Abstract
Plasma polymerization experiments are typically conducted by sustaining a non-thermal plasma in a gas flow containing monomer molecules. Recently, it was however observed that plasma polymerization can also occur in a nitrogen/2% ethanol vapor dielectric barrier discharge operated at medium pressure (5.0 kPa). To obtain a better understanding of this peculiar plasma polymerization behavior, a detailed parameter study is conducted in this work. The influence of plasma exposure time at fixed discharge power on the coating properties is investigated as well as the influence of discharge power applying a fixed energy density. Results reveal that at a discharge power ≥ 3.0 W, very hydrophilic (WCA values < 11°) coatings are deposited possessing N/C ratios in the range 32–38% and O/C ratios in the range 21–40%. XPS analysis shows that the coatings mainly consist out of amides, amines, imines and nitril/isonitrile groups. FTIR results confirm this conclusion and also show that the C≡N groups are mainly present as isonitriles. With increasing discharge power, a small decrease in O/C ratio is observed combined with an increasing amount of isonitriles. The investigated treatment times also do not affect the surface chemical composition nor the surface wettability suggesting excellent in-depth coating homogeneity. The plasma exposure time does however strongly affect the coating thickness: a linear increase in coating thickness with plasma exposure time is observed in this work. The obtained results thus prove that a nitrogen/ethanol vapor DBD operated at medium pressure is capable of depositing highly hydrophilic nitrogen- and oxygen-rich deposits, which can have applications in different research areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-thermal plasma activation is a promising technique for the surface modification of polymeric materials and improvement of polymeric material’s biocompatibility [1, 2]. In the last years, plasma activation has been gaining popularity as a versatile and biocompatible technique for surface modification of materials [3,4,5,6,7]. Plasma activation treatments can be used for the grafting of different surface groups, including COOH and OH functionalities through air [8,9,10,11], O2 [12] or inert gas (Ar, He) plasmas [8, 13] as well as amines by using nitrogen containing plasmas such as N2, NH3, N2/H2,… [14, 15]. The use of plasma activation offers several major advantages over conventional chemical methods; first of all, plasma only modifies the surface of the exposed materials without affecting their physical and chemical bulk properties. Secondly, plasma does not alter the mechanical properties of the outer material layer, as observed with other surface modification techniques: for example, wet chemical treatment of a surface will cause a partial degradation of the polymer chains at the surface, leading to a decrease of the mechanical strength and to a faster degradation. Thirdly, plasma activation treatments enable to uniformly modify material surfaces, regardless of the geometry, and can thus be applied on complex objects. Finally, the use of hazardous solvents and/or chemicals can be avoided, rendering the plasma-activated material compatible with biological systems. An extremely wide range of surface modifications can be realized by performing plasma activation experiments in different low-pressure and atmospheric pressure plasmas. Hence, plasma surface activation has been employed in many technological fields, such as lubrication surfaces, bio-absorbable polymers, biocompatibility enhancement, bone internal fixation devices, diagnostic biosensors,… [16,17,18,19,20,21].
Besides plasma activation, non-thermal plasmas can also be used for the deposition of thin coatings, which is known as plasma polymerization. Plasma polymerization enables a single-step, solvent-free and rapid fabrication of pinhole-free thin films on a wide range of substrates by introducing a monomer carried by a gas flow into a non-thermal plasma reactor [22]. Previously conducted plasma activation experiments however also revealed that plasma polymerization can occur at particular conditions when ethanol vapor is added to a dielectric barrier discharge (DBD) sustained in nitrogen at medium pressure (5.0 kPa) [23]. In this former study, it was observed that when a nitrogen/2% ethanol vapor plasma was ignited at a fixed discharge power of 11.8 W above low density polyethylene (LDPE) substrates, it was possible to deposit very stable nitrogen-rich coatings with a high wettability consisting of a mixture of nitrogen- and oxygen-containing groups. At other investigated ethanol concentrations, plasma activation however occurred leading only to the surface grafting of nitrogen- and oxygen-functionalities on the LDPE substrates [23]. This paper intends to reveal more information on the observed peculiar plasma polymerization behavior on LDPE substrates when sustaining a DBD in a nitrogen/2% ethanol vapor mixture at 5.0 kPa. More particular, the influence of discharge power and plasma exposure time on the plasma polymerization process is investigated in detail in this work. For this purpose, the surface properties of LDPE are probed as a function of power and deposition time using water contact angle (WCA) measurements for wettability determination, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) for chemical composition determination and atomic force microscopy (AFM) for surface morphology determination. In addition, optical reflectance spectroscopy (OPS) is used to examine the influence of power and deposition time on the thickness of the plasma-polymerized layers.
Materials and Methods
LDPE
As deposition substrates, samples of an LDPE film with a thickness of 0.18 mm are used. This additive-free commercially available LDPE foil was purchased from Goodfellow Cambridge Ltd. and is not subjected to any pre-treatment step prior to plasma modification.
DBD Set-Up
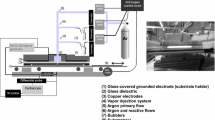
The medium pressure DBD reactor used in this study was built in the lab and is presented in Fig. 1. The discharge occurs between two copper electrodes (diameter = 4 cm), placed within a cylindrical enclosure. Both electrodes are covered with a ceramic plate (Al2O3) (5 cm × 5 cm − thickness = 0.7 mm) and the inter-electrode distance is fixed at 4 mm, resulting in a discharge gap of 2.6 mm. The upper electrode is connected to a high frequency AC power source, while the lower electrode is connected to earth. The frequency of the applied high voltage is kept constant at 50 kHz.
Before starting the experiment, the LDPE film is placed on the lower ceramic plate. The discharge chamber is then pumped down to 0.5 kPa and filled with nitrogen (Air Liquide—Alphagaz 1). After reaching near-atmospheric pressure, the plasma reactor is flushed with nitrogen at a flow rate of 3.0 l/min for 3 min. After this purging step, the pressure in the plasma reactor is again lowered to 5.0 kPa while the nitrogen flow rate is at the same time lowered to 0.5 l/min. After reaching a chamber pressure of 5.0 kPa, 2% ethanol vapor is added to the nitrogen carrier gas while maintaining a total gas flow rate of 0.5 l/min. The ethanol vapor content in the discharge mixture is ensured using an innovative liquid delivery system (CEM®—Controlled Evaporation and Mixing) purchased from Bronkhorst. This system consists of a liquid flow controller for ethanol, a mass flow controller for the carrier gas nitrogen and an evaporation and mixing device. With the use of the flow controllers, the predefined amount of ethanol and nitrogen is distributed to the evaporation device where the temperature of the gas mixture is brought to 30 °C to ensure complete evaporation of the supplied ethanol. Compared to a bubble system, the selection of the gas/liquid ratio is more flexible. In addition, the CEM® system ensures a very stable vapor flow and can accurately control the gas/vapor mixture. In this study, to reach a nitrogen/2% ethanol vapor gas mixture, the liquid ethanol flow is set to 1.2 g/h and the nitrogen flow rate set to 0.49 l/min. After the introduction of ethanol vapor to the discharge gas, the AC power source is turned on to ignite the plasma and modify the LDPE surface while the pressure in the discharge chamber is maintained at 5.0 kPa by slightly pumping during plasma treatment. Plasma deposition experiments in this work are conducted at varying discharge powers and multiple deposition times.
Electrical and Optical Characterization
The most common electrical diagnostic of a DBD consists of the measurement of the voltage applied to the electrodes and the resultant discharge current [24]. The voltage V(t) applied to the high-voltage electrode is measured using a 1000:1 high voltage probe (Tektronix P6015A), whereas the discharge current I(t) is monitored by measuring the voltage over a 50 Ω resistor, which is connected in series with the lower electrode and the ground. The voltage–current waveforms are then recorded using a Tektronix TDS 1002 digital oscilloscope. Using these voltage–current waveforms, the average power P of the discharge is calculated according to the following equation (T = period of the discharge): [25]
To identify the reactive species generated in the discharge, optical emission spectra (OES) are recorded by means of an Ocean Optic S2000 spectrometer in the range 200–900 nm with a low resolution of 0.7 nm.
Optical Reflectance Spectroscopy
To determine the deposition rate of the plasma-polymerized layers, the thickness of the layers is measured as a function of deposition time, using optical reflectance spectroscopy (OPS). For better results, OPS measurements are performed on plasma-polymerized films deposited on silicon wafers (Siegert Wafers) instead of LDPE films making use of a Filmetrics F20-UV device. This device can measure thicknesses between 1 nm and 40 µm with an accuracy of 0.2% (or 1 nm) using wavelengths between 190 and 1100 nm. Using the FILMeasure software, the thickness of the deposited layers can be automatically obtained as a function of plasma exposure time. For each plasma condition, 5 points on a single sample are measured.
Water Contact Angle Measurements
The wettability of the untreated and plasma-coated LDPE films is evaluated using static water contact angle (WCA) measurements. The static WCA values of the foils are obtained at room temperature using a Krüss Easy Drop system. Within a few minutes after plasma treatment, a 1 µl drop of distilled water is deposited on each sample and after a few seconds, the image of the droplet is captured. Based on this imaged water drop profile, the static WCA value is obtained using Laplace–Young curve fitting. Every reported WCA in this work is the average of 5 droplets placed at different positions on a single sample.
X-Ray Photoelectron Spectroscopy
The chemical composition of the LDPE samples is obtained by X-ray Photoelectron Spectroscopy (XPS). This technique provides us with an insight in the surface chemical composition of the untreated and plasma-coated LDPE samples. XPS measurements are performed on a PHI 5000 Versaprobe II spectrometer employing a monochromatic Al Kα X-ray source (hν = 1486.6 eV). Survey scans and high resolution C1s, O1s and N1s peaks are recorded at a take-off angle of 45° relative to the sample surface with a pass energy of 187.85 and 23.5 eV respectively. XPS survey scans are processed using Multipak (9.3) software and from the peak area ratios, the elemental composition of the LDPE samples can be determined. Multipak is also used to curve-fit high resolution C1s, O1s and N1s peaks of the LDPE films: the hydrocarbon component of the C1s spectrum (285.0 eV) is used as calibration of the energy scale. After this calibration step, the C1s, O1s and N1s peaks are deconvoluted using Gaussian–Lorentzian peak shapes making use of an iterated Shirley background and the full-width at half maximum (FWHM) of each line shape is maintained below 1.9 eV.
Fourier Transform Infrared Spectroscopy
Besides XPS, Fourier transform infrared spectroscopy (FTIR) can also be used to gather information on the chemical structure of the pristine and plasma-coated polymers. FTIR analysis is performed using a Bruker Tensor 27 spectrometer equipped with a single reflection ATR (attenuated total reflectance) accessory (MIRacle™ Pike technology) using a germanium crystal as internal reflection element. FTIR spectra are recorded using a liquid nitrogen cooled MCT-detector (mercury–cadmium–telluride) with a resolution of 4 cm−1. The spectra shown in this paper are the result of the integration of 64 scans on a single point location per sample.
Atomic Force Microscopy
The surface morphology of the LDPE samples is visualized using an XE-70 (Park Systems) atomic force microscope (AFM). 15 µm × 15 µm scans are recorded in non-contact mode with a silicon cantilever (Nanosensors™ PPP-NCHR) and XEP software is used for surface roughness analysis after the recorded images are modified with an X- and Y-plane autofit procedure. Average root mean square roughness (Rrms) values are also determined for different samples by averaging the roughness values obtained on 4 different AFM images per sample condition.
Results and Discussion
Electrical and Optical Characterization of the Discharge
The first step in this investigation is the electrical characterization of the discharge to evaluate in which mode the DBD is operating: filamentary or glow mode. In previous work, it has already been shown that the used DBD works in “pseudoglow” mode, especially when ethanol vapor is added to the discharge gas [23]. This regime is characterized by the presence of successive short current pulses with a width of a few µs and is also called the “multi-glow” mode, since the current–voltage characteristic for each current pulse is similar to the one obtained in the glow mode [26, 27]. It is however known that this pseudoglow mode can be easily disturbed by, for example, just introducing a sample into the plasma region. Therefore, the voltage–current waveforms in this work are recorded with no sample and with an LDPE film and a silicon wafer in the plasma discharge. In Fig. 2, the voltage–current waveforms for a 6.3 W discharge are presented. From Fig. 2 can be seen that (with and without introduced samples) the applied voltage has a sinusoidal form while the current waveform consists of 2 broad pulses, one with a duration of 2 µs and another one with a longer duration of 5 µs consisting of a sharp current pulse followed by a less intense, but broad current peak. Therefore, one can conclude that the DBD used in this work still works in multi-glow mode, even when a sample is introduced in the discharge region [27,28,29,30]. This multi-glow mode is a very desirable mode for surface modification as it is known to homogeneously treat surfaces [28,29,30]. In a next step, the voltage–current waveforms of the nitrogen/2% ethanol plasma discharge are recorded using different applied voltages (results not shown here due to their similarities with the waveforms shown in Fig. 2), from which the according discharge power is calculated using Eq. (1). The obtained discharge powers for different applied voltages, with and without a sample in the discharge region, are summarized in Table 1. This table clearly indicates that applying a higher voltage to the upper electrode results in a higher discharge power. In this work, the discharge power was only varied between 1.0 and 10.4 W: at discharge powers below 1.0 W, the electrode is not completely covered with plasma or even no plasma ignition occurs, while at discharge powers above 10.5 W, a plasma arc starts to form which can damage the samples placed in the plasma region or even damage the plasma set-up.
Table 1 also reveals that introducing a polymer or silicon sample in the plasma discharge region does not significantly influence the value of the plasma discharge power. Taking this into account, the influence of the discharge power on the surface characteristics of LDPE is further investigated in this work. For this purpose, the discharge power is varied while maintaining a constant energy density value of 100 J/cm2 to enable an objective comparison. This energy density value is calculated by multiplying the plasma exposure time with the discharge power and by dividing this value by the area of the electrodes. To reach such a constant energy density value, longer plasma exposure times are used at lower discharge powers as clearly indicted in Table 1. In a second set of experiments, the influence of plasma exposure time is examined using 2 fixed discharge powers (2.1 and 6.3 W).
In a second step of this investigation, the excited plasma species present in the discharge will be examined making use of optical emission spectroscopy (OES).
Figure 3 shows the optical emission spectra observed using 2 different powers. The most intensive emission lines can be found in the spectral region between 310 and 440 nm, which can be assigned to different excited states of N2 [31]. Apart from the previous mentioned lines, the spectral line from CO (456 nm) can also be identified. There is however no indication of the formation of C2 as the Swan band doesn’t appear in the spectra. This is mostly desired for polymer modification as it is preferred to incorporate reactive species as oxygen and nitrogen on the surface and not carbon species. Excited OH radicals and oxygen molecules are not observed in the OES spectrum which can be explained as follows: the electron temperature will be too low to dissociate the oxygen and ethanol molecules and excites the oxygen. It can also be expected that the oxygen and OH species occur in their ground state, but to detect this is a very challenging task which is out of the scope of this work. As seen in Fig. 3, an increase discharge power results in an increase of all spectral line intensities, which can be explained by an increase in electron temperature [32]. No new peaks are detected which is another evidence of the increase of the electron temperature.
Deposition Rate Determination
As described above, to determine the thickness of the deposited thin films, plasma polymerization experiments are performed on silicon wafers. In this work, the thickness of the deposited layers is determined for two different discharge powers, 2.1 W and 6.3 W, as a function of plasma treatment time using OPS and the obtained results are shown in Fig. 4. From this figure, it can be seen that for both powers there is a clear linear relation between the layer thickness and the plasma exposure time as linear regression analysis reveals a correlation coefficient R2 of 99.4% and 99.5% for the 2.1 W and 6.3 W discharge power respectively. Increasing the discharge power also results in a higher deposition rate as can be concluded from the steeper slope of the curve at 6.3 W. Using linear regression, the deposition rate is also determined: 35 ± 1 nm/min and 59 ± 2 nm/min for a discharge power of 2.1 W and 6.3 W respectively. From literature, it is well known that the deposition rate is highly influenced by the so-called Yasuda parameter, P/FM, with P the discharge power (W), F the ethanol flow rate (mol/s) and M the molecular weight of ethanol (kg/mol) [33]. For this particular polymerization process, FM remains constant at 33.3 10−6 kg/s. As such, in this work, the deposition rate thus increases with increasing P/FM value as this rate increases with increasing discharge power P. This result reveals that the activated species in the discharge likely have a much lower concentration than the ethanol molecules in the plasma, or, that the DBD works in the so-called monomer sufficient region. In this region, as the discharge power per molecule increases, extra initiation sites are formed resulting in an increased polymerization rate [28, 33]. In the next section, the effects of discharge power and deposition time on the surface characteristics of the deposited coatings will be investigated in detail using WCA, XPS and FTIR.
Coating Surface Characterization as a Function of Discharge Power
In this section, the influence of the discharge power on the surface characteristics of the LDPE film after plasma treatment in a nitrogen/2% ethanol DBD is profoundly studied. For this purpose, LDPE films are plasma treated at a fixed energy density of 100 J/cm2 with a discharge power range between 1.0 and 9.0 W (see Table 1). To characterize the pristine and plasma-coated surfaces, both WCA and XPS measurements are performed.
The results of the WCA measurements as a function of discharge power are summarized in Fig. 5. An untreated LDPE sample has a WCA of 102.4° ± 0.3° but this value is not presented in Fig. 5 for sake of clarity. From Fig. 5, it can be seen that the WCA extremely decreases after conducting the plasma polymerization process. A reduction of more than 80% in WCA value compared to the untreated sample is obtained for all powers under study, with a maximal WCA reduction of 92% obtained at a discharge power of 2.9 W. Figure 5 also reveals that WCA values below 10° are obtained at discharge powers ≥ 2.9 W suggesting the deposition of highly hydrophilic coatings. Lower discharge powers are found to result into less hydrophilic surfaces and also less homogeneous coatings as seen from the larger error bars obtained at discharge powers below 2.9 W.
The diminution of the WCA, as shown in Fig. 5, demonstrates the strongly increased wettability of LDPE as a result of the deposition of a thin film most likely containing a large amount of hydrophilic groups [10, 11, 34, 35]. To investigate which hydrophilic groups are present on the surface of the thin films deposited on the LDPE substrates, XPS measurements are performed on the plasma-coated LDPE samples. In this case, the energy density of the plasma exposure is again maintained at 100 J/cm2 for each sample. Based on XPS survey scans, the atomic composition of the LDPE samples can be determined from which the O/C and N/C ratio can be calculated. These ratios are shown in Fig. 6 for different discharge powers, while the O/C and N/C ratio of an untreated LDPE sample is equal to 1.2 ± 0.9% and 0.2 ± 0.3% respectively. This very small nitrogen and oxygen incorporation on the untreated sample is most likely due to surface contamination. Figure 6 reveals very high O/C and N/C ratios for the plasma-coated samples compared to the pristine samples and thus clearly indicates that the performed plasma polymerization experiments lead to the deposition of nitrogen- and oxygen-rich thin films as O/C ratios of more than 20% and N/C ratios of more than 30% are obtained. Especially this high N/C ratio is largely unexpected as this cannot be achieved using a pure nitrogen discharge and also taking into account the fact that the used liquid (ethanol) does not contain nitrogen atoms in its chemical structure [23]. Figure 6 also reveals that the N/C ratio does not follow a clear trend as a function of discharge power with values remaining close to 35%. In contrast, the O/C ratio first seems to decrease with increasing discharge power up to 6.3 W, after which this ratio remains constant at approximately 22% at higher discharge powers. When simultaneously examining Figs. 5 and 6, no clear relationship between the WCA value on the one hand and the O/C or N/C ratio on the other hand can be found. Indeed, the lowest WCA value is not observed on the sample possessing the highest O/C and N/C ratio, which is obtained at a discharge power of 2.1 W. This discrepancy might be due to the inhomogeneity of the deposited layer at 2.1 W, which may in turn explain the higher mean WCA value and less uniform WCA distribution as evidenced by the higher standard deviation. After excluding the results obtained at 2.1 W, a clear correlation can be found between the WCA and the total (N + O)/C ratio with a Pearson correlation coefficient of − 0.98. This result suggests that the increased LDPE surface wettability is due to the simultaneous deposition of both oxygen- and nitrogen-containing groups as the correlation between the WCA and the O/C or N/C ratio separately is significantly lower.
The high resolution C1s peak of the XPS spectra is also investigated in detail to evaluate which oxygen and nitrogen-containing functional groups are present at the surface of the thin films deposited on the LDPE substrates by plasma exposure. Figure 7 shows the curve fitting of the high resolution C1s peak of the LDPE foil before and after plasma polymerization at an energy density of 100 J/cm2 in a nitrogen/2% ethanol DBD for different discharge powers (2.1, 3.8, 6.3 and 9.0 W). The exact identification of the functional carbons based on the binding energy of the peaks, while both nitrogen and oxygen-containing groups are present, is unfortunately rather difficult, since the energy difference between some nitrogen- and oxygen-related groups is too small to allow separation by mathematical reconstruction within error bars. Taking this into account, the C1s envelope of the LDPE samples will only be decomposed into four distinct peaks: a peak at 285.0 ± 0.1 eV corresponding to C–C and C–H bonds, a peak at 286.5 ± 0.1 eV which can be attributed to C–O, C–NHx C=N or C≡N functional groups, a peak at 287.7 ± 0.1 eV attributed to C=O, O–C–O or N–C=O groups and a peak at 289.1 eV due to O–C=O or N–C(=O)–N groups [14, 36, 37]. As shown in Fig. 7, tremendous changes in the profile of the C1s peaks can be observed after conducting the plasma polymerization experiments at different discharge powers. After plasma treatment, the relative area of the peak at 285 eV strongly decreases while the relative area of all other peaks, namely these at 286.5 eV, 287.7 eV and 289.1 eV, strongly increases. Additionally, Fig. 7 also shows that when the discharge power increases from 2.1 to 3.8 W, the relative area of the peaks at 287.7 and 289.1 eV strongly decreases. When increasing the discharge power to 6.3 and 9.0 W however, no clear visual differences in relative peak area can be observed.
Curve fitting of the N1s peak is also performed in this work as it can indicate whether there are highly oxidized nitrogen groups present at the surface of the deposited thin films. ONO2, NO2 and ONO groups are situated at an energy between 405 and 408 eV while C–N, N–C=O, C–N–O, C=N and C≡N groups are situated between 399 and 401 eV. In this work, the high resolution N1s peaks were found to be located in the energy range 399–401 eV (not shown here), thereby excluding the presence of ONO2, NO2 and ONO groups at the surface of the deposition coatings. Taking this into account, the N1s envelopes of the LDPE samples are decomposed in this study into three distinct peaks: a peak at 398.5 eV attributed to C–N groups, a peak at 399.7 eV corresponding to N–C=O, C=N and C≡N bonds and a peak at 401 eV due to C–N–O groups [14, 36]. Finally, in this work, curve fitting of the high resolution O1s peaks is also performed as it enable the separation between N–C=O and C–O/C=O groups. The O1s envelopes (not shown here) are decomposed into two distinct peaks: a peak at 531.5 eV due to N–C=O groups and a peak at 532.7 eV corresponding to C–O and C=O groups [14].
Based on the deconvoluted C1s, O1s and N1s peaks, the relative concentration of the different carbon bonds at the surface of the plasma-deposited coatings can be calculated and the obtained results are summarized in Table 2. From this table, it can be seen that the relative concentrations of the different carbon bonds do not significantly change when the discharge power is increased from 3.8 to 9.0 W, which is consistent with the results presented in Fig. 7. However, at the lowest discharge power of 2.1 W, considerably less C–C/C–H bonds are present in combination with larger relative amounts of C=O/N–C=O and O–C=O/N2–C=O groups. From the deconvolution of the O1s peaks, it can also be seen that the peak at 287.7 eV can be mainly attributed to N–C=O bonds taking into account its large relative abundance compared to C=O groups, which also contribute to the peak at 287.7 eV. Deconvolution of the high resolution N1s peaks also reveals that nitrogen is mainly incorporated as N–C=O, C=N and C≡N groups at the surface of the deposited thin films. By comparing the results of the C1s, N1s and O1s deconvoluted peaks, it can thus be concluded that the most abundant oxygen-containing functional groups present in the deposited thin films are amide groups (N–C=O), while the most abundant non-oxidized nitrogen-containing functional groups are C–N, imine (C=N) and nitrile/isonitrile (C≡N) groups. More specific identification of these chemical functional groups can also be obtained from FTIR spectroscopy, of which the results will be presented in “FTIR Spectroscopy Results” section. Before showing the FTIR results however, the influence of plasma exposure time on the surface characteristics of the plasma-coated LDPE samples will be first examined in the next section.
Coating Surface Characterization as a Function of Plasma Treatment Time
The influence of plasma treatment time on the plasma polymerization process in a nitrogen/2% ethanol plasma DBD on LDPE substrates is investigated in this section at 2 fixed discharge powers (2.1 W and 6.3 W). Exposure times varying between 10 s and 20 min are taken into account and the evolution of the water contact angle as a function of treatment time is presented in Fig. 8. From this figure, it can be seen that already after a plasma treatment of 10 s, the surface wettability of LDPE is extremely increased, which can be attributed to the deposition of a very thin highly hydrophilic nitrogen- and oxygen-rich coating, as previously shown. Figure 8 also reveals that treatment times above 10 s do not significantly affect the obtained WCA values anymore, which suggest a good in-depth homogeneity of the deposited coatings. Figure 8 also shows the rather high standard deviations found for the coatings deposited at 2.1 W, which is in agreement with the previously obtained WCA results at this particular power.
Besides surface wettability, also the chemical composition of the surface of the plasma-polymerized thin films is investigated as a function of plasma treatment time using XPS measurements. Based on XPS survey scans, the surface atomic composition of the coated LDPE samples as a function of plasma treatment time is determined for a fixed discharge power of 2.1 and 6.3 W. From these results, the O/C and N/C ratios are calculated and their evolution as a function of treatment time is represented in Fig. 9. This figure shows no significant variations (95% confidence level) in O/C and N/C ratios as a function of plasma exposure time, except for the shortest plasma exposure time (10 s) at 2.1 W. In this case, a significantly lower N/C ratio can be observed, which may be attributed to the very limited coating thickness and/or the rather inhomogeneous coating deposition in this particular case. When comparing the results obtained in Figs. 8 and 9, excellent correlations between the WCA on the one hand and the (O + N)/C ratio on the other hand can again be obtained, with Pearson correlation coefficients of − 0.58 and − 0.81 for the 2.1 W and 6.3 W discharge respectively. From the obtained XPS results, it can thus again be concluded that the enhanced LDPE wettability is due to the simultaneous incorporation of nitrogen- and oxygen -containing groups and that the plasma deposition time has no significant influence on the surface chemical composition of the coatings. As such, plasma deposition time thus mainly affects the thickness of the coatings, as already shown in “Deposition rate determination” section.
Similar as was done as a function of discharge power, the concentrations of the different carbon bonds at the coating surface have also been determined as a function of treatment time by deconvoluting high resolution C1s, O1s and N1s peaks. The obtained results are summarized in Tables 3 and 4 for a discharge power of 2.1 W and 6.3 W respectively. From these 2 tables, in agreement with the obtained atomic composition results, it can also be concluded that the treatment time has no significant influence on the relative concentrations of the different surface carbon-containing bonds. Only for the 2.1 W discharge at the shortest plasma exposure time, less nitrogen-containing groups are present at the surface, which is in good agreement with the lower N/C ratio obtained in Fig. 9 for this particular condition.
Tables 3 and 4 also reveal similar chemical information as was found in Table 2: the most abundant functional groups present in the deposited thin films are amides (N–C=O), imines (C=N), nitriles/isonitriles (C≡N) and amines (C–N). More specific identification of the chemical functional carbon groups can be obtained from FTIR spectroscopy, of which the results will be discussed in the next section.
FTIR Spectroscopy Results
Since the penetration depth of FTIR is much higher (600 nm) compared to XPS (3.4 nm), FTIR can provide valuable information on the bulk composition of the deposited coatings. Similar as for WCA and XPS analysis, FTIR measurements are conducted as a function of discharge power at a fixed energy density of 100 J/cm2 and as a function of plasma exposure time at a constant discharge power of 2.1 W and 6.3 W. The resulting FTIR spectra are shown in Figs. 10 and 11 respectively. These figures reveal that after conducting the plasma polymerization experiments, a very broad absorption peak centered at approximately 3300 cm−1 appears, which can be assigned to OH- and NH-stretching vibrations of alcohols, amines, amides and carboxylic acids. Additionally, a broad absorption peak in the region 1750–1550 cm−1 consisting of a sharp peak with a maximal value at 1660 cm−1 and a shoulder centered at approximately 1550 cm−1 can also be seen for the plasma-coated samples. This first sharp peak at 1660 cm−1 can be attributed to the C=O stretching of amides (N–C=O), aldehydes/ketones (C=O), esters (O–C=O) and carboxylic acids (HO–C=O) and also to the C=N stretching of imines, while the shoulder can be assigned to the N–H deformation vibrations of secondary amines and the N–H deformation and C–N stretching vibrations of secondary amides. The FTIR spectra of all plasma-coated LDPE samples also present a new peak centered at 1370 cm−1, which might be due to the C-H deformation vibrations of CH3 groups. For some plasma-coated samples, an additional peak at 2160 cm−1, which can be assigned to the C≡N stretching of isonitriles (–N≡C), can also be observed. The FTIR spectra of the plasma-polymerized films also present some peaks, which are also observed for the LDPE substrates, which can be assigned to CH2 stretching vibrations (2914 and 2850 cm−1) and C–H deformation vibrations (1460 cm−1). The obtained FTIR results thus clearly confirm the conclusions drawn from XPS analysis and even also reveal that C≡N groups are mainly present as isonitriles in the coatings as the absorption peak of nitriles located at 2230–2260 cm−1 is not present in the measured FTIR spectra [38].
In Fig. 10, the FTIR spectra of the thin coatings deposited at different discharge powers are represented. This figure clearly shows that the applied discharge power mainly affects the intensity of the absorption peaks at 2160 cm−1, possessing larger peak areas at higher discharge power. This result thus indicates that the amount of isonitriles in the deposited coatings increases with increasing discharge power. Figure 11a, b show the FTIR spectra of the plasma-coated samples as a function of plasma exposure time for a fixed discharge power of 2.1 W and 6.3 W respectively. From Fig. 11 can be mainly concluded that an increasing plasma treatment time results in an increase of all absorption peaks present in the FTIR spectra, which can be attributed to the deposition of a thicker coating with increasing treatment time, as evidenced from Fig. 4.
The area of the FTIR peak located at 2160 cm−1 has also been determined by integration as it can provide information on the amount of isonitrile groups present in the deposited coatings. The results of this integration step as a function of discharge power and as a function of plasma treatment time, here indicated using the layer thickness, for a fixed discharge power of 2.1 W and 6.3 W are presented in Fig. 12. This figure reveals a clear linear relation between the layer thickness and the area of the absorption peak at 2160 cm−1 indicating the detection of more isonitriles with increasing layer thickness resulting from the large penetration depth of the FTIR measurements. The increase of the area under the 2160 cm−1 peak is also higher for the 6.3 W discharge compared to the 2.1 W discharge as can be seen by the steeper slope of the curve. This means that not only the coating thickness influences the amount of detected –N≡C groups but also the applied discharge power. This conclusion is further confirmed by the linear relation between the peak area at 2160 cm−1 and the discharge power when conducting plasma polymerization experiments at a fixed energy density of 100 J/cm2 (see Fig. 12). With increasing discharge power, the peak area at 2160 cm−1 linearly increases suggesting a higher incorporation of isonitriles in the coatings at higher discharge powers. When looking at the coatings thicknesses obtained at 100 J/cm2, a thickness of 203 ± 8 nm can be found for deposition at 6.3 W and a thickness of 343 ± 13 nm for deposition at 2.1 W. As the deposited layer is thus smaller at higher discharge power, the plasma polymerization process itself is thus responsible for the higher amount of incorporated isonitriles at higher power. This is most likely the result of more pronounced ethanol fragmentation at elevated discharge powers.
Surface Morphology Results
As it is clear from the previous sections that plasma treatment in a nitrogen/2% ethanol DBD results in the deposition of thin coatings on the LDPE surfaces, the surface morphology of these thin layers is also investigated by AFM. Figure 13 shows the AFM images for a fixed plasma exposure time of 5 min using different discharge powers. From the depicted AFM images, it can be seen that the surface morphology considerably changes when the LDPE sample is exposed to a nitrogen/2% ethanol discharge sustained at different powers. The surface of the deposited coating is rather smooth when deposition occurs at 2.1 W, but become more rough when applying higher discharge powers. The latter observation may be due to the more pronounced etching/fragmentation effects at higher discharge powers.
Morphology changes of the coated LDPE samples can be more accurately quantified by Rrms values and the results are summarized in Table 5. This table clearly confirms the previously drawn conclusions: the surface of the thin films deposited at 2.1 W is smoother than the surface of the pristine sample, while the surface of the coatings deposited at higher discharge powers has a similar roughness as the surface of the pristine LDPE sample.
Ageing
Ageing studies have already revealed that the high LDPE wettability can be obtained during 45 days of ageing in air, although significant changes in chemical composition occur. Upon storage in air, the oxygen content on the samples increases while a decrease in nitrogen content appears [23]. This result is in good agreement with previously published papers focusing on the ageing of nitrogen containing plasma polymerized films in which also a strong oxidation of the nitrogen groups is observed upon storage in air [21, 39]. However, the stability of the layer in aqueous environment is important to improve the biocompatibility. Therefore, the ageing behavior in water of the plasma treated LDPE films is also investigated in this work. Both the influence of the discharge power and the treatment time is taken into account. After plasma treatment, the LDPE samples are stored in water at a temperature (T) of 37 °C for a period of 7 days. The influence of the power is investigated after plasma treatment at a fixed energy density of 100 J/m2 for 3 different powers. The influence of the treatment time is investigated after plasma treatment for 1 min, 2.7 min and 5 min in a discharge of 7.6 W. After the appropriate ageing time, the films were analyzed using static WCA and XPS measurements. The results are summarized in Table 6. This table clearly reveals that after exposure at an energy density of 100 J/m2, the higher the discharge power is during the plasma treatment, the less ageing occurs. The WCA remains the lowest after 7 days of ageing when the LDPE is plasma treated at 10.4 W, although no significant difference in chemical composition can be detected. Taking into account the treatment time, Table 6 shows that the longer the treatment time, meaning the thicker the deposited layer, the less ageing occurs looking at the WCA. Here also, no significant difference in chemical composition can be detected after 7 days of ageing in water.
Conclusions
In this paper, a detailed parameter study of the plasma polymerization behavior in a nitrogen/2% ethanol vapor medium pressure dielectric barrier discharge has been conducted. The influence of exposure time on the coating properties was investigated at a fixed discharge power (2.1 and 6.3 W) while the influence of discharge power when applying a fixed energy density was also studied in this work. First of all, results have shown that the used DBD operated in the so-called monomer sufficient region as the deposition rate increases with increasing power or Yasuda parameter, varying from 35 ± 1 nm/min to 59 ± 2 nm/min for a discharge power of 2.1 W and 6.3 W respectively.
The results obtained in this work have also shown that the deposited thin layers on the LDPE substrates are highly hydrophilic, possessing under most examined experimental conditions WCA values below 10°. XPS and FTIR measurements revealed that this enhanced wettability is due to the deposition of thin films containing a large amount of hydrophilic oxygen- and nitrogen-containing functional groups. From XPS measurements, it was concluded that the main oxygen-containing functional groups present in the deposited thin films were amides, while the most important non-oxidized nitrogen-containing functional groups were C–N, imines (C=N) and nitril/isonitrile (C≡N) groups. FTIR measurements confirmed these results and even revealed that the C≡N groups were mainly present as isonitriles in the deposited coatings.
After examining the influence of discharge power (1.0–9.0 W) on the coating characteristics using a fixed energy density of 100 J/cm2, it could be concluded that discharge powers below 3.0 W resulted in the deposition of coatings, showing a lower surface hydrophilicity, but at the same time, also a higher N/C and O/C ratio than the coatings deposited at higher discharge powers. This discrepancy as well as the high obtained standard deviations on these samples suggested that discharge powers below 3 W are probably not sufficient to homogeneously deposit the envisioned coatings. When applying discharge powers ≥ 3 W, no significant influence of discharge power on surface wettability and surface chemical composition could be found besides a small decrease in O/C ratio with increasing discharge power and an increasing amount of isonitriles with increasing discharge power as revealed by FTIR. AFM measurements also indicated that a low discharge power of 2.1 W resulted in the deposition of a rather smooth coating, while discharge powers ≥ 3.0 W led to the deposition of rougher coatings.
Besides the influence of discharge power, also the effect of the applied treatment time on the plasma polymerization process was studied in this paper at 2 discharge powers (2.1 and 6.3 W). The examined treatment times (besides the shortest plasma treatment time of 10 s at 2.1 W which resulted in the deposition of very thin, inhomogeneous coatings) were found to not affect the surface chemical composition nor the surface wettability suggesting excellent in-depth coating homogeneity. The applied treatment time did however strongly affect the coating thickness as evidenced from OPS results, where a linear increase in coating thickness with plasma exposure time was found for both examined discharge powers. The results obtained in this work thus clearly prove that it is possible to deposit highly hydrophilic oxygen- and nitrogen-rich thin films using a nitrogen/2% ethanol vapor medium pressure dielectric barrier discharge, which may have applications in multiple research fields.
References
Schroder K et al (2002) On the applicability of plasma assisted chemical micropatterning to different polymeric biomaterials. Plasmas Polym 7(2):103–125
Ratner BD et al (2004) Biomaterials science: an introduction to materials in medicine. Elsevier Science, New York
Siow KS et al (2006) Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—a review. Plasma Processes Polym 3(6–7):392–418
Desmet T et al (2009) Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromolecules 10(9):2351–2378
Zanini S et al (2007) Polyethylene glycol grafting on polypropylene membranes for anti-fouling properties. Plasma Chem Plasma Process 27(4):446–457
Zanini S et al (2010) Plasma-induced graft-polymerization of polyethylene glycol acrylate on polypropylene films: chemical characterization and evaluation of the protein adsorption. J Colloid Interface Sci 341(1):53–58
Riccardi C, Roman HE, Ziano R (2010) Attachment of polymer chains on plasma-treated surfaces: experiments and modeling. New J Phys 12:073008
Borcia G, Anderson CA, Brown NMD (2003) Dielectric barrier discharge for surface treatment: application to selected polymers in film and fibre form. Plasma Sources Sci Technol 12(3):335–344
Borcia G, Anderson CA, Brown NMD (2004) The surface oxidation of selected polymers using an atmospheric pressure air dielectric barrier discharge. Part I. Appl Surf Sci 221(1–4):203–214
Morent R et al (2008) Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf Interface Anal 40(3–4):597–600
De Geyter N, Morent R, Leys C (2008) Surface characterization of plasma-modified polyethylene by contact angle experiments and ATR-FTIR spectroscopy. Surf Interface Anal 40(3–4):608–611
Morent R et al (2008) Non-thermal plasma treatment of textiles. Surf Coat Technol 202(14):3427–3449
De Geyter N et al (2007) Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf Coat Technol 201(16–17):7066–7075
Guimond S et al (2002) Biaxially oriented polypropylene (BOPP) Surface modification by nitrogen atmospheric pressure glow discharge (APGD) and by air corona. Plasmas Polym 7(1):71–88
Maurau R et al (2012) Nitrogen introduction in pp-HMDSO thin films deposited by atmospheric pressure dielectric barrier discharge: an XPS study. Plasma Processes Polym 9(3):316–323
Chan CM, Ko TM, Hiraoka H (1996) Polymer surface modification by plasmas and photons. Surf Sci Rep 24(1–2):3–54
Malmsten M, Muller D, Lassen B (1997) Sequential adsorption of human serum albumin (HSA), immunoglobulin G (IgG), and fibrinogen (Fgn) at HMDSO plasma polymer surfaces. J Colloid Interface Sci 193(1):88–95
Zhang Z et al (2003) Surface plasmon resonance studies of protein binding on plasma polymerized di(ethylene glycol) monovinyl ether films. Langmuir 19(11):4765–4770
Schiller S et al (2002) Chemical structure and properties of plasma-polymerized maleic anhydride films. Chem Mater 14(1):235–242
Jenkins ATA et al (2000) Pulsed plasma deposited maleic anhydride thin films as supports for lipid bilayers. Langmuir 16(16):6381–6384
Harsch A et al (2000) Pulsed plasma deposition of allylamine on polysiloxane: a stable surface for neuronal cell adhesion. J Neurosci Methods 98(2):135–144
Inagaki N (1996) Plasma surface modification and plasma polymerization. Taylor & Francis, London
Van Deynse A et al (2017) Influence of ethanol vapor addition on the surface modification of polyethylene in a dielectric barrier discharge. Appl Surf Sci 419:847–859
Borcia G, Brown NMD (2007) Hydrophobic coatings on selected polymers in an atmospheric pressure dielectric barrier discharge. J Phys D Appl Phys 40(7):1927–1936
Massines F, Gouda G (1998) A comparison of polypropylene-surface treatment by filamentary, homogeneous and glow discharges in helium at atmospheric pressure. J Phys D Appl Phys 31(24):3411–3420
Sublet A et al (2006) Atmospheric and sub-atmospheric dielectric barrier discharges in helium and nitrogen. Plasma Sources Sci Technol 15(4):627–634
Brandenburg R (2017) Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci Technol 26(5):29
De Geyter N et al (2009) Deposition of polymethyl methacrylate on polypropylene substrates using an atmospheric pressure dielectric barrier discharge. Prog Org Coat 64(2–3):230–237
Morent R et al (2009) Organic-inorganic behaviour of HMDSO films plasma-polymerized at atmospheric pressure. Surf Coat Technol 203(10–11):1366–1372
Trunec D et al (2004) Deposition of thin organosilicon polymer films in atmospheric pressure glow discharge. J Phys D Appl Phys 37(15):2112–2120
Bornholdt S, Wolter M, Kersten H (2010) Characterization of an atmospheric pressure plasma jet for surface modification and thin film deposition. Eur Phys J D 60(3):653–660
Bruggeman P et al (2010) Electronic quenching of OH(A) by water in atmospheric pressure plasmas and its influence on the gas temperature determination by OH(A-X) emission. Plasma Sources Sci Technol 19(1):7
Yasuda H (1981) Glow-discharge polymerization. Macromol Rev Part D J Polym Sci 16:199–293
Morent R et al (2007) Surface modification of non-woven textiles using a dielectric barrier discharge operating in air, helium and argon at medium pressure. Text Res J 77(7):471–488
Morent R, De Geyter N, Leys C (2008) Effects of operating parameters on plasma-induced PET surface treatment. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 266(12–13):3081–3085
Briggs D (1998) Surface analysis of polymers by XPS and static SIMS. Cambridge University Press, Cambridge
Borcia G, Chiper A, Rusu I (2006) Using a He+ N-2 dielectric barrier discharge for the modification of polymer surface properties. Plasma Sources Sci Technol 15(4):849–857
Socrates G (2004) Infrared and raman characteristic group frequencies: tables and charts. Wiley, New York
Mangindaan D et al (2011) Plasma polymerization of amine-containing thin films and the studies on the deposition kinetics. Surf Coat Technol 206(6):1299–1306
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Program (FP/2007-2013)/ERC Grant Agreement No. 335929 (PLASMATS). The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. The data will be made available on request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van Deynse, A., Leys, C., Morent, R. et al. Plasma Polymerization in a Nitrogen/Ethanol Dielectric Barrier Discharge: A Parameter Study. Plasma Chem Plasma Process 39, 1317–1342 (2019). https://doi.org/10.1007/s11090-019-10007-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10007-8