Modern analytical methods used to study the pharmacokinetics of quercetin and other flavonols, i.e., biologically active compounds that exhibit various therapeutic properties, were reviewed. The preparation of biological fluids and tissues for analysis, chromatography conditions, and mass spectrometric detection for various flavonols as the aglycons and glycosides and their metabolites were discussed. Quantitative analyses of flavonol concentrations in biological samples and their mass spectrometric identification in in vitro and in vivo studies during tests with pure compounds and multi-constituent plant extracts were presented as typical examples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Flavonols are a subclass of flavonoids, a varied and broad class of polyphenolic compounds that includes the flavonol quercetin, one of the most widely distributed and studied representatives. These compounds were well known over 100 years ago as plant pigments. However, the discovery in the 1990s of the antioxidant properties of flavonoids stimulated renewed interest in them. They are capable of neutralizing free radicals, which is responsible for their various therapeutic properties [1, 2].
A pharmacokinetic (PK) study in vivo in humans and experimental animals, i.e., absorption, distribution, metabolism, and elimination, is a necessary component of the evaluation of the mechanisms of possible therapeutic or toxic action of a biologically active compound. The PK parameters of drug candidates are related to the effectiveness and administration mode in order to establish the optimum concentrations.
The properties of quercetin and other flavonols were briefly characterized and the development in the last decade of methods for determining the concentrations and identifying flavonols in biological media during PK studies were discussed in the review.
Structure, properties and natural sources of flavonols
Flavonoids are secondary plant metabolites and share common biosynthetic pathways. They are responsible for the color of fruits and leaves. Surface tissues of plants are especially rich in them [3]. They are involved in the photosynthesis, proliferation, and death (through apoptosis) of plant cells. It is assumed that the main biological role of flavonoids is to protect plants from aggressive environmental factors (UV radiation, infectious agents).
The variety of flavonoids is explained by the fact that most of them occur in plants as O- or C-glycosides. The aglycon is a 15-carbon skeleton, the principal elements of which are two aromatic rings joined by a pyran ring. The last determines to which subclass one flavonoid or another belongs. The classification includes the subclasses flavones, flavonols, flavanones (catechins), anthocyanidins, isoflavones, and flavanonols [4].
Quercetin belongs to the flavonol subclass (Fig. 1), which comprises compounds with a hydroxyl in the third position and a C2=C3 double bond, and contains hydroxyls in the 3, 5, 7, 3′, and 4′ positions. Other flavonol representatives are kaempferol (hydroxyls in the 3, 5, 7, and 4′ positions), myricetin (hydroxyls in the 3, 5, 7, 3′, 4′, and 5′ positions), morin (hydroxyls in the 3, 5, 7, 2′, 4′ positions), fisetin (hydroxyls in the 3, 7, 3′, and 4′ positions), etc. [5]. One of the hydroxyls can be methylated or acetylated. The O-methyl quercetin derivatives include rhamnetin (7-position), isorhamnetin (3′-position), azaleatin (5-position), and tamarixetin (4′-position); an O-methyl kaempferol derivative is kaempferide (4′-position).
Flavonol glycosides (Table 1) differ in the position, type, and number of bonded glycosides. O-Glycosides are usually bonded through the 3- or 7-hydroxyls; C-glycosides, to the 6- or 8-C atom. The carbohydrate residues are most often rhamnose, glucose, and arabinose [6].
Flavonols occur in many food products. These include onion, broccoli, tea, red wine, olive oil, grapefruit, various berries (cranberry, strawberry, blueberry, etc.). It is thought that the daily requirement in the central European diet is 20 – 35 mg [7]. The flavonols quercetin and kaempferol were observed in the medicinal plants Azadirachta indica, Cannabis sativa (cultivated hemp), and Desmodium canadense; flavonol glycosides, in Bauhinia monandra, Ginkgo biloba, Acalypha indica, Betula pendula (silver birch), etc. [8–10].
The ability to bind directly to free radicals to form less reactive compounds is the principal mechanism of flavonoid antioxidant activity. The following structural elements of quercetin are considered to be most important for antiradical activity [8]: 1) two OH groups in the C3′- and C4′-positions; 2) a C2=C3 double bond together with a C4 carbonyl, which is required to delocalize the unpaired electron from ring B; and 3) C3-OH and C5-OH groups together with a carbonyl.
Furthermore, flavonols, especially quercetin, readily form complexes with iron, which can explain the altered activity of many Fe-containing enzymes after reaction with quercetin [11]. Thus, quercetin structural features (several electron-donating groups) mediate both the pronounced antioxidant activity and the ability to influence the synthesis and activity of various enzymes, in particular, to inhibit inflammation mediators.
Flavonols exhibit various therapeutic effects [12] including anti-inflammatory, neuroprotective, anti-atherosclerotic, anticarcinogenic, and others. The anti-inflammatory effect is one of the principal ones and induces other effects.
Analytical methods for biological samples from PK studies of flavonols
The properties and structures of flavonoids have been studied for over 70 years. Such interest is fully understandable considering their broad spectra of biological activity. The number of publications focused on the analysis of plant and animal biological matrices in order to identify and determine quantitatively the contents of flavonoids and their metabolic products increased in parallel with the discovery of new pharmacological properties in various representatives of this compound class [13].
Input data for calculating the PK parameters (absolute and relative bioavailability, maximum concentration and time to reach it, clearance, elimination half-life) are the concentration of the compound and/or its metabolite that are measured at various time points in one biological fluid or another or tissue. The main analytical method for studying PK that provides sufficient sensitivity and selectivity for analyzing complicated biological matrices has been for the last decade liquid chromatography, i.e., high-performance (HPLC) and ultra-performance (UPLC) liquid chromatography; for the last 10 – 15 years, a hybrid method combining chromatography and mass spectrometry [14].
Sample preparation. The first step in biological sample analysis is sample preparation. The principal biological matrix for studying PK in vivo in animals and man is blood plasma and less often urine. In some instances, the contents of the test drugs in liver, kidneys, brain, stomach, etc. must be determined. The matrix in in vitro studies can be culture medium containing cells (hepatocytes, microsomes, intestinal epithelial cells) or biomolecules (proteins, DNA).
Three main sample preparation methods are used before injection into the chromatographic system. These are precipitation of proteins (PP), liquid—liquid extraction (LLE), and solid-phase extraction (SPE) [15]. The PP method uses denaturation by a solvent and is universal and simple. The solvent (MeOH, MeCN) is added. The mixture is stirred vigorously on a vortex stirrer and centrifuged. Then, the supernatant is either injected immediately into the instrument or concentrated further by evaporation and dissolution in another solvent with a composition close to the eluent in the subsequent analysis. The PP gives satisfactory results in most instances for routine analyses. The degree of flavonol extraction is ~75% (Table 2) [16–19]. The version involving precipitation by an inorganic compound that was used before [20] (HClO4, 115% degree of extraction) is encountered extremely rarely.
LLE is based on the distribution of molecules between immiscible liquids and is carried out by approximately the same scheme. The extractant for quercetin and related compounds is primarily EtOAc, which is acidified with ascorbic acid, HCl, or other acids in order to stabilize the constituents. The EtOAc evaporation step and dissolution in a solvent with a composition similar to the chromatography eluent is essential [21–25]. The extract from LLE is usually purer than that from PP. The degree of extraction is about 70%.
SPE is based on adsorption on a solid sorbent in chromatographic mode. It gives an even purer extract. However, it is more labor-intensive because is usually includes several sequential elutions of the sorbent with aqueous organic solvents. These are 1) conditioning; 2) sample deposition; 3) elution of interfering constituents by a weak solvent; and 4) elution of the desired constituents by a strong solvent, e.g., MeOH. Sorbents based on C18 are used most often. The process can be carried out on cheap short columns packed with sorbent (cartridges) and in 96-well plates. Rat serum was prepared on Oasis HLB cartridges during screening of bioactive constituents from Moutan Cortex plant preparation, which contained flavonols among others [26]. Sep-Pak C18 cartridges were used to prepare rat urine samples for analysis after oral administration of cranberry extract, which contained quercetin, kaempferol, quercetin glycosides, and other flavonoids [19].
SPE is often used as a secondary and additional purification step, e.g., after preliminary ultrafiltration [27]. Three isoflavonoid glycosides were determined in rat plasma after oral administration of Astragalus mongholicus extract [28]. Plasma proteins were first precipitated by MeOH. Then, the supernatant was purified over a SPE cartridge.
Tissues are the most complicated matrix to prepare. Not only plasma but also liver and kidney tissues and stomach contents and tissues of rats were analyzed during a detailed study of quercetin metabolism [29]. GI tract tissues were rinsed and homogenized in MeOH and phosphate buffer (1:1, 0.1 M, pH 7.0) with added diethyldithiocarbamate antioxidant (20 mM). The homogenate was extracted (3×) with this solution. The three supernatants after centrifugation were combined and evaporated in vacuo. The remaining aqueous solution was extracted with EtOAc and purified by SPE over C18 Sep cartridges using MeOH. The MeOH and EtOAc fractions were combined and evaporated to 500 μL.
The sample preparation requirements can be relaxed because of the more selective detection of tandem mass spectrometry (see below). Rat urinary bladder tissue was frozen in liquid N2, homogenized, and extracted once with H2O:MeOH (20:80) with HOAc (0.1%) [19]. Analysis of the supernatant detected non-conjugated quercetin and isorhamnetin after prolonged administration of cranberry extract although these components were not observed in the urine.
Chromatography. The next step required to obtain an analysis is good chromatographic separation of the desired constituents [30]. Primarily C18 reversed-phase sorbents are used for analytical determination of flavonols and other polyphenol compounds. Table 2 presents the commercial names of the sorbents that were used by various researchers. They all were C18 sorbents although their properties could vary somewhat. In several instances, in particular during determination of flavonol metabolites, more polar phases are recommended. Thus, a mixture of metabolites of four constituents (quercetin, rutin, isoquercitrin, and taxifolin), which was obtained by incubation in vitro with two types of human and rat hepatocytes as a cell suspension or primary culture, was separated [31]. Better results were obtained over silica gel chemically modified with cyanopropyl or phenyl groups than over a sorbent modified with C8 or C18 groups. Typical HPLC analysis conditions were column length 50 – 250 mm, diameter 2 or 4.6 mm, sorbent of standard size 5 or 3 μm, aqueous organic eluent (MeOH, MeCN) with added formic acid (0.1%) with a mass spectrometric detector or HOAc or PA if a diode-matrix detector was used (Table 2). If one or two known compounds are analyzed, then isocratic elution (with a constant solvent composition) is sufficient.
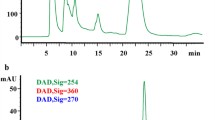
Figures 2 and 3 show HPLC chromatograms of human and rat plasma, respectively. The former was from administering tea, which contains quercetin, to a volunteer; the latter, after oral administration of H. japonicum extract, which contains bioflavonoids. The analyses took 30 – 40 min for quantitative determination in the first instance of one constituent; in the second, two (data published in 2004 and 2008).
Gradient elution with the content of the organic component varying over broad values was used to analyze mixtures of compounds with highly different polarities (plant pharmaceutical preparations, food products) or to determine different metabolites [27]. The HPLC analysis time could reach 120 min [29].
Recently, HPLC methods are gradually being supplanted by the next generation of chromatographic methods, UPLC, which are performed over columns of finer sorbents (1.7 μm). As a result, the efficiency, sensitivity, and time of the chromatographic analysis are improved. However, the back-pressure to eluent flow increases simultaneously. For this reason, solvent delivery systems (pumps) with increased working pressures (>1000 atm, HPLC maximum pressure is 400 atm) are required.
The new capabilities of UPLC (in combination with mass spectrometric detection) are being applied to the analysis of food and drug plant raw material containing flavonoids and biological fluids and tissues containing constituents of this raw material after assimilation into the organism. Thus, constituents from complex mixtures of plant drugs in biological samples were identified using the separating power of UPLC systems. The analysis time was reduced by 3 – 5 times and was 10 – 15 min [19, 26, 32].
Figure 4 shows a UPLC chromatogram with a QqTOF mass detector. Rat serum was analyzed after oral administration of Moutan Cortex extract. A total of 46 individual peaks including the flavonols quercetin, kaempferol, and others were recorded during 18 min of analysis. Of these, 16 were identified as starting constituents in the raw-material extract; 7, as their metabolites.
Detection. All flavonoid aglycons absorb well UV radiation. Therefore, spectrophotometric or diode-matrix detectors provide good sensitivity. These were for a long time the most common methods for quantitation. However, they are still sometimes used if the analytical requirements are not too high, e.g., the determination of one or two constituents or several at detectable concentrations. For example, the mutual influence of quercetin and kaempferol on transport through cellular membranes in vitro was examined [7]. The detection limit of 50 ng/mL for a UV detector was adequate for the problem. A diode-matrix detector was used to study in vitro the permeability of pig skin stratum corneum for quercetin, rutin, and other flavonoids [35].
A lower detection limit of 1.5 pg (recalculated as 15 mg/mL of sample) was reached with electrochemical detection using the redox properties of the phenolic compounds [24]. Figure 2 shows a chromatogram of blood plasma from volunteers who drank green tea after enzymatic hydrolysis of glucuronides and quercetin sulfates.
However, the sensitivity and selectivity of the usual detection methods are often insufficient. The composition of biological samples can vary. Certain constituents can interfere with the determination of the principal compounds. This is especially applicable to bioflavonoids because plant raw material containing them contains a large amount of varied chemical compounds [36, 37]. It often becomes necessary to determine immediately low therapeutic concentrations of several minor constituents. Metabolites, the concentrations of which are less than the starting compounds, are especially difficult to identify.
LC-MS is capable of double selectivity and specificity for this type of analysis. Analyte molecules in the solvent stream from the chromatography column are ionized in the device source. The resulting ions are directed into the mass analyzer operating under high vacuum [30]. Flavonoids are usually ionized well to form negative molecular ions [M –H]−. Positive-ion mode for [M + H]+ is used only for a few derivatives [20, 33].
Electrospray sources are used most often because of their high stability and efficiency. The effect of the mobile-phase composition, namely the formic acid content in it, on the efficiency of forming negative ions by electrospray was studied using quercetin and kaempferol as examples [21]. It was found that the sensitivity for detecting the constituents (triple quadrupole in MRM mode) was directly related to their ionizability and increased several times for acid concentration 0.001%, i.e., two orders of magnitude less than the usual value of 0.1%. In other work [38], the ionization conditions in sources from various equipment manufacturers were compared. The apparatus conditions in addition to the formic acid concentration were found to affect the sensitivity for flavonols and lactones in blood plasma. It was shown that ascorbic acid added to stabilize the samples interfered.
The ions pass from the source into the mass analyzer, where they are separated according to m/z values, where m is the mass of the ion and z, its charge. The most important of existing analyzers for biological samples are the quadrupole (Q), time-of-flight (TOF), and ion trap (IT) [30]. The analyzer can record all produced ions (scanning) or only single ones (selective ion monitoring, SIM), for which the selected ions are detected with higher sensitivity than for scanning.
Figure 5 shows chromatograms from analyses of rat small-intestine tissue using a diode-matrix detector and ion trap in [M – H]− negative-ion SIM mode for quercetin (301 Da) and its sulfate (381 Da). The mass spectrometric detection allowed peaks to be identified and the selectivity to be increased although the sensitivity was not increased very significantly.
Tandem mass spectrometry (MS/MS) represented a further advancement of mass spectrometric detection. Such detectors contain not one but two sequentially connected mass analyzers with an ion-fragmentation chamber between them. The triple quadrupole (two quadrupoles and a co-impact chamber, QqQ), quadrupole with ion trap (QqIT), and quadrupole with time-of-flight (QqTOF) analyzer are the most important for analysis of biological samples. Ions are separated in both analyzers. This allows special recording modes to be used, including scanning of fragment ions. It is used to identify and determine ion structures.
The so-called multiple reaction monitoring (MRM) mode, which is performed in MS/MS triple quadrupole detectors, records fragment ions in the second analyzer that are formed from certain (in the first analyzer) parent molecular ions. Double selectivity, for the parent ion and its daughter fragment, occurs under these conditions. This enables lower detection limits of 1 ng/mL and lower to be obtained in routine analyses while satisfying the requirements for precision and accuracy. Analysis in MRM mode has been for 20 years and remains today the “gold standard” for quantitative analysis of biological samples.
Quantitative analysis by LC-MS/MS
Examples of the use of MRM modes for quantitative analysis of pure substances and flavonols are numerous and varied. For example, culture liquids were analyzed in in vitro studies of flavonol transport through cell membranes (mutual influence of the components [17]). Animal and human plasmas were analyzed in preclinical and clinical PK studies, respectively, including for various administration pathways [18, 20] and also for new synthetic flavonols [33].
However, most recent analyses have been connected with studies of bioavailability and other PK parameters of biologically active flavonols ingested with food products and plant medicinal preparations such as traditional Chinese medicines. Methods for simultaneous quantitative analysis of biological fluids containing many components of plant raw material including flavonol aglycons (quercetin, kaempferol) and glycosides (rutin, quercitrin, etc.) were developed. Thus, quercetin and kaempferol concentrations were determined in dog plasma after oral administration of Tian-Bao-Ning capsules (based on Gingko biloba) [21]; quercetin, after administration of Feng-Liao-Chang-Wei-Kang granules [39]; quercetin, kaempferol, rutin, isoquercitrin, isorhamnetin, and astragalin, after oral administration of raspberry leaf extract [37]; and quercetin, kaempferol, rutin, and other constituents after administration of Fructus sophorae fruit [40].
A method for quantitative analysis of two flavonols (quercetin and kaempferol) and three phenolic acids (oleanolic, p-coumaric, and ferulic) in rat plasma was reported [25]. The method was used to study the PK of compounds after oral administration of Oldenlandia diffusa extract. Figure 6a and 6b show mass spectra of the fragmentation of negative molecular ions [M – H]− of quercetin and kaempferol; Fig. 6c and 6d, MRM chromatograms of rat plasma 45 min after oral administration of the extract. The MRM transitions 300.9 > 151.0 and 284.8 > 184.9 were selected for the quantitative analysis. Interference from other sample components was minimal in this mode. The lower limit of quantitation in plasma was 0.5 ng/mL.
MS/MS negative-ion fragmentation spectra of quercetin (a) and kaempferol (b); fragmentation energy 26.7 and 38 eV, respectively. MRM-chromatograms of rat plasma 45 min after oral administration of Oldenlandia diffusa extract, quercetin (c) and kaempferol (d), injected equivalent quercetin (17.1 mg/kg) and kaempferol (2.4 mg/kg) [25].
All quantitative analytical methods used for PK studies were completely validated. Calibration curves were constructed in the required concentration range. The specificity, sample stability, precision, accuracy (better than 15%), and degree of extraction during sample preparation were determined [20, 25, 37, 40]. Special attention was paid to confirming that matrix effects (effect of ballast endogenous constituents on the results) were not important and to selecting an internal standard (a compound with a structure close to that of the analyte or the same molecule labeled with a stable isotope) required for the quantitative measurements.
Identification in biological samples of starting plant raw material constituents and their metabolites
UV detection and single-stage mass spectrometers have rather limited capabilities for identifying compounds. NMR spectroscopy is attractive for reliable identification and structure elucidation, for example, for identifying 14 quercetin phase II mono- and mixed conjugates in in vitro metabolic studies [41].
The metabolism of quercetin and kaempferol in rat hepatocyte culture was the subject of one of the first studies (2002) where LC-MS was used to establish flavonol metabolites [42]. It was shown that both compounds were almost completely metabolized after incubation. Four quercetin glucuronides and two kaempferol glucuronides were detected. It was found that the same compounds were formed during incubation with hepatocytes as during incubation with the UDP-glucuronosyltransferase isoform UGT1A9. Also, plasma from volunteers after administration of capsules with G. biloba, a plant rich in flavonol glycosides, was analyzed. Glycosides and not glucuronides of quercetin and kaempferol were observed, i.e., it was confirmed that unaltered flavonol glycosides were absorbed without cleaving them to the aglycons.
Metabolic transformation pathways of pure flavonol were established not only during research on in vitro models, animal and human hepatocytes [33, 41, 42] and bacterial medium [34], but also in vivo using experimental animals [16, 19] and man [16, 43]. Not only plasma and urine but also internal tissues from the GI tract, liver, kidneys, etc. were analyzed in the animal experiments [29].
Quercetin metabolism in rats was compared for different administration pathways, intragastric and with food [16]. HPLC-UV analysis detected a greater variety of metabolites after intragastric injection than after ingestion with food. Subsequent analysis of the isolated fractions on an ion-trap mass spectrometer identified three metabolites from their MS2 and MS3 fragmentation spectra. These were methylquercetin sulfoglucuronide, quercetin sulfoglucuronide, and methylquercetin sulfate.
Researchers turn their attention to more complicated problems of studying multi-constituent plant raw material as data accumulate on the metabolism of pure compounds [26, 44]. As a rule, the composition of the administered mixture, e.g., medicinal plant extract, is established in the first stage. Only then are the confirmed starting constituents and resulting metabolites identified in plasma or another matrix. In general, the identification and structure elucidation of flavonoids is a separate problem to which considerable effort is applied [13, 45].
Metabolites of cranberry polyphenols were determined by analyzing rat plasma in a triple-quadrupole mass spectrometer [19]. MRM-data of synthesized standards of proposed metabolites (characteristic pairs of parent—daughter ions were found and the analytical conditions were optimized) were obtained first in order to identify the metabolites. Then, experimental samples were analyzed under the same conditions (Fig. 7). Quercetin, 3′-O-methylquercetin (isorhamnetin), myricetin, kaempferol, and 13 conjugates of quercetin and methylquercetin with glucuronic acid were detected in animal urine. Quercetin was not detected in plasma although quercetin and isorhamnetin were found in the MeOH extract of urinary bladder tissue after multiple administrations of cranberry extract. This suggested that this raw material could be used to inhibit carcinogenesis in the urinary bladder.
MRM chromatograms of quercetin and isorhamnetin glucuronides: synthesized standards (A-D), observed in rat urine after administration of cranberry concentrate (E-F). Transitions m/z: 477 > 301 (A, E, quercetin monoglucuronide), 653 > 301 (B, F, quercetin diglucuronide), 491 > 315 (C, G, isorhamnetin monoglucuronide), and m/z 667 > 491 (D, H, isorhamnetin diglucuronide) [19].
The previous example showed that compounds can be identified by low-resolution mass spectrometers, which includes the triple quadrupole. However, the studies are lengthy and labor-intensive because the proposed metabolites must be synthesized and the parameters for recording each constituent must be established. Furthermore, it was reported that false-positive results can be obtained [46].
Tandem high-resolution mass spectrometers (HRMS) of the QqTOF type, which have a time-of-flight analyzer as a second stage, can expand significantly the ability to identify compounds. (High resolution is considered a ratio of ion mass to its spectral line width at half-height of >10,000.) Mass in HRMS can be measured to an accuracy of four to five decimal places. This enables the elemental formula of an ion to be determined highly accurately. Thus, assumptions about the structures of unknown metabolites can be made from a single exact-mass analysis of the molecular ion and its fragments. Metabolic studies are becoming routine.
A method using HRMS of the QqTOF type was developed to study the metabolism of several flavonols including quercetin, rutin, isoquercitrin, and taxifolin. The studies were conducted in vitro using human and rat hepatocyte models as both primary cultures and suspensions [29]. Phase I and II metabolites were identified using elemental analyses. Furthermore, fragmentation patterns and UV absorption spectra were used. It was shown that methylated flavonols and their glucuronides were formed preferentially. Quercetin and taxifolin were transformed more than rutin and isoquercitrin glycosides.
HRMS was used to identify the active constituents responsible for the therapeutic activity of Hibiscus sabdariffa [44]. The composition of the enriched aqueous plant extract was studied first. Then, the amount of one constituent (quercetin) or a group of compounds (quercetin and kaempferol derivatives) administered to laboratory rats was calculated. The constituents present in the rat plasma after intragastric injection were characterized and analyzed quantitatively. A total of 17 polyphenols (phenolic acids and flavonols) were detected. Of these, 11 were metabolites, including kaempferol and quercetin glucuronides (Table 3). The elimination half-lives of the flavonol glucuronides were greater than those of the acids. Also, the antioxidant activity of the plasma was measured after administration of the extract. It was hypothesized that the flavonols themselves were responsible for the inhibition of LPO in cell membranes and the antioxidant activity of the plant extract.
A UPLC-QqTOF system was used to identify isoquercitrin (quercetin 3-D-glucoside) metabolites that were formed during incubation with a mixture of human intestinal bacteria [32]. The mixture was extracted with EtOAc and then analyzed using gradient elution (0.1% formic acid and MeCN with the MeCN concentration varying from 10 to 90% during a 10-min analysis cycle). Five metabolites and the starting compound were detected. Exact-mass measurements and the structure of the starting compound suggested the metabolic pathway was hydrolysis (for form quercetin), acetylation and dehydroxylation of quercitrin (quercetin glycoside), and hydroxylation and hydroxymethylation of quercetin.
The traditional Chinese medicine Moutan Cortex, the root extract of Paeonia suffruticosa Andrews, was studied [23]. A high-throughput method was developed to screen constituents in rat serum after oral administration of the extract. The analytical system included a fast automated UPLC chromatographic method, exact-mass determination of negative fragment ions in a HRMS (QqTOF), and computer processing of the data using MetaboLynxTM software. Analysis of the plant extract characterized 41 of 46 recorded peaks (Fig. 4), including flavonols and other polyphenolic compounds. A total of 23 peaks were identified in serum. Of these, 16 were starting raw material constituents; 7, metabolites. It was hoped that the proposed simple and sensitive method for screening and identifying bioactive constituents could be used to develop new drugs based on any complex plant raw material.
Thus, hybrid LC-MS/MS has become the dominant routine method for analyzing biological samples. More and more attention is being paid to processes related to administration of a multi-constituent plant raw material containing this class of compounds and other biologically active compounds as data on the PK and metabolism of pure compounds accumulates. Methods are being improved by using UPLC and tandem HRMS as a general trend for increasing the analytical sensitivity and selectivity.
References
E. B. Men'shchikova, V. Z. Lankin, N. K. Zenkov, et al., Oxidative Stress. Pro-oxidants and Antioxidants [in Russian], Firma Slovo, Moscow (2006).
V. Yu. Bogachev, O. V. Golovanova, A. N. Kuznetsov, and A. O. Shekoyan, Angiol. Sosud. Khir., 19(1), 1 – 8 (2013).
E. Yu. Bakhtenko and P. B. Kurapov, Variety of Higher Plant Secondary Metabolites [in Russian], Izd. VGPU, Vologda (2008).
D>Chemical Encyclopedia, Vol. 5, BSE, Moscow (1998), p. 104.
M. Materska, Pol. J. Food Nutr. Sci., 58(4), 407 – 413 (2008).
J. Cao, Y. Zhang, W. Chen, and X. Zhao, Br. J. Nutr., 103(2), 249 – 255 (2010).
S. Kumar and A. K. Pandey, Sci.World J., 2013, 162750 (2013).
G. Puodzhyunene, V. Kairite, V. Yanulis, et al., Khim.-farm. Zh., 45(2), 26 – 28 (2011); Pharm. Chem. J., 45(2), 88 – 90 (2011).
A. Z. Abyshev, E. M. Agaev, and A. B. Guseinov, Khim.-farm. Zh., 41(8), 23 – 26 (2007); Pharm. Chem. J., 41(8), 419 – 423 (2007).
H. El Hajji, E. Nkhili, V. Tomao, and O. Dangles, Free Radical Res., 40(3), 303 – 320 (2006).
G. S. Kelly, Altern. Med. Rev., 16(2), 172 – 194 (2011).
E. H. Liu, L. W. Qi, J. Cao, et al., Molecules, 13(10), 2521 – 2544 (2008).
J. K. Prasain and S. Barnes, Mol. Pharm., 4(6), 846 – 864 (2007).
P. L. Kole, G. Venkatesh, J. Kotecha, and R. Sheshala, Biomed. Chromatogr., 25(1 – 2), 199 – 271 (2011).
Y. Kawai, S. Saito, T. Nishikawa, et al., Biosci. Biotechnol. Biochem., 73(3), 517 – 523 (2009).
G. An, J. Gallegos, and M. E. Morris, Drug. Metab. Dispos., 39(3), 426 – 432 (2011).
X. Yao, G. Zhou, Y. Tang, et al., Molecules, 18, 3050 – 3059 (2013).
R. Rajbhandari, N. Peng, R. Moore, et al., J. Agric. Food Chem., 59(12), 6682 – 6688 (2011).
F. Liu, Y. Xu, L. Rui, et al., Rapid Commun. Mass Spectrom., 20(23), 3522 – 3526 (2006).
Y. Zhao, L. Wang, Y. Bao, and C. Li, Rapid Commun. Mass Spectrom., 21(6), 971 – 981 (2007).
J. Li, Z.-W. Wang, X. Zhang, et al., Biomed. Chromatogr., 22(4), 374 – 378 (2008).
N. Li, C. Liu, S. Mi, et al., J. Chromatogr. Sci., 50(10), 885 – 892 (2012).
D. Jin, H. Hakamata, K. Takahashi, et al., Biomed. Chromatogr., 18(9), 662 – 666 (2004).
N. Li, C. Liu, and S. Mi, J. Chromatogr. Sci., 50(10), 885 – 892 (2012).
J. Liu, H. Sun, A. Zhang, et al., Biomed. Chromatogr., 28(4), 500 – 510 (2014).
J. L. Zhou, Z. M. Qian, and Y. D. Luo, Biomed. Chromatogr., 22(10), 1164 – 1172 (2008).
X. Zhang, Y. G. Sun, M. C. Cheng, et al., Anal. Chim. Acta, 602(2), 252 – 258 (2007).
B. A. Graf, C. Ameho, and G. G. Dolnikowski, J. Nutr., 136(1), 39 – 44 (2006).
J.-S. Kang, in: Tandem Mass Spectrometry – Applications and Principles, J. Prasain (ed.); [Electronic resource] INTECH [Official website] URL http: //www.intechopen.com/books/tandem-mass-spectrometry-applications-and-principles (accessed:12.07.2013).
J. Vacek, B. Papouskova, P. Kosina, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 899, 109 – 115 (2012).
S. Gao, W. Jiang, T. Yin, and M. Hu, J. Agric. Food Chem., 58(11), 6650 – 6659 (2010).
S. E. Saad, D. J. Jones, L. M. Norris, et al., Biomed. Chromatogr., 26(12), 1559 – 1566 (2012).
L. Lu, D. Qian, J. Yang, S. Jiang, et al., Biomed. Chromatogr., 27(4), 509 – 514 (2013).
O. V. Zillich, U. Schweiggert-Weisz, K. Hasenkopf, et al., Biomed. Chromatogr., 27(11), 1444 – 1451 (2013).
M. J. Gray, D. Chang, Y. Zhang, et al., Biomed. Chromatogr., 24(1), 91 – 103 (2010).
J. He, Y. Feng, H. Z. Ouyang, et al., J. Pharm. Biomed. Anal., 84, 189 – 195 (2013).
W. Niu, X. Zhu, K. Yu, et al., J. Mass Spectrom., 47(3), 370 – 380 (2012).
J. Zhang, X. Liu, N. Fu, et al., J. Ethnopharmacol., 133(2), 911 – 913 (2011).
L. Chang, Y. Ren, L. Cao, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 904, 59 – 64 (2012).
H. van der Woude, M. G. Boersma, J. Vervoort, and I. M. Rietjens, Chem. Res. Toxicol., 17, No. 11, 1520 – 1530 (2004).
E. J. Oliveira, D. G. Watson, and M. H. Grant, Xenobiotica, 32(4), 279 – 287 (2002).
L. Wang and M. E. Morris, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 821(2), 194 – 201 (2005).
S. Fernandez-Arroyo, M. Herranz-Lopez, and R. Beltran-Debon, Mol. Nutr. Food Res., 56(10), 1590 – 1595 (2012).
R. March and J. Brodbelt, J. Mass Spectrom., 43(12), 1581 – 1617 (2008).
T. Rousu, J. Herttuainen, and A. Tolonen, Rapid Commun. Mass Spectrom., 24(7), 939 – 957 (2010).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 48, No. 8, pp. 3 – 16, August, 2014.
Rights and permissions
About this article
Cite this article
Guglya, E.B. Pharmacokinetics of Quercetin and Other Flavonols Studied by Liquid Chromatography and LC-MS (a Review). Pharm Chem J 48, 489–498 (2014). https://doi.org/10.1007/s11094-014-1137-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-014-1137-0