The chromatographic analysis of hydrochlorothiazide (HCT) has been carried out by simple high-performance liquid chromatography (HPLC) procedure, which has been developed for determining HCT in pharmaceutical tablets using different mobile phases in certain ratios. The chromatographic studies of variation of the HPLC detector signal depending on the mobile phase, flow rate, detection wavelength, and column temperature have been analyzed fin order to find the optimal experimental conditions for HCT determination. The developed HPLC technique has been successfully applied to quantitative analysis of HCT in its commercial tablets with a mean recovery of 94.96 ± 1.004%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
Hydrochlorothiazide, abbreviated HCT, HCTZ or HZT, belongs to the class of thiazides – drugs that act by inhibiting the ability of kidneys to retain water. This reduces the volume of the blood, decreasing blood return to the heart and thus cardiac output and, by other mechanisms, is believed to lower peripheral vascular resistance [1, 2]. HCT is mainly used for the treatment of hypertension, congestive heart failure, symptomatic edema, diabetes insipidus, and renal tubular acidosis, and for the prevention of kidney stones [3]. Chemically, hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide. The chemical formula of HCT is C7H8ClN3O4S2 and its structure is illustrated in Scheme 1.
Many published articles have been devoted to the determination of HCT in different biological fluids and pharmaceutical formulations, using various instrumental analytical techniques including spectrophotometry [4–6], chromatography [6, 7], liquid chromatography with UV detection [8], HPLC [9–16], and electrochemical methods [6, 17, 18]. However, no special articles were devoted to analysis of HCT by HPLC using a mobile phase containing methanol: water (50: 50 v/v%), other mobile phases, and studied conditions as reported in the literature. In addition, this work was done to analyze the HCT drug in order to develop a precise, reproducible, effective and sensitive HPLC method for determination HCT in pharmaceutical tablet.
2. EXPERIMENTAL PART
2.1. Apparatus
The chromatographic measurements were carried out using HPLC system (with UV–vis detector and auto sampler) UltiMate 3000 (Thermo Scientific Dionex, United States) with a 20 μL manual loop injector and C18 (5 μm) column, connected to Dell Optiplex 3010 computer (China). The chromatograms were printed via HP laser jet Pro 200 color M251n, Hewlett-Packard development company, l.p. (China). Oxford adjustable micropipette (Ireland) was used to inject micro-liter volumes of standard solutions and commercial samples of the HCT drug studied.
2.2. Materials and Reagents
In general, all HPLC solvents were of HPLC grade and other chemicals were used without additional purification. Hydrochlorothiazide was prepared as stock solution to be suitable for HPLC study. Methanol HPLC-grade 0.2 micron filtered (Fisher Scientific, product of Trinidad, New Jersey) was used for a mobile phase. Water that was used as solvent for HCT standard, HCT tablet and in the mobile phase was always distilled to be suited for this chromatographic study.
2.3. HPLC Conditions
The mobile phase initially represented a mixture of methanol, water and acetonitrile. Results showed that H2O : MeOH (50 : 50 v/v%) was the best mobile phase for the chromatographic determination of HCT. The instrumental settings were as follows: flow rate, 1 mL/min; column oven temperature, 30°C; UV detector wavelength, 254 nm; injection volume, 20 μL; HPLC column, C-18 (5 μm). Equal volumes (10 μL) of prepared solutions and samples were injected into the column. The initial HPLC running time was 5.0 min. As a result, 2.83 min was selected as optimum operator for HCT drug determination at all chromatographic measurements.
2.4. Stock Solution Preparation
Hydrochlorothiazide (Qassim Pharmaceutical Plant Buraydah, Spimaco Addwaeih, Buraydah, Saudi Arabia) stock solution of 1 × 10– 3 mol/L was prepared by dissolving the appropriate volume of HCT in distilled water in a 50 mL volumetric flask. This solution was stored in a dark place. The lower concentrations of standard solutions for HCT drug were prepared by diluting the stock solution using distilled water through analysis processes.
2.5. Tablet Sample Preparation
The HCT content of commercially available tablets (Monozide 25 containing 25 mg of HCT, Jordanian Pharmaceutical Manufacturing Co., Jordan) was purchased from local pharmacies in Saudi Arabia. Ten tablets containing 250 mg HCT were digressed and dissolved by distilled water, then transferred to 50-mL volumetric flask. When the solution was kept for enough time for HCT to be completely dissolved, it was filtered so as to be suitable for HPLC study. All chromatographic preparations and measurements were carried out at room temperature (25°C).
3. RESULTS
3.1. Chromatographic Parameters
3.1.1. Effect of mobile phase ratio. A study of the mobile phase ratio is a very important parameter for the chromatographic determination of HCT in drugs. Herein, 6 × 10– 4 M of HCT was analyzed by HPLC at different ratios of water, methanol and acetonitrile in the mobile phase for 254 nm detector wavelength as shown in Table 1.
3.1.2. Effect of flow rate. Flow rate is considered also a very important parameter for the chromatographic study of analyzed drugs. Aliquots of 6 × 10– 4 M HCT were monitored by HPLC technique over the range of flow rates from 0.5 to 3.5 mL/min (see Table 2).
3.1.3. Effect of detector wavelength. A wavelength is added as an important parameter for obtaining a chromatographic signal of the studied drug. By use of HPLC method, aliquots of 6 × 10– 4 M HCT were analyzed over the range from 200 to 254 nm (see Table 3). The HPLC instrument employed could not record signals outside this range.
3.1.4. Effect of Column Temperature. The temperature of chromatographic column is also considered an important operator for the sensitive HPLC determination of HCT. The chromatographic signal from 6 × 10– 4 M HCT was evaluated over the range from 25 to 40°C as shown in Table 4.
3.2. Optimization of the HPLC Conditions
According to the preliminary study of experimental conditions, a mobile phase containing methanol : water (50 : 50 v/v%) at 254 nm wavelength of UV detector and 1 mL/min flow rate for 2.83 min retention time, were adopted as optimum conditions for further chromatographic studies. This investigation of HCT drug analysis indicated that it was separated effectively by HPLC at these parameters and yielded a well-defined chromatographic peak for 6 × 10– 4 M HCT as shown in Fig. 1.
Thus, experiments were performed by changing the ratio of mobile phase, flow rates, detector wavelength, and column temperature. No obvious effect on the HPLC signal and retention time was produced by changing the wavelength, while changes of the mobile phase ratio, flow rate. and column temperature affected the chromatographic signals. As a result, H2O : MeOH (50 : 50 v/v%) mobile phase gave the highest absorbance at 254 nm, and it was selected as the optimum value for further chromatographic studies.
4. DISCUSSION
4.1. Validation of HPLC method
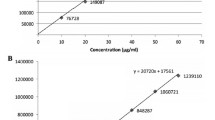
4.1.1. Linearity and calibration curve. According to optimum experimental conditions, a good linear relationship was obtained between the chromatographic signal of HCT and its concentration over the range from 2 × 10– 4 to 1 × 10– 3 mol/L (see Fig. 2). The parameters of HCT concentration – absorbance straight line were calculated by using the least-squares method. The regression equation of the calibration curve is as follows:
where Abs is the absorbance [mAU] of analyte, C is the HCT molar concentration, r 2 is correlation coefficient, and n is the number of chromatographic measurements.
4.1.2. Limit of detection. The limit of detection (LOD), defined as three times the signal-to-noise ratio (S/N = 3) reached under the optimum experimental conditions, for the monitored HCT peak was 3.2 × 10– 7 mol/L (0.095 ppm).
4.1.3. Precision (reproducibility). The precision of the developed method was evaluated from reproducibility of ten determinations of 6 × 10– 4 mol/L HCT under the optimum conditions. Relative standard deviation (RSD) of 0.181% was calculated, which indicated the reproducible separation and monitoring of the analyzed HCT drug (see Table 5).
4.1.4. Stability of HCT solution. The chromatographic signal of 6 × 10– 4 mol/L HCT was investigated by monitoring repeated HPLC measurements every 30 min. The measured chromatographic response seemed to be fixed over the studied period of time (24 h). This study demonstrates the stability of the analyzed drug under the optimum experimental conditions.
5. HPLC ANALYTICAL APPLICATION
The proposed HPLC method has been applied to determine HCT in pharmaceutical tablets. The HCT content of commercially available tablets was analyzed directly by HPLC after the dissolution and filtration steps. Five tablet samples with 5 × 10– 4 mol/L HCT content were prepared, diluted by water as solvent, and filtered to be suited for HPLC analysis. Results of this study are given in Table 6.
6. CONCLUSION
The validation HPLC method can be used for determination of hydrochlorothiazide in its commercial tablet using new operators such as mobile phase, flow rate and so forth under optimum conditions. The used chromatographic method was analytical performed in terms of linearity, detection limit, precision and stability. HPLC can be also successfully used in quality analytical control for the dissolution of this tablet consisting of HCT content and application to analysis this drug in pharmaceutical formulation.
References
B. Beermann, M. Groschinsky-Grind and A. Rosén, Clin. Pharmacol. Ther., 19, 531–537 (1976).
J. D. Duarte and R. M. Cooper-DeHoff, Expert Rev. Cardiovasc. Ther., 8, 793 – 802 (2010).
Hydrochlorothiazide, American Society of Health-System Pharmacists (2011).
N. Erk, Pharmazie, 58, 543 – 548 (2003).
R. Gangola, S. Kaushik, and P. Sharma, J. Appl. Pharm. Sci., 1, 46 – 49 (2011).
B. Burcin, G. Mehmet, T. B. Dogan, et al., J. AOAC Intern., 96, 42 – 51 (2013).
D. Ivanovic, M. Medenica, B. Jancic, et al., Acta Chromatogr., 18, 143 – 156 (2007).
S. S. Qutab, S. N. Razzaq, M. Ashfaq, et al., Acta Chromatogr., 19, 119 – 129 (2007).
I. Violeta, Z. Dragica, and S. Marina, Macedonian Pharm. Bull. , 51, 23 – 28 (2005).
G. N. Menon and L. B. White, J. Pharm. Sci., 70, 1083 – 1085 (1981).
D. Farthinq, I. Fakhry, E. B. Ripley, et al., J. Pharm. Biomed. Anal., 17, 1455 – 1459 (1998).
M. J. Cooper, A. R. Sinaiko, M. W. Anders, et al., Anal. Chem., 48, 1110 – 1111 (1976).
S. Bhagwate and N. J. Gaikwad, J. Appl. Pharm. Sci., 3, 88 – 92 (2013).
I. F. Al-Momani, Turk. J. Chem., 25, 49 – 54 (2001).
S. Sahoo, P. K. Panda, S. K. Mishra, et al., Asian J. Pharm. Clin. Res., 5, 136 – 138 (2012).
S. B.Wankhede, M. R. Tajne, K. R. Gupta, et al., Ind. J. Pharm. Sci., 69, 298 – 300 (2007).
M. B. Gholirand and M. Khodadadian, Electroanalysis, 25, 1263 – 1270 (2013).
O. Abdel Razak, J. Pharm. Biomed. Anal., 34, 433 – 440 (2004).
ACKNOWLEDGEMENTS
The author would like to thank Mr. Awad Alqarni and Ahmad Alribdy at Qassim Pharmaceutical Plant Buraydah (Spimaco Addwaeih, Saudi Arabia) for their assistances and supplying standard drug.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alghamdi, A.F. Quantitative Analysis of Hydrochlorothiazide and Its Determination in a Pharmaceutical Preparation by HPLC. Pharm Chem J 48, 843–847 (2015). https://doi.org/10.1007/s11094-015-1207-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1207-y