Abstract
Polyethylene glycol (PEG) chains with different lengths were covalently bonded to polypropylene membranes by means of RF plasma polymerisation of acrylic acid (pp-Aac) followed by mono-amino PEG attachment in liquid phase. Two reactor configurations were tested for the plasma deposition of ppAAc in order to obtain high retention of carboxylic groups in the deposited thin films. A best configuration was assessed evaluating the membrane surface modifications by means of water droplet adsorption time and contact angles measurements, attenuated total reflection (ATR) spectroscopy and X-ray photoelectron spectroscopy (XPS) analysis. PEG chains were covalently bonded to the best plasma modified membranes and the resulting anti-fouling properties were evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a growing interest for the creation of surfaces resistant to unspecific protein adsorption for medical devices (catheters, dialysers and vascular grafts) and filtration (food industries, pharmaceutical, water treatment) applications. It has been found, that polyethylene glycol (PEG) surfaces resist to adsorption of plasma proteins and this results in a high interest in attaching PEG to different substrates for the creation of anti-fouling surfaces [1–4]. Many techniques have been used to prepare PEG surfaces for biomaterials applications. The simplest technique is the adsorption of Polyethylene-polypropylene-polyethylene (PEG/PPG/PEG) copolymers onto a hydrophobic substrate [5, 6]. A problem of PEG surfaces prepared by a simple adsorption technique is their tendency of elution or competitive adsorption. Another technique to obtain PEG-surfaces is to apply a stabilising post-treatment to the PEG adsorbed surface (for instance an ion beam treatment) [7].
However, more stable systems have been made by wet chemical covalent bonding of a functional PEG to an activated substrate. For instance a hydroxyl end group of an oligomeric PEG can be reacted by means of an active coupling agent with a corresponding functional group introduced on the surface. Alternatively, the hydroxyl end group requires a derivatisation with an appropriate coupling agent for the attachment to the substrate surface. In some cases both of these approaches can be combined. Desai and Hubbell grafted cyanuric chloride-activated PEG to an amine-derivatised PET surface [2]. Wang and Hsiue prepared polyacrylic acid grafted PE films by surface graft copolymerisation [8]. Covalent immobilisation of bis-amino PEG onto the pp-AAc-grafted PE surface in the presence on a coupling agent was carried out by Gong et al. [9]. PEG has been grafted onto a FEP surface by plasma polymerisation of acetaldehyde and acrolein and reaction with bis- and mono-amino PEG. PEG can also be grafted to a surface via a backbone polymer in which case pendant PEG chains do exist [10]. Methoxy PEG monomethacrylate is commonly used to synthesise these type of PEG surface and gels [11]. Non-fouling films can also be produced in single step processes, via RF plasma polymerisation of volatile, low-molecular weight ethylene oxide (EO) containing monomers, as demonstrated by Wu et al. with diethylene glycol vinyl ether and cyclic ethers [12, 13].
Although it is commonly accepted, that PEG surface show a low degree of protein adsorption, the underlying mechanisms are not fully understood. However, it seems likely, that the performance of the PEG-grafted films would be improved by grafting high-molecular weight PEG with high-surface density [14, 15]. As a prerequisite, high concentrations of the active coupling agent at the substrate surface are needed in order to covalently bond PEG chains with a high-surface density.
In this work, PEGs of different chain lengths were covalently attached to polypropylene (PP) membranes by means of a two step procedure. Plasma polymerisation of acrylic acid (pp-AAc) was carried out on PP membranes and mono-amino PEG attachments were performed in liquid phase in presene of a coupling agent (1-ethyl-3- (3-dimethylaminopropyl) carbodiimide). Surface modifications were investigated by means of contact angle measurement, water droplet absorption time, attenuated total reflection (ATR) spectroscopy and X-ray photoelectron spectroscopy (XPS) analysis. The adsorption of bovine serum albumine (BSA) and fibrinogen on untreated and PEG grafted PP membrane was evaluated in order to test the effect of PEG grafting on the protein adsorption.
Experimental
Materials
The PP membranes with 0.2 μm pore size were supplied by Schleicher and Schuell GmbH Germany and used as delivered. Amino PEG (750 D) and amino PEG (2000 D) were supplied by Rapp-Polymere, Tübingen, Germany. Acrylic acid was supplied by Fluka, München, Germany, and degassed prior to in plasma polymerisation. BSA ≥98%, Mw 67000, was supplied by Fluka. Human fibrinogen, Mw 340000, was supplied by Serva, Heidelberg, Germany.
Plasma Deposition of Acrylic Acid and Grafting of Amino-PEG
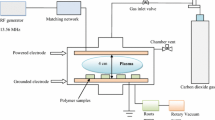
Plasma polymerisation of acrylic acid on PP membranes was performed in a cylindrical, inductively coupled plasma reactor (diameter 5 cm, length 120 cm, Fig. 1). The plasma was ignited using RF energy at 13.56 MHz (Eni Co., Berkshire, UK, model ACG-5, matching network Nye Viking Co., Priest River, ID, USA, model MB-IV-A using symmetric output) connected to two ring electrodes placed around the tubular reactor. A sample holder orients the membranes perpendicular to the ring electrodes and to the gas flow. Such a plasma reactor configuration allows maximal exposure and penetration of the activated gas through the membranes. This is an important requirement for an efficient treatment of such porous materials. Another requirement is the realisation of plasma deposits with high retention of carboxylic groups, in order to immobilise PEG chains with high-surface density. To reach this goal, two reactor configurations were tested. In the first configuration (Fig. 1a) the by-pass, which connects the right and the left side of the reactor, was kept closed and all the gas flow is forced through the pores of the membrane. In this configuration, plasma was switched on in the upstream side of the reactor (right side). In the second configuration (Fig. 1b), the by-pass was maintained open and plasma was ignited on the downstream side of the membrane sample (left side).
Amino-PEG grafting was carried out in water at room temperature in presence of a coupling agent (1-ethyl-3- (3-dimethylaminopropyl) carbodiimide, EDC). Concentration of amino-PEG and of the EDC and reaction time were varied in order to optimise the PEG grafting.
Characterisation Techniques
Horizontal attenuated total reflection (HATR) spectroscopy was performed with a Perkin Elmer, Fulerton, CA, USA, FTIR spectrometer (Spectrum 1000). A 45° ZnSe crystal was used. Spectra were repeatedly scanned 32 times between 4,000 and 650 cm−1 with a resolution of 1 cm−1. Before analysis, a ZnSe crystal was cleaned with ethanol and dried with nitrogen. The sample was pressed with a defined pressure onto the ZnSe crystal to get reproducible measurements.
The chemical composition and binding types were determined by XPS (KRATOS Axis Ultra).
The hydrophilic modification of the treated samples were tested by measuring the water droplet contact angle and the water droplet adsorption time (time needed for a total adsorption of a 20 μl water droplet). Contact angle was measured with the sessile drop method using a goniometer.
In order to test the effect of PEG grafting on the protein adsorption of the PP membranes, the adsorption of BSA and fibrinogen on untreated and PEG grafted PP membranes were evaluated. A solution of 100 ml of 0.2 g l−1 BSA in phosphate buffer saline (PBS, pH 7.4) was passed through the membrane at a flow rate of 50 ml h−1. The membrane was then rinsed with distilled water and analysed by means of XPS spectroscopy. The relative intensity of the nitrogen signal from the peptide bonds of the protein was employed as an indicator for the relative amount of the protein adsorbed onto the membrane. The same procedure was used with 100 ml of 0.2 g l−1 fibrinogen solution in PBS.
Results and Discussion
Plasma Polymerisation of Acrylic Acid and Grafting of Amino-PEG
Acrylic acid was plasma polymerised in presence of hydrogen, according to a procedure described in the literature [16]. After a H2 plasma treatment (5 s), the acrylic acid vapour was added during the desired plasma polymerisation time.
For the plasma polymerisation two reactor configurations were tested (see Experimental section). We prepared a set of samples using the first configuration (with by-pass closed and plasma zone on the upstream side of the reactor) by varying the relevant plasma parameters (power input, total pressure, H2 flux and exposure time). Surface modifications were tested by measuring the corresponding contact angle and water droplet adsorption times. In all experiments no surface modifications due to the plasma treatment could be observed. A water droplet putted on the surface of the membranes evaporates without adsorption. Since pp-AAc with high retention of carboxylic groups should exhibit a significant wetting effect, we conclude that this reactor configuration does not result in a sufficient retention of the monomer structure. The reason of this result could be, that in this configuration the acrylic acid monomers are forced to pass through the plasma zone before reaching the substrate. During the residence time in the plasma zone the monomer can undergo decarboxylation due to the strong fragmentation in the plasma zone resulting in the deposition of an hydrophobic carbohydrate layer.
In the second reactor configuration, the by-pass was maintained open and the plasma zone was shifted to the downstream side of the reactor. With this configuration the monomer is not forced to pass through the plasma zone before reaching the PP membrane surface. With this setup it is possible to retain the carboxylic functionality. Moreover, there is a continuous activation of the PP membrane (also inside the pores) due to the plasma on the left side and diffusion of active species into the porous structure of the membrane. Hence, a formation of radicals on the PP membrane surface are grafting points for the acrylic acid molecules diffusing from the plasma averted side of the membrane towards the plasma side of the membrane
The plasma polymerisation was optimised at first by keeping the total pressure \( {\left( {P_{{\text{t}}} = P_{{{\text{H}}_{{\text{2}}} }} + P_{{{\text{AAc}}}} } \right)} \) constant and equal at 0.059 mbar and varying the H2 flow (and consequently the H2 pressure and the PH2/Pt ratio). Contact angles and water droplet absorption times were measured on untreated sample and on plasma treated samples, priority washed with distilled water to remove adsorbed residual not polymerised AAc. ATR spectra were also recorded on the washed samples in order to confirm the plasma polymerisation and the stability of the deposited layer. The power input was choose very similar to that obtained from analytical calculations following the up-scaling rules described by Sciarratta et al. [16]. For this calculation we started with the optimal power input value, found for pp-Aac in the cylindrical parallel plate reactor used by Sciarratta (22 W). If we calculate the optimal power input for our tubular reactor by keeping the ratio W/V (power input/plasma volume) constant, we obtained 3.5 W. Instead, if we calculate the power input by keeping the ratio W/A (power input/reactor section area) constant we obtained 1.4 W. For our treatments we choosed a power input slightly greater than the calculated values (5 W).
Table 1 and Fig. 2 show the influence of H2 flux on the contact angle of the plasma treated PP membrane (power input = 5 W, treatment time = 300 s). As a consequence of the plasma deposition of acrylic acid, there is a decrease of the water contact angle on the treated samples. The minimum in contact angle was obtained at lower H2 flux (2 sccm), when the ratio between the partial pressure of hydrogen and the total pressure is equal to 0.57. Higher values of the PH2/Pt ratio lead to higher contact angle values, indicating greater fragmentation of the monomer in the plasma phase and hence lower retention of carboxylic groups. The same trend was observed by measuring the absorption time of a 20 μl water droplet put on the PP membrane surface (see Fig. 2).
The best H2 flow and PH2/Pt ratio found with this preliminary study was used for the further preparations. In these experiments the vacuum system of the reactor was throttled in order to keep the H2 pressure constant at 0.05 mbar and the total pressure constant at 0.1 mbar. In this way, we used conditions very similar to that used by Sciarratta in a different reactor [16]. It is very interesting to note that in this way we reach good results of monomer functionalities retention and layer stability.
Figure 3a shows the ATR spectrum of a PP membrane treated with acrylic acid plasma using the best deposition conditions (power input 5 W; treatment time 30 min; H2 flux 2 sccm; H2 partial pressure 0.052 mbar and total pressure 0.1 mbar without plasma). The ATR spectrum was recorded after the stability test.
The typical band characteristics of pp-AAc and the PP membrane without treatment can be demonstrated in this figure. In particular, the typical band of pp-AAc: the O–H stretching (3,700–3,000 cm−1), the C = O stretching (1,715 cm−1), the C–O bending (1,150–1,250 cm−1) are observed.
The unmodified and the plasma treated membrane was analysed by ESCA in order to evaluate the surface modification due to the plasma treatment and to characterise the structure of the deposited layer. The C1s spectra of the unmodified PP membrane shows only the component at 284.6 eV, which can be attributed to C–C and C–H bonds (Fig. 4a). In contrast to this, the spectra of the modified membrane (Fig. 4b) shows different components, that can be attributed to C–C (284.6 eV), C–O–C and C–OH (286.0 eV), C = O (287.3 eV), and O–C = O (288.8 eV) functionalities. The O–C = O peak represents 12.2% of the total carbon (see Table 2), instead of the 33% theoretical value, indicating that there is not total carboxylic group retention.
In a second step, after the optimisation of the pp-AAc, we optimised the liquid phase reaction with amino-PEG. Amino-PEG grafting was carried out in water solution at room temperature in presence of a coupling agent (1-ethyl-3- (3-dimethylaminopropyl) carbodiimide, EDC). Concentration of amino-PEG, of the EDC and reaction time were varied in order to optimise the PEG grafting density. Surface modifications were investigated by measuring the water droplet absorption times and by recording the corresponding ATR-IR spectra of all the samples. The grafting effect was evaluated by comparing the only plasma treated sample with the plasma treated and PEG grafted ones.
We find, that the water droplet absorption time of a 20 μl put on the modified PP membrane remains unchanged after the PEG grafting compared to that measured on the only plasma modified membrane. In case of the sample treated with the best plasma polymerisation and PEG grafting, the droplet absorption time was 3 min.
Figure 3b shows the ATR spectra of PEG 2000 grafted PP membrane. Reaction with amino PEG results in a decrease of the band at 1,710 cm−1 and a shift of this band to lower wave numbers, which is a consequence of the formation of amidic bonds. The PEG grafting is also confirmed by the presence of the band at 1,100 cm−1, which is due to C–O–C functional groups. Reaction times of 4 h are sufficient to reach a good level of the PEG grafting density. A further prolongation of the reaction time results only in a very small further increase of the band at 1,100 cm−1. The influence of the amino PEG concentration on PEG grafting was investigated both for amino PEG 750 and for amino PEG 2000. By increasing the PEG concentration from 3 to 20 g l−1, there is an increase of the PEG grafting, which is confirmed by the increase of the band at 1,100 cm−1. The same trend was observed for the grafting of both PEG 2000 and PEG 750.
The XPS analyses was performed on PP membranes after the pp-Aac and the grafting of amino PEG 750 or amino PEG 2000. In Table 2 and Fig. 4c, d, we find, that the PEG grafting leads to an increase of the C–O–C and N–C = O components of carbon and to the increase of nitrogen (derived from the amidic bond). Figure 5 shows the C–O/C ratios for four sample of PP membrane:
-
Untreated.
-
After the pp-AAc.
-
After the grafting of amino PEG 750.
-
After the grafting of amino PEG 2000.
It can be seen, that the PEG grafting leads to an increase of the ratio C–O/C. This increase is very similar for grafting with amino PEG 750 and grafting with amino PEG 2000.
Evaluation of Protein Adsorption on Modified PP Membrane
Modified and unmodified PP membranes were tested for protein adsorption by filtration of BSA and fibrinogen solutions. After the filtration experiments, the membranes were rinsed with distilled water and analysed by means of XPS spectroscopy.
Figure 6 shows the relative intensity of the nitrogen signal for untreated, only acrylic acid plasma treated, acrylic acid plasma treated and amino PEG 750 grafted and acrylic acid plasma treated and amino PEG 2000 grafted PP 0.2 membrane, before and after BSA filtration. The untreated PP membrane surface does not contain any nitrogen.
As it can be seen in Fig. 6, the acrylic acid plasma treatment leads to an insertion of nitrogen on the PP surface, probably due to air contamination in the plasma chamber. The reaction with amino PEG leads to an increase of the nitrogen atomic percentage, owing to the formation of amide bonds. The atomic percentage of nitrogen after the reaction is 3%. BSA filtration leads to a further increase of the nitrogen percentage only on the unmodified PP surface, due to the protein adsorption. On the unmodified membrane the percentage of nitrogen after BSA filtration reaches 7.8%. On modified PP membranes the atomic percentage of nitrogen does not increase after BSA filtration, but remains constant or slightly decreases. The reason may be, that there is no BSA adsorption on the modified membrane. When BSA filtration is done, it is not possible to distinguish the effect of PEG grafting from the effect of poly acrylic acid deposition. In fact, also the acrylic acid plasma treated sample shows no increase of the nitrogen atomic percentage, hence no BSA adsorption. This albumin adsorption prevention by acrylic acid may be due to electrostatic effect (at neutral pH BSA molecule and acrylic acid modified membrane are negatively charged) [17]. In order to confirm the anti-fouling properties and to verify the effect of the PEG grafting, we test the unmodified and the modified membrane by fibrinogen filtration. Figure 7 shows the relative intensity of the nitrogen signal for untreated, only acrylic acid plasma treated, acrylic acid plasma treated and amino PEG 750 grafted and acrylic acid plasma treated and amino PEG 2000 grafted PP 0.2 membrane, before and after fibrinogen filtration.
For the atomic percentage of nitrogen on membrane before fibrinogen filtration we have to keep in mind the consideration previously written. The acrylic acid plasma treatment leads to an insertion of nitrogen on the PP surface, due to residual air in the plasma chamber. The reaction with amino PEG leads to an increase of the nitrogen atomic percentage, owing to the formation of amide bond. The atomic percentage of nitrogen after the reaction is 3%. On unmodified membranes, the filtration of a 100 ml fibrinogen solution leads to an additional increase of the nitrogen atomic percentage. The increase was higher than for the filtration of BSA, as a consequence of the higher tendency of fibrinogen to adsorb to the membrane surface. The atomic percentage of nitrogen on unmodified PP membrane after the fibrinogen filtration was 13.9%. Plasma deposition of acrylic acid on PP membrane leads to a reduction of the protein adsorption and the atomic percentage of nitrogen on only plasma modified PP membrane after the fibrinogen filtration was 8.8%. PEG grafted membranes show a further decreased protein adsorption. In particular, PEG 750 grafted membrane shows an atomic percentage of nitrogen of 4.6% after fibrinogen filtration. More effective is a grafting of PEG 2000, which shows an atomic percentage of nitrogen of 3.4% after fibrinogen filtration only. Before fibrinogen adsorption, the PEG 750 and PEG 2000 grafted membranes shows an atomic percentage of nitrogen of 2.8 and 2.9%, respectively. We can conclude, that there is a higher reduction of the protein adsorption, particularly for the PEG 2000 grafted membrane. This result is consistent with literature data, where longer PEG chains are resulting in better anti-fouling properties. PEG 2000 modified membrane shows a 95% protein adsorption reduction which respect to the untreated sample. This reduction is comparable with the best results for PEO-like surfaces obtained with single-step or multi-step processes [3, 13].
Conclusions
Surface of PP membranes were modified by pp-AAc in a cylindrical, inductively coupled plasma reactor. Best results for a carboxylic group retention and stability of the plasma deposited layer was obtained with a configuration in which the monomer is not forced to pass through the plasma zone before reaching the PP membrane surface. PEG chains were successfully covalently attached on the plasma modified membranes and the resulting anti-fouling properties were confirmed by BSA and fibrinogen filtration experiments. Pp-AAc modified membranes exhibit a reduced protein adsorption, probably due to electrostatic repulsion between negatively charged deposited layer and protein molecules. The anti-fouling properties increase with PEG grafting. Consistently with literature data, a 95% protein adsorption reduction is obtained with longer PEG chains.
References
Elbert DL, Hubbell JA (1996) Annu Rev Mater Sci 26:365
Desai NP, Hubbell JA (1991) J Biomed Mater Res 25:829
Bergstrom K, Holmberg K, Safranj A, Hoffman AS, Edgell MJ, Kozlowski A, Hovanes BA, Harris JM (1992) J Biomed Mater Res 26:779
Gombotz WR, Guanghui W, Horbett TA, Hoffman AS (1991) J Biomed Mater Res 25:1547
Sheu MS, Hoffman AS, Feijen J (1992) J Adhes Sci Technol 6:995
Green RJ, Davies MC, Roberts CJ, Tendler SJB (1998) J Biomed Mater Res 42:165
Manso-Silvan M, Valsesia A, Gilliland D, Ceccone G, Rossi F (2004) Surf Interface Anal 36:733
Wang CC, Hsiue GH (1993) J Appl Polym Sci 50:1141
Gong X, Dai L, Griesser HJ, Mau AWH (2000) J Polym Sci B: Polym Phys 38:2323
Nagaoka S, Mori Y, Takiuchi H, Yokoata K, Tanzawa H, Nishiumi S (1985) In: Shalaby SW, Hoffman AS, Ratner BD, Horbett TA (eds) Polymers as biomaterials. Plenum Press, New York, pp 361–374
Zou XP, Kang ET, Neoh KG (2002) Surf Coatings Technol 149:119
Wu YJ, Timmons RB, Jen JS, Molock FE (2000) Colloids Surf B: Biointerfaces 18:235
Wu YJ, Griggs AJ, Jen JS, Monolache S, Denes FS, Timmons RB (2001) Plasmas Polym 6:123
Jeon SI, Lee JH, Andrade JD, De Gennes PG (1991) J Colloid Interface Sci 142:149
Jeon SI, Andrade JD (1991) J Colloid Interface Sci 142:159
Sciarratta V, Hegemann D, Müller M, Vohrer U, Oehr C (2003) In: Proceedings of the “transfer of plasma results to different reactors and substrates”, ISPC16 symposium, Taormina, Italien
Zhan J, Liu Z, Wang B, Ding F (2004) Sep Sci Technol 39:2977
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zanini, S., Müller, M., Riccardi, C. et al. Polyethylene Glycol Grafting on Polypropylene Membranes for Anti-fouling Properties. Plasma Chem Plasma Process 27, 446–457 (2007). https://doi.org/10.1007/s11090-007-9094-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-007-9094-y