Abstract
Small-scale thermal treatment of municipal solid waste (MSW) was investigated using mass and energy balances based on the assumption of thermodynamic equilibrium. A typical average MSW composition from the literature was used as basis for modelling of a one ton per day waste gasification facility (plant). Syngas production by pyrolysis, stoichiometric O2 addition and auto-thermal (gasification with oxygen and/or air where no external heat input is required) combustion were considered. These cases were evaluated for production of electricity only, and steam. From purely thermodynamic considerations, it was observed that auto-thermal oxygen gasification produces the most electricity (47.00 kWe) and oxygen plasma gasification produces a positive net amount (4.07 kWe) on a 1 ton per day scale. Although auto-thermal air gasification also produces a net positive amount, the calorific value of the syngas is too low to fuel an internal combustion engine. As expected, the amount of steam generated by the different scenarios is high due to higher process efficiencies. The close-coupled auto-thermal oxygen process proved to be the most efficient. The cost of additional oxygen generation was however not taken into account, and may change the picture significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal solid waste (MSW) as a source of recoverable energy is widely utilised by incineration or auto-thermal gasification (gasification with oxygen and/or air where no external heat input is required). This, together with pre-sorting and recycling, reduces the amount of material consigned to landfill. In addition energy can also be recovered from existing landfills in the form of landfill gas [1].
Thermal waste treatment can be broadly divided into conventional and plasma-assisted processes [2]. Commercial waste treatment facilities vary considerably in size, usually processing between 10,000 and 800,000 t/a MSW [3–5].
The United States, China and Japan are increasingly making use of waste to energy (WtE) in order to get rid of MSW mainly for energy production and land reforming. Every country has its own policies and government incentives, and depending on the economic stance, access to different WtE technologies. Steam and electricity production are the two main products according to Themelis and Mussche [6, 8, 12]. Conventional moving grid and fluidised bed gasification reactors are used. Panepinto et al. [7] evaluated different gasification and pyrolysis processes according to environmental performances and energy efficiency. They concluded that gasification as a technology can be considered as a competitive thermal waste treatment option for MSW.
Small-scale (1–100 t/d) gasification of non-hazardous organic waste is being investigated in South Africa with the aim to reduce land-fill requirements and recover energy. MSW must be sorted and prepared beforehand to obtain a refuse-derived fuel (RDF) of close to uniform composition [2, 8]. The syngas obtained can be tailored according to the intended energy product such as local electricity [11] generation, steam supply, or Fischer–Tropsch synthesis.
In all cases the syngas stream will be quenched to prevent the formation of dibenzo-dioxins and dibenzo-furans and treated to remove other impurities, e.g. fly ash and acidic gases. The ensuing clean syngas stream could be saturated with water vapour after quenching and may need to be dried if required.
This paper presents a theoretical study to define the operating window for a typical small scale gasification process for MSW. A method is developed for a 1 kg/h process by thermodynamic modelling of: pyrolysis; gasification with stoichiometric amounts of oxygen and air; auto-thermal gasification with oxygen and/or air where no external heat input is required.
The following five feed streams were then considered by way of illustration, for a 40 kg/h gasification process:
-
pure oxygen plasma gasification;
-
stoichiometric oxygen/nitrogen plasma gasification;
-
stoichiometric air plasma gasification;
-
auto-thermal oxygen gasification; and
-
auto-thermal air gasification.
Three different application systems were considered:
-
electricity generation by internal combustion (IC) engine. The syngas is quenched and scrubbed beforehand. The process sensible heat is dissipated in the quench, scrubber and cooling tower;
-
steam generation with cleaned syngas in a down-stream boiler. The boiler feed water is pre-heated by partial process heat recovery; and
-
steam generation in a close-coupled boiler followed by flue gas clean-up.
The techno-economic viability is application- and location specific and is not discussed here.
Energy Flow Model
Process Description
MSW is introduced into a gasification reactor operating at 1000–1100 °C. For the purposes of this study, 1050 °C was chosen as an acceptable average. The waste is net oxygen deficient with respect to the carbon content, therefore oxygen is introduced in the form of either O2 or air to ensure complete gasification. Any additional heat requirement is provided either by a non-transfer arc plasma or partial combustion by addition of excess oxygen. Energy can be harvested from the syngas content or the sensible heat of the full product stream.
Energy Balance
The energy balance, Eq. (1), is the sum of the chemical and sensible energy outputs of the process, minus the energy required for processing and losses due to inefficiencies.
Here \(H_{net}\) is the net energy obtainable from the process; \(H_{{c \left( {syngas} \right)}}\) is the heat of combustion of the syngas produced; \(\sum H_{{\eta \left( {combustion} \right)}}\) are losses due to the thermal inefficiency of syngas combustion; \(\sum H_{{sensible \left( {1050 - 25} \right)}}\) represents the sensible (in principle, recoverable) heat of the product gas stream; \(\sum H_{{\eta \left( {heat\;usage} \right)}}\) are heat losses due to inefficiencies of heat utilisation; \(H_{{r\left( {gasification} \right)}}^{{}}\) is the heat of reaction for the gasification; \(H_{{added\;sensible \left( { \to 1050} \right)}}\) is the additional sensible heat required to heat the reactants and products to the processing temperature (1050 °C); and \(\sum H_{{\eta \left( {gasification} \right)}}\) represents the heat losses due to inefficiencies of the gasification process.
For gasification, combustion of the recovered syngas, and recovery of sensible heat, the inefficiencies have to be taken into account. The energy required for processing is the sum of the heat of reaction for gasification, the sensible heat required to raise the temperature of the products to 1050 °C, and losses due to various inefficiencies. The quantity of sensible heat required to raise the temperature to 1050 °C (\({\text{H}}_{{{\text{added}}\;{\text{sensible }}\left( { \to 1050} \right)}}\)) obviously decreases as the energy contributed by combustion increases. The harvestable energy sources consist of chemical energy contained in the product stream (calorific value) and the sensible heat recoverable by cooling the products from 1050 °C.
Model Implementation
Calculation Method
The model was developed using the arithmetic outlined in “Energy Flow Model”. Proximal and ultimate analysis data for typical MSW are essential for the evaluation of each term. This is discussed in “Composition and Energy Content of MSW” below. As mentioned above, the following processing conditions were considered at the 1 kg/h scale:
-
Pyrolysis, where the material is partially gasified and some of the carbon content remains as soot due to the sub-stoichiometric oxygen and moisture content in the feed;
-
Stoichiometric gasification where a stoichiometric amount of oxygen (in the form of O2 or air) is added to convert all carbon to CO. Being a net endothermic process, it requires some form of additional energy source such as a plasma;
-
Auto-thermal gasification (with O2 and air) where enough energy is obtained by partial incineration to sustain the gasification process (without recourse to additional energy sources);
Equilibrium compositions and mass and energy balances were calculated using the commercial software package HSC Chemistry 5.11® (HSC) [9]. Equilibrium compositions were obtained for each scenario, with an MSW heat of formation estimated from the mean dry, ash free (daf) heat of combustion (H c ) of the waste and that of the constituting elements. The mean composition was normalised to a 1 kg/h feed rate. The ash was not taken into account, since its speciation in the feed is unknown. However, it’s sintering and/or slag formation properties will have to be taken into account in a final, complete energy balance.
The operating temperature of 1050 °C ensured complete conversion of the MSW including tars and volatiles to CO and H2 and avoided thermal degradation of the refractory. Equilibrium compositions were calculated over the range of 25–1500 °C. The results showed that in most cases the yield and composition of the syngas become stable at around 1050 °C. This temperature and the resulting gas composition were used to calculate \(H_{{c \left( {syngas} \right)}}\), \(\sum H_{{sensible \left( {1050 - 25} \right)}}\), \(H_{{r\left( {gasification} \right)}}^{{}}\), and \(H_{{added\;sensible \left( { \to 1050} \right)}}\). The process inefficiencies, \(\sum H_{{\eta \left( {combustion} \right)}}\), \(\sum H_{{\eta \left( {heat\;usage} \right)}}\) and \(\sum H_{{\eta \left( {gasification} \right)}}\), are discussed in "Process Inefficiencies" section below.
Composition and Energy Content of MSW
The composition of MSW in South Africa may vary widely depending on the prevailing socio-economic conditions. Assuming that MSW in the metropolitan areas is typical of similar cities elsewhere, data such as those published by the Pacific Northwest National Laboratories (PNNL) [8] can be used for modelling and process design purposes. Similar data for Europe is available from the Dutch Phyllis 2 database [10]. The PNNL mean values for proximate and ultimate analyses are given in Tables 1 and 2, and were used to derive the composition on an as received basis (Table 3, column 7 and 8) for input to equilibrium calculations.
This composition was then normalised, related to the mean moisture and ash content found in Table 1 and the projected ash production calculated (Table 2).
The mean elemental composition in Table 2 was normalised to a 1 kg dry ash free (daf) basis, followed by normalisation to account for the ash- and the moisture contents. Division of the dry ash free (daf) values (X i(daf)), by the total affords the normalised values (X i(n)), expressed as mass fractions, Eq. (2). To express the composition on a dry material basis (ash included), the corresponding mass fractions X i(d) are calculated by Eq. (3) where X a is the mass fraction of ash (0.17) in the dry material presented in Table 3. The element mass fractions X i(m) in the moist feed material as received are calculated using Eq. (4), where X w denotes the mass fraction of water (0.146) in the as-received feed material (Table 3).
The results are summarised in Table 3. Each 1 kg of MSW, as received, including moisture and ash, contains 0.855 kg of matter that could be gasified which is expressed as molar composition (kmol/kg of feed material) required for the equilibrium calculations. The ash was not taken into account, although in a practical process it has to be accounted for in the energy balance, the properties of the solid residue and eventual waste disposal. The element mass fractions in the moist feed material, including ash is denoted as X i(ma).
Equilibrium Composition Calculations
Equilibrium composition for each of the scenarios considered, are described below. For simplification, only the major components, C, H, O and N are considered further.
Pyrolysis
Figure 1 below shows the equilibrium composition diagram for pyrolysis. At temperatures above 1000 °C the composition of the gas phase stays roughly constant—syngas with a high solid char residual. In practice the syngas composition will be strongly influenced by the moisture content of the material up to the point where enough is present to ensure complete gasification.
Stoichiometric Oxygen Addition
In this scenario a stoichiometric amount of oxygen is introduced as oxidizing reagent into the process in the form of O2 or steam to convert all the carbon to CO. The yield of syngas is maximised, as is its calorific value (CV). The choice of oxygen or steam will be determined by the intended use of the syngas—CO-rich gas (oxygen addition) as fuel for internal combustion (IC) gas engines and H2-rich gas (steam addition) for Fischer–Tropsch (F–T) syntheses. The overall process is still endothermic and an additional source of energy has to be supplied. An oxygen or steam plasma would be the optimal solution, but nitrogen or air plasmas may be more economically viable. The H2:CO molar ratio of the syngas can be manipulated by varying the amounts of steam used in the process. Stoichiometric steam gasification (0.018 kg steam per kg MSW) results in a H2-rich syngas stream with a H2:CO ratio of about 1.4:1. Syngas for F–T synthesis should have a H2:CO molar ratio of about 2.1:1. This requires gasification with about 1.44 kg steam per kg MSW. Only oxygen addition will be considered further.
The stoichiometric amount of oxygen required was obtained from the equilibrium composition at 1050 °C for increasing oxygen feed rates (Fig. 2), with the stoichiometric amount defined at the point where maximum CO yield is obtained (0.005 kmol O2/kg MSW).
The equilibrium composition diagram is illustrated in Fig. 3. This shows complete gasification of the carbon content at temperatures above about 1000 °C, yielding syngas with an approximate H2:CO mole ratio of about 1.1:1, suitable to fuel a gas engine or turbine. A lower H2:CO ratio may be achieved by blending or using a different feed material.
Auto-Thermal Processing with O2
Although the classic definition of “auto-thermal” refers to fermentation processes, it is understood here as the situation where the required energy for the process is obtained by partial combustion of the feed stock. Thermal losses excluded, and with only the heat of formation taken into account, this would require 0.015 kmol (0.48 kg) O2 per kg MSW.
The equilibrium composition diagram is shown in Fig. 4. It is clear that the mole ratio of H2:CO in the syngas could be manipulated by changing the operating temperature within the range of 700–1100 °C. The syngas stream now contains appreciable amounts of CO2 and water vapour.
Auto-Thermal Processing with Air
The equilibrium composition diagram is shown in Fig. 5 amounts of CO2, water vapour and nitrogen from air. Nitrogen has a negative effect on the CV of the process gas since it act as a diluent and is present in significant amounts.
Process Inefficiencies
Mass and energy balance calculations included heat of formation, a plasma to supply the energy short-fall, and process-gas composition. Estimated process energy requirements and efficiencies for the plasma torch, generating equipment and steam boiler are taken into account.
Gasification
Processing inefficiencies as listed below have to be considered
-
Thermal efficiency of the plasma torch. The cold cathode non-transfer arc plasma torches routinely used at Necsa have an efficiency of around 65–80% when run on nitrogen. Plasma enthalpy will influence torch efficiency. An enthalpy of 10 MJ/kg is selected to be a reasonable compromise between the need to supply energy to the process with the least dilution of the syngas, cooling water losses and electrode lifetimes. Higher enthalpies will cause the electrode lifetimes to decrease and thereby increasing the running cost. A lower enthalpy will cause dilution of the process gas and thereby decreasing the energy value of the syngas.
-
Choice of plasma gas. As mentioned a pressure swing absorption unit (PSA) and the attendant power requirement will be mandatory for the gas supply. However, it will allow a choice of gas compositions for the plasma, oxidant and instrumentation ranging from pure nitrogen to pure oxygen.
-
Process heat losses. These losses are to cooling water and due to convection from hot surfaces.
-
Gasification efficiency. Good reactor design (geometry, adequate residence time, temperature and flow profiles) can mitigate processing inefficiencies. According to the equilibrium modelling, soot will be eliminated at temperatures above ~850 °C. However, ensuring complete cracking of tars and condensables will require operating temperatures in excess of 1000 °C, with the attendant increase of thermal losses to the reactor walls. Some sensible heat could be recovered before and/or during quenching the product stream.
The choice of processing/plasma gas influences the electrode lifetimes and the required syngas composition and heating value.
Product-Gas Utilization
There are several options for syngas product utilization. This includes synthesis chemistry, steam and/or hot water production, methanol and ethanol production end electricity production. For the purpose of this paper the two simplest and best proven technologies are chosen.
-
Power generation. Gas composition and calorific value are directly dependent on processing conditions and can be tailored, using plasma, to obtain maximum efficiency from internal combustion (IC) engines, gas turbines, and combined cycle set-ups.
-
Steam Generation. Different efficiencies for steam generation could be observed depending on the type of boiler used for example close-coupled or down-stream configuration.
Theoretical Energy Yields
Results
The modelling results for the ideal case, with no inefficiencies taken into account, are summarised in Table 4. Here 1 kg/h “as received” MSW feed, i.e. containing ash and moisture as defined in Table 3, is used as basis. The total amount of matter taking part in gasification, is 0.855 kg/kg, the remainder being ash.
Effect of Oxygen Addition
The two curves presented in Fig. 6 are: the heat of combustion of the syngas obtained from the process, top; and the amount of energy required to run the process, bottom. To the left of point G the process is endothermic, while to its right, it is exothermic. The values are taken from Table 4 above.
Point pair A and E represents pyrolysis, i.e. no oxygen is added; the carbon content is fully converted to CO, but with some solid carbon residue in the equilibrium composition. Point pairs B and F, C and G, and D and H represent stoichiometric addition of oxygen, auto-thermal, and full incineration respectively. Stoichiometric addition implies that the carbon content of the feed has been fully converted to CO, with no solid residue and with no CO2 forming. The auto-thermal point is where the process is self-sustaining, i.e. no additional energy has to be added and the energy of combustion is sufficient for the process to proceed. The carbon here is in the form of both carbon monoxide and carbon dioxide. Full incineration is the point where the carbon content is converted to carbon dioxide, with no excess oxygen in the system.
The area traced by points A, B, C, G, F, and E represents the energy that can in principle be harvested by combustion of the syngas. Here the reaction is endothermic, and the difference between line EFG and the zero line is the energy that has to be supplied by the plasma torch to maintain the process at each gas composition. The area C, D, H, G is the regime where no addition of external energy is required, and where there is CO present in the equilibrium composition, which can in principle be cooled, stored, and used as energy source. Area CDG is the chemical energy extractable in this regime, while GDH is the sensible heat that may be harvested.
These results show that, in principle, there is a large operating window in which transportable energy in the form of syngas may be obtained from gasification. The efficiencies of gas use and gasification will determine whether this is possible in practice, as is discussed in "Application to a Pilot Scale Process" section below. The pyrolysis process will not be discussed further since the aim of this paper is to evaluate a plant that efficiently gasify all of the carbon values contained in the waste feed material.
Application to a Pilot Scale Process
Application Options
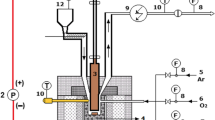
The calculation method outlined in "Model Implementation" was applied to the modelling of a 40 kg/h pilot plant scale gasification process. Simplified process flow diagrams for the respective syngas application systems considered are shown in Fig. 7, 8 and 9. The utilities and auxiliary equipment were not considered in the overall process energy balances.
For electricity generation (Fig. 7), the syngas stream is quenched and scrubbed and fuels an IC generator set. All sensible heat in the gas stream is dissipated in the quench, scrubber, cooling tower and the engine exhaust gas and cooling water. The efficiency of the generator set is assumed to be about 35%.
For steam generation by a down-stream boiler (Fig. 8), a steam boiler is fired with cleaned and cooled syngas. Part of the sensible heat is recovered in a high-temperature heat exchanger at the reactor exit to warm the boiler feed water.
In the case of steam generation by the use of a close-coupled boiler (Fig. 9), the hot syngas from the gasifier is fed directly into a steam boiler which utilises both the sensible and combustion heat. The flue gas is cleaned before releasing it into the atmosphere.
MSW Feed Composition
Equilibrium compositions were calculated using the MSW composition in Table 5 together with the relevant oxidiser requirements from Table 6.
Oxygen addition can vary from 6.4 kg/h (stoichiometric O2 processing) to 29.1 kg/h (auto-thermal processing with air). The nitrogen feed can vary between 0 kg/h (auto-thermal gasification with O2) to 101.9 kg/h (auto-thermal gasification with air). The processing gas feeds for the scenarios under consideration are summarised in Table 6.
Gasification Processes
The oxidiser feed streams as listed in Table 6 were considered.
Gasification with a stoichiometric pure oxygen (6.4 kg/h) plasma will require 97 kWe (63 kWth) and have an enthalpy of 9.85 kWh/kg (35 MJ/kg). This cannot be achieved in the non-transfer arc torches in use at Necsa. Thermal efficiency and electrode lifetimes will also be compromised. Therefore, if an oxygen plasma is to be considered, the plasma gas flow must be increased to lower the enthalpy until the desired enthalpy (2.8 kWh/kg) is achieved. The oxygen contributes combustion energy to the process, and simultaneously decreases the amount of external (plasma) energy required. In this case the amount of oxygen required is twice the stoichiometric amount. In this case CO and H2 are produced in almost equimolar amounts at the reference temperature of 1050 °C, together with some CO2 and water vapour due to partial oxidation of the products.
In the case of a stoichiometric nitrogen–oxygen plasma the plasma gas flow for stoichiometric oxygen gasification (6.4 kg/h) is augmented by addition of N2 to adjust the enthalpy to the required 2.8 kWh/kg. As the results show this plasma gas composition will be very close to that of air. The syngas is slightly hydrogen-rich and yield is improved over the pure oxygen case above. However, it is now diluted with N2.
For a stoichiometric air plasma, (2.8 kWh/kg) the air stream is split between the plasma requirement and an auxiliary feed to the reactor.
For auto-thermal processing with pure O2 addition, enough combustion energy is generated to sustain the process at 1050 °C. No additional external energy input will be required, and in this case \(H_{{added\;sensible \left( { \to 1050} \right)}}\) becomes zero.
For auto-thermal processing with air, the process is sustained at 1050 °C by the partial combustion of the feed with air. \(H_{{added\;sensible \left( { \to 1050} \right)}}\) is once more zero.
Syngas Energy Content
Water is condensed from the syngas stream during quench and scrubbing. The syngas compositions shown in Table 7 are for the dry gas.
The total heat of combustion (H c ) obtainable from the syngas is in favour of the stoichiometric oxygen treatment if the gas is to be used for heating but for fuelling gas engines and turbines a high LCV is beneficial as long as the CO:H2 mol ratio is close to 1. Auto-thermal air gasification is not an option (LCV 2.430 MJ/kg) and one might as well go for full incineration.
There is not much to be gained by using a stoichiometric N2/O2 plasma over using an air plasma.
Energy Balance Results
The energy balance data for the different processing scenarios are summarised in Tables 8, 9 and 10. The terms are defined in Eq. (1).
Power generated electrical power is assumed to be generated in an internal combustion (IC) generator set with an efficiency of 35% (η = 0.35). Exhaust gas sensible energy is not recovered as would be in the case of co-generation.
Net electrical power the net amount of electrical power balance for the gasification process (power generated minus plasma power requirement), plant auxiliary power requirements for pumps, compressors, fans, etc. excluded. Positive values indicate net electrical gain and negative values indicate net electrical power shortfall for the process. A zero value indicates a self-sustaining gasification process.
The summarised overall energy balance data for Tables 8 and 10 are plotted in Fig. 10. The values on the category axis, 1–5, indicate the respective gasification scenarios.
In three of the cases net energy can, in principle be extracted: with a pure oxygen plasma; by auto-thermal combustion with pure oxygen; and by auto-thermal combustion with air. In the case of stoichiometric nitrogen/oxygen and air plasmas, more energy is required than is extractable. In the auto-thermal air gasification case the calorific value of the process gas is 2.430 MJ/kg (Table 7). This is too low for electricity production or steam generation. Thus only two viable options remain: oxygen plasma and auto-thermal combustion with pure oxygen. The auto-thermal combustion with pure oxygen case however requires twice as much oxygen as for a pure oxygen plasma. The production energy associated with this additional amount was not taken into account. In addition no heat recovery was done. This aspect may reduce the net energy produced by the auto-thermal oxygen gasification.
In the case of steam generation (Figs. 11, 12) significantly more energy is extractable as expected. For both cases of steam production and for each of the feed streams a net amount of energy (>60 kW) is available.
Conclusions
An energy balance was used to structure a thermodynamic investigation into the most efficient process and operating conditions for MSW gasification. Electricity generation, close-coupled and down-stream steam generation processes were evaluated. These processes were evaluated according to the following process parameters: 1050 °C; ambient pressure; excess energy needed supplied by plasma technology; and necessary oxidant introduced by pure O2 addition, oxygen and nitrogen, or air addition.
From energy considerations only, and taking process inefficiencies into account, the auto-thermal O2 gasification process for electricity generation was the most efficient, with 47.00 kW net electricity produced. The oxidant cost has, however, not been taken into account. The oxygen plasma process also produced a net positive amount of electricity. These two processes also produced the highest amount of steam. At first glance the auto-thermal air process also looks promising, but the CV of the process syngas is only 2.430 MJ/kg, which is too low for generation of either of the products.
References
Ethekwini Municipality (2016). http://www.kznenergy.org.za/durban-landfill-gas-to-electricity-project/. October 2016
Young GC (2010) Municipal solid waste-to-energy conversion processes, economic technical and renewable comparisons. Wiley, Hoboken
Ramazzini N, Malvezzi S, Zaniboni L (2012) Experience from operating the waste-to-energy facility in Naples. The ISWA world solid waste congress Florence, 17 September 2012
AlterNRG Corporation (2016). http://www.alternrg.com/waste_to_energy/projects/. October 2016
Plasco Energy Group, Trail Road, Ottawa (2016). http://www.plascoenergygroup.com/our-technology/the-plasco-process/. October 2016
Themelis NJ, Mussche C (2013) Municipal solid waste management and waste-to-energy in the United States, China and Japan, Earth Engineering Centre, Columbia University
Panepinto D, Tedesco V, Brizio E, Genon G (2015) Environmental performances and energy efficiency for MSW gasification treatment. Waste Biomass Valor 6:123–135
Valkenburg C, Gerber MA, Walton CW, Jones SB, Thompson BL, Stevens DJ (2008) Municipal solid waste (MSW) to liquid fuels synthesis, volume 1: availability of feedstock and technology. PNNL report no. 18144, December 2008
Roine A (2011) HSC Chemistry 5.11, Outokumpu Research Centre
Energie Centrum Nederland (ECN) (2016) Phyllis 2 database. https://www.ecn.nl/phyllis2/. October 2016
Siemens syngas turbines (2016). http://www.energy.siemens.com/nl/pool/hq/energy-topics/pdfs/en/gas-turbines-power-plants/ModernGasTurbineswithHighFuelFlexibility.pdf. October 2016
Rabou LPLM, van Leijenhorst RJC, Hazewinkel JHO (2008) High-efficiency energy production from biomass and waste. Energie Centrum Nederland (ECN), doc no. ECN-E-08-086
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Walt, I.J., Jansen, A.A. & Crouse, P.L. Plasma-Assisted Treatment of Municipal Solid Waste: A Scenario Analysis. Plasma Chem Plasma Process 37, 763–782 (2017). https://doi.org/10.1007/s11090-017-9786-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9786-x