Conversion of sugars into furans in acidic solution was used to quantify the carbohydrate contents in plant medicines using juice from fresh greater burdock (Arctium lappa L.) leaves as an example. Hexoses (fructose, glucose) and inulin degraded in acidic solution to form 5-hydroxymethylfurfurol (HMF) and other compounds. Fructose and sucrose could be quantified by spectrophotometry and HPLC and could also be determined spectrophotometrically after UV degradation by using the formation by acid hydrolysis of HMF and its derivatives with chromophores at 283 – 285 nm. It was found experimentally that furan derivatives (HMF) with absorption maximum at 283 nm, which was taken as the analytical wavelength, were formed via acid degradation of the juice and a solution of fructose. The carbohydrate contents in the test samples varied from 1.05 to 1.36%. The relative error of the mean of the result from the proposed method was 7.5%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acid-catalyzed conversion of hexoses into furans was described in detail in the 1960s [1]. Hexoses (fructose, glucose) and inulin were observed to degrade in acidic solution to the major derivatives 5-hydroxymethylfurfurol (HMF), 5-(hydroxymethyl)-2-furaldehyde, and 2-(2-hydroxyacetyl)-furan in addition to furfurol, 5-methyl-2-furaldehyde, isomaltol, 2-(2-hydroxyacetyl)furan formate, and 4-hydroxyhexane-2,3,5-trione [2,3,4,5]. The degradation products also included formic, acetic, and levulinic acids that were produced via HMF destruction. The component composition varied with pH [6,7,8,9].

Furans can be formed from fructose, glucose, sucrose, and ascorbic acid. Small quantities are obtained from malic and citric acids. Their amounts increase sharply as the pH decreases from 8 to 3 [10].
Two alternative hypotheses regarding the degradation mechanism of fructose to HMF were reported. In particular, these are a sequence of reactions starting with retention of the intact fructo-furanose ring (1) and one occurring mainly through open-chain intermediates (2). Existing evidence for both hypotheses was verified and argued in favor of (1). It was found that the intermediate fructo-furanosyl cation that was necessary to synthesize HMF was formed directly from sucrose via hydrolysis [11].
HMF and other components are known to form in aqueous phytoextracts and also after heat treatment. Figure 1 shows the structural formula of HMF and the mass and UV-Vis spectra that were obtained on an Agilent 1200 liquid chromatograph – mass spectrometer (HPLC-MS, LC/MS, Agilent, USA) with a diode-array detector (DAD) [12].
5-Hydroxymethylfurfurol (HMF): UV-Vis (a) and MS spectra (b ) [12].
The quantitative contents of fructose and sucrose could be estimated from the formation of HMF and its derivatives with chromophores at 283 – 285 nm that resulted from acid hydrolysis of fructose and sucrose by using spectrophotometry and HPLC [13,14,15] and also by spectrophotometry after UV degradation [16].
Alkaline degradation of the sugars differed qualitatively from acidic degradation. Acid salts were obtained from alkaline degradation of monosaccharides (D-glucose, D-fructose, D-mannose, and D-xylose) and fructo-furanosides [17, 18].
For example, alkaline degradation of D-glucose and fructo-furanosides in aqueous Ca(OH)2 solution at 100°C produced a mixture consisting of >50 components (para-saccharinic, 2-C-methylpentonic, hexametasaccharinic, isosaccharinic, glycolic, lactic, glyceric, 2-C-methylglyceric, deoxytetronoic, deoxypentonic acids, etc.). The presence in the mixture of para-saccharinic acid argued in favor of the existence of glucose-3,4-enediol as an intermediate [18,19,20].
The major components from alkaline degradation of inulin were α-D-glucosaccharinic acid in addition to α-D-isosaccharinolactone, α-D-glucosaccharinolactone, 2-deoxy-Dglycerotetronic, lactic, and lactyllactic acids [21].
Pyrolysis of inulin, glucose, and fructose in neutral and alkaline media was studied. The transformation products were furan derivatives, hexasaccharinolactones, anhydrosugars, glycolaldehyde, acetol, and dihydroxyacetone and acetic, formic, and lactic acids [22].
The goal of the research was to use acid degradation of sugars for their quantitative spectrophotometric assay in juice from fresh leaves of burdock (Arctium lappa L. and A. tomentosum L., Asteraceae). Juice obtained from fresh leaves of greater burdock that were collected in 2010 at the VILAR Botanical Garden (Moscow) were studied. EtOH up to 20% was added to the juice as a preservative. UV spectra were recorded on a Cary 100 Scan spectrophotometer (Varian, USA).
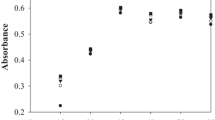
Furan derivatives (HMF) resulting from acid degradation of the juice and a fructose solution were detected experimentally (Fig. 2) at 283 nm, which was taken as the analytical wavelength.
The effect of hydrolysis time on the concentration of formed furan derivatives in fructose solution (Fig. 3) and burdock juice (Fig. 4) was investigated. The absorption maximum at 283 nm increased with increasing hydrolysis time up to 2.5 h and then fell at 3 h. This was related to subsequent HMF destruction. Thus, the optimum time for acid degradation of burdock juice and fructose was determined to be 2.5 h.
Experimental Part
Total carbohydrates recalculated as fructose in burdock juice were quantitatively determined as follows. A sample (5 g, accurate weight) was placed into a 100-mL round-bottomed flask, treated with dilute HCl (1 mL), connected to a reflux condenser, heated on a boiling-water bath for 2.5 h, cooled to room temperature, disconnected from the condenser, transferred into a 50-mL volumetric flask, adjusted to the mark with H2O, and stirred (solution A). Solution A (1 mL) was placed into a 50-mL volumetric flask, adjusted to the mark with H2O, and stirred (solution B). Optical densities of solutions were measured in a 10-mm cuvette at the absorption maximum at 283 nm. Water was used as the reference solution. The optical density of a solution of fructose working standard sample (WSS) was measured in parallel. An aqueous solution of fructose WSS (2 mL, 0.05 – 0.1%) was placed into a 100-mL round-bottomed flask, treated with dilute HCl (1 mL), connected to a reflux condenser, heated on a boiling-water bath for 2.5 h, cooled to room temperature, disconnected from the condenser, transferred into a 50-mL volumetric flask, adjusted to the mark with H2O, and stirred.
The carbohydrate contents were recalculated as fructose (X, %) using the formula:
where D is the optical density of solution B; D 0, optical density of fructose WSS solution; m WSS, mass of fructose (g); m, sample mass (g).
The carbohydrate contents in the test samples varied from 1.05 to 1.36%.
The method was validated using criteria such as linearity, precision, intralaboratory reproducibility, and accuracy. Linearity was determined for six fructose WSS concentrations in the range 6.4 – 38.6 μg/mL. The correlation coefficient was 0.989. The percent recovery varied from 97.4 to 102.17% and averaged 101.09%. This met acceptance criteria requirements. The metrological characteristics of the method were X av = 1.239; S 2 = 5.603 × 10–3; S x = 7.458 × 10–2; P x = 0.95; t(p, f) = 2.78; E av = ± 7.5%; ΔX av = 0.093; and ΔX = 0.208.
Thus, a method based on degradation of sugars into furans followed by their spectrophotometric assay was proposed for estimating the carbohydrate content in burdock juice. The method had rather simple analytical operations and satisfied validation requirements.
References
C. J. Moye, Aust. J. Chem., 19(12), 2317 – 2320 (1966).
P. E. Shaw, J. H. Tatum, and R. E. Berry, Carbohydr. Res., 5(3), 266 – 273 (1967).
M. J. Antal, Jr., and W. S. L. Mok, Carbohydr. Res., 199, 91 – 109 (1990).
H. E. Van Dam, A. P. G. Kieboom, and H. Van Bekkum, Starch / Starke, 38, 95 – 101 (1986).
B. F. M. Kuster, Starch / Starke, 42(8), 314 – 321 (1990).
J. Horvat, B. Klaic, B. Metelko, et al., Tetrahedron Lett., 26, 2111 – 2114 (1985).
S. McKibbins, J. F. Harris, J. F. Saeman, et al., For. Prod. J., 12, 17 – 23 (1962).
B. Girisuta, L. P. B. M. Janssen, and H. L. Heeres, Chem. Eng. Res. Des., 84, 339 – 349 (2006).
B. Girisuta, L. P. Janssen, B. M. Janssen, et al., Green Chem., 8, 709 (2006).
X. Fan, J. Agric. Food. Chem., 53(20), 7826 – 7831 (2005).
M. J. Antal, Jr., and W. S. L. Mok, Carbohydr. Res., 199(1), 91 – 109 (1990).
M. Herrera, M. Castro-Puyana, L. Rocamora, et al., Food Res. Int., 47(1), 31 – 37 (2012).
E. R. Garrett and J. Blanch, Anal. Chem., 39(10), 1109 – 1113 (1967).
J. Serra-Bonvehi, Sci. Aliments, 11, 547 – 557 (1995).
P. B. Andrade, M. T. Amaral, A. P. Cunha, et al., Acta Technol. Legis Medicamenti, 6, 289 – 293 (1995).
B. O. T. Roig, Anal. Chim. Acta, 477, 325 – 329 (2003).
J. M. De Bruijn, A. P. G. Kieboom, and H. van Bekkum, Recl. Trav. Chim. Pays-Bas, 106, 35 – 43 (1987).
Y. Y. Byung and R. Montgomery, Carbohydr. Res., 280(1), 47 – 57 (1996).
Y. Y. Byung and R. Montgomery, Carbohydr. Res., 280(1), 4, 27 – 45 (1996).
Y. Y. Byung and R. Montgomery, Bioresour. Technol., 98(16), 3084 – 3089 (2007).
J. N. BeMiller, T. R. Steinheimer, and E. E. Allen, Jr., Clin. Chem., 13(4), 261 – 269 (1967).
G. R. Ponder and G. N. Richards, Carbohydr. Res., 244(2), 341 – 359 (1993).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 4, pp. 38 – 40, April, 2017.
Rights and permissions
About this article
Cite this article
Kopytko, Y.F. Quantitative Determination of Total Carbohydrates (Recalculated for Fructose After Conversion to Furans) in Burdock Juice. Pharm Chem J 51, 285–287 (2017). https://doi.org/10.1007/s11094-017-1599-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1599-y