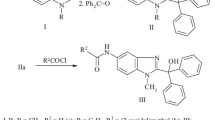
Alkylation of 2-aminobenzimidazole by methyliodide and benzyl- or 2-aryloxyethylbromides produced 1-substituted 2-aminobenzimidazoles that were quaternized by chloroacetamide to previously undescribed 3-aryloxyethyl(benzyl)-1-carbamoylmethyl-2-iminobenzimidazoline hydrochlorides. These compounds were shown to possess antibacterial activity against several pathogenic Gram-positive and Gram-negative microbes (Staphylococcus aureus, Escherichia coli) combined with pronounced protistocidal activity against the protozoa Colpoda steinii that was on the level of the clinical reference drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Resistance of pathogenic microorganisms to antibiotics qualified by the WHO is one of the greatest threats to human health [1–5]. About 25,000 patients per year die of multidrug resistant infections, including nosocomial ones, even in the relatively safe European Union. The problem is exacerbated by the emergence of multi-drug resistant bacterial strains that are unaffected by the majority of commercial antibiotics. Therefore, the discovery of new natural and synthetic antibacterial drugs is one of the most important tasks of modern science. It combines the efforts of organic chemists, microbiologists, physicians, and other specialists.
Benzimidazoles are known to include many compounds with antimicrobial activity [6–11]. They comprise 2-unsubstituted 1-ω-aryloxyalkylbenzimidazoles that are active against Staphylococcus aureus and Salmonella typhi [12]. Recently, 2-ureidobenzimidazoles were used as examples to study the mechanism of antimicrobial action of benzimidazoles that were highly effective against Gram-positive pathogenic microbes. The activity was related to inhibition of two key bacterial enzymes, DNA-gyrase and DNA-topoisomerase [6, 7].
We patented earlier salts of 1-β-aryloxyethyl-3-benzyl-2-iminobenzimidazolines, which exhibited high antimicrobial activity against Gram-positive bacteria (S. aureus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus sp., Corynebacterium sp.). Several of them were more active against S. aureus than antibiotics such as ampicillin and oxytetracycline [13].
In continuation of this research, herein we report new functionalized derivatives of 2-iminobenzimidazoline, salts of 3-aryloxyethyl(benzyl)-1-carbamoylmethyl-2-iminobenzimidazolines (I), and their activity against S. aureus, Escherichia coli, and the ciliate Colpoda steinii.
Amides Ib-i were synthesized in 70-80% yields by quaternization of already known [13–15] or newly synthesized 1-aryloxyethyl(benzyl)-2-aminobenzimidazoles (II) using chloroacetamide in DMF at 120°C. The simplest 3-substituted 1-carbamoylmethyl-2-iminobenzimidazoline, 3-methyl-1-carbamoylmethyl-2-iminobezimidazoline hydrochloride (Ia), was also synthesized and studied for comparison.
The structures of amides I were confirmed by PMR spectra and mass spectrometry.

Experimental Chemical Part
PMR spectra of the synthesized compounds in DMSO-d6 or CDCl3 were recorded on a Varian Unity-300 (300 MHz) spectrometer. Chemical shifts of protons were given relative to the residual deuterated solvent resonance. Mass spectra were measured on a Finnigan MAT INCOS 50 instrument with direct sample introduction into the source at ionization energy 70 eV. Elemental analyses of amines II and salts I agreed with those calculated.
2-Amino-1-methylbenzimidazole (IIa) was prepared by the literature method [14]; 2-amino-1-(2-phenoxyethyl)-benzimidazole (IIc), by the literature method [15]; 1-[2-(4-methylphenoxy)ethyl]-2-aminobenzimidazole (IId) and 1-[2-(4-chlorophenoxy)ethyl]-2-aminobenzimidazole (IIg), by the literature method [13].
2-Amino-1-(2-fluorobenzyl)benzimidazole (IIb). A mixture of 2-aminobenzimidazole (1.31 g, 10 mmol) and KOH (0.67 g, 10 mmol) (calculated as 85% KOH) in DMSO (15 mL) was stirred at 35°C for 15 min, treated with 2-fluorobenzylbromide (2.08 g, 11 mmol) at a rate such that the temperature remained <50°C, held for 30 min at 50°C, cooled, and treated stepwise with H2O (40 mL). The resulting voluminous precipitate of amine IIb was filtered off and washed with KOH solution (5%, 2 mL) and H2O. Yield 1.74 g (72%). White plates, mp 184-185°C (MeCN). C14H12FN3. PMR spectrum (300 MHz), δ, ppm (CDCl3)1: 4.65 (br.s, 2H, NH2), 5.19 (s, 2H, CH2), 7.03 – 7.16 (m, 6H, 3′-H, 4′-H, 5′-H, 6′-H, 5-H, 6-H), 7.26 (d, 1H, 7-H), 7.44 (d, 1H, 4-H)
2-Amino-1-(2-chlorobenzyl)benzimidazole (IIc) was synthesized analogously to amine IIb from 2-minobenzylimidazole and 2-chlorobenzylbromide in 77% yield. Beige crystals, mp 157 – 159°C (EtOH). C14H12ClN3. PMR spectrum (300 MHz), δ, ppm (CDCl3): 4.70 (br.s, 2H, NH2), 5.22 (s, 2H, CH2), 7.00 – 7.22 (m, 6H, 3′-H, 4′-H, 5′-H, 6′-H, 5-H, 6-H), 7.25 (d, 1H, 7-H), 7.48 (d, 1H, 4-H).
2-Amino-1-[2-(4-methoxyphenoxy)ethyl]benzimidazole (IIf). A solution of 2-aminobenzimidazole (1.33 g, 10 mmol) and KOH (1.3 g, 20 mmol) (calculated as 85% KOH) in H2O (1.5 mL) was stirred vigorously, treated with Me2CO (10 mL) and in portions with 2-(4-methoxyphenoxy)-ethylbromide (2.54 g, 11 mmol) so that the temperature remained <40°C, stirred for 2.5 h at 45°C, and cooled. The acetone layer was separated, evaporated to half the volume, and poured into H2O (20 mL). The resulting precipitate was filtered off and washed with H2O (5 mL). Yield, 1.90 g (67%). Pale-pink crystals, mp 150 – 151°C (i-PrOH). C16H17N3O2. PMR spectrum (300 MHz), δ, ppm (CDCl3): 3.72 (s, 3H, Me), 4.24 (t, 2H, NCH2), 4.34 (t, 2H, OCH 2), 5.05 (s, 2H, NH2), 6.74 – 6.80 (m, 4H, ArO), 7.05 – 7.18 (m, 3H, 5-H, 6-H, 7-H), 7.45 (d, 1H, 4-H).
2-Amino-1-[2-(4-bromophenoxy)ethyl]benzimidazole (IIh) was prepared analogously to IIf from 2-aminobenzimidazole and 2-(4-bromophenoxy)ethylbromide in 79% yield. Colorless crystals, mp 167 – 168°C (i-PrOH). C15H14BrN3O. PMR spectrum (300 MHz), δ, ppm (CDCl3): 4.25 (t, 2H, NCH2), 4.38 (t, 2H, OCH2), 5.20 (br.s, 2H, NH2), 6.74 (2H, 3′-H, 5′-H), 6.82 – 6.95 (m, 2H, 5-H, 6-H), 7.20 – 7.38 (m, 3H, 2′-H, 6′-H, 7-H), 7.45 (d, 1H, 4-H).
2-Amino-1-[2-(1-naphthoxy)ethyl]benzimidazole (IIi) was prepared from 2-aminobenzimidazole and 2-(1-naphthoxy) ethylbromide analogously to IIb in 79% yield. Colorless crystals, mp 234°C (nitromethane). C19H17N3. PMR spectrum (300 MHz), δ, ppm (CDCl3): 4.48 (s, 4H, 2CH2), 4.90 (br.s, 2H, NH2), 6.80 (d, 1H, 8′-H), 7.06 – 7.20 (m, 3H, 5-H, 6-H, 2′-H), 7.30 – 7.48 (m, 5H, 3′-H, 4′-H, 5′-H, 6′-H, 7′-H), 7.78 (d, 1H, 7-H), 8.05 (d, 1H, 4-H).
Hydrochlorides of 3-aryloxyethyl(benzyl)-1-carbamoylmethyl-2-iminobenzimidazolines (I). General method. A solution of 1-substituted 2-aminobenzimidazole (II, 10 mmol) in DMF (20 mL) at 80°C was treated with chloroacetamide (0.94 g, 10 mmol) and held at 120°C for 1 h. The precipitate that formed on cooling was filtered off, washed with EtOH, and recrystallized from DMF (Ia-c and Ii) or a mixture (1:1) of DMF and EtOH (Id-h). Table 1 presents the yields, melting points, and PMR spectra of the synthesized compounds.
Experimental Biological Part
Antimicrobial activity of Ia-i was studied using standard strains S. aureus ATCC 25923 and E. coli ATCC 25922 and literature methods [16, 17] for double serial dilutions in Luria—Bertani (LB) liquid agar growth medium. A suspension of bacteria (2 mL) at a concentration of 5 × 105 microbes/mL was placed into tubes with a solution (2 mL) of the test compound at various concentrations. Thus, the calculated microbe load was 250,000 microbes per mL. The tubes were incubated in a thermostat for 18 h at 37°C. The controls were tubes containing growth medium with bacteria at a concentration of 250,000 microbes per mL (positive control). The negative controls were tubes with growth medium (without bacteria). The test organisms were bacteria strains E. coli 078 (field strain) and S. aureus P-209. Table 2 lists the antibacterial activity of amides I.
Protistocidal activity of amides I against the protozoa C. steinii was studied using the previously developed method [18].
The activity of the compounds was determined in decreasing dilutions from 1000.0 to 0.5 g/mL with a constant load of 3-day protozoa culture. The viability control of the culture was medium used to prepare the dilutions. The reference drug was Baycox (Bayer AG), which is a widely used anticoccidial. Table 3 presents the protistocidal activities.
The antibacterial activity of amides I was significantly less than that of the reference drugs and the 1-β-aryloxyethyl-2-iminobenzimidazolines reported previously [13]. Amides Ie and Ig-i were the most active against S. aureus; amide If, which contained a 3-aryloxyethyl substituent, against E. coli. Amide Ig with a p-chlorophenoxyethyl group had the most pronounced protistocidal activity, which was at the level of the known protistocidal drug Baycox (Bayer AG).
Thus, the results led to the conclusion that phenoxyethyl-(benzyl)-substituted 2-iminobenzimidazolines were promising for discovering antimicrobial drugs, including for veterinary use, with combined (antibacterial and protistocidal) activity for treating diseases caused by simultaneous infection by bacteria and protozoa.
References
Urgently needed: new antibiotics (Editorial), Lancet, 374, 1868 (2009).
S. Vento and F. Cainelli, Lancet, 375, 637 (2010).
L. C. Panasevich, Nation’s Health, 34(7), 8 (2004).
D. Bald and A. Koul, Drug Discovery Today, 18, 250 – 255 (2013).
R. G. Wax, K. Lewis, A. A. Salyers, and H. Taber (eds.), Bacterial Resistance to Antimicrobials, 2nd Ed., CRC Press, Taylor & Francis Group, Boca Raton, FL (2008).
P. S. Charifson, et al., US Pat. Appl. No. 20050256136 A1, Nov. 17, 2005; http://docs.google.com/a/google.com/viewer?url=www.google.com/patents/US20050256136.pdf; [https://www.google.com/patents/US20050256136?dq=us+20050256136&hl=en&sa=X&ei=8vBcVKm2BND0oATzvILIDw&ved=0CB4Q6wEwAA]
P. S. Charifson, A.-L. Grillot, T. H. Grossman, et al., J. Med. Chem., 51, 5243 – 5263 (2008).
N. Singh, A. Pandurangan, K. Rana, et al., Int. Curr. Pharm. J., 1(5), 119 – 127 (2012).
Y. Bansal and O. Silakari, Bioorg. Med. Chem., 20, 6208 – 6236 (2012).
B. L. Fuente, T. B. Sonawane, B. Arumainayagam, et al., Br. J. Pharmacol., 149, 551 – 559 (2006).
R. Walia, M. Hedaitullah, S. F. Naaz, et al., Int. J. Res. Pharm. Chem., 1(3), 565 – 574 (2011).
A. Khalafi-Nezhad, M. N. Soltani Rad, H. Mohabatkar, et al., Bioorg. Med. Chem., 5(13), 1931 – 1938 (2005).
A. S. Morkovnik, L. N. Divaeva, et al., RU Pat. No. 2,423,355, Jul. 10, 2011; Byull. Izobret., No. 19 (2011).
A. F. Pozharskii, V. A. Anisimova, and E. B. Tsupak, Practical Studies in Heterocyclic Chemistry [in Russian], Izd. Rost. Univ., Rostov-on-Don (1988), p. 105.
A. S. Morkovnik, L. N. Divaeva, K. A. Lyssenko, et al., Mendeleev Commun., 133 – 136 (2005).
L. S. Strachunskii and S. N. Kozlov, Modern Antimicrobial Chemotherapy: Handbook for Physicians [in Russian], Moscow (2002).
G. N. Pershin, Methods of Experimental Chemotherapy (Practical Handbook), Moscow (1971), pp. 100 – 106.
A. A. Zubenko, L. N. Fetisov, A. N. Bodryakov, et al., in: Abstracts of Papers of the All-Russian Scientific-Practical Conference “Science-based Innovative Development for Domestic Animal Husbandry” [in Russian], Novocherkassk (2011), pp. 162 – 165.
Acknowledgments
The work used equipment at the CCU “Molecular spectroscopy” of the IPOC, SFU, and was performed in part under the auspices of State Task, Project No. 4.196.2014/K.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 48, No. 10, pp. 29 – 32, October, 2014.
Rights and permissions
About this article
Cite this article
Divaeva, L.N., Morkovnik, A.S., Zubenko, A.A. et al. Synthesis, Antimicrobial, and Protisticidal Activity of 3-Aryloxyethyl(Benzyl)-1-Carbamoylmethyl-2-Iminobenzimidazoline Hydrochlorides. Pharm Chem J 48, 661–664 (2015). https://doi.org/10.1007/s11094-015-1165-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1165-4