. Ding, X. C. Fu, and W. Q. Liang, Pharmazie, No. 4, 298 – 300 (2006).
K. S. Warner, S. K. Li, andW. I. Higuchi, J. Pharm. Sci., 90(6), 1143 – 1153 (2001).
N. Roy, M. Agrawal, S. Chaudhary, et al., Int. J. Pharm. Sci. Res., 8(3), 1001 – 1011 (2019).
C. Padula, S. Pescina, S. Nicoli, et al., Pharmaceutics, 10(4), 201 – 211 (2018).
S. A. Ibrahim and K. Li, Pharm. Res., 1(27), 115 – 126 (2010).
O. G. Strusovskaya, S. V. Poroiskii, A. G. Strusovskaya, Khim.-Farm. Zh., 52(11), 3 – 8 (2018); Pharm. Chem. J., 52(11), 879 – 884 (2018);.
D. W. Osborne and J. Musakhanian, AAPS Pharm. Sci. Tech., 19(8), 3512 – 3533 (2018).
D. W. Sullivan, Jr. and S. C. Gad, M. Julien, Food Chem. Toxicol., 72, 40 – 50 (2014).
D. Prasanthi and P. K. Lakshmi, J. Adv. Pharm. Technol. Res., 3(4), 216 – 223 (2012).
M. Kopeèná, M. Macháèek, A. Novaèková, G. Paraskevopoulos, et al., Sci. Rep., 9(1), 14,617 (2019).
Q. Jiang, Y. Wu, H. Zhang, et al., Pharm. Biol., 55(1), 1592 – 1600 (2017).
A. Herman and A. P. Herman, J. Pharm. Pharmacol., 67, 473 – 485 (2014).
I. D. Gulyakin, N. A. Oborotova, V. M. Pechennikov, Khim.-Farm. Zh., 48(3), 46 – 50 (2014); Pharm. Chem. J., 48(3), (2014).
I. Som, K. Bhatia, and M. Yasir, J. Pharm. Bioallied Sci., 4(1), 2 – 9 (2012).
A. Pandey, A. Mittal, N. Chauhan, et al., Mol. Pharm. Org. Process Res., 2(113) (2014); https://doi.org/10.4172/2329-9053.1000113
N. Wonglertnirant, T. Ngawhirunpat, and M. Kumpugdee-Vollrath, Biol. Pharm. Bull., 35(4), 523 – 531 (2012).
S. A. V. Morris, K. P. Ananthapadmanabhan, and G. B. Kasting, J. Pharm. Sci., 108(11), 3640 – 3648 (2019).
S. A. V. Morris, R. T. Thompson, R. W. Glenn, K. P. Ananthapadmanabhan, et al., Int. J. Cosmet. Sci., 41(1), 55 – 66 (2019).
Q. D. Pham, S. Bjorklund, J. Engblom, et al., J. Control Rel., 232, 175 – 187 (2016).
P. Jansook, N. Ogawa, and T. Loftsson, Int. J. Pharm., 535(1 – 2), 272 – 284 (2018).
T. Loftsson, P. Jarho, M. Másson, T. Jarvinen, Expert Opin. Drug Deliv., 2(2), 335 – 351 (2005).
R. Ghanghoria, P. Kesharwani, H. B. Agashe, and N. K. Jain Drug Deliv. Transl. Res., 3(3), 272 – 285 (2013).
S. A. Al-Suwayeh, E. I. Taha, F. M. Al-Qahtani, et al., Sci. World J., 2014, 127495 (2014); https://doi.org/10.1155/2014/127495.
J. Yang, C. J. Wiley, D. A. Godwin, and L. A. Felton, Eur. J. Pharmacol. Biopharm., 69(2), 605 – 612 (2008).
M. Lundborg, C. L. Wennberg, A. Narangifard, et al., J. Control Rel., 283, 269 – 279 (2018).
N. Kanikkannan, K. Kandimalla, S. S. Lamba, et al., Curr. Med. Chem., 7(6), 593 – 608 (2000).
K. Vávrová, J. Zbytovská, A. Hrabálek, Curr. Med. Chem., 12(19), 2273 – 2291 (2005).
K. Kubota, A. Shibata, T. Yamaguchi, Eur. J. Pharmacol. Sci., 86, 75 – 83 (2016).
T. Haque, K. M. Rahman, D. E. Thurston, et al., Eur. J. Pharmacol. Sci., 121, 59 – 64 (2018).
T. Furuishi, S. Takahashi, N. Ogawa, et al., Eur. J. Pharm. Sci., 106, 79 – 86 (2017).
S. Fiala, S. A. Jones, and M. B. Brown, Int. J. Pharm., 393(1 – 2), 68 – 73 (2010).
S. Mohammadi-Samani, G. Yousefi, F. Mohammadi, F. Ahmadi, Iran J. Basic Med. Sci., 17(2), 112 – 118 (2014).
C. Liu, J. Hu, H. Sui, et al., Drug Deliv. Transl. Res., 7(2), 325 – 332 (2017).
T. Shukla, N. Upmanyu, M. Agrawal, S. Saraf, et al., Biomed Pharmacother., 108, 1477 – 1494 (2018).
A. Kogan and N. Garti, Adv. Colloid Interface Sci., 123 – 126, 368 – 385 (2016).
A. Azeem, Z. I. Khan, M. Aqil, et al., Drug Dev. Ind. Pharm., 35(5), 525 – 547 (2009).
C. M. R. R. Nastiti, T. Ponto, E. Abd, et al., Pharmaceutics, 9(4), 37 (2017).
J. Che, Z. Wu, W. Shao, et al., Eur. J. Pharmacol. Biopharm., 93, 136 – 148 (2015).
N. B. Hopf, P. Spring, N. Hirt-Burri, et al., Toxicol. Lett., 287, 122 – 130 (2018)
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 11, pp. 45 – 53, November, 2020.
Rights and permissions
About this article
Cite this article
Anurova, M.N., Demina, N.B. & Bakhrushina, E.O. Permeability Enhancers in Transdermal Delivery System Technology (Review). Pharm Chem J 54, 1162–1168 (2021). https://doi.org/10.1007/s11094-021-02336-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02336-w